Abstract
Cocaine is a commonly abused central nervous system stimulant that enhances dopamine (DA) neurotransmission through its ability to block dopamine transporters (DATs). Recent evidence suggests there may be an interaction between DATs and D2/D3 autoreceptors that modulates cocaine's effects. The purpose of this study was to explore how D2/D3 autoreceptors modulate the ability of cocaine to inhibit DA uptake through DATs on presynaptic DA terminals. Using fast-scan cyclic voltammetry in brain slices containing the nucleus accumbens core from male and female C57BL/6J mice, we first sought to examine the effects of global autoreceptor blockade using the nonselective D2/D3 autoreceptor antagonist, raclopride. We found that the ability of cocaine to inhibit DA uptake was increased by raclopride and that this effect was consistent across sexes. Further, using D2 (L-741,626) or D3 (SB-277011-A) autoreceptor selective antagonists, we discovered that blockade of D3, but not D2, autoreceptors was responsible for the increased cocaine potency. Alterations in cocaine potency were attributable to alterations in uptake inhibition, rather than cocaine effects on vesicular DA release, suggesting that these results may be a product of a functional D3/DAT interaction apart from the canonical inhibitory actions of D3 autoreceptors on DA release. Additionally, application of D2 (sumanirole) and D3 (PD 128907) autoreceptor specific agonists had inverse effects, whereby D2 autoreceptor activation decreased cocaine potency and D3 autoreceptor activation had no effect. Together, these data show that dopamine autoreceptors dynamically regulate cocaine potency at the DAT, which is important for understanding cocaine's rewarding and addictive properties.
Keywords: Voltammetry, sex differences, nucleus accumbens, mouse, D2 autoreceptor, addiction
Introduction
Cocaine is a commonly abused psychostimulant that produces its reinforcing effects primarily by enhancing dopamine (DA) neurotransmission in the brain, specifically in regions such as the nucleus accumbens (NAc) (Wu et al. 2001b). The NAc is critically involved in guiding the selection and execution of motivated behaviors based on previous experience, in which DA plays a key role (Humphries and Prescott 2010; Day and Carelli 2007). Further, DA within the NAc plays a major role in mediating the reinforcing effects of addictive drugs (Di Chiara and Imperato 1988; Koob 1992). Cocaine increases extracellular DA levels by binding to and blocking the function of the dopamine transporter (DAT) in presynaptic terminals of DA neurons, thereby inhibiting the reuptake of synaptic DA (Mortensen and Amara 2003; Kahlig and Galli 2003) and leading to a prolonged intensity and duration of DA signaling in the brain.
DAT function as well as DA release and synthesis are modulated by feedback-inhibitory autoreceptors. In addition to serving as classical postsynaptic receptors, D2 and D3 receptors located on DA nerve terminals function as presynaptic autoreceptors. As autoreceptors, they regulate extracellular DA levels through the modulation of DA firing rate, DA synthesis, DA uptake rate and DA release as part of a negative feedback loop (Ford 2014; Chen et al. 2009; Diaz et al. 2000; Beaulieu and Gainetdinov 2011; Rice et al. 2011). Mice deficient in D2 autoreceptors show increased DA release and synthesis (Bello et al. 2011). Additionally, mice with the selective loss of D2 autoreceptors exhibit increased place preference for cocaine (Bello et al. 2011) as well as heightened acquisition of cocaine self-administration and intensified reactivity to cocaine-paired cues (Holroyd et al. 2014), providing strong evidence implicating D2 autoreceptors in the modulation of cocaine effects. Further, a physical interaction between the N-terminus of the DAT and the third intracellular loop of D2 autoreceptors has been reported, which influences DAT surface localization and activity (Lee et al. 2007; Bolan et al. 2007; Chen et al. 2013). However, the role of D3 autoreceptors in cocaine actions is less well understood.
Previous studies suggest that D3 autoreceptors also regulate DA neurotransmission and interact with the DAT, suggesting that they may play a role in modulation of cocaine potency. For example, DA D3 agonists inhibit DA release in the NAc and caudate-putamen (Maina and Mathews 2010), and a physical interaction between D3 autoreceptors and DATs has been demonstrated following prolonged treatment with a D3 agonist, which results in reduced DA uptake in the striatum (Castro-Hernández et al. 2015). Our lab and others have previously shown that chronic cocaine exposure reduces DA terminal autoreceptor function (Mateo et al. 2005; Yi and Johnson 1990; Edwards et al. 2007; Park et al. 2013; Jones et al. 1996; Gifford and Johnson 1992), but whether autoreceptors in turn may modulate cocaine effects at the DAT is unknown.
As is the case with several other drugs of abuse, there is evidence suggesting that females may be more vulnerable to some aspects of psychostimulant abuse than males (Becker and Hu 2008). For example, female rats form conditioned place preference for cocaine after a fewer number of pairings and at a lower dose, and acquire cocaine self-administration more rapidly and self-administer greater amounts of cocaine than male rats (Hu et al. 2004). For these reasons, it is important to study both sexes. In the present study, we used fast-scan cyclic voltammetry (FSCV) in brain slices containing the NAc core to assess the effects of autoreceptor blockade and activation on the ability of cocaine to inhibit the DAT in male and female mice. Given the contribution of D2/D3 autoreceptors to DAT function and cocaine's addictive properties, it is important to understand these functional relationships.
