SUMMARY
The striatum contains neurochemically defined compartments termed patches and matrix. Previous studies suggest patches preferentially receive limbic inputs and project to dopamine neurons in substantia nigra (SNc), whereas matrix neurons receive sensorimotor inputs and do not innervate SNc. Using BAC-Cre transgenic mice with viral tracing techniques we mapped brain-wide differences in the input-output organization of the patch/matrix. Findings reveal a displaced population of striatal patch neurons termed “exo-patch”, which reside in matrix zones but have neurochemistry, connectivity, and electrophysiological characteristics resembling patch neurons. Contrary to previous studies, results show patch/exo-patch and matrix neurons receive both limbic and sensorimotor information. A novel inhibitory projection from bed nucleus of the stria terminalis to patch/exo-patch neurons was revealed. Projections to SNc were found to originate from patch/exo-patch and matrix neurons. These findings redefine patch/matrix beyond traditional neurochemical topography and reveal new principles about their input-output connectivity, providing a foundation for future functional studies.
In Brief
Using BAC-Cre transgenic mice with Cre-dependent viral tracing, electrophysiology and optogenetics, Smith, Klug, Ross et al. reveal new striatal circuitry and redefine the input-output organization of striatal patch and matrix compartments.
INTRODUCTION
The basal ganglia are subcortical nuclei involved in action selection and motor learning (Hikosaka, 1999; Yin, 2006; Jin and Costa, 2015). The striatum, the main input nucleus of the basal ganglia, contains neurochemically defined compartments termed patches (striosomes) and matrix (rodent: Pert et al., 1976; Herkenham and Pert, 1981; Gerfen, 1984; cat, primate, human: Graybiel and Ragsdale, 1978). The patch compartment is identified as mu opioid receptor (MOR) enriched compared to the surrounding matrix (Pert et al., 1976), among other neurochemical differences including acetylcholinesterase (Graybiel and Ragsdale, 1978), calbindin (Gerfen et al., 1985; Kincaid and Wilson, 1996), enkephalin (Koshimizu et al., 2008), and others (Gerfen and Young, 1988; Besson et al., 1990; Graybiel, 1990). Neurons within patch and matrix compartments respect these boundaries with regards to their dendrites and local axonal collaterals (Gerfen, 1985; Kawaguchi et al., 1989).
While the function of the patch and matrix compartments has not been clearly determined, studies suggest roles in motor learning (Canales and Graybiel, 2000; Lawhorn et al., 2009), reward-guided learning (White and Hiroi, 1998) and biasing decisions during cost-benefit conflict (Friedman et al., 2015). Furthermore, patch/matrix are differentially affected in neurological pathologies such as Parkinson’s disease (Koizumi et al., 2013), Huntington’s disease (Lawhorn et al., 2008), drug addiction (Hurd and Herkenham, 1993), and others (Crittenden and Graybiel, 2011).
Many studies infer function of patch/matrix compartments based on differences in their input-output organization. Specifically, patches are thought to receive preferential limbic innervation (rodent: Gerfen, 1984; Donoghue and Herkenham, 1986; Friedman et al., 2015; cat, primate: Goldman-Rakic, 1982; Eblen and Graybiel, 1995), and project to the dopamine neurons in the substantia nigra pars compacta (SNc), as well as to the substantia nigra pars reticulata (SNr) and other basal ganglia nuclei (rodent: Gerfen, 1984; 1985; Fujiyama et al., 2011; Watabe-Uchida et al., 2012; primates: Jimenez-Castellanos and Graybiel, 1989). In contrast, matrix neurons are preferentially innervated by sensorimotor regions and project only to SNr and other basal ganglia nuclei, but not SNc (cat, primate: Ragsdale and Graybiel, 1981; rodent: Gerfen, 1984; 1985).
Traditionally, the patch and matrix were defined largely by immunostaining and thus based on rough spatial boundaries, not genetic or molecular markers. The development of bacterial artificial chromosomal Cre-recombinase transgenic mice (BAC-Cre) has allowed new methods of dissecting neural circuits with cell subtype specificity (Gong et al., 2003; Gerfen et al., 2013). In combination with Cre-dependent adeno-associated viruses (AAVs) and modified rabies viruses (Wickersham et al., 2007a, b), it is possible to map the input-output organization of specific cell-types with precision (Wall et al., 2012) and investigate functional connectivity via Cre-dependent expression of channelrhodopsin-2 (Boyden et al., 2005).
Here, using BAC-Cre transgenic mice preferential to patch and matrix compartments of striatum we map their input-output organization. Results reveal the existence of striatal neurons, termed “exo-patch”, which are physically located in the matrix but have genetic, neurochemical and electrophysiological properties, as well as connectivity similar to patch neurons. Contrary to previous studies, both patch/exo-patch and matrix neurons integrate limbic and sensorimotor information from identical layers of cortex. However, limbic subcortical inputs have a stronger preference for patch/exo-patch neurons, including a novel inhibitory projection from the bed nucleus of the stria terminalis (BNST). Finally, contrary to previous claims, the predominant input to dopamine neurons in the SNc originates outside of traditionally defined patches, presumably from exo-patch and matrix neurons. These results suggest a critical reappraisal of patch/matrix organization, and provide further insight into these subregion functions.
RESULTS
Validating striatal patch and matrix BAC-Cre mouse lines
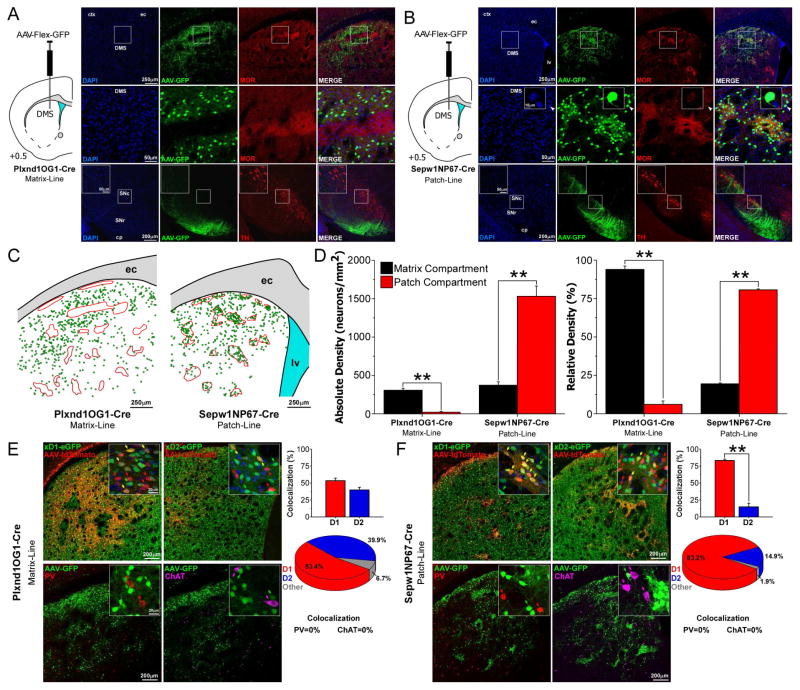
To investigate the input-output organization of striatal patch and matrix compartments, we used BAC-Cre transgenic mice that preferentially express Cre in each compartment (Gerfen et al., 2013). To characterize their specificity, we injected AAVs that express green fluorescent protein (GFP) in a Cre-dependent manner (Figure 1). Injections in matrix-Cre mice (Plxnd1-OG1) revealed GFP-positive neurons distributed throughout the matrix, avoiding the patch compartments as determined by MOR staining (Figure 1A top and middle). Inspection of the midbrain (Figure 1A bottom) revealed dense terminal innervation of SNr, but not SNc. In contrast, GFP expression in striatum following injections in patch-Cre mice (Sepw1-NP67) was extremely dense in MOR-enriched patches (Figure 1B), yet GFP-expression in a subgroup of neurons, scattered throughout the matrix was observed. At higher magnification, these neurons showed significantly elevated perisomatic expression of MOR (Figure S1; Mann-Whitney test, 45 exo-patch neurons from 3 patch-Cre mice, 47 matrix neurons from 3 matrix-Cre mice; U = 2040, Z = 7.67, p = 1.7 × 10−14). These MOR+ cells that reside in the matrix compartment of patch-Cre mice were termed “exo-patch” spiny projection neurons (SPNs). Labeling in the midbrain showed innervation of both SNr and SNc (Figure 1B bottom).
