Abstract
Apolipoprotein E (apoE), involved in cholesterol and lipid metabolism, also influences cognitive function and injury repair. In humans, apoE is expressed in three isoforms. E4 is a risk factor for age-related cognitive decline and Alzheimer’s disease, particularly in women. E4 might also be a risk factor for developing behavioral and cognitive changes following 56Fe irradiation, a component of the space environment astronauts are exposed to during missions. These changes might be related to enhanced generation of reactive oxygen species (ROS). In this study, we compared the behavioral and cognitive performance of sham-irradiated and irradiated wild-type (WT) mice and mice expressing the human E3 or E4 isoforms, and assessed the generation of ROS in hippocampal slices from these mice. E4 mice had greater anxiety-like and conditioned fear behaviors than WT mice, and these genotype differences were associated with greater levels of ROS in E4 than WT mice. The greater generation of ROS in the hippocampus of E4 than WT mice might contribute to their higher anxiety levels and enhanced fear conditioning. In E4, but not wild-type, mice, PMA-treated hippocampal slices showed more DHE oxidation in sham-irradiated than irradiated mice and hippocampal HO-1 levels were higher in irradiated than sham-irradiated E4 mice.
Keywords: anxiety, cognition, superoxide, DHE, hippocampus, irradiation
Graphical Abstract
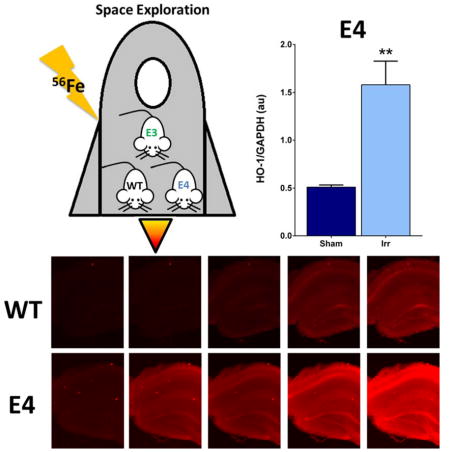
Mice with apolipoprotein E4 (E4), a risk factor for Alzheimer’s disease, have greater anxiety-like and conditioned fear behaviors than wild-type (WT) mice. Generation of reactive oxygen species (ROS, in red) three months following 56Fe irradiation, a component of the space environment astronauts are exposed to, is more pronounced in the hippocampus of E4 than WT mice. In E4, but not WT, mice, hippocampal levels of the oxidative stress-relevant marker heme oxygenase-1 are higher in irradiated than sham-irradiated E4 mice.
Introduction
Among the major human isoforms of apolipoprotein E (apoE) involved in cholesterol and lipid metabolism, apoE4 (E4) is a risk factor to develop age-related cognitive decline and Alzheimer’s disease, particularly in women (Farrer et al. 1997, Raber et al. 2004, Spinney 2014). Effects of cranial 56Fe irradiation (3 Gy, 600 MeV) on hippocampus-dependent cognition in C57BL/6J WT mice three months following irradiation (Villasana et al. 2010) and in human apoE mice thirteen months following irradiation (Villasana et al. 2011a) are sex-dependent, with female mice being more susceptible to radiation-induced cognitive impairments than male mice. The increased risk of cognitive decline in E4 carriers might be related to a reduced ability to protect against reactive oxygen species (ROS)-related lipid peroxidation (Pedersen et al. 2000) and may involve genotype differences in brain levels of ROS. Although acute increases in ROS may be important for learning and memory (Kishida et al. 2005a, Kishida & Klann 2007, Hidalgo et al. 2007a), a prolonged increase in ROS can lead to injury through oxidation of cellular components such as lipids, proteins and DNA (Knapp & Klann 2002a). The brain is particularly vulnerable to ROS damage, as it has a high oxygen consumption but relatively low levels of antioxidants.
Space radiation exposure, including 56Fe exposure, poses a significant risk to the central nervous system. In rodents, 56Fe irradiation might affect hippocampus-dependent cognitive function (Haley et al. 2012a, Villasana et al. 2010, Rola et al. 2004, Rabin et al. 2004, Rabin et al. 2002, Rabin et al. 2009, Haley et al. 2013, Yeiser et al. 2013). ROS are altered by irradiation (Manda et al. 2008, Mizumatsu et al. 2003, Monje et al. 2003, Limoli et al. 2007, Tseng et al. 2013) and, in this way, affect brain function as well.
Most in vivo irradiation rodent studies have focused on oxidative damage (Raber et al. 2011, Suman et al. 2013), levels of antioxidant enzymes, mice lacking or overexpressing a particular antioxidant enzyme (Rola et al. 2007, Raber et al. 2011), or compounds scavenging ROS (Manda et al. 2007, Manda et al. 2008, Villasana et al. 2013). Although ROS levels have been studied in both the context of aging (Driver et al. 2000) and of brain function thirty days after radiation exposure that occurred between 24 and 48 hours after birth (Caceres et al. 2010), less is known about the levels of ROS generated in the tissues of mice long after irradiation. Dihydroethidium is a fluorescent dye that is oxidized by superoxide into the stable dihydroxy ethidium (DHE). Based on its ability to detect intracellular and extracellular superoxide, DHE is used for in vitro and in vivo analysis of superoxide (Peshavariya et al. 2007, Hall et al. 2012), including hippocampal slices of mice (Haley et al. 2012a) and rats (D’agostino et al. 2007).
Heme oxygenase-1 (HO-1) catalyzes the degradation of heme to generate carbon monoxide, biliverdin and free iron (Dwyer et al. 1992, Vincent et al. 1994). Increased HO-1 levels are seen in many neurological conditions associated with increased oxidative stress and inflammation, as well as following X-Ray irradiation (Rugo & Schiestl 2004). While elevated HO-1 levels can restore redox homeostasis and reduce inflammation (Bergeron et al. 1997, Ewing et al. 1992, Jernigan et al. 2001, Lee et al. 2011, Ndisang & Jadhav 2009, Zhang et al. 2004), excessive heme degradation may result in toxic levels of CO, bilirubin and iron (Cuadrado & Rojo 2008). Based on these results, alterations in DHE oxidation might be related to changes in HO-1 levels.
In the current study, we compared behavioral and cognitive performance of sham-irradiated and 56Fe-irradiated wild-type (WT), E3, and E4 mice and analyzed whether behavioral and cognitive changes are associated with changes in hippocampal superoxide and HO-1 levels.
Material and Methods
Animals and irradiation
Human E3 and E4 targeted replacement mice, generated by Dr. Patrick Sullivan and bred in our colony, were used for the present study. C57BL/6J (WT) mice were purchased from Jackson Laboratories and also bred in our colony for the present study. Two-month-old E4 (sham-irradiated: n = 10 mice; irradiated: n = 14 mice), E3 (sham-irradiated: n = 4; irradiated: n = 6), and WT (sham-irradiated: n = 16 mice; irradiated: n = 16 mice) female mice bred in our mouse colony at OHSU were shipped to Brookhaven National Laboratories (BNL) in Upton, NY for cranial 56Fe irradiation (3 Gy, 600 MeV/n) or sham-irradiation. Following acclimatization for 1 week at the animal facility, the mice were transferred to the NASA Space Radiation Laboratory (NSRL) on the day of the sham-irradiation or 56Fe-irradiation and weighed. All mice received i.p. anesthesia, (ketamine (Sigma), 80 mg/kg and xylazine (Sigma), 20 mg/kg), and ophthalmic solution was placed on the eyes for protection. The mice designated for irradiation were placed in positional cradles to stabilize the head during irradiation. Sham-irradiated mice received the same procedure except that they were not irradiated. Upon recovery from anesthesia, the mice were returned to the animal facility. One week after irradiation, the mice were shipped to Oregon Health and Science University (OHSU) for behavioral and cognitive testing starting three months after irradiation. Four months after irradiation, the mice were weighed again. At BNL and prior to behavioral testing at OHSU, the mice were housed under a constant 12 hr light: 12 hr dark cycle. Food (PicoLab Rodent Diet 20, no. 5053; PMI Nutrition International, St. Louis, MO), and water were provided ad libitum. All procedures were approved by Institutional Animal Care and Use Committee at OHSU and BNL.