Methods and Materials
Animals
Adult male and female C57BL/6J mice aged 9-12 weeks (Jackson Laboratories; Bar Harbor, ME) were maintained on a 12:12 hour light/dark cycle (6:00 am lights on; 6:00 pm lights off) with food and water ad libitum. Female mice were not examined for their estrous cycle phase when voltammetry experiments were conducted in the present study. However, previous in vivo and in vitro analyses of dopamine release, maximal velocity of dopamine uptake, and the affinity of the dopamine transporter for dopamine reveal that none of these parameters vary across the female estrous cycle (Walker et al. 2000). All animals were maintained in accordance with the National Institutes of Health guidelines in Association for Assessment and Accreditation of Laboratory Animal Care accredited facilities. The experimental protocol was approved by the Institutional Animal Care and Use Committee at Wake Forest School of Medicine.
In Vitro Voltammetry
FSCV was used to characterize presynaptic DA signaling and cocaine potency in the NAc core. A vibrating tissue slicer was used to prepare 400 μm thick coronal brain sections, as previously described (Siciliano et al. 2014). The tissue was immersed in oxygenated artificial cerebrospinal fluid (aCSF) containing (in mM): NaCl (126), KCl (2.5), NaH2PO4 (1.2), CaCl2 (2.4), MgCl2 (1.2), NaHCO3 (25), glucose (11), L-ascorbic acid (0.4) at a pH of 7.4. Once sliced, the tissue was transferred to testing chambers containing bath aCSF (32°C), which flowed at 2 ml/min. A carbon fiber microelectrode (50-150 μM length, 7 μM radius) and bipolar stimulating electrode were placed in close proximity in the NAc core. Extracellular DA was recorded by measuring changes in current at the oxidation and reduction potentials for dopamine (0.6 and −0.2V, respectively). A triangular voltage waveform (−0.4 to +1.2 to −0.4V vs Ag/AgCl, 400 V/s) was applied to the recording electrode every 100ms. DA release was evoked by a single electrical pulse (350 μA, 4 ms, monophasic) applied to the tissue every 3 minutes until a stable baseline was established (3 collections within 10% variability in peak height).
To explore the possibility of an interaction between D2/D3 autoreceptors and cocaine-induced inhibition of the DAT, raclopride (100 nM), a D2/D3 receptor antagonist, L-741,626 (30 nM), a D2 receptor-selective antagonist, SB-277011- A (300 nM), a D3 receptor-selective antagonist, sumanirole (300 nM), a D2 receptor-selective agonist, or (+)-PD 128907 (10 nM), a D3 receptor-selective agonist, were bath applied to separate slices after pre-drug baseline measures of peak dopamine concentration of the electrically evoked signal were stable. Once stability was reestablished following agonist/antagonist application, increasing cocaine concentrations (0.3 - 30 μM) were cumulatively bath applied to each slice. Stimulations were repeated until evoked DA levels reached stability at each concentration (approximately 30 minutes). Control slices were stimulated for the same amount of time using the same cocaine concentrations, except no agonist/antagonist was applied.
Data Analysis
For analysis of FSCV data, Demon Voltammetry and Analysis software was used (Yorgason et al. 2011). Recording electrodes were calibrated by recording responses (in electrical current; nA) to a known concentration of DA (3 μM) using a flow-injection system. Calibrations were used to convert electrical current to peak DA concentration. Michaelis-Menten modeling parameters were used to determine the maximal rate of DA uptake and cocaine-induced uptake inhibition (Wightman et al. 1988). Michaelis–Menten modeling provides parameters that describe the amount of DA released following electrical stimulation, the maximal rate of DA uptake (Vmax), and alterations in the ability of DA to bind to the DAT, or apparent Km. For pre-drug modeling, we followed standard voltammetric modeling procedures by setting the baseline Km parameter to 160 nM based on the affinity of DA for the DAT (Wightman et al. 1988; Wu et al. 2001a), whereas Vmax values were allowed to vary as the pre-drug measure of the rate of DA uptake. Following drug application, apparent Km was allowed to vary to account for changes in drug-induced DA uptake inhibition while the respective Vmax value determined for that subject at baseline was held constant. The apparent Km parameter models the amount of DA uptake inhibition following a particular concentration of drug (Ferris et al. 2013).
Statistics
Graph Pad Prism (version 5, La Jolla, CA, USA) was used to statistically analyze data sets and create graphs. DA kinetics were compared with a two-tailed paired-samples t-test to test for differences between baseline (pre-drug) and antagonist/agonist application. Uptake inhibition and release data were subject to a mixed-model repeated measure two-way ANOVA with cocaine concentration (within) and drug treatment (between) as the factors. When main effects were obtained, differences between groups were tested using Bonferroni's post hoc test. Following the raclopride experiments, all subsequent data comparisons were a priori. All p values of < 0.05 were considered to be statistically significant.
