Abstract
Protein expression of major hepatic uptake and efflux drug transporters in human pediatric (n=69) and adult (n=41) livers was quantified by LC-MS/MS. Transporter protein expression of OCT1, OATP1B3, P-gp and MRP3 was age-dependent. Particularly, significant differences were observed in transporter expression (p <0.05) between the following age-groups: neonates vs. adults (OCT1, OATP1B3, P-gp), neonates or infants vs. adolescents and/or adults (OCT1, OATP1B3 and P-gp), infants vs. children (OATP1B3 and P-gp) and adolescents vs. adults (MRP3). OCT1 showed the largest increase, of almost 5-fold, in protein expression with age. Ontogenic expression of OATP1B1 was confounded by genotype and was revealed only in livers harboring SLCO1B1*1A/*1A. In livers > 1 year, tissues harboring SLCO1B1*14/*1A showed 2.5-fold higher (P<0.05) protein expression than SLCO1B1*15/*1A. Integration of these ontogeny data in physiologically based pharmacokinetic (PBPK) models will be a crucial step in predicting hepatic drug disposition in children.
Keys: Transporter ontogeny, developmental expression of transporters, transporter protein quantification, hepatic transporters, pharmacogenetics, transporter proteomics
INTRODUCTION
Drug transporters are important in drug disposition and response. The International Transporter Consortium (ITC) and regulatory agencies (e.g., FDA, EMEA, PMDA) now recommend assessment of a new drug as a substrate or inhibitor of multiple drug transporters, including organic anion-transporting polypeptide transporters (OATPs; SLCOs), P-glycoprotein (P-gp, MDR1; ABCB1) and breast cancer resistant protein (BCRP or ABCG2) (1–3). Many drugs that are substrates of transporters, such as ondansetron, morphine, methotrexate, valsartan, pravastatin and irinotecan, are administered to children. Age-dependent or ontogeny data for the hepatic and intestinal transporters are limited, and primarily confined to mRNA expression of these transporters. However, the poor correlation between mRNA and ex-vivo protein expression (4) does not allow translation of such data to predict transporter-related differences in drug disposition in children vs. adults. Moreover, most of these human transporter mRNA expression data are limited to fetal livers (5–8). Proteomics quantification of 10 drug transporters (excluding OCT1 and OATP1B3) in human liver is recently reported (9). However, because this study was primarily based on the limited number of fetal and infant samples, the developmental patterns are not conclusive.
Due to the lack of data on the ontogeny of the expression of transporters, it is not feasible to predict transporter-mediated disposition in children based on adult clinical data. Unlike adults, where hepatocytes are available for conducting functional assays for drug transporters, such resources are not routinely available from pediatric donors (10). Moreover, changes in transporter expression in cultured hepatocytes over time are highly dependent on the culture conditions used (11, 12). For example, BSEP and MRP2 protein expression decreases while that of BCRP increases with time in sandwich cultured human hepatocytes (12). A recent whitepaper by the Pediatric Transporter Working Group critically highlighted the knowledge gap with respect to human ontogeny of drug transporters (10). To fill this crucial knowledge gap, we describe in this paper the expression of drug transporters in pediatric and adult liver tissues using LC-MS/MS. In addition, because OATP1B1, BCRP and MRP2 are polymorphic, we also studied the effect of genotype on protein expression of these transporters.
RESULTS
Effect of ontogeny on hepatic uptake transporter protein expression
The expression of OCT1 and OATP1B3 was significantly lower (Table 1, Figs. 1 and 2 and Supplementary Fig. 1S) in neonates or infants compared to adolescents and adults. For example, a 5- and 3-fold difference in the mean OCT1 and OATP1B3 expression was observed between neonates and adults, respectively. OATP1B3 expression was also significantly lower in infants vs. children. When all the samples were considered in the analysis, OATP1B1 expression was found to be independent of age. However, age-dependent OATP1B1 expression became clearly evident in the carriers of the SLCO1B1*1A/*1A reference allele (Fig. 3A). Expression of OATP2B1 and NTCP was not different across age (Figs. 2 and 4 and Supplementary Figs. 1S and 2S). Interestingly, the relative protein expression of Na+K+ATPase was lower (almost 2-fold) in neonates and infants vs. adults (Fig. 2). Sex did not affect protein expression of the uptake drug transporters or Na+K+ATPase. Transporter abundance data in individual livers are presented in Supplementary Figs. 1S and 2S.
Table 1.
Key parameters describing ontogenetic trajectories of transporter proteins that showed significant age-dependent protein expression
| Parameters/ transporters |
OCT1 | OATP1B3 | P-gp | |
|---|---|---|---|---|
| E0 | Mean ± SE | 0.58±0.20 | 0.50±0.08 | 0.15± 0.05 |
| 95% CI | 0.19 to 0.96 | 0.35 to 0.65 | 0.05 to 0.24 | |
| Emax | Mean ± SE | 3.98±0.37 | 1.14±0.11 | 0.41±0.14 |
| 95% CI | 3.23 to 4.72 | 0.92 to 1.36 | 0.14 to 0.69 | |
| Age50 | Mean ± SE | 0.47±0.17 | 0.58±0.66 | 2.94±1.33 |
| 95% CI | 0.10 to 0.90 | −0.20 to 0.33 | −0.16 to 4.8 | |
| h | Mean ± SE | 0.92±0.20 | 4.87±3.10 | 0.78±0.44 |
| 95% CI | 0.44 to 1.40 | −1.34 to 11.08 | −0.10 to 1.66 |
The model used was Sigmoidal Emax model with baseline expression [E = E0+Emax*Ageh/(Age50h + Ageh)], where E is the protein expression; E0 is the transporter expression at birth; Emaxis the maximum protein expression from the baseline, Age50 is the age in years at which 50% of adult expression is observed and h is the Hill constant. The units of E0 and Emax are fmol/µg membrane protein. SE, standard error; CI, confidence interval
Fig. 1.