Behavioral and cognitive testing
Three months following sham- or 56Fe irradiation, mice were behaviorally and cognitively tested. They were first tested in the open field, light-dark, elevated zero maze, and elevated plus maze (on successive days in week 1); subsequently tested for hippocampus-dependent spatial learning and memory in the water maze (week 2); and finally tested for hippocampus-dependent contextual fear memory and hippocampus-independent cued fear memory (week 3) (detailed methods are described below). The experimenter testing the mice was blinded to the treatment of the mice. All tests with the exception of water maze were conducted in the morning. Water maze test sessions were conducted in the morning and early afternoon (beginning at approximately 8:00 am and 1 pm, respectively).
Open field
The open field was used to evaluate measures of anxiety and locomotor behavior. Mice were placed in a 40.64cm × 40.64 cm brightly lit (luminescence: 200 lux) open arena equipped with infrared photocells interfaced with a computer (Kinder Scientific, Poway, CA). Active times (single beam breaks within 1 second) and distance moved were recorded for a single 10-minute session. In the open-field, the center zone (20.3 × 20.3 cm) is more anxiety-provoking than the peripheral zone; therefore, mice that are more anxious in the open field spend less time in the center (Choleris et al. 2001, Clement et al. 2002). Total distance moved was used as an index of exploratory behavior and percent of time spent in the center of the open field as a measure of anxiety.
Light-dark
The light-dark test was also used to assess anxiety levels. In the light-dark test, mice were placed in the open field enclosure (described above) containing black plastic inserts that covered the sides and the top fifty percent of the open field (Hamilton-Kinder, Poway, CA). A single opening in the wall of the insert adjacent to the open area allowed the mice to enter or exit the more anxiety-provoking light area of the maze (luminescence: 200 lux). Active times and distance moved were recorded for a single 10-minute session. Breaks in the photo beams were used to calculate path length, active times, and rest time in the open and closed compartments of the enclosure. Mice with increased measure of anxiety spend less time in the light side of the enclosure (Bhatnagar et al. 2004).
Elevated zero maze
The elevated zero maze was also used to assess measures of anxiety and exploratory behavior. The custom built elevated zero maze (Kinder Scientific) consisted of two enclosed areas with two adjacent open areas. Mice were placed in the closed part of the maze and allowed free access for 10 minutes (luminescence: 200 lux). Mice could spend their time either in the closed or open area of the maze. A video tracking system (Noldus Information Technology, Sterling, VA, set at six samples/second) was used to calculate the time spent in the open areas and distance moved throughout the maze. Mice that are more anxious in the elevated zero maze spend less time in the open areas (Shepherd et al. 1994). Outcome measures included the percent of time spent in the open areas and distance moved.
Water maze
The water maze test was used to assess spatial learning and memory (Morris 1984). A circular pool (140 cm diameter) was filled with water (22°C ± 2°C). The water was made opaque with white chalk in order to hide the platform. The platform (20 cm wide) was located approximately 1 cm below the water level. On the first two days of water maze testing, the mice were trained to locate a visible platform (flagged with a visible beacon). There were three trials per session (5-minute inter-trial interval (ITI) and two sessions (two hours apart) per day. The platform was moved to a new quadrant for each of the four visible platform sessions. Trials ended when the mice reached the platform and remained on it for 3 seconds or when 60 seconds elapsed. In trials in which the mice did not find the platform, they were guided to the platform and allowed to remain on it for 3 seconds. Upon removal from the maze, the mice were dried with absorbent towels and returned to their home cages.
After visible platform training, the mice were trained to locate a hidden platform in three trials per session (5-minute ITI) and two sessions (two hours apart) per day. The location of the hidden platform remained constant although the drop location varied for each trial. Mice were allowed to remain on the platform for 3 seconds before they were removed from the pool. Performance measures for visible and hidden platform training included swim speeds and cumulative distance to the platform. Cumulative distance to the platform measures how far the mice are located from the platform over the duration of the trial. The lower the cumulative distance, the better the performance. Thigmotaxis, defined as the percent of time spent in the outer 20 cm perimeter of the pool, was analyzed as an anxiety measure in the water maze.
Probe trials (platform removed) were conducted exactly 1 hour after the last trial of each day of hidden platform training in order to assess spatial memory retention. Percent of time spent in the target quadrant (learned location of the platform during prior hidden platform trials) and cumulative distance to the platform location were used as measures of spatial memory retention. The swimming patterns of the mice were analyzed using the Ethovision video tracking system set at 6 samples/sec.
Contextual and cued conditioned fear
Conditioned fear was used to assess hippocampus-and amygdala-dependent associative memory. In this task, mice learn to associate the environmental context (fear conditioning chamber) or cue (tone) with a mild foot shock (unconditioned stimulus, US). When mice are re-exposed to the context or the tone (conditioned stimuli, CS), conditioned fear results in freezing behavior which is characterized by cessation of all movement except for respiration. Contextual fear conditioning is thought to be hippocampal- and amygdala-dependent, whereas cued fear conditioning is amygdala-dependent, but not hippocampal-dependent (Phillips & LeDoux 1992). On the first day of the conditioned fear test, each mouse was placed in a fear conditioning chamber (Med Associates, Inc, St. Albans, VT) and allowed to explore it for 2 minutes before the delivery of a 30 second tone (80 dB) which was immediately followed by a 2 second foot shock (0.35 mA). Two minutes later, a second tone-shock pair was delivered. Mice were removed from the testing chambers 10 seconds after the second shock and were returned to their home cages. Chambers were cleaned with 0.5% acetic acid between animals. The pre-tone time, which was the first 2 minutes of the trial, was used as the baseline measure for freezing behavior. Mice were removed from the testing chambers 10 seconds after the end of the second shock and returned to their home cages. On day 2, each mouse was first placed in the fear conditioning chamber containing the exact same context but without delivery of a tone or foot shock. Freezing was analyzed for 3 minutes. The context of the chambers was changed by adding a smooth floor texture over the grid floor, inserting the shape of a triangle, adding a new scent (hidden vanilla soaked nestlets), and by cleaning the chamber with 70% ethanol rather than acetic acid. One hour after the last contextual test for each mouse, the mice were assessed for cued fear conditioning. Mice were placed in the chambers containing the modified context and were allowed to explore for 3 minutes before they were re-exposed to the fear conditioning tone for 3 minutes. Freezing behavior was analyzed for the first and last 3 minutes of the cued fear conditioning test. Eight mice (WT mice: three sham-irradiated and two irradiated mice; E4 mice: one sham-irradiated and two irradiated mice) were excluded from the complete fear conditioning tests due to experimental error. An additional eighteen mice (WT mice: five sham-irradiated and four irradiated mice; E4 mice: four sham-irradiated and five irradiated mice) were excluded from the cued fear conditioning test due to a technical error.
Freezing was measured using a motion index, calculated based on a proprietary motion analysis algorithm in the Med Associates Video Freeze Software (Med Associates Inc). Briefly, the software analyzes and acquires videos of the trials at a frequency of 30 frames/second. The motion index is based on the sum of the pixel changes in a frame compared to those of a reference frame and to those of successive frames. The reference frame is based on a video capture when the mouse is not in the chamber. The motion index threshold used in the current study was 18. This means that the motion index had to remain below 18 pixel changes to be considered freezing.
Analysis of group differences in freezing before delivery of the first tone during fear conditioning training on day 1 were analyzed as measure of baseline freezing. This allowed us to determine whether there were potential pre-conditioning group differences in behaviors such as immobility, which could contribute to freezing scores. Potential group differences in the motion index during the two shocks on day 1 were also analyzed. This allowed us to determine whether there were possible group differences in sensory response to the shocks. Finally, the percent time freezing during contextual and cued testing on day 2 were analyzed.