Results
The ability of cocaine to inhibit DA uptake was increased with autoreceptor blockade
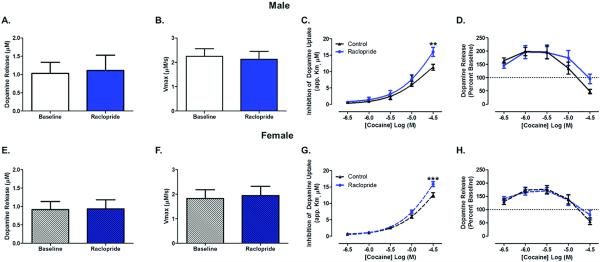
To determine whether D2/D3 autoreceptors interact with cocaine inhibition of the DAT, we used the D2/D3-receptor antagonist, raclopride (100 nM), to block D2 and D3 autoreceptors in the NAc core. In males, there was no change in DA release (Figure 1A) or uptake (Figure 1B) following bath application of raclopride, confirming previous findings that there is no constitutive DA release, or DA tone, present in an acute slice preparation (Phillips et al. 2002; Wilson et al. 2004). Following bath application of cocaine, we found that cocaine-induced increases in apparent Km for DA uptake were greater in the presence of raclopride than in control slices which were run under raclopride-free bath conditions (Figure 1C): repeated measures two-way ANOVA (cocaine concentration X raclopride treatment, n=5), cocaine concentration (F4,32 = 172.3, p < 0.0001), interaction (F4,32 = 4.689, p = 0.0043), Bonferroni's posttest: control vs raclopride (cocaine 30 μM) p < 0.01. In addition to enhancing DA transmission through inhibition of the DAT, which blocks DA uptake, cocaine can mobilize reserve pools of DA-containing vesicles, resulting in increased exocytotic/stimulated release of DA (Venton et al. 2006). We found that cocaine induced effects on release (Figure 1D): repeated measures two-way ANOVA (cocaine concentration X raclopride treatment, n=5), cocaine concentration (F4,32 = 56.99, p < 0.0001), interaction (F4,32 = 4.501, p = 0.0053). However, there was no difference in cocaine-induced changes in DA release between raclopride treatment and control recordings. These data demonstrate that autoreceptors act to regulate cocaine potency at the DAT.
Figure 1. Global autoreceptor blockade enhances cocaine's ability to inhibit dopamine uptake at the dopamine transporter.
Male data (A-D) showing (A) no change in dopamine release or (B) maximal rate of dopamine uptake (Vmax) between baseline and the D2/D3 antagonist, (raclopride, 100 nM), application. (C) Cocaine concentration-response curve (CRC) demonstrating an increase in the inhibition of dopamine uptake at the dopamine transporter (apparent Km) in the presence of raclopride. (D) Cocaine altered dopamine release over concentrations but there were no differences between control and raclopride groups. The 0 μM concentration (baseline) to which the data is normalized is denoted by a dotted line at 100%. Female data (E-H) showing (E) no change in dopamine release or (F) maximal rate of dopamine uptake between baseline and the D2/D3 antagonist application. (G) Cocaine CRC depicting an increase in the apparent Km in the presence of raclopride. (H) Dopamine release across cocaine concentrations indicating no change in cocaine-induced dopamine release between groups. **, p < 0.01; ***, p < 0.001 as compared to control. Male, n=5, female n=6.
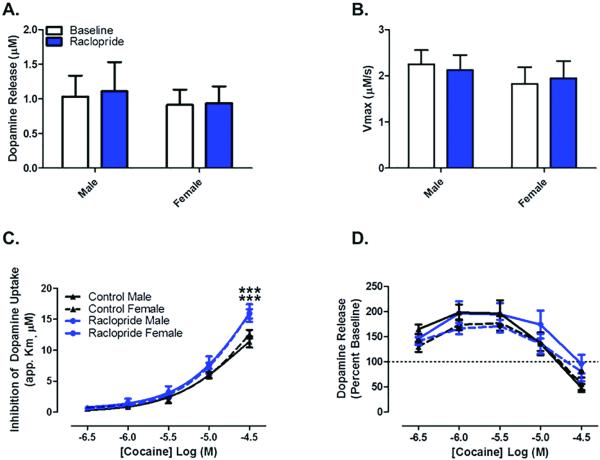
Similarily, in females, there was no change in DA release (Figure 1E) or Vmax (Figure 1F) following raclopride bath application. Following bath application of cocaine we found that cocaine-induced uptake inhibition was augmented in the presence of raclopride (Figure 1G): repeated measures two-way ANOVA (cocaine concentration X raclopride treatment, n=6), cocaine concentration (F4,40 = 400.8, p < 0.0001), raclopride treatment (F1,40 = 9.765, p = 0.0108), interaction (F4,40 = 6.465, p = 0.0004), Bonferroni's posttest: control vs raclopride (cocaine 30 μM) p < 0.001. Cocaine altered release in both groups but there were no differences in cocaine-induced changes in DA release between raclopride treatment and control recordings (Figure 1H): repeated measures two-way ANOVA (cocaine concentration X raclopride treatment, n=6), cocaine concentration (F4,40 = 18.80, p < 0.0001). Additionally, there were no differences between males and females in any of the parameters measured (Figure 2AD). The effects of increased cocaine-induced uptake inhibition in the presence of raclopride remained for raclopride vs control of the same sex (Figure 2C): repeated measures two-way ANOVA (cocaine concentration X raclopride treatment, n=5-6), cocaine concentration (F4,72 = 518.7, p < 0.0001), interaction (F12,72 = 3.800, p = 0.0002), Bonferroni's posttest: control male vs raclopride male (cocaine 30 μM) p < 0.001, control female vs raclopride female (cocaine 30 μM) p < 0.001. Similarly, the effect of cocaine on dopamine release was consistent across group or sex (Figure 2D): repeated measures two-way ANOVA (cocaine concentration X raclopride treatment, n=5-6), cocaine concentration (F4,72 = 66.72, p < 0.0001). Since there were no sex differences in any of the parameters measured, we chose to complete the remainder of the study in male subjects.