Figure 1.
Characterization of BAC-Cre transgenic mice for studying patch/matrix organization (A) Cre-dependent viral expression in striatum of a Plxnd1OG1 mouse (top and middle rows) that labels neurons in the matrix as defined by lower mu opioid receptor staining (MOR). Midbrain labeling appears in substantia nigra reticulata (SNr) but not pars compacta (SNc). (B) Cre-dependent viral expression in striatum of a Sepw1NP67 mouse reveals labeled neurons clustered densely in MOR-patches as well as a less dense group of neurons in the matrix, termed “exo-patch” neurons. Exo-patch neurons have elevated MOR expression compared to the surrounding matrix cells (inset in middle row, see also Figure S1). Patch-line injections showed dense terminal labeling in SNr and SNc, which closely apposed dopaminergic neurons (bottom row). (C) Digital reconstruction of striatal labeling in panels A and B. (D) Absolute (left graph) and relative (right graph) density measures of GFP-labeled neurons shows labeling was more dense in the matrix compartment of matrix-Cre mice (n = 3, two-tailed t-test, p < 0.01), whereas labeling was denser in the patch compartment of patch-Cre mice (n = 3, two-tailed t-test, p < 0.01). (E) Crossing matrix-Cre mice with D1- and D2-eGFP mouse lines (top row, n = 3 in each group) and injecting AAV-tdTomato in striatum reveals that matrix neurons are both direct and indirect spiny projection neurons (SPN). Counter staining for parvalbumin (PV) or choline acetyltransferase (ChAT) showed no colocalization (bottom row). (F) Patch-Cre striatal neurons are predominantly direct pathway SPNs (n = 2 D1-eGFP x Patch-Cre; n = 3 D2-eGFP x Patch-Cre; two-tailed t-test, p < 0.01; see also Figure S2). Patch (and exo-patch) neurons are born predominantly before matrix neurons (see Figure S3). Values are mean ± SEM. ** p < 0.01; (ec, external capsule; lv, lateral ventricle; cp, cerebral peduncle)
Digital reconstructions of GFP+ cells with respect to MOR defined boundaries of patches (Figure 1C) revealed denser cell labeling in the matrix of matrix-Cre mice (Figure 1D left; paired sample t-test; t2 = 12.16, p = 0.0067), and in the patches of patch-Cre mice (Figure 1D right; paired sample t-test; t2 = 13.16, p = 0.0057). This result was confirmed by relative cell density analysis in matrix-Cre (paired sample t-test; t2 = 19.75, p = 0.0025) and patch-Cre (paired sample t-test; t2 = 47.60, p = 0.00044).
We determined the identity of these neurons relative to direct or indirect pathways based D1- or D2-dopamine receptor expression, respectively (Figure 1E, F top). Patch- and matrix-Cre mice were crossed with D1-eGFP and D2-eGFP mice and injected with AAV-FLEX-tdTomato in striatum to identify Cre+ neurons. Matrix-Cre mice contained both direct (53.4 ± 3.9%) and indirect pathway (39.9 ± 3.9 %) neurons. In contrast, patch-Cre mice were predominantly D1 expressing (83.2 ± 1.9 %; t3 = 9.36, p = 0.0026), matching previous reports (Gerfen and Young, 1988; Besson et al., 1990; Fujiyama et al., 2011; Banghart et al., 2015). Patch and exo-patch SPNs were found to have similar distributions of D1 (Figure S2; patch: 80.3 ± 1.8%; exo-patch: 80.7 ± 2.1%; paired sample t-test; t1 = 0.94, p = 0.52) and D2 SPNs (patch: 14.7 ± 7.1%; exo-patch: 10.1 ± 2.4%; paired sample t-test; t2 = 0.91, p = 0.46). Co-staining for parvalbumin (PV) and choline acetyltransferase (ChAT) revealed no colocalization with GFP+ neurons in patch- or matrix-Cre mice (Figure 1E, F bottom).
Patch neurons develop earlier than a majority of matrix neurons (Graybiel and Hickey, 1982; van der Kooy and Fishell, 1987; Mason et al., 2005). To determine when patch, matrix, and exo-patch neurons develop, we birth dated these cells using prenatal injections of bromodeoxyuridine (BrdU; see Supplemental Experimental Methods) in patch-Cre mice crossed to a Cre-dependent CAG-tdTomato-reporter line (Ai14) on embryonic days ten through twelve (E10–E12). Patch and exo-patch SPNs were observed to develop concomitantly during this embryological period (Figure S3), beginning E10, with matrix neurons largely born in the following days.
Together these results suggest that these BAC-Cre mice are appropriate tools for targeting patch/exo-patch versus matrix SPNs. They also reveal exo-patch SPNs, which are patch-like neurons residing in the striatal matrix.
Electrophysiological characterization of patch and matrix SPNs
To determine whether exo-patch SPNs resemble patch or matrix SPNs, we compared electrophysiological membrane properties, cell excitability, basal synaptic transmission, and responses to the opioid receptor agonist, enkephalin, in patch, matrix and exo-patch SPNs.
Using Cre-dependent viral expression of tdTomato in patch-Cre mice (crossed with D1-eGFP mice), we performed whole-cell patch clamp recordings of patch, exo-patch and matrix D1-SPNs. This experimental approach (Figure 2D), restricted our sampling to eGFP+ D1-SPNs, to avoid known differences in excitability in D1 and D2 SPNs (Gertler et al 2008). TdTomato+ patch-SPNs were found in clusters, with obvious fluorophore expression in somas and neuropil (Figure 2A). TdTomato+ exo-patch SPNs were targeted at least 100μm from nearby patches (Fig 2B). Matrix SPNs were defined as tdTomato-negative neurons, located outside of patch regions (Figure 2C).
Figure 2.
Electrophysiological characterization indicates exo-patch neurons are similar to patch neurons in membrane properties, excitability, basal inhibitory synaptic transmission and enkephalin-mediated suppression of inhibition. (A–C) Patch, exo-patch, and matrix 40× DIC (top) and epifluorescence (bottom) images of dorsal striatal brain slice. White arrows call out example whole-cell recorded neuron. Scale bars, 200μm. (B) Schematic of injection of Cre-dependent AAV expression of tdTomato allowing visualization of patch and exo-patch neurons patch-Cre mice. +0.5 represents rostrocaudal distance from bregma for injection site. (E–G) Representative traces of patch, exo-patch and matrix SPN responses to hyperpolarizing and depolarizing current injection. (H) Plot of number of spikes versus current injection. * p < 0.05; ** p < 0.01; n.s. not significant; Repeated measures-Two-way ANOVA, Tukey’s multiple comparisons. (I–P) Plot of cell capacitance, membrane resistance, membrane time constant (tau), resting membrane potential, rheobase current (current injection to elicit first action potential), firing threshold, action potential (AP) amplitude, and AP area for patch, exo-patch and matrix neurons, respectively. (Q) Representative spontaneous IPSCs (sIPSCs) for patch, exo-patch, and matrix SPNs with plots of sIPSC frequency (R) and amplitude (S) across all cells. (T–V) Electrically evoked IPSC traces for control and post 30μM leu-enkephalin application for patch (red), exo-patch (red), and matrix (black) SPNs. Yellow arrow denotes electrical stimulation artifact. (W) Baseline normalized IPSC amplitude plot for leu-enkephalin bath application. Black rectangle indicates drug wash on. Grey box denotes summary window for quantification relative to 5 minute baseline. (X) Summary data showing average baseline normalized IPSC amplitude. *p < 0.05; ****p < 0.0001. n.s. denotes not significant. Two-way ANOVA, Tukey’s multiple comparisons. All data are shown mean ± SEM.