DHE oxidation analysis
DHE oxidation levels were assessed approximately one week after fear conditioning in slices from E4 (sham-irradiated: n = 5 sections; irradiated: n = 9 sections), E3 (sham-irradiated: n = 2 sections; irradiated: n= 4 sections) and WT (sham-irradiated: n = 6 sections; irradiated: n = 5 sections) mice. Hemibrains were placed in 4°C oxygenated (95% oxygen 5% carbon dioxide) cutting solution (in mM: 110 sucrose, 60 NaCl, 3 KCl, 1.25 NaH2PO4, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2, 5 glucose). Acute brain coronal sections (150 μm) were generated using a Vibratome (Leica Microsystems, St. Louis MO) containing an oxygenated bath. Sections from each mouse were collected and kept separate in a 12-well plate bath containing ice cold and oxygenated cutting solution. Once all sections were collected, the bath solution was replaced with half cutting and half artificial cerebrospinal fluid (ACSF) solution (in mM: 125 NaCl, 2.4 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2 CaCl2, 1 MgCl2 and 25 glucose). Thirty minutes later, the bath solution was changed to a 100% ACSF solution and the bath temperature was gradually increased to 34°C. Sections were transferred and allowed to equilibrate for one hour in a multi-bath chamber placed on an Olympus spinning disk confocal microscope. The multi-bath chamber was situated on the confocal stage and received 36°C oxygenated ACSF using a gravity fed perfusion system and a multiple in-line heater (Warner Instruments, Hamden, CT). The rate of perfusion was 1ml/minute. A superfusion pump was used to perfuse out solution from the chambers, making this an open perfusion system. Each separate chamber contained representative sections from each group of mice. This allowed all the sections from the different treatment groups and two genotypes to be examined under the exact same conditions. Images of the hippocampus (crux, enclosed blade and free blade of the dentate gyrus; areas CA1 and CA3) were acquired for background reference (Ex λ 488 nm; Em λ > 590 nm) before the addition of DHE (10 μM, Molecular Probes, Eugene, OR). Phorbol-12-Myristate-13-Acetate (PMA) induces generation of superoxide via stimulation of the NADPH-oxidase complex (Tejada-Simon et al. 2005). Because induction of superoxide is important for learning, memory, and synaptic plasticity (Kishida et al. 2006, Kishida et al. 2005b, Knapp & Klann 2002b, Thiels et al. 2000), we used PMA as a functional assay to assess whether irradiation alters the ability to generate superoxide in E4, E3, and WT mice. PMA (1 μM) or DMSO was added to separate ACSF solution reservoirs approximately 5 min following the addition of DHE. Images were acquired every 2 minutes for up to 20 minutes at a 4× magnification. The optimal x, y, and z coordinates for each slice and each region were selected and programmed before the DHE experiment began. This allowed us to determine the best focal plane before the experiment began, and it also allowed us to label the sections with their corresponding mouse ID number. Images were acquired after addition of DHE using a 4× water objective. As there were slight variations in the focal plane within each slice, several images (3–6) were acquired for optimal image quality. An Olympus confocal microscope and Slidebook 6 Digital Microscopy Software (Intelligent Imaging Innovations Inc.) were used to collect the images and analyze the intensity of oxidized DHE. The experimenter that prepared and analyzed the DHE-oxidation of the slices was blind to the treatment and genotype. There were a total of 3 experiments. Each experiment consisted of 2–3 replicate slices for each mouse from each treatment group and for each drug treatment. The mean temperature was 35.9°C with a range of 34.3 – 37.3°C between individual experiments. The mean location of the hippocampal slices from Bregma was −2.0 with a range of −1.58mm–−2.4mm. This anatomical range was selected because previous data suggest that the dorsal hippocampus is more involved in hippocampus-dependent spatial memory compared to the ventral hippocampus (Moser et al. 1993).
Western Blot Analysis
Hippocampal tissues were homogenized in RIPA lysis buffer [0.1% SDS, 0.5% sodium deoxycholate, 1% NP-40, 150 mM NaCl, 50 mM Tris pH 8.0] containing the phosphatase inhibitor sodium vanadate [NaV, 1mM]. Protein lysates were extracted by centrifugation and protein concentrations were calculated using the MicroBCA protein assay. Equal amounts of protein were separated on sodium dodecyl sulfate polyacrylamide (10% SDS-PAGE) gels and transferred to a polyvinylidene difluoride (PVDF) membrane. Membrane blots were then blocked in tris-buffered saline and tween 20 (TBST) [1 × TBS, 0.1% Tween 20] containing 5% bovine serum albumin (BSA). The membranes were then incubated with primary antibody diluted (1:1,000) in TBST containing 1% bovine serum albumin (BSA) overnight at 4°C. Membranes were then washed in TBST [3×10min] before being incubated in secondary antibody [1:10,000 dilution] for one hour. Membranes were incubated in enhanced chemiluminescence (ECL) reagent before being exposed to CL-XPosure Film to detect protein changes. A representative blot is shown in Fig 1.
Figure 1.
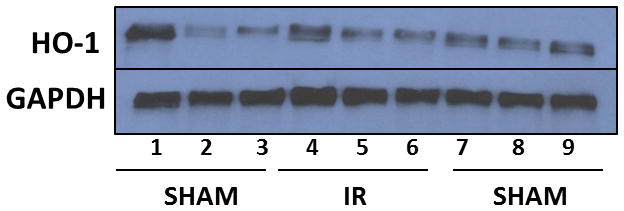
Representative Western blot of hippocampal HO-1 levels. Western blot analysis showing protein levels of HO-1 (higher band) and GAPDH (lower band, to ensure equal loading) in hippocampal tissues of WT and E4 mice. Lanes 1–3: sham-irradiated WT; lanes 4–6: irradiated WT; lanes 7–9: sham-irradiated E4.
Statistical analyses
All statistical tests were conducted using SPSS 22.0 (SPSS Inc, Chicago, IL, USA) or GraphPad Prism software (GraphPad Software, La Jolla, CA) and were considered significant at P < 0.05. All figures were generated using GraphPad Prism software. Where relevant, post-hoc corrections for multiple comparisons were applied. Data are reported as averages ± the standard error of the mean. For all statistical analyses, data were first assessed for normality and homogeneity of variance to determine whether to use parametric or non-parametric statistical analyses. The data distribution was considered normal at a significance of p > 0.01 (Shapiro-Wilk test).
RESULTS
General heath and body weights
There were no signs of illness or sick-like behaviors as a result of 56Fe irradiation in either genotype. There were no effects of 56Fe irradiation or genotype on activity or measures of anxiety in the light-dark test (not shown).
Activity and anxiety measures
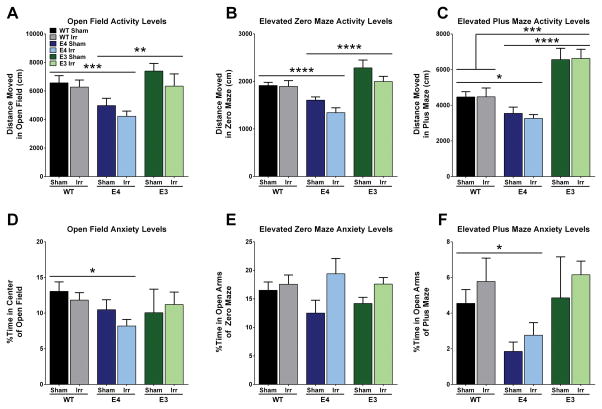
Effects of genotype on activity and anxiety measures were seen in the elevated zero maze, elevated plus maze, and open field. Genotype differences were seen for the activity measures of the open field (F2,58 = 9.172, p < 0.001; Fig. 2A), the elevated zero maze (F2,59 = 15.44, p < 0.0001; Fig. 2B), and the elevated plus maze (F2,59 = 18.76, p < 0.0001; Fig. 2C). Compared to WT and E3 mice, E4 mice explored less in the open field (p < 0.001; p < 0.01, respectively; Sidak’s post-hoc), the elevated zero maze (p < 0.0001, both; Tukey’s post-hoc), and the elevated plus maze (p < 0.05; p < 0.0001, respectively; Tukey’s post-hoc). E3 mice explored more than WT mice in the elevated plus maze (p <0.001; Tukey’s post-hoc). Similarly, genotype differences were found in anxiety measures of the open field (F2,58 = 3.197, p < 0.05; Fig. 2D) and the elevated plus maze (F2,59 = 5.177, p < 0.01; Fig. 2F). WT mice spent more time in the center of the open field (p < 0.05; Tukey’s post-hoc) and in the open arms of the elevated plus maze (p < 0.05; Tukey’s post-hoc) compared to E4 mice. Finally, there was a trend towards a main effect of irradiation in anxiety measures in the elevated zero maze (F2,57 = 3.972, p = 0.0511, Fig. 2E) and in the activity measures in the elevated zero maze (F1,59 = 3.526, p = 0.065), with irradiated mice showing lower anxiety levels and spending more time in the open areas of the maze, yet moving less than their sham counterparts.