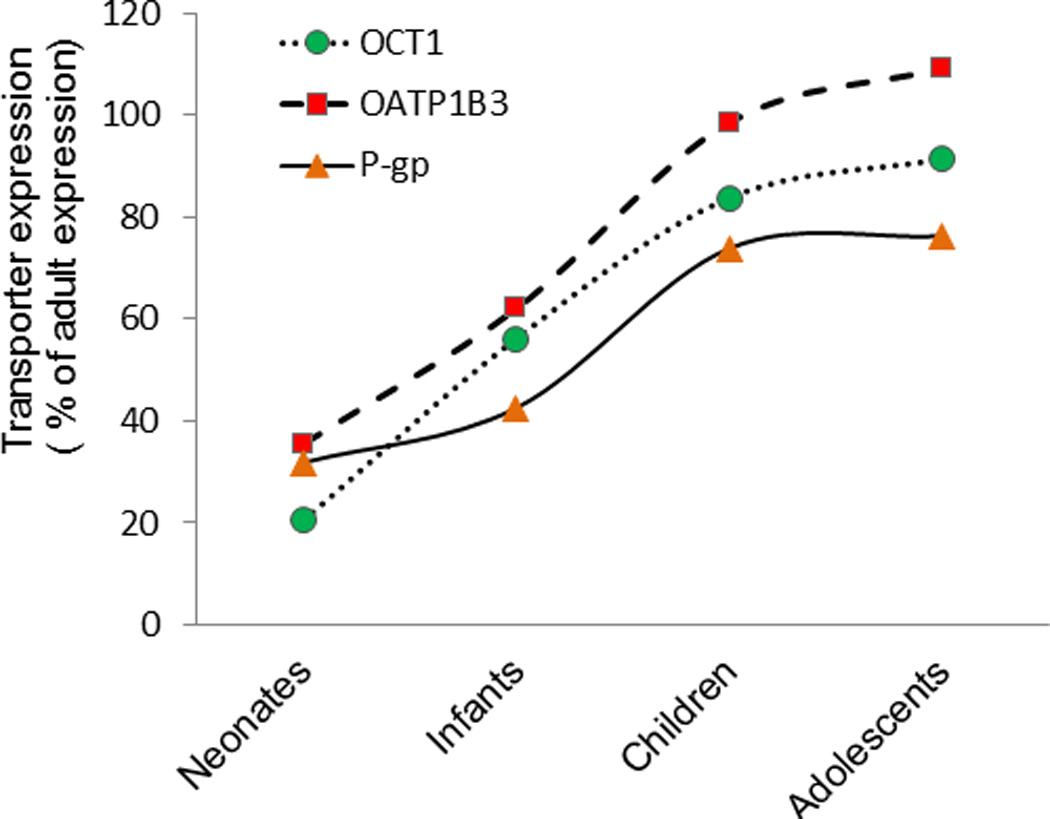
Ontogeny of protein expression of OCT1, OATP1B3 and P-gp in liver tissue from neonates to adults.
Fig. 2.
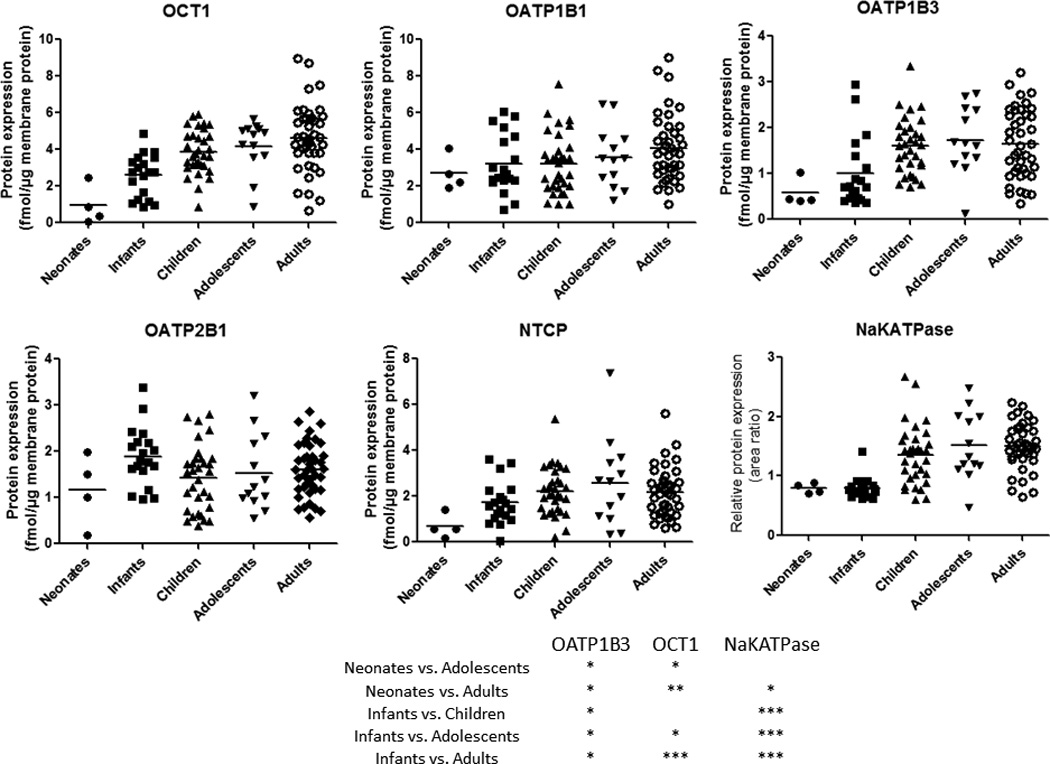
Ontogeny of protein expression of uptake transporters and Na+K+ATPase in liver tissue from neonates to adults. The dots represent the observed values. Horizontal line represents the mean. *, ** and *** indicate p values of <0.05, <0.01 and <0.001, respectively.
Fig. 3.
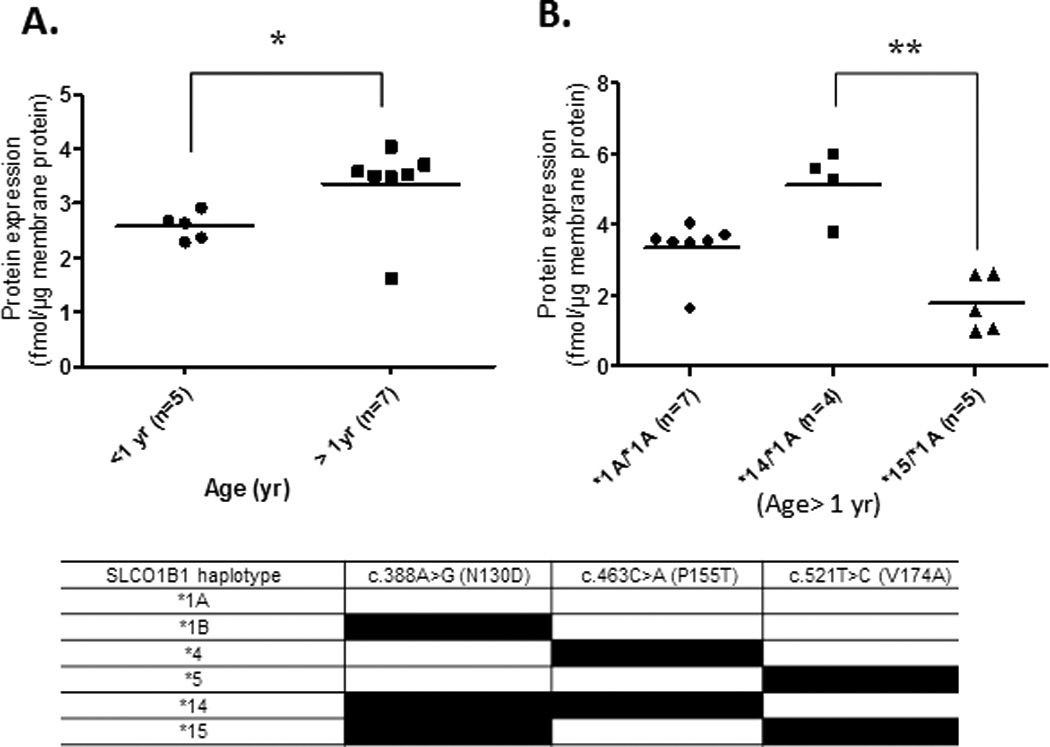
Ontogeny of OATP1B1 protein expression in subjects harboring the SLCO1B1*1A/*1A reference allele (A). Effect of diplotype on OATP1B1 expression in subjects age > 1 year, i.e., where OATP1B1 expression was not age-depedent (B). For this analysis, subjects were classified based on their SLCO1B1 diplotypes which were determined using three previously described key SNPs. Horizontal line represents the mean.* and ** indicate p values of <0.05 and <0.01, respectively.