Figure 2. Sex has no impact on dopamine kinetics and cocaine effects at the dopamine transporter.
Data showing no sex differences in (A) dopamine release, (B) maximal rate of dopamine uptake, (C) cocaine-induced inhibition of dopamine uptake (although there is still a difference between raclopride and control of same sex), or (D) cocaine-induced dopamine release, under drug-free bath conditions or in the presence of raclopride. ***, p < 0.001 vs same sex control group. Male, n=5, female n=6.
D3, but not D2, autoreceptor inhibition increases the ability of cocaine to inhibit DA uptake
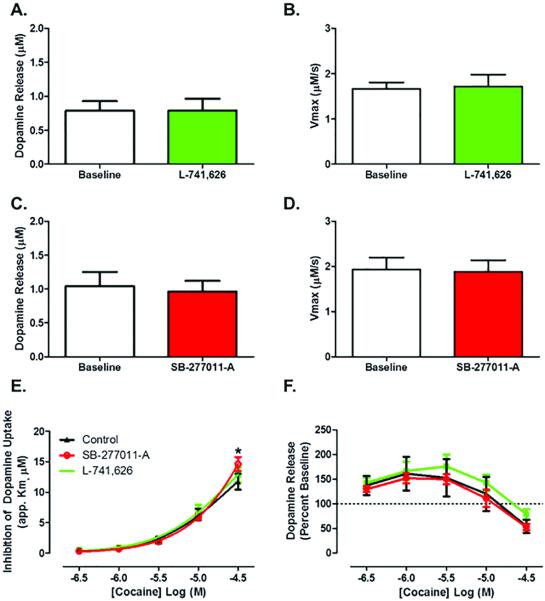
Using the D2-receptor selective antagonist, L-741,626 (30 nM), and the D3-receptor selective antagonist, SB-277011-A (300 nM), we sought to determine whether the increase in cocaine potency that was observed in the presence of raclopride was mediated through DAT functional interactions with the D2 or D3 autoreceptor. In line with what we found upon raclopride application, neither L-741,626 nor SB-277011-A bath application resulted in a change in DA release (Figure 3A, C) or Vmax (Figure 3B, D) between baseline and antagonist application. However, we found that cocaine-induced uptake inhibition was amplified in the presence of the D3-receptor selective antagonist, SB-277011-A (Figure 3E): repeated measures two-way ANOVA (cocaine concentration X antagonist treatment, n=6), cocaine concentration (F4,56 = 273.1, p < 0.0001), Bonferroni's posttest: control vs SB-277011-A (cocaine 30 μM) p < 0.05. Interestingly, there was no difference in cocaine effects in the presence of the D2-receptor selective antagonist, L-741,626, as compared to controls. This demonstrates that autoreceptor modulation of cocaine potency was mediated through DA D3 receptors. Consistent with the raclopride experiment, there were no differences in the observed cocaine-induced increases in DA release between D2 or D3 antagonist treatment and control (Figure 3F): repeated measures two-way ANOVA (cocaine concentration X antagonist treatment, n=5-6), cocaine concentration (F4,56 = 64.26, p < 0.0001).
Figure 3. D3, but not D2, autoreceptor blockade increases the ability of cocaine to inhibit dopamine uptake.
(A) No effect of the D2 selective antagonist L-741,626 (30 nM) on dopamine release or (B) maximal rate of dopamine uptake as compared. (C) No effect of the D3 selective antagonist SB-277011-A (300 nM) on dopamine release or (D) maximal rate of dopamine uptake as compared. (E) D3 autoreceptor antagonist augments cocaine-induced increases in Km but the D2 antagonist has no effect. (F) No effect of D2 or D3 autoreceptor blockade on cocaine-induced dopamine release. *, p < 0.05 as compared to control. Male, n=6.
D2, but not D3, autoreceptor activation decreases the ability of cocaine to inhibit DA uptake
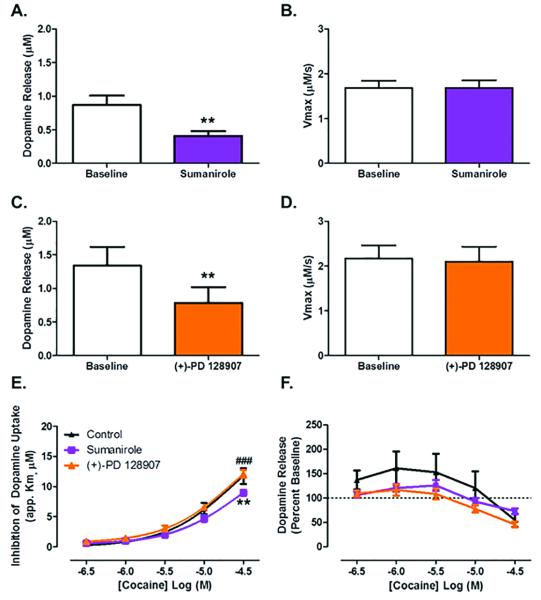
Having demonstrated that D3, but not D2, autoreceptor blockade augmented cocaine potency at the DAT, we hypothesized that activation of D3, but not D2 autoreceptors, would have the opposite effect of the antagonist and result in a decrease in the ability of cocaine to inhibit DA uptake. To test this hypothesis, we used the D2-receptor selective agonist, sumanirole (300 nM), and the D3-receptor selective agonist, (+)-PD 128907 (10 nM). Drug concentrations were selected based on pilot data demonstrating that the IC50 for DA release was 300 nM and 10 nM for sumanirole and (+)-PD 128907, respectively. As expected, following bath application of 300 nM sumanirole, we saw a 53% decrease in DA release as compared to baseline (Figure 4A): paired-samples t-test, t (5) = 5.862, p = 0.002. Similarly, following bath application of (+)-PD 128907, we saw a 41% decrease in DA release as compared to baseline (Figure 4C): paired-samples t-test, t (4) = 5.259, p = 0.0063. There was no change in Vmax with either agonist (Figure 4B, D).