We assessed cell excitability by hyperpolarizing and depolarizing current injections (Figure 2E–H). Previous studies conflict regarding differences in membrane resistance and resting membrane potential in patch versus matrix SPNs (Kawaguchi et al., 1989, Miura et al. 2007). Patch SPNs displayed increased spiking compared to matrix cells, which was significant at larger current injections (Figure 2H; F(2,13) = 1.757, p = 0.2112; Tukey’s multiple comparison test, 400pA: q = 3.897, p < 0.05; 450pA: q = 4.327, p < 0.01). Exo-patch and patch SPNs did not significantly differ in current injection elicited spiking (Figure 2H). No differences were observed in other membrane properties including capacitance, membrane resistance, membrane time constant and resting membrane potential across patch, exo-patch and matrix SPNs (Fig 2I–L), nor in the depolarizing current sufficient to elicit first action potential (rheobase current), firing threshold voltage, action potential amplitude or action potential area (Figure 2M–P). These results indicate that excitability, but not membrane properties, are different between patch and matrix SPNs, but not patch and exo-patch SPNs.
Next, we isolated spontaneous inhibitory synaptic currents (sIPSCs) and compared the frequency and amplitude of these events. In young mice (2–4 weeks), no difference was observed between patch and matrix SPNs in basal inhibitory synaptic transmission (Miura et al. 2007). Similarly, in adult mice we found no difference in sIPSC frequency or amplitude across patch, exo-patch and matrix SPNs (Figure 2Q–S), indicating basal inhibitory synaptic transmission is similar across these cell-types.
We next tested the responses of patch, exo-patch, and matrix SPNs to opioid agonists. Patch and exo-patch cells show increased staining for MORs (Figure S1), suggesting that enkephalin, the endogenous ligand for MORs (and delta opioid receptors, DOR), could modulate synaptic transmission on exo-patch SPNs. MOR (and DOR) agonists suppress excitatory synaptic transmission on patch and matrix SPNs (Atwood et al. 2014, Blomeley et al. 2011; Jiang and North, 1992; Miura et al. 2007), but MOR (and DOR) agonists selectively suppress IPSCs in patch SPNs (Miura et al. 2007, Banghart et al. 2015). The source of these enkephalin-sensitive inhibitory afferents is believed to be from local D2- SPNs (Banghart et al., 2015), but may arise from as yet unknown extrastriatal sources as well. Therefore, we determined the effect of enkephalin on local, electrically evoked IPSCs. Example IPSC traces in patch, exo-patch and matrix cells are shown before and after wash on of 30μM leu-enkephalin (Figure 2T–V). Matrix SPNs were insensitive to leu-enk bath application, while both patch and exo-patch SPNs showed significant IPSC reduction 2–7mins following leu-enk bath application compared to baseline (Figure 2W, X; F(5,100) = 12.33, p < 0.0001; Tukey’s multiple comparison test, patch: IPSC % baseline = 79.08 ± 3.80, q = 4.515, p < 0.05; exo-patch: 62.92 ± 2.23, q = 8.0801, p < 0.0001; matrix: 98.92 ± 3.983, q = 0.3412, n.s.). These data demonstrate that only patch and exo-patch SPNs show IPSC inhibition to leu-enk application, indicating they should be considered a single population, separate from matrix SPNs.
Mapping whole-brain inputs to patch and matrix compartments with dG-rabies virus
To determine differences in inputs to matrix and patch (including exo-patch) SPNs, we used the EnvA(ΔG-RV-eGFP) G-deletion rabies virus (Wickersham et al., 2007; Wall et al., 2013). This technique expresses GFP in neurons that synapse directly onto the subpopulation of Cre-expressing neurons within the injection site (see Supplemental Experimental Procedures). Unilateral virus injections were carried out in both patch- and matrix-Cre mice (n = 6 per group).
Representative labeling from matrix-Cre (top) and patch-Cre (bottom) injections are shown in Figure 3. In both cases, prominent labeling was observed in the cortex (including limbic, motor, sensory and associative regions), intralaminar and higher order thalamic nuclei, other basal ganglia nuclei, and regions of the brainstem (SNc, Raphe nucleus, and pedunculopontine area). Patch-Cre mice showed additional inputs from subcortical structures including the BNST, claustrum (CLA), central amygdala (CeA), and others.
Figure 3.
Representative examples of labeling throughout the brain following dG-rabies injections in patch- and matrix-Cre mice. Injection method shown in cartoons on the left. Top rows show the dominant inputs to the matrix from many regions of cortex, thalamus and brainstem neuromodulatory centers. Bottom rows show inputs to the patch/exo-patch neurons arise from the same regions, with additional inputs from subcortical limbic regions (pink arrows), especially the claustrum (CLA), central amygdala (CeA), anterior thalamus, and bed nucleus of the stria terminalis (BNST). Abbreviations: M2, secondary motor cortex; Cg, cingulate cortex; GPe, globus pallidus external; AD, anterior dorsal thalamus; AV, anterior ventral thalamus; AM, anterior medial thalamus; S1, primary somatosensory cortex; PC, paracentral thalamus; CL, centrolateral thalamus; Po, thalamic posterior nucelus; Pf, parafasicular thalamic nucleus; STN, subthalamic nucleus; SNc, substantia nigra pars compacta; PPN, pedunculopontine nuclei.
Labeling in both hemispheres throughout the entire brain was counted relative to boundaries for brain regions. The total number of input neurons labeled in patch- and matrix-Cre injections was not significantly different (matrix inputs: 4,943 ± 1,263; patch inputs: 14,899 ± 6,607; Mann-Whitney test, z28 = 1.52, p = 0.13). The normalized distribution of labeled neurons for both hemispheres and the contralateral proportion of cortical labeling were analyzed (Figure 4).
Figure 4.
Analysis of brain-wide direct inputs to patch/exo-patch and matrix SPNs. (A) Normalized distribution of labeling across grouped brain regions following dG-rabies injections in patch- and matrix-Cre mice (n = 6 in each group). The matrix compartment of the striatum showed significantly more inputs from cortex (two-tailed t-test, p = 0.05), whereas patch neurons had significantly more subcortical inputs (excluding thalamus and basal ganglia inputs), labeled as “Other subcortex” group (two-tailed t-test, p = 0.01). In both hemispheres, cortical inputs were significantly more than subcortical inputs (two-tailed t-test, p < 0.01). (B) Bilateral distribution of cortical inputs to patch/exo-patch and matrix compartments of striatum. Dominant inputs were from the limbic, motor and sensory cortices (two-tailed t-test, p < 0.01) (C) Percentage of labeling in the contralateral cortex reveals intratelencephalic projecting corticostriatal neurons (IT-type) go to both patch and matrix. (D) IT-type neurons are more numerous in limbic cortices compared to sensory and associative (two-tailed t-test, p < 0.05). (E) Analysis of individual brain regions showed many limbic subcortical regions are patch/exo-patch preferring, including anterior thalamic nuclei, thalamic reticular nucleus, Bed nucleus of the stria terminalis, substantia innominata, hypothalamus, and septum (two-tailed t-test, p < 0.05). Values are mean ± SEM. In blue, * p < 0.05; ** p < 0.01; In black, * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001. In gray, n.s. means non-significant.