Fig. 4.
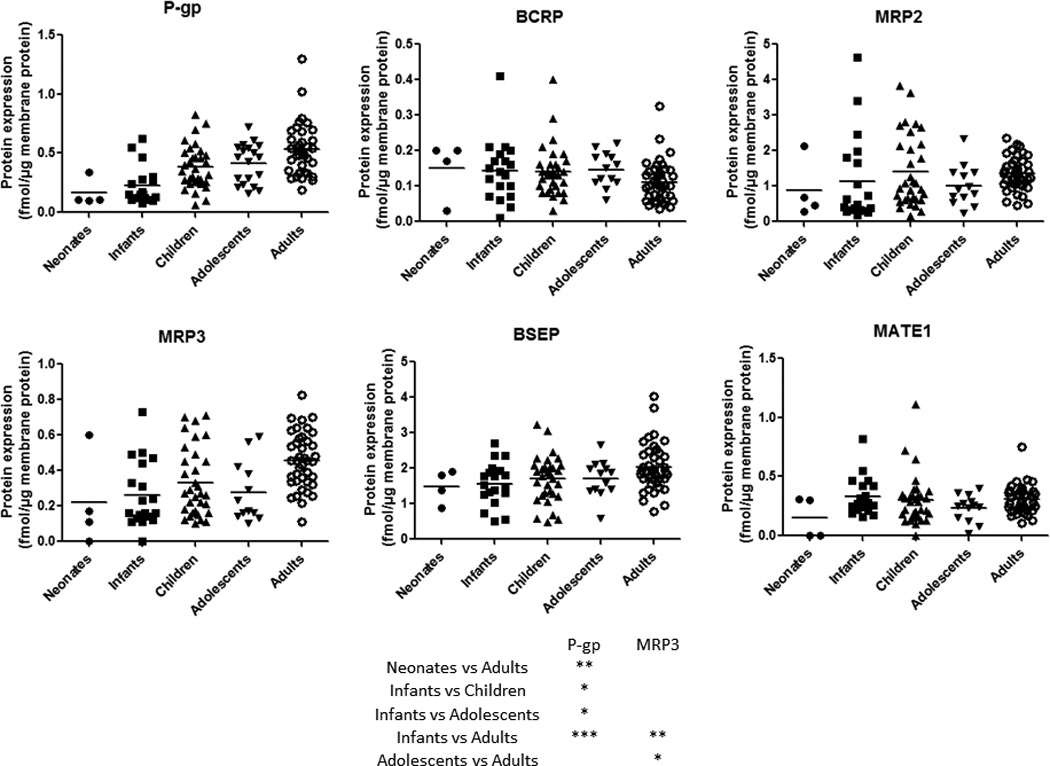
Ontogeny of protein expression of efflux transporters in liver tissue from neonates to adults. The dots represent the observed values. Horizontal line represents the mean. *, ** and *** indicate p values of <0.05, <0.01 and <0.001, respectively.
Because we used a modified methodology of sample preparation, OATP1B1 and OATP1B3 expression values reported in this study are ~1.5-2 fold higher than our previously reported data (13, 14). This modification consisted of an additional step of methanol-chloroform-water extraction of the membrane extraction buffer and inclusion of 1% sodium deoxycholate as solubilizer (15). This difference in OATP1B1 and OATP1B3 protein expression between the two methods was likely due to the interference of trypsin digestion by membrane extraction buffers. Therefore, our expression values presented here are more reliable as the refined method decreased the potential matrix effect by the methanol-chloroform-water desalting procedure.
Effect of ontogeny on hepatic efflux transporter protein expression
Age-dependent expression was observed for P-gp and MRP3 (Fig. 4). For example, P-gp expression was significantly lower in neonates or infants vs. children, adolescents and adults (Figs.1 and 4). Similarly, MRP3 was significantly lower in infants and adolescents when compared to adults. While Age50 could not be estimated with confidence for MRP3 due to significant interindividual variability, P-gp reached 50% of adult expression from baseline at the 2.9 years of age (Table 1). The expression of BCRP, MRP2, BSEP and MATE1 was not affected by age. Similar to uptake transporters, the expression of the efflux transporters was independent of sex.
Effect of genotype on hepatic transporter protein expression in children
We have previously reported the effect of genotype on protein expression of hepatic drug transporters in the adult livers (13–16). Therefore, we determined if genetic polymorphism was a confounding factor in revealing the age-dependent effect on transporter expression of OATP1B1, BCRP and MRP2. The frequency (n) of SLCO1B1, ABCG2 and ABCC2 SNPs detected in the pediatric livers investigated is shown in Table 2. Since the most dramatic developmental changes in transporter expression occurred in neonates and infants, these samples were pooled in one pediatric category (<1 yr age) while children and adolescents were grouped into another pediatric category (>1 yr). This categorization allowed us to achieve power in each genotype category for statistical analysis. Individual SNPs of SLCO1B1 show significant linkage disequilibrium with each other (14). For this reason, our analysis focused only on the diplotypes, *1A, *14 and *15. Interestingly, in carriers of reference allele, SLCO1B1*1A/*1A, we observed a statistically significant age-dependent increase (p <0.05) in OATP1B1 protein expression in children >0 to 1 year of age (Figs. 3A and 3B). However, this effect of age (individuals with age <1 yr vs. >1 yr) was eliminated when all the samples were analyzed without considering the genotype. The sample number was too small to analyze effect of age expression of OATP1B1 in *14/*1A and *15/*1A diplotypes. However, in individuals >1 year of age (a category where age did not affect OATP1B1 expression) the influence of genotype on protein expression was observed, where carriers of SLCO1B1*14/*1A had significantly higher (2.5-fold) protein expression than those with SLCO1B1*15/*1A (Fig. 3B). In this limited number of pediatric samples, BCRP and MRP2 protein expression was not associated with genotype even when neonatal and infant data were excluded (Fig. 5).
Table 2.