Figure 4. D2, but not D3, autoreceptor activation decreases the ability of cocaine to inhibit dopamine uptake.
(A) ~50% decrease in dopamine release following application of the D2 receptor agonist sumanirole (300 nM) as compared to baseline. (B) No effect of sumanirole on maximal rate of dopamine uptake. (C) ~40% decrease in dopamine release following application of the D3 receptor agonist (+)-PD 128907 (10 nM). (D) No effect of (+)-PD 128907 on maximal rate of dopamine uptake. (E) Cocaine's ability to inhibit dopamine uptake at the dopamine transporter is decreased in the presence of sumanirole, with no effect in the presence of (+)-PD 128907. (F) No change in cocaine effects on dopamine release with sumanirole or (+)-PD 128907. **, p < 0.01 as compared to control; ###, p < 0.001 sumanirole vs. (+)-PD 128907. Male, n=6.
Contrary to our hypothesis, we found that following bath application of cocaine, there was no change in the inhibition of DA uptake in the presence of (+)-PD 128907, and a decrease in uptake inhibition in the presence of sumanirole (Figure 4E): repeated measures two-way ANOVA (cocaine concentration X agonist treatment, n = 5-6), cocaine concentration (F4,52 = 293.1, p < 0.0001), agonist treatment (F2,52 = 4.146, p = 0.0405), interaction (F8,52 = 2.891, p = 0.0096), Bonferroni's posttest: control vs sumanirole (30 μM) p < 0.01, (+)-PD 128907 vs sumanirole (30 μM) p < 0.001. Additionally, cocaine altered dopamine release but there was no difference in cocaine-induced increases in DA release between D2 or D3 agonist treatment and control recordings (Figure 4F): repeated measures two-way ANOVA (cocaine concentration X agonist treatment, n = 5-6), cocaine concentration (F4,52 = 41.64, p < 0.0001), interaction (F8,32 = 2.230, p = 0.0398). Taken together, these data suggest that there is modulation of cocaine potency at the DAT by both D3 and D2 autoreceptors, such that when D3 autoreceptors are blocked, there is an increase in cocaine-induced inhibition of DA uptake and when D2 autoreceptors are activated, there is a decrease in the inhibition of DA uptake.
Discussion
In the present study, we show that in the presence of the D2/D3 autoreceptor antagonist, raclopride, the ability of cocaine to inhibit the DAT was increased in the NAc core of both male and female mice. We did not observe any changes in DA release or the rate of DA reuptake (Vmax) between baseline and raclopride application, and these effects were consistent across sex. Further, we found that raclopride-induced augmentation of cocaine potency was mediated by blockade of D3 type autoreceptors. Finally, we show that D2 and D3 type autoreceptors dynamically modulate cocaine potency, with D2 activation and D3 blockade producing attenuated and augmented cocaine potency, respectively. These data are consistent with previous results showing a synergistic increase in extracellular DA concentration when cocaine is administered following nonselective autoreceptor blockade with raclopride (Aragona et al. 2008). These findings suggest that D2/D3 autoreceptors function to negatively modulate cocaine potency; however, if autoreceptors are “offline” then cocaine can maximally exert its effects.
When D3 autoreceptors were blocked using the selective antagonist, SB-277011-A, the ability of cocaine to inhibit dopamine reuptake at the DAT was increased. However, it does not appear that this effect was occurring through antagonist-inhibition of canonical autoreceptor actions on dopamine release. This is evidenced by our data indicating there was no change in DA release upon antagonist application, which coincides with other studies that note that there is minimal dopamine tone, and thus no tonic agonist activation of autoreceptors, in an acute slice preparation (Phillips et al. 2002; Wilson et al. 2004). Additionally, we found no change in cocaine-induced increases in dopamine release in the presence of raclopride or SB-277011-A, again indicating that the D3 receptor-mediated effect on cocaine potency was not through loss of feedback inhibition on dopamine release. Thus, it is more likely through an allosteric mechanism which may involve direct interactions between D3 autoreceptors and DAT. This idea is supported by a recent study which reported a physical interaction between D3 receptors nd DAT, as assessed by co-immunoprecipitation and in situ proximity ligation assay (Castro-Hernández et al. 2015). It is therefore possible that a D3 receptor antagonist may disrupt this interaction to produce effects on cocaine potency. Similar physical interactions have been seen between D2 receptors and DAT (Bolan et al. 2007; Sullivan et al. 2013; Lee et al. 2007), as well as between D3 and D2 receptors (Maggio and Millan 2010), suggesting that a disruption of the physical interaction between D3 autoreceptors and DATs is a potential explanation for our findings.