Grouped at a gross anatomical level (cortex, thalamus, basal ganglia, and other subcortical regions), the matrix was found to receive significantly more inputs from cortex (independent t-test, t10 = 2.22, p = 0.050), whereas the group “other subcortical” preferentially innervated patches (independent t-test, t10 = 3.16, p = 0.010). The percentage of ipsilateral labeling was much stronger than contralateral (patch inputs: t10 = 49.65, p = 2.65e−13; matrix inputs: t10 = 45.49, p = 6.35e−13). In both hemispheres, cortex provided the strongest input to striatum (two tailed t-tests, p < 0.01). In the ipsilateral hemisphere, thalamic inputs to striatum were larger than basal ganglia inputs (two tailed t-tests, p < 0.05).
Labeling patterns in cortex were grouped into limbic, motor, sensory, and associative regions (Figure 4B). No significant differences between patch and matrix inputs were observed across cortical groups in either the ipsilateral (F(1,43) = 0.037, p = 0.85) or contralateral hemisphere (F(1,43) = 1.87, p=0.19). A significant difference in labeling between hemispheres was observed (F(1,43) = 72.8, p = 8.4e−11), wherein labeling in the ipsilateral hemisphere was far larger than in the contralateral hemisphere for both patch and matrix injections (patch inputs: t46 = 7.07, p = 7.08e−9; matrix inputs: t46 = 7.49, p = 1.68e−9). In the ipsilateral hemisphere, limbic, motor, and sensory cortices showed similar proportions of labeling, but were larger than associative cortices (two tailed t-tests, p < 0.01, p < 0.0001 and p < 0.001, respectively). The same was true in the contralateral hemisphere (two tailed t-tests, p < 0.01), where limbic cortex also had more labeling than sensory cortex (two tailed t-tests, p < 0.05).
We next analyzed the relative proportion of neurons in the contralateral hemisphere. Matrix cases tended to have more contralateral labeling (Figure 4C), but this difference was not significant (t10 = 1.39, p = 0.19). We then investigated contralateral labeling in different regions of cortex (Figure 4D), as these represent intratelencephalic projecting (IT-type) corticostriatal neurons (Reiner et al., 2010). We observed no significant difference between patch and matrix inputs (F(3,43) = 2.63, p = 0.11). Differences in the number of IT-type neurons were observed across cortical regions (F(3,43) = 11.18, p = 0.000015). Limbic cortices had significantly more IT-type inputs compared to sensory cortex (patch: t10 = 2.70, p = 0.022; matrix: t10 = 2.85, p = 0.017) and associative cortex (patch: t10 = 2.68, p = 0.02; matrix: t10 = 3.85, p = 0.0032).
Differences in specific brain regions are plotted in Figure 4E, which shows regions having greater than 0.4% of total labeling (the remaining regions combined as “other”). In the ipsilateral cortex, significantly larger proportions of matrix-projecting neurons were observed in secondary motor cortex (t10 = 2.39, p = 0.038) and visual cortex (t10 = 2.40, p = 0.038). Additionally, a few subcortical areas had more inputs to the patch compartments, specifically in the anterior thalamic nuclei (AV: t10 = 3.19, p = 0.0096; AD: t10 = 2.98, p = 0.014), thalamic reticular nucleus (t10 = 2.41, p = 0.037), septum (t10 = 2.77, p = 0.020), hypothalamus (t10 = 3.12, p = 0.011), substantia innominata (t10 = 3.14, p = 0.011) and BNST (t10 = 2.69, p = 0.023).
Characterizing striatal input from the BNST
The most prominent difference in inputs to patch and matrix regions revealed by dG-rabies virus tracing was from limbic subcortical structures (Figure 4A, E). Among these regions there were differences in the amount of neuronal inputs (F(6,35) = 4.89, p = 0.001). Specifically, the BNST provided the largest contribution (Figure 5A); significantly more than the claustrum (paired sample t-test; t5 = 2.92, p = 0.033), septum (t5 = 2.96, p = 0.031), hypothalamus (t5 = 3.66, p = 0.015), and substantia innominata (t5 = 3.25, p = 0.023), but not the amygdala (t5 = 2.15, p = 0.084).
Figure 5.
Bed nucleus of the stria terminals (BNST) innervates patch/exo-patch in dorsal striatum as well as SNc. (A) Normalized distribution of patch/exo-patch inputs from limbic subocortical regions are dominated predominantly from BNST (two-tailed t-test, p<0.05). (B) Plxnd1OG1-Cre mice also express Cre in BNST. (C) Trajectory of viral injection in BNST. (D) Cre-dependent viral expression of GFP in BNST reveals a dense population of inhibitory projection neurons as determined by glutamic acid decarboxylase (GAD) co-staining (see inset and Fig. S4). (E) BNST sends dense projections to SNc, as well as weaker inputs to the ventral tegmental area (VTA). (F) and (G) BNST injections also showed labeling in known targets including paraventricular thalamic nucleus (PVN) and lateral hypothalamus (LH). (H–L) Fibers were observed terminating in both patch and matrix compartments (presumably on exo-patch neurons), confirming our dG-rabies tracing. (M) Optogenetic evoked IPSC traces in the presence of AMPAR antagonist NBQX (10μM) and NMDAR antagonist DL-APV (50μM). Application of picrotoxin, a GABAAR antagonist, completely blocks the IPSC. Plot on right shows IPSC amplitudes for patch (circles) and exo-patch neurons (triangles). All values are mean ± SEM.
To confirm connections between BNST and striatum, we made AAV-FLEX-GFP injections in BNST. Fortuitously, Plxnd1OG1-Cre mice (matrix-Cre) also express Cre in BNST (Figure 5B). Injections were made via the contralateral hemisphere (Figure 5C) to avoid any potential leakage in other regions along the track of the injection needle. Injections were cytoarchitecturally confirmed to be located in the BNST using DAPI staining, and co-staining with GAD identified these neurons as GABAergic (Figure 5D and Figure S4).
The case shown in Figure 5D had terminal labeling in the ventral tegmental area (VTA, Figure 5E), the paraventricular nucleus of the thalamus (PVN, Figure 5F), and lateral hypothalamus (LH, Figure 5G), as well as the periaqueductal gray (not shown). These projections are consistent with known projections of the BNST (Stamatakis et al., 2014), verifying viral expression specifically in the BNST. Furthermore, inspection of GPe and SNr (Figure 5E) revealed no GFP expression, indicating no leakage into the surrounding striatum. Surprisingly, we observed extraordinarily dense innervation of tyrosine hydroxylase (TH) positive neurons in SNc, far stronger than in the VTA (Figure 5E), indicating a strong projection to the dopamine neurons that innervate dorsal striatum.
Consistent with our dG-rabies injections in striatum, we also observed BNST projections terminating in striatum (Figure 5H–L). These fibers were small, with en passant boutons indicative of functional synapses (Kincaid and Wilson, 1996), and terminated in both patch and matrix compartments (Figure 5I–L). Rabies tracing from matrix-Cre mice showed almost no labeling in the BNST, suggesting that BNST targets both patch and exo-patch SPNs.
To confirm functional connectivity between BNST and patch/exo-patch SPNs, we injected BNST of patch-Cre mice with AAVs expressing channelrhodopsin-2 (ChR2-eYFP, Boyden et al. 2005) and Cre-dependent AAVs expressing tdTomato in striatum to label patch and exo-patch SPNs (Figure 5M). Fast latency IPSCs were observed in both patch and exo-patch SPNs following laser stimulation (n=8), but not in matrix SPNs. These currents were not diminished by bath addition of AMPAR and NMDAR antagonists, but were completely blocked by the GABAA antagonist, picrotoxin (Figure 5M). These results align with our GAD-positive anterograde-AAV and retrograde-rabies tracing, confirming a strong inhibitory functional connection from BNST to patch and exo-patch SPNs.