Number of livers from carriers of SLCO1B1, ABCG2 and ABCC2 SNPs present in the studied pediatric livers (Age, 0–16 yr)
| Gene SNP ID |
Variant | Protein Amino acid change |
Number of livers (n) | ||
|---|---|---|---|---|---|
| Homozygous variant |
Heterozygous variant |
Wild- type |
|||
| SLCO1B1# | |||||
| rs2306283 | 388A>G (*1A) | N130D | 18 | 22 | 28 |
| rs11045819 | 463C>A (*4) | P155T | 2 | 13 | 54 |
| rs4149056 | 521T>C (*5) | V174A | 2 | 17 | 51 |
| ABCG2 | |||||
| rs2231142 | 421C>A | Q141K | 1 | 11 | 57 |
| ABCC2 | |||||
| rs2273697 | 1249G>A | V417I | 2 | 25 | 42 |
Number of livers harboring haplotype SLCO*1A/*1A, *14/*1A and *15/*1A were 12, 4 and 8, respectively.
Fig. 5.
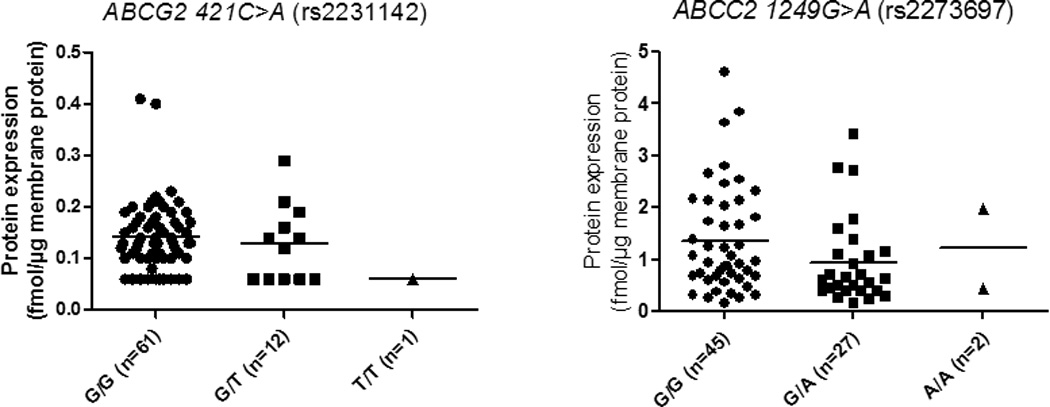
Genotype-dependent protein expression of BCRP and MRP2 in human livers (frequencies (n) in parenthesis). Dots indicate the observed values. Horizontal line represents the mean.
DISCUSSION
We present here for the first time a comprehensive analysis of the ontogeny of protein expression of hepatic transporters in a unique resource of pediatric and adult livers. While the total membrane yield (37.3±11.3 mg/gram of liver tissue, Supplementary Fig. 3S) was consistent across all ages (13, 16), when analyzed in a categorical manner, protein expression of OCT1, OATP1B3, P-gp, and MRP3 was found to be age-dependent (Fig. 2 and 4).
However, when analyzed in a continuous manner (Fig. 1S and 2S), only the expression of OCT1, OATP1B3, P-gp showed distinct ontogeny. This difference is not surprising as the continuous analysis is weighted towards the older age group due the larger number of data points in this category. As a result, although the Sigmoid Emax model (Table 1) best described the trend in the data, the 95% CI for some of the parameters were wide indicating low confidence in their estimates (e.g., Age50 and h). Given the variability in protein expression and that the ontogeny of transporters is most pronounced at the younger age, a larger number of subjects in these groups will be needed to estimate these parameters with greater confidence (Fig 2). Irrespective of the method of analysis, OCT1 expression showed the most dramatic age-dependent change, where approximately 5-fold increase in protein expression was observed between neonates vs. adults. Interestingly we also observed age-dependent expression of NaKATPase, which implies that Na+K+ATPase cannot be used as housekeeping protein. While there are no historical data on age-dependent expression of Na+K+ATPase in human liver, age-dependent expression of Na+K+ATPase has been reported in other tissues in both humans (28) and rodents (29, 30).
When compared with the published human mRNA data (8, 9, 17, 18), our results show similar directional change in OATP1B3 and P-gp protein expression but of smaller magnitude. Similarly, our data are in good agreement with preliminary observations reported in a review article by Klaassen et al., where mRNA data indicated developmental patterns for OCT1, OATP1B1, OATP1B3, P-gp and MRP3. However, our protein expression data do not agree with the ontogenic mRNA expression reported for NTCP, BCRP and MATE1 in individuals 0–4 yr vs. >7 yr (8). This discrepancy could be a result of difference in the mRNA and protein expression patterns or/and difference in the quality and number of samples used. The only published proteomics data do not conclusively show the effect of age on transporter expression due to the limited sample size (9). Interestingly in this study, OCT1 and OATP1B3, which showed developmental patterns in our study, were not studied.
In contrast to human data, considerably more data are available on ontogeny of mRNA expression of selected transporters in rat and mice. In the rat, P-gp expression increases from virtually zero before birth to ~50–60% of the adult level in the first week post birth followed by 1.5–2 times the adult level between the 3rd and 4th week of age, with a subsequent decrease to adult levels at 7th week of age (31). Oct1 mRNA levels in the rat increase from ~30% of the adult level around birth to a plateau at about one to two times the adult level between the 3rd and 4th week of age. Oatp1a4 mRNA expression increases from negligible at day 1 to ~1.5 times the adult level in week 3, followed by a decrease to adult levels at week 7. In mice, Oct1 mRNA levels at 45th day of age were ~6 times higher than the level immediately after birth (32). Both rat and mouse data are generally consistent with our data. Rat Bcrp and Bsep are known to be expressed in high abundance before or at birth and then decrease gradually (31). Although, compared with other ages, we also observed a small decrease in mean BCRP expression in adult human livers (Fig. 4), this did not reach statistical significance.
Consistent with our OCT1 protein expression data, clearance of ondansetron, an OCT1 substrate (19), increases with age in children aged 1–48 months (20). Other substrates of OCT1, such as tramadol (21) and morphine (22, 23), also show age-related changes in hepatic clearance in neonates but this effect can be partly explained by maturation of drug metabolizing enzymes. Consistent with our data, in older children, no change is observed in age-dependent pharmacokinetics of typical OATP and OCT1 substrates such as atorvastatin, pravastatin and metformin (24–26). With respect to ontogeny, P-gp is the best-studied transporter where mRNA expression of P-gp in fetuses, neonates, and infants increases until 1 year of age with no difference between children > 1 year and adults (6, 27). However, clinical studies in younger children, where we found a clear trend, are needed to elucidate the impact of age-dependent expression of OATPs and P-gp in the disposition of drugs that are substrates of these transporters. Clinical data on drugs that are substrates of other transporters are, as yet, not available.
At first sight the protein expression of OATP1B1 appeared to be independent of age, but this was found not to be true when the effect of age on OATP1B1 protein expression was analyzed in only carriers of the reference allele SLCO1B1*1A/*1A. This highlights the importance of genotyping prior to assessing the influence of other factors (e.g. age, disease) on protein expression. Protein expression of all the hepatic transporters was independent of sex.