Figure 2.
Anxiety and activity measures in sham- and 56Fe-irradiated WT, E3, and E4 female mice. E4 mice moved less in A. the open field (***p < 0.001; **p < 0.01), B. the elevated zero maze (****p < 0.0001), and C. the elevated plus maze (*p < 0.05) than WT and E3 mice. E4 mice also spent less time in D. the center of the open field (*p < 0.05), and F. the open arms of the elevated plus maze (p < 0.05) but not the elevated zero maze (E). Bars represent the group means ± SEM. N = 4–16 mice per group.
Cognitive performance
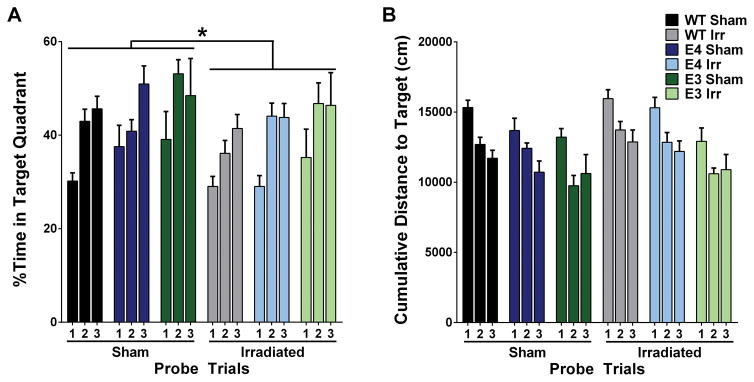
Next spatial learning and memory were assessed in the water maze. There were no significant effects of genotype or radiation on ability of the mice to locate the visible or hidden platform locations (Table 1). However, irradiation affected spatial memory retention in the water maze probe trials (Fig. 3). To compare performance across probe trials, we analyzed performance as percent time in the target quadrant in the three probe trials using a repeated measures ANOVA. There were main effects of irradiation (F2,58 =4.693, p < 0.05) and genotype (F2,58 = 4.831, p < 0.05) (Fig. 3A). Independent of genotype, sham-irradiated mice spent more time in the target quadrant than irradiated mice. E3 mice spent more time in the target quadrant than WT mice (p < 0.05; Bonferroni’s post-hoc). Genotype differences were also observed when cumulative distance to the target was calculated (Fig. 3B). There was a main effect of genotype (F2,58 = 6.85, p < 0.01); E3 mice swam closer to the platform location than WT mice (Bonferroni’s post-hoc).
Table 1.
Cognitive and anxiety measures of sham-irradiated and 56Fe irradiated E4, E3, and WT female mice in the water maze test.1
| Measure | Visible | Hidden | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Sham-Irradiated | Irradiated | Sham-Irradiated | Irradiated | |||||||||
| WT | E4 | E3 | WT | E4 | E3 | WT | E4 | E3 | WT | E4 | E3 | |
| Mean Cumulative Distance to target (cm) | 6648.89 ± 790.08 | 5973.12 ± 734.88 | 5514.02 ± 1080.81 | 7572.34 ± 892.13 | 6935.45 ± 732.78 | 6205.69 ± 1214.5 | 6465.46 ± 421.39 | 5963.24 ± 517.24 | 5482.66 ± 698.72 | 7479.77 ± 530.61 | 6951.99 ± 532.69 | 4447.47 ± 546.21 |
| Thigmotaxis (%) | 15.45 ± 2.05 | 13.56 ± 1.82 | 14.66 ± 3.27 | 17.16 ± 2.25 | 15.46 ± 1.99 | 9.87 ± 2.32 | 4.82 ± 0.46 | 4.86 ± 0.91 | 2.23 ± 0.42 | 6.84 ± 0.75 | 9.46 ± 1.66 | 2.96 ± 0.85 |
| Swim Speeds (cm/s) | 17.49 ± 0.36 | 17.97 ± 0.50 | 18.16 ± 0.58 | 17.78 ± 0.26 | 16.40 ± 0.27 | 17.71 ± 0.45 | 18.44 ± 0.31 | 17.51 ± 0.35 | 18.55 ± 0.33 | 18.34 ± 0.28 | 17.16 ± 0.34 | 17.89 ± 0.35 |
Data are shown as group averages ± SEM. N = 10–16 per group. All data were analyzed by ANOVA, using genotype and treatment as between-group factors. There were no significant effects in any outcome measure.
Figure 3.
Spatial memory retention of sham and 56Fe-irradiated WT, E3, and E4 mice in the Morris Water Maze. A. Percent of time spent in target quadrant in the three probe trials of the water maze. The percent of time spent in target quadrant differed across probe trials and between genotypes and treatment. Sham-irradiated mice spent more time in the target quadrant than irradiated mice (p < 0.05) and E3 mice spent more time in the target quadrant than WT mice (p < 0.05). B. Cumulative distance to the target location in the three probe trials of the water maze. E3 mice swam closer to the platform than WT mice (p < 0.01). Bars represent group average ± SEM. N = 4–16 mice per group. *p < 0.05; ^p < 0.01; #p < 0.001; +p < 0.0001.
Finally, mice were tested for contextual and cued fear conditioning. There were no effects of genotype or irradiation on average baseline motion (prior to the first tone) (Fig. 4A). There were also no effects of irradiation in response to the shocks. However, there was a main effect of genotype (F2,45=6.194, p < 0.01) in response to the two shocks, with E3 and E4 mice showing a greater response than WT mice (p < 0.01, p < 0.05, respectively; Tukey’s post-hoc; Fig. 4B). Similarly, there was an effect of genotype in response to the two tones (F2,45 = 9.573, p < 0.001) (Fig. 4C) and in freezing during the interval between the two tone-shock pairings (F2,45= 7.366, p < 0.01) (Fig. 4D). E4 mice had higher immobility during the tones than WT mice (p < 0.001; Bonferroni’s post-hoc) and higher immobility during the interval than WT (p < 0.01) and E3 mice (p < 0.05; Tukey’s post-hoc). Twenty four hours after training, genotype differences were also seen in freezing during the hippocampus-dependent contextual memory test (F2,49 = 7.401, p < 0.01); freezing was higher in E4 than WT mice (p < 0.001; Tukey’s post-hoc, Fig. 4E). This genotype difference was not limited to the contextual memory test. Freezing during the hippocampus-independent cued memory test was also influenced by genotype (F2,28 = 15.035, p < 0.001, Fig. 3F). E4 mice showed higher freezing levels both before and during the tone than WT mice (p < 0.000; Bonferroni’s post-hoc). In contrast to these genotype differences and the effects of 56Fe irradiation on spatial memory retention in the water maze, there were no effects of irradiation on freezing levels during the contextual or cued memory tests.
Figure 4.
Acquisition and memory of conditioned fear in sham and 56Fe-irradiated WT, E3, and E4 mice A. Average baseline motion, prior to the first tone on the training day. There were no effects of radiation or genotype. B. Response to the average of the two shocks on the training day. WT mice showed lower response levels than E3 (p < 0.01) and E4 (p < 0.05) mice. C. Freezing levels during the two tone-shock pairings on the training day. E4 mice showed higher freezing levels than WT mice (p < 0.001). D. Immobility during the interval between the two tone-shock pairings. E4 mice showed higher freezing levels than WT (p < 0.01) and E3 (p < 0.05) mice. E. Freezing levels during the contextual fear memory test. E4 mice showed higher freezing levels than WT mice (p < 0.001). F. Freezing levels prior to (PT) and during the tone (T) in the cued fear memory test. E4 mice showed higher freezing levels than WT mice prior to and during the tone (p < 0.001). Bars and line graphs represent group average ± SEM. Training day and context test day 2: N = 4 – 12 mice; cued test day 2: N = 3 – 10 mice.