In addition to physical interactions, it is also possible that intracellular mechanical and/or signaling mechanisms are involved in the functional D3-DAT interactions. Members of the D2-like dopamine receptor family, including D2 and D3 receptors, couple to the inhibitory Gαi/o family of heterotrimeric G-proteins for signal transduction. This family is commonly characterized by inhibition of adenylyl cyclase signaling and inactivation by pertussis toxin treatment. Specifically, the D2 receptor can couple to both Gαo and Gαi (Lledo et al. 1992; Gazi et al. 2003). Interestingly, in addition to Gαo and Gαi subtypes, the D3 receptor can also couple to Gαz and Gαq, which activate phospholipase C (Sidhu and Niznik 2000; Lane et al. 2008). Previous studies indicate that various agonists may induce selectivity for one G-protein subtype over another (Cordeaux et al. 2001) thereby leading to differential downstream effects, which could differentially affect the DAT. Additionally, both D2 and D3 receptors are involved in the regulation of protein kinase B, also known as Akt, signaling; however, DA inhibition of Akt is dependent on D2 receptor activation but only modulated by D3 receptors (Beaulieu et al. 2007), providing an example of differential regulation produced by these receptors. Previous studies have also found that D2 and D3 autoreceptors regulate DAT function through different mechanisms such that both phosphoinositide 3-kinase (PI3K) and extracellular signal-regulated kinases (ERK1/2) inhibitors abolish the effect of D3 receptor activation on DAT function (Zapata et al. 2007), whereas there is a role of ERK1/2, but not PI3K, in meditating D2 receptor regulation of DAT (Bolan et al. 2007). Taken together, these data offer another explanation for our results such that differential coupling of D2 vs. D3 autoreceptors with downstream intracellular signaling pathways could directly or indirectly affect DAT activity.
Further, DA activates different DA receptors with various affinities (Beaulieu and Gainetdinov 2011). Specifically, dopamine itself has more than 20 times higher affinity at the D3 than at the D2 receptor (Sokoloff et al. 1990). This is another possible mechanism that could account for the differential effects of D2 vs. D3 autoreceptors on cocaine-regulation of DAT activity. In particular, the D3 antagonist may be eliciting an effect because the D3 receptors have such a high affinity for dopamine that even though there is very low DA tone in a slice, these low concentrations may still be sufficient to activate D3, but not the D2 autoreceptors. On the other hand, activation of D2 receptors would require much higher levels of DA in the slice, which may explain why we see no effect of the D2 antagonist.
Initially our findings may appear contradictory to those that report deleting or blocking D2 autoreceptors alters cocaine actions (Bello et al. 2011; Holroyd et al. 2014). However, these discrepancies are likely due to differences in animal/tissue preparation, and lack of agonist activity at autoreceptors in an acute slice preparation, as discussed above. In both of the studies cited above, the authors used AutoDrd2KO mice, which are generated by crossing Drd2loxP/loxP mice with Dat+/IRES-cre mice. The resulting mice have a selective loss of D2 autoreceptors only in neurons that also express the DAT in the midbrain. Bello et al. (2011) reported that AutoDrd2KO mice displayed elevated DA synthesis and release, hyperlocomotion and supersensitivity to the psychomotor effects of cocaine. These effects are likely due to reduced D2 autoreceptor inhibition, as in wild type animals D2 receptors provide strong inhibition of DA cell firing and DA release during times of elevated DA levels, such as following cocaine administration. Additionally, Holroyd et al. (2014) found that the selective loss of D2 autoreceptors impairs the feedback inhibition of DA release, which allows cocaine to induce an amplified effect on DA transmission in the NAc. In both of these studies, deletion of D2 receptors alters cocaine effects primarily in vivo, where there is constant dopamine activity at the receptor. We show that an agonist at D2 receptors decreases cocaine potency; therefore an antagonist in the presence of an agonist would increase cocaine potency. However, we saw no change in potency with the antagonist because there was no DA tone in our slice preparation. Although the studies above report some alterations in vitro, for example, amplification of the effect of cocaine on DA transmission, these effects may be a consequence of compensatory changes in DA neurons due to the long-term nature of genetic deletion. For example, there is elevated DA synthesis and release in the D2 KO mice as compared to their controls, which is likely responsible for the increased cocaine effect in vitro. Importantly, to our knowledge this is the first study to use D2 and D3 selective drugs to examine the role of autoreceptors on cocaine's effects, as previous studies have used nonselective D2/D3 antagonists (Bello et al. 2011; Holroyd et al. 2014; Aragona et al. 2008).