The diplotypes associated with changes in protein expression in adults have been well characterized by us and others (14, 33). In agreement with the reported data on the effect of genotype on in vivo OATP1B1 activity and expression (34–39), we observed that OATP1B1 protein expression was diplotype-dependent. For example, carriers of the SLCO1B1*14/*1A diplotype had significantly higher OATP1B1 protein levels compared to those with the SLCO1B1*15/*1A diplotype (Fig. 3). While BCRP expression decreases in adults harboring ABCG2 c.421C>A (rs2231142) (13), we did not see this effect in children perhaps due to increased variability in this population. However, we observed that the 50% of donors harboring a variant allele of this SNP showed BCRP expression below the lower limit of quantification (LLOQ), while only 16% of the samples harboring the reference allele showed BCRP expression below LLOQ.
Our study has some limitations. Our pediatric liver samples are autopsy tissues while the majority of our adult liver tissues were obtained from breathing organ donors. Thus, it is possible that the lower expression observed in pediatric livers may be due to degradation of the proteins. For some transporters, the absolute expression of surrogate peptides of hepatic drug transporters in adult livers in our study was lower than that reported by others (40–42). Such inter-laboratory differences could be due to differences in subject demographics or sample characteristics (ethnicity, genotype or quality of tissue), membrane isolation method, sample enrichment method, proteomics method (MRM vs. global) and/or use of different surrogate peptides. However, these inter-laboratory differences do not detract from our conclusions because we controlled for all the above factors by processing and analyzing all the samples in-house using exactly the same protocol. Therefore, the relative changes in the protein expression due to age hold true. While we studied the transporters in pediatric livers that are known to be expressed in adults, pediatric liver may also express other (unknown) transporters poorly expressed in adult livers. We did not observe a significant impact of OCT1 genotype on its expression in our adult livers (15). However, because OCT1 is a highly polymorphic (43), whether OCT1 genotype/haplotype affects its protein expression in pediatric livers can be tested once a larger set of pediatric samples are available. The expression of some transporters (especially BCRP) was below LLOQ in some of the livers. The use of the LLOQ value in analyzing the impact of genetic polymorphism on protein expression, when the peptide for the protein was detectable but not quantifiable, is a conservative approach. That is, such an approach can result in concluding no impact of genetic polymorphism on protein expression even when one exists.
The hepatic transporter protein expression data presented here, together with ontogeny data on drug metabolizing enzymes and PBPK models, will be invaluable to predict age-dependent hepatic clearance of drugs. While impact of other factors such as posttranslational modification (e.g., glycosylation) affecting transporter activity in children are yet to be characterized, the protein expression based PBPK modeling approach could be a first step towards prediction of first-in-children dosing of drugs and the potential for drug-drug interactions.
METHODS
Chemicals and reagents
The ProteoExtract native membrane protein extraction kit was procured from Calbiochem (Temecula, CA). The protein quantification bicinchoninic acid (BCA) assay kit, sequencing grade trypsin, iodoacetamide and dithiothreitol were purchased from Pierce Biotechnology (Rockford, IL). Synthetic surrogate peptide (Supplementary Table 1S) for each protein that were observed to be the most reproducible and sensitive in our previous studies (13–16) were obtained from New England Peptides (Boston, MA), while the corresponding stable isotope labeled internal standards were obtained from Thermo Fisher Scientific (Rockford, IL). Chloroform, HPLC-grade acetonitrile/methanol and formic acid were purchased from Fischer Scientific (Fair Lawn, NJ). Ammonium bicarbonate (98% purity) and sodium deoxycholate (98% purity) were obtained from Thermo Fisher Scientific (Rockford, IL) and MP Biomedicals (Santa Ana, CA), respectively.
Procurement of human liver samples
The pediatric tissues were provided from the liver bank at Children’s Mercy Kansas City (CMKC), and were grouped based on the FDA classification except that the neonates were classified differently: neonates (age 0–28 days, n=4), infants (age 29 days to age ≤1 year (365 days), n=19), children (age >1 to 12 years, n=32) and adolescents (age >12 to 16 years, n=14) (Supplementary Table 2S). These tissue samples were obtained through the Brain and Tissue Bank for Developmental Disorders at the University of Maryland (Baltimore, MD) (n=29) and the Liver Tissue Cell Distribution System (LTCDS) (n=34). Some tissue samples (n=11) were provided by XenoTech LLC (Lenexa, KS). Thirty-six liver tissue samples from the human liver bank of the School of Pharmacy, University of Washington and five adult liver samples from CMKC were used as adult controls (age, >16 yrs). While the adult liver data have been previously reported by us (13–16), adult samples were reprocessed (membrane isolation, trypsin digestion and analysis) in this study to allow direct comparison with the pediatric livers. Subject demographics are provided either in Supplementary Table 2S or published for the UW samples (HL102, HL104, HL105, HL106, HL108, HL109, HL111, HL115, HL118, HL121, HL127, HL128, HL129, HL131, HL133, HL135, HL136, HL144, HL145, HL147, HL148, HL149, HL150, HL153, HL154, HL157, HL158, HL159, HL160, HL162, HL163, HL165, HL166, HL167, HL168 and HL170) (14). Procurement, characteristics and storage of the adult liver samples have been previously described (14). The use of tissues was classified as nonhuman subject research by the Institutional Review Boards of Children’s Mercy Hospital (MO, USA) and the University of Washington (WA, USA).
Membrane protein extraction, trypsin digestion and LC-MS sample preparation
The total membrane protein was isolated from ~100 mg of the liver tissue using ProteoExtract™ native membrane protein extraction kit (Calbiochem, Temecula, CA) as described before (13, 14, 16). Total isolated membrane protein concentration (i.e., the supernatant of second centrifugation step) was determined using the BCA protein assay kit. 2.0 mg/mL (or lower concentration) total membrane protein (100 µL) was denatured, reduced, alkylated as per published protocol (15). Similarly, the denatured/alkylated protein was then desalted and precipitated using methanol:choloform:water precipitation as described before (15). The protein pellet was re-suspended and digested as discussed before (15). The digestion reaction was quenched by 20 µL of labeled peptide internal standard cocktail containing all the heavy peptides listed in Supplementary Table 1S (prepared in 50% acetonitrile in water containing 0.1% formic acid) plus 10 µL of blank solvent (50% acetonitrile in water containing 0.1% formic acid). The samples were centrifuged at 5000 × g for 5 min at 4°C, and 5 µL of the supernatant was injected onto the LC-MS/MS system. The calibrators were prepared by spiking peptide standards into extraction buffer II from the membrane protein extraction kit. The quality control samples were prepared by spiking peptides into extraction buffer II (three concentrations at low, medium, and high level of the calibration curve) or pooled liver membrane (at medium and high level of the calibration curve).