Hippocampal DHE oxidation levels
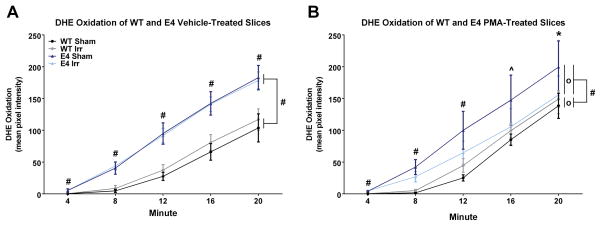
Representative images showing the time course of DHE oxidation in hippocampal slices of WT and E4 mice are displayed in Fig. 5. The quantification of all the images showed that there were significant time x genotype (F3.016,1092 = 22.348, p < 0.000), time x irradiation (F1.530,1092 = 4.019, p < 0.05), time x drug (PMA or vehicle) (F1.530,1092 = 15.171, p < 0.000), time x genotype x irradiation (F3.016,1092 = 4.816, p < 0.01), time x genotype x drug (F3.016,1092 = 5.304, p < 0.001), and time x irradiation x drug (F1.530,1092 = 5.304, p < 0.01) interactions for DHE oxidation levels, and a trend towards a time x genotype x irradiation x drug interaction (F3.016,1092 = 2.30, p = 0.075). Because there were no interactions with hippocampal region, the average of the five different regions was used for further analyses. A significant main effect of genotype (F2,273=42.692, p < 0.000) was found, as well as significant genotype x irradiation (F2,273=4.197, p < 0.05) and irradiation x drug (F1,273 = 4.504, p < 0.05) interactions, and a trend toward an interaction of genotype x drug (F2,273 = 2.737, p = 0.067). Bonferroni’s post hoc test revealed significant genotype differences in DHE oxidation between WT and E4 (p < 0.001) and WT and E3 mice (p < 0.001). There was no difference in DHE oxidation between E3 and E4 mice (p = 0.877). Based on these results and the significant behavioral and cognitive differences between E4 and WT mice, we proceeded to investigate in more detail the differences in DHE oxidation between WT and E4 mice. A repeated measures ANOVA of vehicle-treated sections revealed a significant time x genotype interaction (F1.380,428 = 52.492, p < 0.001) and a significant main effect of genotype (F1,115= 105.233, p < 0.001). Sidak’s pairwise comparison revealed that the hippocampal slices of E4 mice showed more DHE oxidation than those of WT mice (Fig. 6A).
Figure 5.
Representative images of time courses of DHE-incubated hippocampal slices from WT (top), E4 (middle), and E3 (bottom) mice demonstrating higher background levels of ROS in E4 than WT mice. Vehicle-treated slices from WT mice had lower levels of DHE-oxidation compared to slices from E4 mice.
Figure 6.
Baseline and pharmacologically-induced levels of ROS in acute hippocampal slices from sham-irradiated and 56Fe-irradiated WT and E4 female mice. A. Baseline levels of DHE oxidation in vehicle-treated hippocampi from E4 mice were higher than those from WT mice at each time point (#p < 0.001 each) and across the average of the time points (#p < 0.001). However, there were no differences in DHE oxidation in vehicle-treated slices from sham and irradiated mice at any time point in either genotype B. Following pharmacological induction of ROS with PMA, hippocampal slices from E4 mice showed greater DHE oxidation than those from WT mice at each time point and across the average of the time points (#p < 0.001; ^p < 0.01; *p < 0.05). In E4 mice, DHE oxidation levels were attenuated in PMA-treated hippocampal slices from irradiated mice compared to those from sham-irradiated E4 mice across the average of the time points (0p < 0.05). In contrast, DHE oxidation in slices from PMA-treated irradiated WT mice was higher than those from sham-irradiated WT mice across the average of the time points (0p < 0.05). Data are displayed as the estimated marginal means for each group ± SEM. N = 5 – 7 sections per group.
Finally, we analyzed the response of hippocampal slices of E4 and WT mice to PMA, an inducer of ROS. PMA-treated hippocampal slices showed a significant time x genotype interaction (F1.30,428 = 7.008, p < 0.01) and a significant main effect of genotype (F1,107 = 18.062, p < 0.001; Fig. 6B). Additionally, PMA-treated sections revealed a significant time x genotype x irradiation interaction (F1.30,428 = 6.041, p < 0.01) and a genotype x irradiation interaction (F1.,107 = 8.441, p < 0.01). Therefore, we ran a repeated-measures ANOVA in E4 and WT mice separately to examine the differing irradiation effects. The effect of irradiation was significant in both E4 (F1,57 = 5.613, p < 0.05) and WT mice (F1,50 = 5.275, p < 0.05). However, irradiation affected the genotypes differently. Hippocampal slices of irradiated WT mice showed more DHE oxidation than those of sham-irradiated WT mice. In contrast, hippocampal slices of E4 irradiated mice showed less DHE oxidation levels than those of E4 sham-irradiated mice (Bonferroni’s pairwise comparison).
Hippocampal HO-1 levels
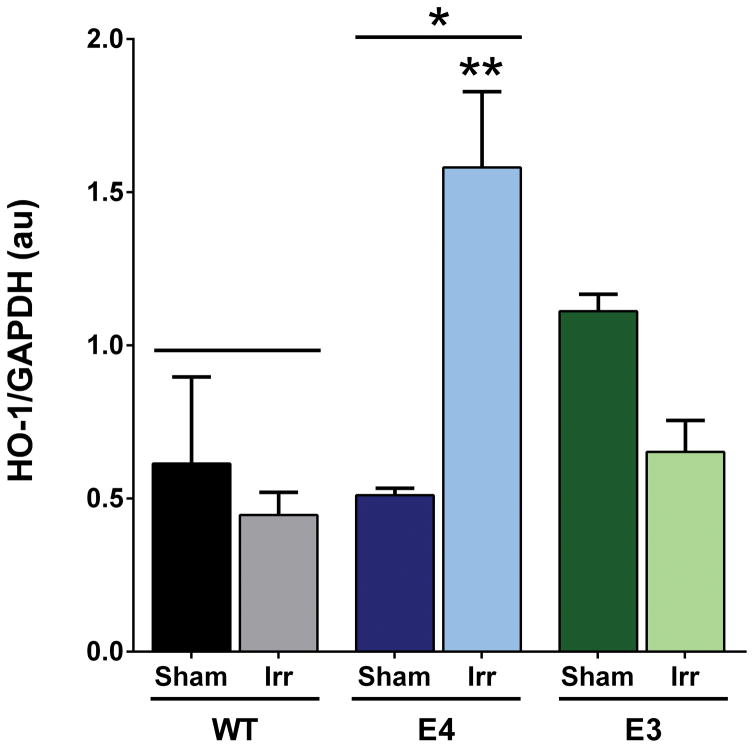
Hippocampal HO-1 levels were analyzed in sham-irradiated and irradiated WT, E4, and E3 mice. There was an effect of genotype (F2,12 = 5.184, p < 0.05; Fig. 7) with higher hippocampal HO-1 levels in E4 than WT mice (p = 0.023). There was also a genotype x radiation interaction (F2,12 = 12.31, p = 0.0012). Hippocampal HO-1 levels were higher in irradiated than sham-irradiated E4 mice (p = 0.0018, Sidak’s post-hoc). No effect of irradiation on HO-1 levels was seen in WT mice (p = 0.92). Finally, there was a trend towards lower HO-1 levels in irradiated than sham-irradiated E3 mice (p = 0.08).
Figure 7.
Hippocampal HO-1 levels in sham-irradiated and irradiated Wt, E4, and E3 mice. There was a genotype x radiation interaction (F2,12 = 12.31, p = 0.0012) and an effect of genotype (F2,12 = 5.184, p < 0.05) (Fig. 7). Hippocampal HO-1 levels were higher irradiated than sham-irradiated E4 mice (p = 0.0018, Sidak’s multiple comparisons test). No effect of irradiation on HO-1 levels was seen in WT (p = 0.92) mice and there was a trend towards lower HO-1 levels in irradiated than sham-irradiated E3 mice (p = 0.08). Hippocampal HO-1 levels were also higher in E4 than WT mice. N = 3 mice/genotype/treatment. *p < 0.05; **p < 0.01 versus sham-irradiated E4.