Together, we propose a model whereby presynaptic dopamine autoreceptors dynamically modulate cocaine potency through two separate mechanisms. D2 receptors, as noted by Bello et al. (2011), Holroyd et al. (2014), and in the current study, bi-directionally modulate cocaine potency with receptor activation decreasing and receptor inhibtion (in the presense of an agonist) augmenting cocaine potency, respectively. On the other hand, D3 receptors most likely modulate cocaine potency through an allosteric mechanism which can be disrupted by an antagonist or through differential coupling with downstream signaling pathways that have differential effects on cocaine-regulation of DAT activity. This is the first investigation to show that D3 autoreceptors play a role in cocaine potency. Future studies are needed to examine the exact mechanism in which D3 is modulating DAT to determine if it is a physical interaction or if it is operating through a separate mechanism such as intracellular signaling.
Acknowledgements
This work was funded by NIH grants R01 DA021325, R01 DA030161, P50 DA006634 (SRJ), F31 DA037710 (CAS), and T32 AA007565 (CAS and MMM).
Abbreviations
- DA
dopamine
- DAT
dopamine transporter
- FSCV
fast scan cyclic voltammetry
- NAc
nucleus accumbens
- PI3K
phosphoinositide 3-kinase
- ERK1/2
extracellular signal-regulated kinases 1/2
Footnotes
Conflict of Interest: The authors have no conflicts to report.
References
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J. Neurosci. 2008;28:8821–31. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J-M, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Beaulieu J-M, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR, Borrelli E, Caron MG. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J. Neurosci. 2007;27:881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front. Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello EP, Mateo Y, Gelman DM, Noaín D, Shin JH, Low MJ, Alvarez VA, Lovinger DM, Rubinstein M. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat. Neurosci. 2011;14:1033–8. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolan E. a, Kivell B, Jaligam V, Oz M, Jayanthi LD, Han Y, Sen N, et al. D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol. Pharmacol. 2007;71:1222–1232. doi: 10.1124/mol.106.027763. [DOI] [PubMed] [Google Scholar]
- Castro-Hernández J, Afonso-Oramas D, Cruz-Muros I, Salas-Hernández J, Barroso-Chinea P, Moratalla R, Millan MJ, González-Hernández T. Prolonged treatment with pramipexole promotes physical interaction of striatal dopamine D3 autoreceptors with dopamine transporters to reduce dopamine uptake. Neurobiol. Dis. 2015 doi: 10.1016/j.nbd.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Chen PC, Lao CL, Chen JC. The D3 dopamine receptor inhibits dopamine release in PC-12/hD3 cells by autoreceptor signaling via PP-2B, CK1, and Cdk-5. J. Neurochem. 2009 doi: 10.1111/j.1471-4159.2009.06209.x. [DOI] [PubMed] [Google Scholar]
- Chen R, Daining CP, Sun H, Fraser R, Stokes SL, Leitges M, Gnegy ME. Protein kinase Cβ is a modulator of the dopamine D2 autoreceptor-activated trafficking of the dopamine transporter. J. Neurochem. 2013;125:663–72. doi: 10.1111/jnc.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara G, Di, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. U. S. A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeaux Y, Nickolls SA, Flood LA, Graber SG, Strange PG. Agonist regulation of D2 dopamine receptor/G protein interaction. Evidence for agonist selection of G protein subtype. J. Biol. Chem. 2001;276:28667–28675. doi: 10.1074/jbc.M008644200. [DOI] [PubMed] [Google Scholar]
- Day JJ, Carelli RM. The nucleus accumbens and Pavlovian reward learning. Neuroscientist. 2007;13:148–59. doi: 10.1177/1073858406295854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J, Pilon C, Foll B, Le, Gros C, Triller A, Schwartz JC, Sokoloff P. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J. Neurosci. 2000 doi: 10.1523/JNEUROSCI.20-23-08677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Whisler KN, Fuller DC, Orsulak PJ, Self DW. Addiction-related alterations in D1 and D2 dopamine receptor behavioral responses following chronic cocaine self-administration. Neuropsychopharmacology. 2007;32:354–366. doi: 10.1038/sj.npp.1301062. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Yorgason JT, Jones SR. Examining the complex regulation and drug-induced plasticity of dopamine release and uptake using voltammetry in brain slices. ACS Chem. Neurosci. 2013;4:693–703. doi: 10.1021/cn400026v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282C:13–22. doi: 10.1016/j.neuroscience.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazi L, Nickolls SA, Strange PG. Functional coupling of the human dopamine D2 receptor with G alpha i1, G alpha i2, G alpha i3 and G alpha o G proteins: evidence for agonist regulation of G protein selectivity. Br. J. Pharmacol. 2003;138:775–86. doi: 10.1038/sj.bjp.0705116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford AN, Johnson KM. Effect of chronic cocaine treatment on D2 receptors regulating the release of dopamine and acetylcholine in the nucleus accumbens and striatum. Pharmacol. Biochem. Behav. 1992;41:841–846. doi: 10.1016/0091-3057(92)90236-9. [DOI] [PubMed] [Google Scholar]