Corticostriatal projections target both patch and matrix
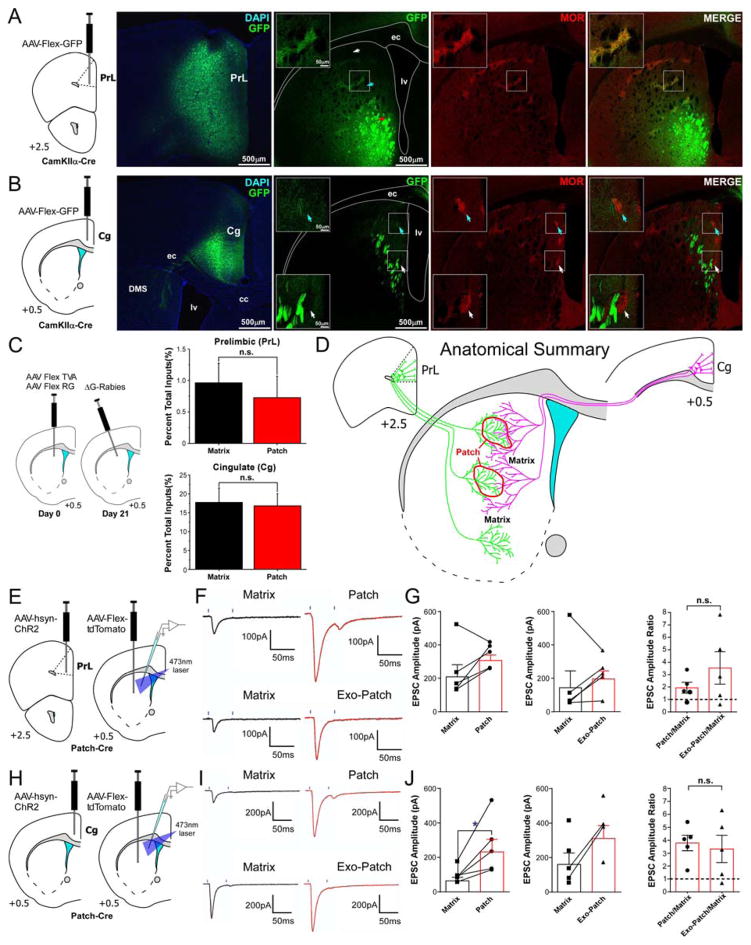
The striatal patch compartment is thought to be preferentially innervated by limbic cortical regions, whereas sensorimotor cortex targets the matrix (Gerfen, 1984; Donoghue and Herkenham, 1986; Eblen and Graybiel, 1995; Friedman et al., 2015). For instance, prelimbic (PrL) and cingulate (Cg) cortex have been previously shown to have a strong preference for patch and matrix, respectively (Donoghue and Herkenham, 1986; Friedman et al., 2015). However, our dG-rabies tracing experiments showed similar patch and matrix innervation by limbic, motor, sensory, and associative inputs (Figure 4B). Specifically, no significant difference was observed between patch and matrix inputs (Figure 4E) for either PrL (t10 = 0.51, p = 0.62) or Cg (t10 = 0.19, p = 0.86), in contrast to previous studies.
To further investigate corticostriatal selectivity for patch/matrix compartments, we injected AAV-FLEX-GFP into PrL and Cg of CamKIIα-Cre mice. Injections into PrL (Figure 6A) revealed dense innervation of patches in dorsal striatum, but also showed terminal labeling in the surrounding matrix, albeit less densely. More ventral regions of striatum showed equal preference for patch and matrix compartments. Injections into Cg (Figure 6B) showed even innervation of patch and matrix in dorsal areas but preferential termination in matrix more ventrally, as previously reported in cats (Ragsdale and Graybiel, 1990). These results, though discrepant with our rabies tracing data (Figure 6C), do show that both patch and matrix compartments are directly innervated by both limbic and sensorimotor cortex (Figure 6D).
Figure 6.
Patch and matrix compartments integrate both limbic and sensorimotor cortex. (A) Virus injections into prelimbic cortex (PrL) of CamKIIα-Cre mice show labeled terminals in both patch and matrix compartments. White arrow shows a region without terminal labeling, blue arrow and inset shows a region with strong patch labeling and weaker matrix labeling, and red arrow shows a region of equal innervation of patch and matrix. (C) Corticostriatal projections from cingulate cortex (Cg). Top inset with blue arrow indicates where Cg projects equally to both patch and matrix. Bottom inset shows a region where Cg avoids a patch and primarily innervates the matrix. (C) Results from dG-rabies tracing in patch- and matrix-Cre mice show no significant difference in labeling between PrL and Cg. (D) Summary cartoon of corticostriatal innervation of patch and matrix by PrL and Cg. (E) Experimental design for whole-cell recordings in striatum following viral expression of channelrhodopsin-2 (ChR2) in PrL with Cre-dependent tdTomato expression in striatum of patch-Cre mice. (F) Example traces of patch/matrix or exo-patch/matrix paired recordings with paired-pulse laser evoked stimulation of PrL terminals in striatum. (G) Plot of EPSC amplitude of patch (circles) and matrix (squares) cell pairs, as well as exo-patch (triangles) and matrix (squares). Far right panel shows EPSC amplitude ratios. (H) Schematic of experiment design with ChR2 in Cg. (I) Paired-pulse laser evoked Cg terminal stimulation on striatal patch/matrix or exo-patch/matrix cell pairs. (J) Plot of EPSC amplitude with same conventions as for PrL in (G). *p < 0.05; n.s., not-significant. All values are mean ±SEM.
To investigate the relationship between corticostriatal terminal density (revealed by viral anterograde tracing) and functional synaptic strength, paired whole-cell recordings were made following viral expression of ChR2 in PrL or Cg (Figure 6E–G, H–J). Patch and exo-patch SPNs were paired with neighboring matrix cells and the ratio of light-evoked EPSC amplitudes was compared between patch/matrix and exo-patch/matrix pairs.
Our anterograde viral tracing indicated that PrL synapses more densely in the patch compartment. Surprisingly, no significant difference was observed in light evoked EPSC amplitudes between patch and neighboring matrix SPNs (Figure 6G left; t4 = 1.676, p = 0.1691), nor between exo-patch and matrix pairs (Figure 6G middle; t4 = 0.7241, p = 0.5091). The overall patch/matrix and exo-patch/matrix ratios were not significantly different (Figure 6G right; p = 0.2834), suggesting that the strength of excitatory inputs from PrL to patch and exo-patch SPNs is similar. This suggests that despite input differences to patch and matrix compartments from PrL, the functional connection strength on individual neurons in these compartments is similar.
We next repeated this experiment for corticostriatal inputs from Cg. Our anterograde tracing data indicated that Cg had somewhat denser input to the matrix compartment. Strikingly, recordings from patch/matrix cell pairs showed significantly larger EPSCs in patch SPNs compared to local matrix SPNs (Figure 6J left; t4 = 2.965, p = 0.0413). Similarly, many exo-patch SPNs showed larger EPSCs compared to their matrix SPN neighbor, though not significantly (Figure 6J middle; t4 = 2.344, p = 0.0791). The ratios of EPSC amplitude for patch/matrix and exo-patch/matrix were not significantly different from each other (Figure 6J right; p = 0.7872), suggesting that both patch and exo-patch SPNs have similar input strengths from Cg.
Cortical layer innervation of patch and matrix compartments
We next investigated specificity of cortical layer projections to patch/matrix. Previous literature has suggested differences in innervation of patch and matrix by cortical layers (Gerfen 1989; Kincaid and Wilson, 1996) or layer 5 IT-type and pyramidal tract projecting (PT-type) corticostriatal neurons (for review, see Reiner et al., 2010).