DISCUSSION
We found that compared to WT mice, E4 mice explored less in the open field, the elevated zero maze, and the elevated plus maze. Similarly, E4 mice showed higher measures of anxiety in the open field and elevated plus maze than WT mice. Together with the isoform-dependent effects of apoE on measures of anxiety in mice (Raber et al. 1998, Robertson et al. 2005) and humans (Robertson et al. 2005), increased measures of anxiety in apoE-deficient mice (Raber et al. 2000, Bongers et al. 2004), and isoform-dependent effects of apoE on PTSD-like behaviors (Johnson et al. 2015, Kimbrel et al. 2015), these data highlight the importance of these effects in the neurobiological effects of apoE (for a review, see Raber 2007, Raber 2008). Genotype differences were also observed in fear conditioning. E4 mice showed a greater response to the shocks and enhanced freezing during training, and enhanced hippocampus-dependent contextual and cued fear memory compared to WT mice. With the increased shock response, it is conceivable that this genotype difference might have contributed to the higher subsequent freezing responses and contextual and cued fear memories in E4 than WT mice. In all genotypes, irradiation induced hippocampus-dependent impairments in spatial memory retention in the water maze. The increased anxiety levels and enhanced fear learning and memory of E4 and WT were associated with increased DHE oxidation.
In the current study, E4 mice did not show impairments in acquisition or retention of spatial memory. Consistent with these data, E4 mice did not show impairments in spatial learning and memory in previous studies (Siegel et al. 2010, Haley et al. 2012b, Johnson et al. 2014, Villasana et al. 2011b). However, the experimental conditions might modulate these results as impaired acquisition and retention of spatial memory was reported in E4 mice. (Reverte et al. 2013).
Both contextual fear memory and spatial memory retention in the water maze are hippocampus-dependent. However, only spatial memory retention in the water maze was affected three months following 56Fe irradiation. A dissociation between spatial memory in the water maze and contextual learning in fear conditioning has been previously reported (Logue et al. 1997, Silva et al. 1998). Consistent with the current study, whole body 56Fe irradiation affected water maze performance but not contextual fear conditioning within one month following radiation exposure in human apoE mice (Haley et al. 2012a) or 13 months following 56Fe irradiation (Villasana et al. 2011a). These data indicate the importance of considering different hippocampus-dependent cognitive tests in assessing effects of irradiation on the brain.
ROS is required for normal synaptic plasticity, learning and memory (Kishida et al. 2006, Kishida et al. 2005b, Knapp & Klann 2002b, Thiels et al. 2000, Hidalgo et al. 2007b). Therefore, enhanced DHE oxidation levels in hippocampal slices of E4 mice might have been associated with enhanced fear conditioning. E4 has reduced antioxidant capacity (Colton et al. 2005) and it is conceivable that this contributed to the higher basal levels of ROS and enhanced fear conditioning in E4 mice. The lower DHE oxidation in PMA-treated slices of irradiated versus sham-irradiated E4 mice and higher HO-1 levels in irradiated than sham-irradiated E4 mice suggest that the enhanced antioxidant capacity of E4 mice following 56Fe irradiation might protect them against increases in oxidative stress and ROS. A similar scenario was seen in mice lacking the extracellular form of superoxide dismutase that showed higher basal levels of oxidative stress. Hippocampal oxidative injury, assessed as 3-nitrotyrosine levels, showed less profound increases in mice lacking the extracellular form of superoxide dismutase than in WT mice (Raber et al. 2011). In contrast to E4 mice, WT mice had lower basal levels of ROS. DHE oxidation was higher in PMA-treated slices of irradiated than sham-irradiated WT mice. Importantly, the effect of irradiation on ROS levels was only observed upon pharmacological stimulation, which may be functionally relevant to synaptic plasticity, learning and memory (Hidalgo et al. 2007b, Kishida et al. 2005b, Tejada-Simon et al. 2005).
These paradoxical effects seen in pharmacologically stimulated slices of irradiated E4 and WT mice, suggest that the level of ROS required for behavioral and cognitive performance is determined by background levels. Thus, the role of ROS in behavioral and cognitive performance may depend on genetic differences in background levels. Further studies, possibly with antioxidants, should help elucidate the role that background levels of ROS play in the ability to induce ROS following irradiation.
In this study, there was a trend towards reduced measures of anxiety in the elevated zero maze three months following 56Fe irradiation. These results are consistent with reduced measures of anxiety in E4 mice thirteen month following cranial 56Fe irradiation (Villasana et al. 2011a). As anxiety, within reasonable limits, serves as a warning signal (Perkinsa et al. 2013), it is hard to determine whether the trend towards reduced measures of anxiety following 56Fe irradiation is beneficial or detrimental. The analysis of performance in the open field, elevated plus maze, and light-dark tests indicates that this trend is task specific. These data are consistent with the notion that distinct anxiety tests differ in detecting anxiety differences in mutant mice (Rizk et al. 2004). In E4 mice, these data are consistent with the studies showing that ROS increase anxiety levels and that attenuation of ROS can ameliorate anxiety levels (Novio et al. 2011, Xu et al. 2014, Bouayed et al. 2009). Although the hippocampus is involved in the regulation of anxiety, the amygdala and other brain regions are involved as well. It is conceivable that DHE oxidation levels are differentially affected in brain regions other than the hippocampus. Future studies are warranted to assess this possibility.
In the current study, the mean cumulative distance to the target during the hidden and visible platform conditions was similar. It is conceivable that the groups’ similar mean cumulative distance to the target in the two days of visible and three days of hidden sessions is due to the fact that they were trained for task learning prior to the hidden sessions, as well as receiving an extra day of training for the hidden location.
In summary, higher hippocampal ROS levels in E4 mice are associated with increased measures of anxiety and enhanced freezing levels during fear learning and memory. The opposite effects of PMA on DHE oxidation in slices of irradiated and sham-irradiated E4 and WT mice highlight the complex role of ROS in behavioral and cognitive performance. Nevertheless, future efforts are warranted to determine whether reducing ROS levels in E4 carriers is able to reduce anxiety levels
Acknowledgments
This work was supported by NIA grant T32 NS007466-05, NIAAA T32 AA007290-33, NIEHS T32 ES007060, NIMH grant MH77647, NASA Grants NNJ05HE63G, NNJ12ZSA001N, and NNX10AD59G, Alzheimer’s Association Grant IIRG-05-14021 and an APA Dissertation Award. We thank Peter Guida and Adam Rusek at BNL for their support with the irradiations.
Footnotes
There are no conflicts of interest to declare.