LC and MS method parameters
Drug transporters (OCT1, OATP1B1, OATP1B3, OATP2B1, NTCP, P-gp, BCRP, MRP2, MRP3, BSEP and MATE1) were quantified using previously reported parameters (13–16) using Waters® Xevo TQS tandem mass spectrometer coupled to Waters® Acquity™ UPLC system (Waters, Hertfordshire, UK) with few modifications, mainly in the LC parameters. Briefly, a UPLC column (Acquity UPLC® HSS T3 1.8 µm, 2.1 × 100 mm, Waters) with a Security Guard column (C18, 4 mm × 2.0 mm) from Phenomenex (Torrance, CA) was eluted (0.3 mL/min) with a gradient mobile phase consisting of water and acetonitrile (with 0.1 formic acid; see below). The injection volume was 5 µL (~10 µg of total protein). The mobile phase gradient conditions were 97% A (water containing 0.1% v/v formic acid) and 3% B (acetonitrile containing 0.1% v/v formic acid) held for 3 min, followed by four steps of linear gradient of mobile phase B concentration of 3% to 13%, 13% to 25%, 25% to 50% and 50% to 80% over 3–10 min, 10–20 min, 20–24 min and 24.1–25 min, respectively. This was followed by a washing step using 80% mobile phase B for 0.9 min, and re-equilibration for 4.9 min. The parent to product ion transitions for the analyte peptides and their respective SIL peptides were monitored using optimized LC-MS/MS parameters (Supplementary Table 1S) in ESI positive ionization mode.
DNA Isolation
Genomic DNA (gDNA) was isolated using mini spin kits from various suppliers including the Qiagen AllPrep DNA/RNA Mini Kit and DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA) and Illustra tissue and cells genomic Prep Mini Spin Kit (GE Healthcare, Piscataway, NJ). At the time of isolation, gDNA quality was assessed by agarose gel electrophoresis and DNA concentration determined spectrophotometrically with a NanoDrop instrument Thermo Scientific (Rockford, IL).
Genotyping
We have previously shown correlation of some high frequency SLCO1B1, ABCG2 and ABCC2 SNPs (Table 2) with transporter protein expression in adult liver samples (13, 14, 16). Therefore, the pediatric samples were genotyped for selected SNPs of these three transporters. Sequence variations residing in the SLCO1B1, ABCG2 and ABCC2 transporter genes encoding the OATP1B1, BCRP and MRP2 transporters, respectively, were carried out on gDNA using commercially available TaqMan genotyping assays from Life Technologies (Foster City, CA, USA) and by a high resolution melt (HRM) assay. All assays were performed along DNAs obtained from the Coriell Institute (www.coriell.org) (Supplementary Table 3S) and a no template control (negative control).
TaqMan genotyping assays were carried out in 5µL reactions containing 10–15 ng gDNA, KAPA PROBE FAST qPCR Kit Master Mix (2×) Universal (KAPA Biosystems, Boston, MA) and TaqMan assay primers and probes as recommended. Cycling and detection was performed on a QuantStudio 12K Flex Real-Time PCR System (Life Technologies). Assay IDs and rs numbers are provided in Supplementary Table 3S.
As no TaqMan assay was commercially available, an HRM assay was designed to detect rs11045819 defining the SLCO1B1*4 allele. HRM was performed on an Illumina Eco Real-Time PCR System (Illumina, San Diego, CA) using the KAPA HRM FAST PCR Kit (KAPA Biosciences, Boston, MA) following the manufacturer’s instructions for assay conditions. Six µL reactions contained forward (5'-CAAATTTTATCACTCAATAGAGCATC) and reverse (5'-CTGTCAATATTAATTCTTACCTTTTCC) primers at 0.3 µM, 1× KAPA reaction mix, 2.5 mM MgCl2, and 10 to 20 ng of gDNA. PCR conditions were as following: initial denaturation at 95°C for 3 min, 45 cycles at 95°C for 10 sec (denaturing) and a combined annealing and extension step of 60°C for 20 sec. Subsequently, the 66 bp long PCR products were ‘melted’ over a 55°C to 95°C gradient and fluorescence detected using preset parameters of the Eco Real-Time instrument. Genotypes were assigned by comparing sample curves with control curves. A representative HRM normalization plot is shown in Supplementary Fig. 4S.
Statistical analysis
Non-parametric tests were used to test age- or genotype-dependent expression because the general trend of the distribution pattern in different age-categories was non-symmetric. For individual categories (neonates to adults), age-dependent data analysis was performed using Kruskal Wallis test and Dunn’s multiple comparison test. To compare two groups (e.g., effect of sex and variant allele) we used the non-parametric Mann Whitney test. A sigmoidal Emax model with baseline protein expression (Hill Equation, Table 1) (GraphPad Prism, San Diego, California) was fitted to the continuous ontogenic protein expression data of the transporters exhibiting age-dependent expression. The goodness of model fit was evaluated by visual inspection, 95% confidence intervals (CIs) of the parameter estimates and residual plots. Weighs of 1/Y2 were used. While the model described OCT1, OATP1B3, P-gp and Na+K+ATPase expression data reasonably well, this approach did not show age-dependent effect on the expression of MRP3 (which showed some age-dependency when analyzed in a categorical manner) potentially due to high inter-individual variability of the expression data. We pooled children and adolescent cohorts in SLCO1B1*1A/*1A group into one group to i) increase number of samples in each category and ii) eliminate confounding impact of ontogeny (which was only observed during neonatal age) on the genotype effect. For this analysis, as a conservative approach, samples that were below LLOQ were assigned the LLOQ value.
Supplementary Material
Study Highlights.
What is the current knowledge on the topic?
Data on ontogeny of hepatic transporter protein expression across entire human age range are not available. Data currently available are either in rodents or limited to gene expression of a few human transporters, such as OATP1B1, OATP1B3 and P-gp.
What question did this study address?
Using quantitative LC-MS/MS, this study determined the protein expression of a panel of hepatic transporters (important in hepatobiliary clearance of drugs) in pediatric and adult human livers.
What this study adds to our knowledge?
This manuscript describes, for the first time, the ontogenic changes in protein expression of multiple hepatic uptake and efflux drug transporters. In addition, the effect of SLCO1B1 diplotype on protein expression was determined.
How this might change clinical pharmacology or translational science?
For ethical and practical reasons it is not feasible to determine the disposition of every drug administered to the pediatric population. The protein abundance data presented here will provide the necessary data to predict, through PBPK modeling and simulation, the disposition of drugs in this population.
Acknowledgments
Supported by UWRAPT (University of Washington Research Affiliate Program on Transporters sponsored by Biogen, Genentech and Merck & Co., Inc), P01DA032507 (JDU) and R01HD08129901 from NICHD (BP). The Liver Tissue Cell Distribution System is funded by NIH Contract #N01-DK-7-0004/HHSN267200700004C. The project entitled “Laboratory of Developmental Biology” was supported by a NIH Award (5R24HD0008836) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health. We are also grateful to XenoTech, LLC for their generous gift of tissue fractions.
Footnotes
Conflict of Interest/Disclosure
There is no conflict of interest
Author contributions
J.U., B.P., A.G., M.V., L.S., X.C., G.X., J.S.L., C.H., R.E., and L.G. wrote the manuscript; J.U., B.P., A.G., L.S., X.C., G.X., J.S.L., C.H., R.E., and L.G. designed the research; B.P., A.G., M.V., and R.G. performed the research; J.U., B.P., A.G., M.V., and J.S.L. analyzed the data.