No differences in the laminar specificity of inputs targeting patch and matrix compartments were observed with our dG-rabies tracing data. Significant differences were found in normalized laminar distribution of corticostriatal neurons, for both the ipsilateral (F(2,66) = 81.73, p < 0.00001) and contralateral hemispheres (F(2,66) = 71.38, p < 0.00001) of patch injections as well as the ipsilateral (F(2,66) = 161.88, p < 0.00001) and contralateral hemispheres (F(2,66) = 82.29, p < 0.00001) of matrix injections (Figure 7A). In limbic cortices, layer 2/3 and layer 5 had similar amounts of labeling, which were significantly larger than layer 6 (two-tailed t-tests, p < 0.01). In motor, sensory, and associative cortices, layer 5 contained significantly more labeling than either layer 2/3 or layer 6 (two-tailed t-tests, p < 0.01). The proportion of labeled neurons in layer 2/3 of limbic cortex was larger than motor cortex, sensory cortex, and associative cortex in both patch and matrix projections (two-tailed t-tests, p < 0.05). Conversely, limbic cortex was found to have a smaller proportion of layer 5 neurons compared to motor cortex, sensory cortex, and associative cortex in both patch and matrix projections (two-tailed t-tests, p < 0.05). Distributions for individual cortical areas in each hemisphere are shown in Figure 7B.
Figure 7.
Laminar analysis of labeled neurons across cortical areas. (A) No significant differences were found between patch and matrix lines, but limbic cortex was found to have significantly more Layer 2/3 labeling than other cortical areas (two-tailed t-test, p<0.05), whereas sensorimotor corticostriatal projections originate predominantly from layer 5 (two-tailed t-test, p<0.05). (B) Normalized distribution of retrogradely labeled neurons across layers of individual cortical areas in each hemisphere. (C–F) Injections of Cre-dependent viruses into secondary motor cortex (M2) of Layer 2/3-Cre, Layer 5 IT-type-Cre, Layer 5 PT-type-Cre, and Layer 6-Cre mice (See Figure S5 for Cre expression and corticostriatal projections to patch and matrix compartments in CamKIIα mice) Labeling in patches denoted by white arrowheads and matrix by blue arrowheads. See Figure S6 for interhemispheric pattern of labeling from these lines.
We next made Cre-dependent viral injections into secondary motor cortex (M2) of mice with Cre in layer 2/3 neurons, layer 5 IT-type, layer 5 PT-type and layer 6 projection neurons (Figure 7 C, D, E, F, respectively, and see Figure S5A–D). Injections across all layers of M2 in a CamKIIα-Cre mouse showed labeling in both patch and matrix compartments, with a slight preference for the matrix (Figure S5E–G) matching our rabies tracing data (Figure 4E). Our anterograde injections targeting layer 2/3, layer 5 IT-type, layer 5 PT-type, and layer 6 projection neurons in M2 showed terminal boutons in both patch and matrix compartments (Figure 7 C, D, E, F). These results demonstrate no differences in patch/matrix input specificity across cortical laminae.
In addition to observations in ipsilateral striatum, we also found a unique pattern of interhemispheric projections to striatum and claustrum (Figure S6). Layer 2/3 neurons projected to the ipsilateral striatum, but sent bilateral projections to the claustrum, whereas layer 5 IT-type neurons project to bilateral regions of striatum, but principally the contralateral claustrum. Layer 5 PT-type and layer 6 neurons only targeted ipsilateral striatum. These results suggest distinct bilateral dissemination of different types of cortical information for movement control (Bauswein et al., 1989; Turner and Delong, 2000).
Revisiting the striatal innervation of SNc dopamine neurons
Patch SPNs, as opposed to matrix SPNs, are thought to project to dopamine neurons in SNc (Gerfen, 1984; Gerfen 1985; Fujiyama et al., 2011; Watabe-Uchida et al., 2012). Our results from AAV injections into matrix- and patch-Cre mice seemed to support this result (Figs. 1A, B bottom). To ascertain whether these projections are truly restricted to patches, we retrogradely labeled striatonigral projections to dopamine neurons using dG-rabies tracing from the SNc of DAT-Cre mice (Figure 8A).
Figure 8.
Striatal projections to dopamine neurons in the SNc originate from both patch and matrix compartments. (A) Injection schema of dG-rabies tracing from SNc of DAT-Cre mice. (B) Retrograde labeling in striatum appears throughout the matrix with some clustering in patches. (C) Digital reconstruction of labeling in striatum with respect to patch boundaries. (D) Most striatal projections to SNc arise from the matrix (n=3 sections, two tailed t-test, p<0.001). (E) Labeling was densest in the patch compartments (two-tailed t-test, p<0.01). (F) Dense retrograde labeling was observed in the BNST, and was somatostatin (SOM) negative. (G) BNST projections to dopamine neurons in the SNc appeared GAD negative.
Inspection of striatal labeling revealed both patch and matrix neurons (Figure 8B,C) that sometimes clustered into patches in dorsal regions (Figure 8B bottom, left and middle), but largely avoided patches in ventral regions (Figure 8B bottom, right). Quantitative analysis revealed that the predominant input to SNc originated from the matrix (Figure 8D, paired sample t-test, t3 = 16.80, p = 0.00046). Previously, neurons could have appeared to be more prominent in the patches due to their higher density (Figure 8E, paired sample t-test, t3 = 6.17, p = 0.0089). Analysis of MOR staining of rabies-GFP+ cells residing in the matrix compartment indicate that these neurons are likely exo-patch and matrix neurons (Figure S1).
From the same injections, dense labeling was observed in the BNST (Figure 8F). These neurons appeared to be GAD-negative (Figure 8G), implying they are likely glutamatergic neurons (Kudo et al., 2012). When considered with our AAV injections into BNST, which were GABAergic, these results suggest that BNST innervation of the SNc might consist of both glutamatergic and GABAergic projections.
DISCUSSION
This study employed BAC-Cre transgenic mice with Cre-dependent viral tracing, ex vivo slice electrophysiology, and optogenetics to investigate the input/output organization of the patch and matrix compartments of striatum. This approach revealed projection neurons in striatum, termed “exo-patch”, which are located in the matrix region but are similar to patch neurons in terms of genetics, neurochemistry, embryogenesis, membrane properties, excitability, basal inhibitory synaptic transmission, response to opioid agonists, and functional connectivity. We found patch and matrix compartments each integrate limbic and sensorimotor information, though patch and exo-patch SPNs are preferentially innervated by subcortical limbic inputs. Among these subcortical inputs, we uncover a new inhibitory circuit from BNST to patch/exo-patch SPNs in striatum. Finally, contrary to dogma, striatal projections to dopamine neurons in SNc originate from cells in both the patch and matrix compartment (predominantly the matrix), and are likely composed of a patch, exo-patch and matrix SPNs. A summary of these findings is graphically illustrated in Figure S7.
Exo-patch striatal projection neurons
Use of BAC-Cre mice in these experiments has called into question the boundaries of the patch and matrix compartments. We have broadened the definition of patches to include “exo-patch” SPNs, which are located in the matrix but are characteristically more similar to patch SPNs. Specifically, patch and exo-patch groups are D1-receptor dominant, as previously shown for patches (Banghart et al., 2015), compared to matrix SPNs. Additionally, exo-patch SPNs appear in the expression profile of multiple patch-enriched genes (see Table 1 in Crittenden and Graybiel, 2011 for list of genes). A review of the available patch-enriched genes with somal expression (n = 8) from the GENSAT database revealed labeling in patches and cells scattered throughout the matrix (Figure S8), resembling the pattern of exo-patch SPNs observed in our Sepw1-NP67-patch-Cre mice.
Interestingly, similar patterns of patch and exo-patch SPNs are observed in prodynorphin expression in striatum of BAC Pdyn-eGFP mice (Figure S8). High levels of Oprm1, the transcript encoding MORs, were found to colocalize with Pdyn-cells (Banghart et al., 2015), supporting the neurochemical relationship of MOR with patch and exo-patch SPNs (Figure S1). Furthermore, our patch clamp recordings confirm that inhibitory transmission in patch and exo-patch SPNs is sensitive to enkephalin, whereas matrix SPNs are not. Finally, our tracing results demonstrate that patch and exo-patch SPNs receive common inputs. For example, BNST inputs were found to preferentially target patch and exo-patch SPNs, but not matrix SPNs (Figure 5M).