References
- Bergeron M, Ferriero DM, Vreman HJ, Stevenson DK, Sharp FR. Hypoxia-ischemia, but not hypoxia alone, induces the expression of heme oxygenase-1 (HSP32) in newborn rat brain. J Cereb Blood Flow Metab. 1997;17:647–658. doi: 10.1097/00004647-199706000-00006. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Sun L, Raber J, Maren S, Julius D, Dallman M. Changes in anxiety-related behaviors and hypothalamic-pituitary-adrenal activity in mice lacking the 5-HT-3A receptor. Physiol Behav. 2004;81:545–555. doi: 10.1016/j.physbeh.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Bongers G, Leurs R, Robertson J, Raber J. Role of H3 receptor-mediated signaling in anxiety and cognition in wild-type and Apoe−/− mice. Neuropsychopharmacology. 2004;29:441–449. doi: 10.1038/sj.npp.1300352. [DOI] [PubMed] [Google Scholar]
- Bouayed J, Rammal H, Soulimani R. Oxidative stress and anxiety: relationship and cellular pathways. Oxid Med Cell Longev. 2009;2:63–67. doi: 10.4161/oxim.2.2.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres L, Aon Bertolino L, Saracero G, Zorilla Zubiele M, Uran S, Capari F, Guelman L. Hippocampal-related memory deficits and histological damage induced by neonatal ionizing radiation exposure. Brain Res. 2010;1312:67–78. doi: 10.1016/j.brainres.2009.11.053. [DOI] [PubMed] [Google Scholar]
- Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiasepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Clement Y, Calayud F, Belzung C. Genetic basis of anxiety-like behaviour: a critical review. Brain Res Bull. 2002;57:57–71. doi: 10.1016/s0361-9230(01)00637-2. [DOI] [PubMed] [Google Scholar]
- Colton C, Brown C, Vitek M. Sex steroids, APOE genotype and the innate immune system. Neurobiol Aging. 2005;26:363–372. doi: 10.1016/j.neurobiolaging.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Cuadrado A, Rojo A. Heme oxygenase-1 as a therapeutic taget in neurodegenerative disease and brain infections. Curr Pharm Des. 2008;14:429–442. doi: 10.2174/138161208783597407. [DOI] [PubMed] [Google Scholar]
- D’agostino D, Putriam R, Dean J. Superoxide (*O2−) production in CA1 neurons of rat hippocampal slices exposed to graded levels of oxygen. J Neurophysiol. 2007;98:1030–1041. doi: 10.1152/jn.01003.2006. [DOI] [PubMed] [Google Scholar]
- Driver A, Kodavanti P, Mundy W. Age-relared changes in reactive oxygen species production in rat brain homogenates. Neurotoxicol Teratol. 2000;22:175–161. doi: 10.1016/s0892-0362(99)00069-0. [DOI] [PubMed] [Google Scholar]
- Dwyer BE, Nishimura RN, de Vellis J, Yoshida T. Heme oxygenase is a heat shock protein and PEST protein in rat astroglial cells. Glia. 1992;5:300–305. doi: 10.1002/glia.440050407. [DOI] [PubMed] [Google Scholar]
- Ewing JF, Haber SN, Maines MD. Normal and heat-induced patterns of expression of heme oxygenase-1 (HSP32) in rat brain: hyperthermia causes rapid induction of mRNA and protein. J Neurochem. 1992;58:1140–1149. doi: 10.1111/j.1471-4159.1992.tb09373.x. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. J Am Med Assoc. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Haley G, LV, CD, MJDJR ApoE Genotype-Dependent Paradoxical Short-Term Effects of 56Fe Irradiation on the Brain. Int J Radiat Oncol Biol Phys. 2012a;84:793–799. doi: 10.1016/j.ijrobp.2011.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley G, Yeiser L, Olsen R, Davis M, Johnson L, Raber J. Early effects of whole body 5Fe irradiation on hippocampal function in C57BL/6J mice. Radiat Res. 2013;179:590–596. doi: 10.1667/RR2946.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley GE, Villasana L, Dayger C, Davis MJ, Raber J. Apolipoprotein e genotype-dependent paradoxical short-term effects of (56)fe irradiation on the brain. Int J Radiat Oncol Biol Phys. 2012b;84:793–799. doi: 10.1016/j.ijrobp.2011.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D, Han SH, Chepetan A, Inui E, Rogers MV, Dugan LL. Dynamic optical imaging of metabolic and NADPH oxidase-derived superoxide in live mouse brain using fluorescne lifetime unmixing. J Cereb Blood Flow Metab. 2012;32:23–32. doi: 10.1038/jcbfm.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo C, Carasso M, Munoz P, Nunez M. A role for reactive oxygen/nitrogen species and iron on neuronal plasticity. Antioxid Redox Signal. 2007a;97:245–255. doi: 10.1089/ars.2007.9.245. [DOI] [PubMed] [Google Scholar]
- Hidalgo C, Carrasco MA, Munoz P, Nunez MT. A role for reactive oxygen/nitrogen species and iron on neuronal synaptic plasticity. Antioxid Redox Signal. 2007b;9:245–255. doi: 10.1089/ars.2007.9.245. [DOI] [PubMed] [Google Scholar]
- Jernigan NL, O’Donaughy TL, Walker BR. Correlation of HO-1 expression with onset and reversal of hypoxia-induced vasoconstrictor hyporeactivity. Am J Physiol Heart Circ Physiol. 2001;281:H298–H307. doi: 10.1152/ajpheart.2001.281.1.H298. [DOI] [PubMed] [Google Scholar]
- Johnson L, Olsen R, Merkens L, DeBarber A, Steiner R, Sullivan P, Maeda N, Raber J. Apolipoprotein E–low density lipoprotein receptor interaction affects spatial memory retention and brain ApoE levels in an isoform-dependent manner. Neurobiol Disease. 2014;64:150–162. doi: 10.1016/j.nbd.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L, Zuloaga D, Bidiman E, Marzulla T, Weber S, Wahbeh H, Raber J. ApoE2 exaggerates PTSD-related behavioral, cognitive, and neuroendocrine alterations. Neuropsychopharmacology. 2015;40:2443–2453. doi: 10.1038/npp.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrel N, Hauser M, Garrett M, Ashley-Koch A, Liu Y, Dennis M, Klein R. Effect of the APOE e4 allele and combat exposure on PTSD among Iraq. Afghanistan-era veterans. Depress Anxiety. 2015;32:307–315. doi: 10.1002/da.22348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida K, Klann E. Sources and targets of reacitive oxygen species in synaptic plasticity and memory. Antioxid Redox Signal. 2007;9:233–244. doi: 10.1089/ars.2007.9.ft-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida K, Pao M, Holland S, Klann E. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA! Neurochem. 2005a;94:299–306. doi: 10.1111/j.1471-4159.2005.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida KT, Hoeffer CA, Hu D, Pao M, Holland SM, Klann E. Synaptic plasticity deficits and mild memory impairments in mouse models of chronic granulomatous disease. Mol Cell Biol. 2006;26:5908–5920. doi: 10.1128/MCB.00269-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida KT, Pao M, Holland SM, Klann E. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA1. J Neurochem. 2005b;94:299–306. doi: 10.1111/j.1471-4159.2005.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp L, Klann E. Role of reactive oxygen species in hippocampal long-term potentiation: contributory or inhibitory. J Neurosci Res. 2002a;70:1–7. doi: 10.1002/jnr.10371. [DOI] [PubMed] [Google Scholar]
- Knapp LT, Klann E. Potentiation of hippocampal synaptic transmission by superoxide requires the oxidative activation of protein kinase C. J Neurosci. 2002b;22:674–683. doi: 10.1523/JNEUROSCI.22-03-00674.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IS, Lim J, Gal J, Kang JC, Kim HJ, Kang BY, Choi HJ. Anti-inflammatory activity of xanthohumol involves heme oxygenase-1 induction via NRF2-ARE signaling in microglial BV2 cells. Neurochemistry International. 2011;58:153–160. doi: 10.1016/j.neuint.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Limoli CL, Giedzinski E, Baure J, Rola R, Fike JR. Redox changes induced in hippocampal precursor cells by heavy iron irradiation. Radiat Environ Biophys. 2007;46:167–172. doi: 10.1007/s00411-006-0077-9. [DOI] [PubMed] [Google Scholar]
- Logue S, Paylor R, Wehner J. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned fear task. Behav Neurosci. 1997;111:104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Manda K, Ueno M, Anzai K. Memory impairment, oxidative damage and apoptosis induced by space radiation: ameliorative potential of alpha-lipoic acid. Beh Brain Res. 2008;187:387–395. doi: 10.1016/j.bbr.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Manda K, Ueno M, Moritake T, Anzai K. Radiation-induced cognitive dysfunction and cerebellar oxidative stress in mice: protective effect of alpha-lipoic acid. Beh Brain Res. 2007;177:7–14. doi: 10.1016/j.bbr.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Mizumatsu S, Monje M, Morhardt D, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Morris RJ. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984:11. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesion, but is hardly present following ventral lesions. J Neurosci. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndisang JF, Jadhav A. Heme oxygenase system enhances insulin sensitivity and glucose metabolism in streptozotocin-induced diabetes. Am J Physiol Endocrinol Metab. 2009;296:E829–841. doi: 10.1152/ajpendo.90783.2008. [DOI] [PubMed] [Google Scholar]