References
- 1.Tweedie D, Polli JW, Berglund EG, Huang SM, Zhang L, Poirier A, et al. Transporter studies in drug development: experience to date and follow-up on decision trees from the International Transporter Consortium. Clin Pharmacol Ther. 2013;94:113–125. doi: 10.1038/clpt.2013.77. [DOI] [PubMed] [Google Scholar]
- 2.Hillgren KM, Keppler D, Zur AA, Giacomini KM, Stieger B, Cass CE, et al. Emerging transporters of clinical importance: an update from the International Transporter Consortium. Clin Pharmacol Ther. 2013;94:52–63. doi: 10.1038/clpt.2013.74. [DOI] [PubMed] [Google Scholar]
- 3.Giacomini KM, Balimane PV, Cho SK, Eadon M, Edeki T, Hillgren KM, et al. International Transporter Consortium commentary on clinically important transporter polymorphisms. Clin Pharmacol Ther. 2013;94:23–26. doi: 10.1038/clpt.2013.12. [DOI] [PubMed] [Google Scholar]
- 4.Maier T, Guell M, Serrano L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009;583:3966–3973. doi: 10.1016/j.febslet.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 5.van Kalken CK, Giaccone G, van der Valk P, Kuiper CM, Hadisaputro MM, Bosma SA, et al. Multidrug resistance gene (P-glycoprotein) expression in the human fetus. Am J Pathol. 1992;141:1063–1072. [PMC free article] [PubMed] [Google Scholar]
- 6.Miki Y, Suzuki T, Tazawa C, Blumberg B, Sasano H. Steroid and xenobiotic receptor (SXR), cytochrome P450 3A4 and multidrug resistance gene 1 in human adult and fetal tissues. Mol Cell Endocrinol. 2005;231:75–85. doi: 10.1016/j.mce.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Fakhoury M, de Beaumais T, Guimiot F, Azougagh S, Elie V, Medard Y, et al. mRNA expression of MDR1 and major metabolising enzymes in human fetal tissues. Drug Metab Pharmacokinet. 2009;24:529–536. doi: 10.2133/dmpk.24.529. [DOI] [PubMed] [Google Scholar]
- 8.Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev. 2010;62:1–96. doi: 10.1124/pr.109.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mooij MG, van de Steeg E, Van Rosmalen J, Windster JD, de Koning BA, Vaes WH, et al. Proteomic analysis of the developmental trajectory of human hepatic membrane transporter proteins in the first three months of life. Drug Metab Dispos. 2016 doi: 10.1124/dmd.115.068577. [DOI] [PubMed] [Google Scholar]
- 10.Brouwer KL, Aleksunes LM, Brandys B, Giacoia GP, Knipp G, Lukacova V, et al. Human ontogeny of drug transporters: Review and recommendations of the pediatric transporter working group. Clin Pharmacol Ther. 2015 doi: 10.1002/cpt.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li N, Singh P, Mandrell KM, Lai Y. Improved extrapolation of hepatobiliary clearance from in vitro sandwich cultured rat hepatocytes through absolute quantification of hepatobiliary transporters. Mol Pharm. 2010;7:630–641. doi: 10.1021/mp9001574. [DOI] [PubMed] [Google Scholar]
- 12.Li N, Bi YA, Duignan DB, Lai Y. Quantitative expression profile of hepatobiliary transporters in sandwich cultured rat and human hepatocytes. Mol Pharm. 2009;6:1180–1189. doi: 10.1021/mp900044x. [DOI] [PubMed] [Google Scholar]
- 13.Prasad B, Lai Y, Lin Y, Unadkat JD. Interindividual variability in the hepatic expression of the human breast cancer resistance protein (BCRP/ABCG2): effect of age, sex, and genotype. J Pharm Sci. 2013;102:787–793. doi: 10.1002/jps.23436. [DOI] [PubMed] [Google Scholar]
- 14.Prasad B, Evers R, Gupta A, Hop CE, Salphati L, Shukla S, et al. Interindividual variability in hepatic organic anion-transporting polypeptides and P-glycoprotein (ABCB1) protein expression: quantification by liquid chromatography tandem mass spectroscopy and influence of genotype, age, and sex. Drug Metab Dispos. 2014;42:78–88. doi: 10.1124/dmd.113.053819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Prasad B, Salphati L, Chu X, Gupta A, Hop CE, et al. Interspecies variability in expression of hepatobiliary transporters across human, dog, monkey, and rat as determined by quantitative proteomics. Drug Metab Dispos. 2015;43:367–374. doi: 10.1124/dmd.114.061580. [DOI] [PubMed] [Google Scholar]
- 16.Deo AK, Prasad B, Balogh L, Lai Y, Unadkat JD. Interindividual variability in hepatic expression of the multidrug resistance-associated protein 2 (MRP2/ABCC2): quantification by liquid chromatography/tandem mass spectrometry. Drug Metab Dispos. 2012;40:852–855. doi: 10.1124/dmd.111.043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mooij MG, Schwarz UI, de Koning BA, Leeder JS, Gaedigk R, Samsom JN, et al. Ontogeny of human hepatic and intestinal transporter gene expression during childhood: age matters. Drug Metab Dispos. 2014;42:1268–1274. doi: 10.1124/dmd.114.056929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgess KS, Philips S, Benson EA, Desta Z, Gaedigk A, Gaedigk R, et al. Age-related changes in microRNA expression and pharmacogenes in human liver. Clin Pharmacol Ther. 2015 doi: 10.1002/cpt.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzvetkov MV, Saadatmand AR, Bokelmann K, Meineke I, Kaiser R, Brockmoller J. Effects of OCT1 polymorphisms on the cellular uptake, plasma concentrations and efficacy of the 5-HT(3) antagonists tropisetron and ondansetron. Pharmacogenomics J. 2012;12:22–29. doi: 10.1038/tpj.2010.75. [DOI] [PubMed] [Google Scholar]
- 20.Li Q, Guo D, Dong Z, Zhang W, Zhang L, Huang SM, et al. Ondansetron can enhance cisplatin-induced nephrotoxicity via inhibition of multiple toxin and extrusion proteins (MATEs) Toxicol Appl Pharmacol. 2013;273:100–109. doi: 10.1016/j.taap.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allegaert K, Anderson BJ, Verbesselt R, Debeer A, de Hoon J, Devlieger H, et al. Tramadol disposition in the very young: an attempt to assess in vivo cytochrome P-450 2D6 activity. Br J Anaesth. 2005;95:231–239. doi: 10.1093/bja/aei170. [DOI] [PubMed] [Google Scholar]
- 22.Knibbe CA, Krekels EH, van den Anker JN, DeJongh J, Santen GW, van Dijk M, et al. Morphine glucuronidation in preterm neonates, infants and children younger than 3 years. Clin Pharmacokinet. 2009;48:371–385. doi: 10.2165/00003088-200948060-00003. [DOI] [PubMed] [Google Scholar]