When taken together, these data suggest that patch and exo-patch SPNs are a characteristically similar group of neurons, born during the same embryological window, which have been spatially separated through subsequent developmental growth of matrix SPNs (Newman et al., 2015).
A BNST-basal ganglia circuit
Our data have also demonstrated a new circuit by which BNST interacts with the basal ganglia. The BNST is known to be an interface between reward and anxiety and by virtue of projections to the VTA can alter activity in the basal ganglia (Kudo et al., 2012; Jennings et al., 2013; Kim et al., 2013; Stamatakis et al., 2015). Here, we describe a novel addition to this circuitry by demonstrating that BNST sends GABAergic projections to patch and exo-patch SPNs in dorsal striatum, which may in turn innervate dopamine neurons in SNc. Striatonigral projections are inhibitory and therefore likely to inhibit dopamine output (Lerner et al., 2015). Activation of inhibitory BNST projections to dorsal striatum may serve to disinhibit dopamine release. However, patch neurons also innervate SNr (Figure 1B), which could in turn inhibit SNc dopamine neurons (Pan et al. 2013). Thus, future mechanistic studies are required to elucidate how BNST-basal ganglia circuits control midbrain dopamine neurons.
Cortical regions and layers similarly innervate patch and matrix compartments
As discussed above, previous models have suggested that patch and matrix compartments are differentially innervated by limbic and sensorimotor cortex, respectively (reviewed in Crittenden and Graybiel, 2011). Our data demonstrate a complicated pattern of patch/matrix innervation by limbic and sensorimotor cortex (Figure 6D), showing no clear exclusivity. Although similar numbers of neurons in limbic and sensorimotor cortex project to patch and matrix compartments, slight preferences were observed in the terminal distributions of their corticostriatal projections. Specifically, PrL more densely terminates in patches compared to the surrounding matrix, and vice versa for Cg projections. However, our electrophysiology results indicate that at the single cell level, the functional strength of synaptic input from PrL to patch and matrix SPNs is similar. Furthermore, Cg projections to patch SPNs were found to be significantly stronger than to matrix SPNs, despite terminal density showing the opposite trend. One potential explanation is that corticostriatal projections to patch SPNs are inherently stronger than corticostriatal inputs to matrix SPNs. Regardless, these current data refute previous hypotheses that limbic and sensorimotor information are largely segregated between patch and matrix compartments.
Previous studies have suggested that different cortical layers or subtypes of corticostriatal projection neurons preferentially innervate patch and matrix compartments. (Gerfen 1989; Kincaid and Wilson, 1996; Reiner et al., 2010). Surprisingly, we observed no clear trends in our data to support these hypotheses, and instead observed patch and matrix innervation by all layers and corticostriatal subtypes (IT- and PT-Type neurons). Evidence exists for cortical neurons in each layer that appear to preferentially target patch and matrix compartments, respectively (Gerfen et al., 2013, see their Figure 8). Previous studies using small deposits of traditional dextran neuroanatomical tracers may have in some cases selectively labeled these neurons, leading to these observations.
Striatal innervation of dopamine neurons in the SNc
Previous observations on patch/matrix output have stressed that patch neurons provide the striatal inputs to dopamine neurons in the SNc (Gerfen, 1984, 1985; Fujiyama et al., 2011; Watabe-Uchida et al., 2012). However, our dG-rabies tracing from SNc of DAT-Cre mice demonstrate that these projections originate in both the patch and matrix compartments, but predominantly from neurons in the matrix (> 75%). Positively determining the identity of these striatonigral neurons relative to the exo-patch and matrix neurons described in our BAC-Cre mice poses a challenge. However, our analysis of MOR expression indicates a mix of exo-patch- and matrix-like rabies-eGFP+ neurons (Figure S1). Our anterograde injections in matrix-Cre mice revealed terminal arborizations in SNr, but seemingly not SNc. However, these terminals may be synapsing on the dendrites of dopaminergic SNc neurons that extend into SNr. Conversely, a small number of TH+ neurons exist in SNr, which may be the target of these rabies-eGFP+ matrix neurons. Alternatively, these neurons may be part of a subset of striatal neurons not captured by either our patch- or matrix-Cre lines. With the evidence available, we suggest that striatal innervation of dopaminergic SNc neurons originates from patch, exo-patch and matrix neurons, contrary to dogma.
Technical considerations of dG-rabies tracing, anterograde tracing and electrophysiology
The use of dG-rabies as a neuroanatomical tracer has been shown to have remarkable specificity for population specific tracing. However, this method has some interpretative limitations that have been previously described (Wall et al., 2010; 2013), but warrant further discussion here. The actual mechanism of rabies virus retrograde transmission remains incompletely known, therefore it is difficult to assess if the rabies efficiency is different for different types of synapses (excitatory, inhibitory, neuromodulatory, etc.), location of synapses (dendritic spine heads, dendritic spine necks, etc.), or strength/structure of synapses (many or few receptors, probability of release, mushroom-like, etc.). We, like others (Wall et al., 2013), found neuromodulatory synapses seem to be underreported in the data presented here. Midbrain dopamine terminals densely innervate the striatum, but only account for a small percentage of total neurons labeled with the dG-rabies approach.
Additionally, the dG-rabies viral tracing technique is sensitive to the size of starter populations. This issue is particularly pertinent to our study since patches represent only 10% of the striatum (Crittenden and Graybiel, 2011). Due to the linear relationship between starter and input labeled neurons (Watabe-Uchida et al., 2011; Wall et al., 2013), we were able to normalize our cell counts for each animal and control for these variations in the size of starter populations across experiments. While the patch represents roughly 10% of the total number of striatal neurons, when that number is combined with exo-patch neurons the numbers of patch/exo-patch versus matrix SPNs in dorsal striatum is not significantly different. As such, we observed no significant difference in the total number of inputs to patch and matrix compartments using the dG-rabies technique. Regardless, because we compare inputs to patch and matrix in different animals, it is impossible to truly ascertain differences in absolute connectivity magnitude to patch and matrix compartments.
To this end, we employed anterograde tracing methods to verify some of our main rabies results. Surprisingly, in the case of PrL and Cg projections, our dG-rabies tracing data suggested equivalent strength of input to patch and matrix, whereas our anterograde tracing data suggested a preference of PrL for patch and Cg for matrix. However, our ex vivo patch clamp recordings with optogenetic stimulation from these regions discovered the strength of the functional connection on a single neuron to be largely equivalent, in line with our rabies data. These results stress the importance of orthogonal approaches to investigate neural circuitry.
One additional complication in our experimental design is that we cannot disambiguate inputs to patch versus exo-patch SPNs using this technique alone. To address this concern, we employed ex vivo patch clamp recordings with optogenetics. This orthogonal approach confirmed our dG-rabies data in the case of the BNST, where we observed functional connectivity with patch and exo-patch SPNs, but not matrix.
Together these results demonstrate that dG-rabies tracing provides invaluable information about the inputs to specific types of neurons, but caution should be taken when interpreting the strength of connection. While there are limitations of the dG-rabies technique, it does not preclude the tremendous advancement in understanding whole brain connectivity evidenced by the numerous findings employing this technique, especially in combination with functional studies using electrophysiology (Kress et al., 2013).
EXPERIMENTAL PROCEDURES
Mouse lines and virus injections
See Supplemental Experimental Procedures for descriptions of mouse Cre-lines and viruses, as well as detailed descriptions of surgical procedures. All methods followed NIH guidelines and were approved by the Institutional Animal Care and Use Committee at Salk Institute for Biological Studies.
Patch clamp electrophysiology
Whole cell ex vivo patch clamp electrophysiology was used to assay functional connectivity (see Supplemental Experimental Procedures).
Microscopy
Tissue was imaged using an Olympus VS-120 Virtual Slide Scanning Microscope with a 10× objective or a Zeiss LSM 710 Laser Scanning Confocal Microscope.