- Holroyd KB, Adrover MF, Fuino RL, Bock R, Kaplan AR, Gremel CM, Rubinstein M, Alvarez V. a. Loss of Feedback Inhibition via D2 Autoreceptors Enhances Acquisition of Cocaine Taking and Reactivity to Drug-Paired Cues. Neuropsychopharmacology. 2014:1–15. doi: 10.1038/npp.2014.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol. 2010;90:385–417. doi: 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Jones SR, Lee TH, Wightman RM, Ellinwood EH. Effects of intermittent and continuous cocaine administration on dopamine release and uptake regulation in the striatum: in vitro voltammetric assessment. Psychopharmacology (Berl) 1996;126:331–338. doi: 10.1007/BF02247384. [DOI] [PubMed] [Google Scholar]
- Kahlig KM, Galli A. Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine. Eur. J. Pharmacol. 2003;479:153–8. doi: 10.1016/j.ejphar.2003.08.065. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol. Sci. 1992;13:177–84. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Lane JR, Powney B, Wise A, Rees S, Milligan G. G protein coupling and ligand selectivity of the D2L and D3 dopamine receptors. J. Pharmacol. Exp. Ther. 2008;325:319–30. doi: 10.1124/jpet.107.134296. [DOI] [PubMed] [Google Scholar]
- Lee FJS, Pei L, Moszczynska A, Vukusic B, Fletcher PJ, Liu F. Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J. 2007;26:2127–36. doi: 10.1038/sj.emboj.7601656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Homburger V, Bockaert J, Vincent JD. Differential G protein-mediated coupling of D2 dopamine receptors to K+ and Ca2+ currents in rat anterior pituitary cells. Neuron. 1992;8:455–463. doi: 10.1016/0896-6273(92)90273-g. [DOI] [PubMed] [Google Scholar]
- Maggio R, Millan MJ. Dopamine D2-D3 receptor heteromers: pharmacological properties and therapeutic significance. Curr. Opin. Pharmacol. 2010;10:100–7. doi: 10.1016/j.coph.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Maina FK, Mathews TA. Functional Fast Scan Cyclic Voltammetry Assay to Characterize Dopamine D2 and D3 Autoreceptors in the Mouse Striatum. ACS Chem. Neurosci. 2010;1:450–462. doi: 10.1021/cn100003u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo Y, Lack CM, Morgan D, Roberts DCS, Jones SR. Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology. 2005;30:1455–1463. doi: 10.1038/sj.npp.1300687. [DOI] [PubMed] [Google Scholar]
- Mortensen OV, Amara SG. Dynamic regulation of the dopamine transporter. Eur. J. Pharmacol. 2003;479:159–170. doi: 10.1016/j.ejphar.2003.08.066. [DOI] [PubMed] [Google Scholar]
- Park K, Volkow ND, Pan Y, Du C. Chronic cocaine dampens dopamine signaling during cocaine intoxication and unbalances D1 over D2 receptor signaling. J. Neurosci. 2013;33:15827–36. doi: 10.1523/JNEUROSCI.1935-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Hancock PJ, Stamford JA. Time window of autoreceptor-mediated inhibition of limbic and striatal dopamine release. Synapse. 2002;44:15–22. doi: 10.1002/syn.10049. [DOI] [PubMed] [Google Scholar]
- Rice ME, Patel JC, Cragg SJ. Dopamine release in the basal ganglia. Neuroscience. 2011;198:112–37. doi: 10.1016/j.neuroscience.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Ferris MJ, Jones SR. Biphasic mechanisms of amphetamine action at the dopamine terminal. J. Neurosci. 2014;34:5575–82. doi: 10.1523/JNEUROSCI.4050-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu A, Niznik HB. Coupling of dopamine receptor subtypes to multiple and diverse G proteins. 2000 doi: 10.1016/s0736-5748(00)00033-2. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–51. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Sullivan D, Pinsonneault JK, Papp AC, Zhu H, Lemeshow S, Mash DC, Sadee W. Dopamine transporter DAT and receptor DRD2 variants affect risk of lethal cocaine abuse: a gene–gene–environment interaction. Transl. Psychiatry. 2013;3:e222. doi: 10.1038/tp.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venton BJ, Seipel AT, Phillips PE, Wetsel WC, Gitler D, Greengard P, Augustine GJ, Wightman RM. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J Neurosci. 2006;26:3206–3209. doi: 10.1523/JNEUROSCI.4901-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Rooney MB, Wightman RM, Kuhn CM. Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 2000;95:1061–1070. doi: 10.1016/s0306-4522(99)00500-x. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience. 1988;25:513–23. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Puntis M, Lacey MG. Overwhelmingly asynchronous firing of rat subthalamic nucleus neurones in brain slices provides little evidence for intrinsic interconnectivity. Neuroscience. 2004;123:187–200. doi: 10.1016/j.neuroscience.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith MEA, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J. Neurosci. Methods. 2001a;112:119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Kuhar MJ, Carroll FI, Garris PA. Preferential increases in nucleus accumbens dopamine after systemic cocaine administration are caused by unique characteristics of dopamine neurotransmission. J. Neurosci. 2001b;21:6338–47. doi: 10.1523/JNEUROSCI.21-16-06338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SJ, Johnson KM. Chronic cocaine treatment impairs the regulation of synaptosomal 3H-DA release by D2 autoreceptors. Pharmacol. Biochem. Behav. 1990;36:457–461. doi: 10.1016/0091-3057(90)90241-9. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J. Neurosci. Methods. 2011;202:158–64. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A, Kivell B, Han Y, Javitch J. a., Bolan E. a., Kuraguntla D, Jaligam V, et al. Regulation of dopamine transporter function and cell surface expression by D3 dopamine receptors. J. Biol. Chem. 2007;282:35842–35854. doi: 10.1074/jbc.M611758200. [DOI] [PubMed] [Google Scholar]