- Novio S, MJN, Amigo G, Freire-Garabal M. Effects of fluoxetine on the oxidative status of peripheral blood leucocytes of restraint-stressed mice. Basic Res Pharmacol Toxicol. 2011;109:365–371. doi: 10.1111/j.1742-7843.2011.00736.x. [DOI] [PubMed] [Google Scholar]
- Pedersen WA, Chan SL, Mattson MP. A mechanism for the neuroprotective effect of apolipoprotein E: isoform-specific modification by the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 2000;74:1426–1433. doi: 10.1046/j.1471-4159.2000.0741426.x. [DOI] [PubMed] [Google Scholar]
- Perkinsa AMS, Ettinger U, et al. Advancing the defensive explanation for anxiety disorders: lorazepam effects on human defense are systemtically modulated by personality and threat-type. Translation Psychiatr. 2013:e246. doi: 10.1038/tp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peshavariya H, Dusting G, Selemidis S. Analysis of dihydroethidium fluorescence for the detection of intracellular and extracellular superoxide produced by NADPH oxidase. Free radical research. 2007;41:699–712. doi: 10.1080/10715760701297354. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Raber J. Role of Apolipoprotein E in anxiety. Neural Plasticity. 2007 doi: 10.1155/2007/91236. in press, published on line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J. Role of apolipoprotein E in anxiety. Future Lipidology. 2008;3:97–103. [Google Scholar]
- Raber J, Akana SF, Bhatnagar S, Dallman MF, Wong D, Mucke L. Hypothalamic-pituitary-adrenal function in Apoe−/− mice: Possible role in behavioral and metabolic alterations. J Neurosci. 2000;20:2064–2071. doi: 10.1523/JNEUROSCI.20-05-02064.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Huang Y, Ashford J. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging. 2004;25:641–650. doi: 10.1016/j.neurobiolaging.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Raber J, Villasana L, Rosenberg J, Zou Y, Huang TT, Fike JR. Irradiation enhances hippocampus-dependent cognition in mice deficient in extracellular superoxide dismuase. Hippocampus. 2011;21:72–80. doi: 10.1002/hipo.20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Wong D, Buttini M, Orth M, Bellosta S, Pitas RE, Mahley RW, Mucke L. Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: increased susceptibility of females. Proc Natl Acad Sci U S A. 1998;95:10914–10919. doi: 10.1073/pnas.95.18.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin B, Buhler L, Joseph J, Shukitt-Hale B, Jenkins D. Effects of exposure to 56Fe particles or protons on fixed ratio operant responding in rats. J Radiat Res (Tokyo) 2002;43(Suppl):S225–S228. doi: 10.1269/jrr.43.s225. [DOI] [PubMed] [Google Scholar]
- Rabin B, Carrihill-Knoll K, Hinchman M, Shukitt-Hale B, Joseph J, Foster B. Effects of heavy particle irradiation and diet on object recognition memory in rats. Adv Space Res. 2009;43:1193–1199. [Google Scholar]
- Rabin B, Joseph J, Shukitt-Hale B. Heavy particle irradiation, neurochemistry and behavior; thresholds, dose-response curves and recovery of function. Adv Space Res. 2004;33:1330–1333. doi: 10.1016/j.asr.2003.09.051. [DOI] [PubMed] [Google Scholar]
- Reverte I, Klein A, Domingo J, Colomina M. Long term effects of murine postnatal exposure to decabromodiphenyl ether (BDE-209) on learning and memory are dependent upon APOE polymorphism and age. Neurotoxicol Teratol. 2013;40:17–27. doi: 10.1016/j.ntt.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Rizk A, Curley J, Robertson J, Raber J. Anxiety and cognition in histamine H3 receptor−/− mice. Eur J Neurosci. 2004;19:1992–1996. doi: 10.1111/j.1460-9568.2004.03251.x. [DOI] [PubMed] [Google Scholar]
- Robertson J, Curley J, Kaye J, Quinn J, Pfankuch T, Raber J. apoE isoforms and measures of anxiety in probable AD patients and Apoe−/− mice. Neurobiol Aging. 2005;26:637–643. doi: 10.1016/j.neurobiolaging.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Rola R, Zou Y, Huang TT, Fishman K, Baure J, Rosi S, Milliken H, Limoli CL, Fike JR. Lack of extracellular superoxide dismutase (EC-SOD) in the microenvironment impacts radiation-induced changes in neurogenesis. Free Radic Biol Med. 2007;42:1133–1145. doi: 10.1016/j.freeradbiomed.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugo RE, Schiestl RH. Increases in oxidative stress in the progeny of X-irradiated cells. Radiat Res. 2004;162:416–425. doi: 10.1667/rr3238. [DOI] [PubMed] [Google Scholar]
- Shepherd VL, Cowan HB, Abdolrasulnia R, Vick S. Dexamethasone blocks the interferon-gamma-mediated downregulation of the macrophage mannose receptor. Arch Biochem Biophys. 1994;312:367–374. doi: 10.1006/abbi.1994.1321. [DOI] [PubMed] [Google Scholar]
- Siegel J, Haley G, Raber J. Apolipoprotein E isoform-dependent effects on anxiety and cognition in female TR mice. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.03.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Giese K, Fedorov N, Frankland PW, Kogan J. Molecular, cellular, and neuroanatomical substrates of place learning. Neurobiol Learn Mem. 1998;70:44–61. doi: 10.1006/nlme.1998.3837. [DOI] [PubMed] [Google Scholar]
- Spinney L. The forgetting gene. Nature. 2014;510:26–28. doi: 10.1038/510026a. [DOI] [PubMed] [Google Scholar]
- Suman S, Rodriguez O, Winters T, Fornace A, Albanese C, Datta K. Therapeutic and space radiation exposure of mouse brain causes impaired DNA repair response and premature senescence by chronic oxidant production. Aging. 2013;5:607–622. doi: 10.18632/aging.100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejada-Simon MV, Serrano F, Villasana LE, Kanterewicz BI, Wu GY, Quinn MT, Klann E. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol Cell Neurosci. 2005;29:97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiels E, Urban NN, Gonzalez-Burgos GR, Kanterewicz BI, Barrionuevo G, Chu CT, Oury TD, Klann E. Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2000;20:7631–7639. doi: 10.1523/JNEUROSCI.20-20-07631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng B, Giedzinski E, Izadi A, et al. Consequences of radiation-induced oxidative stress in neural stem and precursor cells exposed to charged particle irradiation. Antioxid Redox Signal. 2013;20:1410–1422. doi: 10.1089/ars.2012.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasana L, Benice T, Raber J. Long-term effects of 56Fe irradiation on spatial memory of mice: role of sex and apolipoprotein E isoform. Int J Radiat Oncol Biol Phys. 2011a;80:567–573. doi: 10.1016/j.ijrobp.2010.12.034. [DOI] [PubMed] [Google Scholar]
- Villasana L, Rosenberg J, Raber J. Sex-dependent effects of 56Fe Irradiation on contextual fear conditioning in C56BL/6J mice. Hippocampus. 2010;20:19–23. doi: 10.1002/hipo.20659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasana L, Rosenthal R, Doctrow S, Pfankuch T, Zuloaga D, MacColl Garfinkel A, Raber J. Effects of alpha-lipoic acid on associative and spatial memory of shan-irradiaed and 56Fe-irradiated C57BL/6J mice. Pharmacol Biochem Behav. 2013;103:487–493. doi: 10.1016/j.pbb.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasana LE, Benice TS, Raber J. Long-term effects of 56Fe irradiation on spatial memory of mice: role of sex and apolipoprotein E isoform. Int J Radiat Oncol Biol Phys. 2011b;80:567–573. doi: 10.1016/j.ijrobp.2010.12.034. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Das S, Maines MD. Brain heme oxygenase isoenzymes and nitric oxide synthase are co-localized in select neurons. Neuroscience. 1994;63:223–231. doi: 10.1016/0306-4522(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ruan L, Zhang H, O’Donnell J. Stress-induced depression- and anxiety-like behaviors are associated with the imbalance of redox state: the protective effect of PDE2 inhibition. FASEB J. 2014;28:113. [Google Scholar]
- Yeiser L, Villasana L, Raber J. ApoE isoform modulates effects of cranial 56Fe irradiation on spatial learning and memory in the water maze. Beh Brain Res. 2013;237:207–214. doi: 10.1016/j.bbr.2012.09.029. [DOI] [PubMed] [Google Scholar]
- Zhang B, Tanaka J, Yang L, Sakanaka M, Hata R, Maeda N, Mitsuda N. Protective effect of vitamin E against focal brain ischemia and neuronal death through induction of target genes of hypoxia-inducible factor-1. Neuroscience. 2004;126:433–440. doi: 10.1016/j.neuroscience.2004.03.057. [DOI] [PubMed] [Google Scholar]