- 23.Krekels EH, Tibboel D, de Wildt SN, Ceelie I, Dahan A, van Dijk M, et al. Evidence-based morphine dosing for postoperative neonates and infants. Clin Pharmacokinet. 2014;53:553–563. doi: 10.1007/s40262-014-0135-4. [DOI] [PubMed] [Google Scholar]
- 24.Hedman M, Neuvonen PJ, Neuvonen M, Antikainen M. Pharmacokinetics and pharmacodynamics of pravastatin in children with familial hypercholesterolemia. Clin Pharmacol Ther. 2003;74:178–185. doi: 10.1016/S0009-9236(03)00153-X. [DOI] [PubMed] [Google Scholar]
- 25.Knebel W, Gastonguay MR, Malhotra B, El-Tahtawy A, Jen F, Gandelman K. Population pharmacokinetics of atorvastatin and its active metabolites in children and adolescents with heterozygous familial hypercholesterolemia: selective use of informative prior distributions from adults. J Clin Pharmacol. 2013;53:505–516. doi: 10.1002/jcph.66. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Infantes D, Diaz M, Lopez-Bermejo A, Marcos MV, de Zegher F, Ibanez L. Pharmacokinetics of metformin in girls aged 9 years. Clin Pharmacokinet. 2011;50:735–738. doi: 10.2165/11593970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Mooij MG, Nies AT, Knibbe CA, Schaeffeler E, Tibboel D, Schwab M, et al. Development of Human Membrane Transporters: Drug Disposition and Pharmacogenetics. Clin Pharmacokinet. 2015 doi: 10.1007/s40262-015-0328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kjeldsen K, Gron P. Age-dependent change in myocardial cardiac glycoside receptor (Na,K-pump) concentration in children. J Cardiovasc Pharmacol. 1990;15:332–337. doi: 10.1097/00005344-199002000-00022. [DOI] [PubMed] [Google Scholar]
- 29.Aperia A, Larsson L. Induced development of proximal tubular NaKATPase, basolateral cell membranes and fluid reabsorption. Acta Physiol Scand. 1984;121:133–141. doi: 10.1111/j.1748-1716.1984.tb07439.x. [DOI] [PubMed] [Google Scholar]
- 30.Banerjee B, Chaudhury S. Thyroidal regulation of different isoforms of NaKATPase in glial cells of developing rat brain. Life Sci. 2001;69:2409–2417. doi: 10.1016/s0024-3205(01)01314-5. [DOI] [PubMed] [Google Scholar]
- 31.de Zwart L, Scholten M, Monbaliu JG, Annaert PP, Van Houdt JM, Van den Wyngaert I, et al. The ontogeny of drug metabolizing enzymes and transporters in the rat. Reprod Toxicol. 2008;26:220–230. doi: 10.1016/j.reprotox.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Alnouti Y, Petrick JS, Klaassen CD. Tissue distribution and ontogeny of organic cation transporters in mice. Drug Metab Dispos. 2006;34:477–482. doi: 10.1124/dmd.105.006932. [DOI] [PubMed] [Google Scholar]
- 33.Nies AT, Niemi M, Burk O, Winter S, Zanger UM, Stieger B, et al. Genetics is a major determinant of expression of the human hepatic uptake transporter OATP1B1, but not of OATP1B3 and OATP2B1. Genome Med. 2013;5:1. doi: 10.1186/gm405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mwinyi J, Johne A, Bauer S, Roots I, Gerloff T. Evidence for inverse effects of OATP-C (SLC21A6) 5 and 1b haplotypes on pravastatin kinetics. Clin Pharmacol Ther. 2004;75:415–421. doi: 10.1016/j.clpt.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 35.Niemi M, Schaeffeler E, Lang T, Fromm MF, Neuvonen M, Kyrklund C, et al. High plasma pravastatin concentrations are associated with single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide-C (OATP-C, SLCO1B1) Pharmacogenetics. 2004;14:429–440. doi: 10.1097/01.fpc.0000114750.08559.32. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigues AC, Perin PM, Purim SG, Silbiger VN, Genvigir FD, Willrich MA, et al. Pharmacogenetics of OATP Transporters Reveals That SLCO1B1 c.388A>G Variant Is Determinant of Increased Atorvastatin Response. Int J Mol Sci. 2011;12:5815–5827. doi: 10.3390/ijms12095815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sortica VA, Fiegenbaum M, Lima LO, Van der Sand CR, Van der Sand LC, Ferreira ME, et al. SLCO1B1 gene variability influences lipid-lowering efficacy on simvastatin therapy in Southern Brazilians. Clin Chem Lab Med. 2012;50:441–448. doi: 10.1515/cclm.2011.804. [DOI] [PubMed] [Google Scholar]
- 38.Voora D, Shah SH, Spasojevic I, Ali S, Reed CR, Salisbury BA, et al. The SLCO1B1*5 genetic variant is associated with statin-induced side effects. J Am Coll Cardiol. 2009;54:1609–1616. doi: 10.1016/j.jacc.2009.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schipani A, Egan D, Dickinson L, Davies G, Boffito M, Youle M, et al. Estimation of the effect of SLCO1B1 polymorphisms on lopinavir plasma concentration in HIV-infected adults. Antivir Ther. 2012;17:861–868. doi: 10.3851/IMP2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vildhede A, Wisniewski JR, Noren A, Karlgren M, Artursson P. Comparative Proteomic Analysis of Human Liver Tissue and Isolated Hepatocytes with a Focus on Proteins Determining Drug Exposure. J Proteome Res. 14:3305–3314. doi: 10.1021/acs.jproteome.5b00334. [DOI] [PubMed] [Google Scholar]
- 41.Schaefer O, Ohtsuki S, Kawakami H, Inoue T, Liehner S, Saito A, et al. Absolute quantification and differential expression of drug transporters, cytochrome P450 enzymes, and UDP-glucuronosyltransferases in cultured primary human hepatocytes. Drug Metab Dispos. 2011;40:93–103. doi: 10.1124/dmd.111.042275. [DOI] [PubMed] [Google Scholar]
- 42.Sakamoto A, Matsumaru T, Ishiguro N, Schaefer O, Ohtsuki S, Inoue T, et al. Reliability and robustness of simultaneous absolute quantification of drug transporters, cytochrome P450 enzymes, and Udp-glucuronosyltransferases in human liver tissue by multiplexed MRM/selected reaction monitoring mode tandem mass spectrometry with nano-liquid chromatography. J Pharm Sci. 100:4037–4043. doi: 10.1002/jps.22591. [DOI] [PubMed] [Google Scholar]
- 43.Chen L, Takizawa M, Chen E, Schlessinger A, Segenthelar J, Choi JH, et al. Genetic polymorphisms in organic cation transporter 1 (OCT1) in Chinese and Japanese populations exhibit altered function. J Pharmacol Exp Ther. 2010;335:42–50. doi: 10.1124/jpet.110.170159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