Data analysis
Data are presented as mean ± SEM in figures and text. Shapiro-Wilk tests were performed on all data to test for normality. Nonparametric statistics were employed when significant deviations from a normal distribution were observed.
Supplementary Material
Highlights.
Redefines striatal patch compartment to include “exo-patch” SPNs in the matrix zone
Novel GABAergic projections from the BNST targets striatal patch/exo-patch SPNs
Both striatal patch and matrix receive limbic and sensorimotor information
Corticostriatal projections to patch and matrix originate from equivalent layers
Acknowledgments
The authors thank Brian Mathur for comments on the manuscript and acknowledge the laboratory of Fred Gage for conversations regarding BrdU experiments. This work was supported by grants from the US National Institutes of Health (R01NS083815 and R01AG047669), the Dana Foundation, the Ellison Medical Foundation, and the Whitehall Foundation to X.J.
Footnotes
Supplemental information includes 8 figures and Supplemental Experimental Procedures.
AUTHOR CONTRIBUTIONS
X.J., J.B.S., and J.R.K. designed the research. J.B.S., D.L.R., N.G.H. and V.I.K. performed neuroanatomical tracing experiments. J.B.S., D.L.R., C.D.H., N.G.H., H.H., and V.I.K. performed perfusions, histology and immunohistochemistry. J.B.S., D.L.R., and C.R.G. performed microscopy. J.B.S., J.R.K., D.L.R., C.D.H., N.G.H., H.H., and V.I.K. analyzed the data. J.R.K. performed patch clamp electrophysiology and analysis. E.M.C developed and provided the rabies virus. C.R.G. developed and provided the BAC Cre mouse lines. J.B.S. and J.R.K. constructed the figures. J.B.S., J.R.K. and X.J. wrote the manuscript. X.J. supervised all aspects of the work. All authors discussed the results, revised and approved the manuscript.
CONFLICTS OF INTEREST
None of the authors declare any conflict of interest, financial or otherwise.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atwood BK, Kupferschmidt DA, Lovinger DM. Opioids induce dissociable forms of long-term depression of excitatory inputs to the dorsal striatum. Nat Neurosci. 2014;17:540–548. doi: 10.1038/nn.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banghart MR, Neufeld SQ, Wong NC, Sabatini BL. Enkephalin disinhibits mu opioid receptor-rich striatal patches via delta opioid receptors. Neuron. 2015;88:1227–1239. doi: 10.1016/j.neuron.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauswein E, Fromm C, Preuss A. Corticostriatal cells in comparison with pyramidal tract neurons: contrasting properties in the behaving monkey. Brain Res. 1989;493:198–203. doi: 10.1016/0006-8993(89)91018-4. [DOI] [PubMed] [Google Scholar]
- Besson MJ, Graybiel AM, Quinn B. Co-expression of neuropeptides in the cats striatum: an immunohistochemical study of substance p, dynorphin b and enkephalin. Neuroscience. 1990;39:33–58. doi: 10.1016/0306-4522(90)90220-x. [DOI] [PubMed] [Google Scholar]
- Blomeley CP, Bracci E. Opioidergic interactions between striatal projection neurons. J Neurosci. 2011;31:13346–13356. doi: 10.1523/JNEUROSCI.1775-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereotypy. Nat Neurosci. 2000;3:377–383. doi: 10.1038/73949. [DOI] [PubMed] [Google Scholar]
- Crittenden JR, Graybiel AM. Basal ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Frontiers in Neuroanatomy. 2011;5:1–25. doi: 10.3389/fnana.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Ostlund SB, James AS, Park CS, Ge W, Roberts KW, Mittal N, Murphy NP, Cepeda C, Kieffer BL, et al. Targeted expression of m-opioid receptors in a subset of striatal direct-pathway neurons restores opiate reward. Nat Neurosci. 2014;17:254–261. doi: 10.1038/nn.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue JP, Herkenham M. Neostriatal projections from individual cortical fields conform to histochemically distinct striatal compartments in the rat. Brain Res. 1986;365:397–403. doi: 10.1016/0006-8993(86)91658-6. [DOI] [PubMed] [Google Scholar]
- Eblen F, Graybiel AM. Highly restricted origin of prefrontal cortical inputs to striosomes in the macaque monkey. J Neurosci. 1995;15:5999–6013. doi: 10.1523/JNEUROSCI.15-09-05999.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G, Van Der Kooy D. Pattern formation in the striatum: developmental changes in the distribution of striatonigral neurons. J Neurosci. 1987;7:1969–1978. doi: 10.1523/JNEUROSCI.07-07-01969.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A, Homma D, Gibb LG, Amemori K, Rubin SJ, Hood AS, Riad MH, Graybiel AM. A corticostriatal path targeting striosomes controls decision-making under conflict. Cell. 2015;161:1–14. doi: 10.1016/j.cell.2015.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama F, Sohn J, Nakano T, Furuta T, Nakamura KC, Matsuda W, Kaneko T. Eur. J Neurosci. 2011;33:668–677. doi: 10.1111/j.1460-9568.2010.07564.x. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984;311:461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic. I Compartmental organization of projections from the striatum to the substantia nigra in the rat. J Comp Neurol. 1985;22:454–476. doi: 10.1002/cne.902360404. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: striatal patch-matrix organization is related to cortical lamination. Science. 1989;246:385–388. doi: 10.1126/science.2799392. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Baimbridge KG, Miller JJ. The neostriatal mosaic: compartmental distribution of calcium-binding protein and parvalbumin in the basal ganglia of the rat and monky. Proc Natl Acad Sci USA. 1985;82:8780–8784. doi: 10.1073/pnas.82.24.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Paletzki R, Heintz N. GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron. 2013;80:1368–1383. doi: 10.1016/j.neuron.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Young WS. Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 1988;460:161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci. 2008;28:10814–24. doi: 10.1523/JNEUROSCI.2660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cytoarchitectonic heterogeneity of the primate neostriatum: subdivision into island and matrix cellular compartments. J Comp Neurol. 1982;205:398–413. doi: 10.1002/cne.902050408. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends in Neuroscience. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Ragsdale CW. Histochemically distinct compartments in the striatum of human, monkey, and cat demonstrated by acetylthiocholinesterase staining. Proc Natl Acad Sci USA. 1978;75:5723–5726. doi: 10.1073/pnas.75.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Ragsdale CW, Yoneoka ES, Elde RP. An immunohistochemical study of enkephalins and other neuropeptides in the striatum of the cat with evidence that the opiate peptides are arranged to form mosaic patterns in register with the striosomal compartments visible by acetylcholinesterase staining. Neuroscience. 1981;6:377–397. doi: 10.1016/0306-4522(81)90131-7. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Pert CB. Mosaic distribution of opiate receptors, parafasicular projections and acetylcholinesterase in rat striatum. 1981;291:415–418. doi: 10.1038/291415a0. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, Miyachi S, Doya K. Parallel neural networks for learning sequential procedures. Trends Neurosci. 1999;22:464–471. doi: 10.1016/s0166-2236(99)01439-3. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZG, North RA. Pre- and postsynaptic inhibition by opioids in rat striatum. J Neurosci. 1992;12:356–361. doi: 10.1523/JNEUROSCI.12-01-00356.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Castellanos J, Graybiel AM. Evidence that histochemically distinct zone of the primate substantia nigra pars compacta are related to patterned distributions of nigrostriatal projection neurons and striatonigral fibers. Exp Brain Res. 1989;74:227–238. doi: 10.1007/BF00248855. [DOI] [PubMed] [Google Scholar]
- Jin X, Costa RM. Shaping action sequences in basal ganglia circuits. Curr Opin Neurobiol. 2015;33:188–196. doi: 10.1016/j.conb.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J Neurophysiol. 1989;62:1052–1068. doi: 10.1152/jn.1989.62.5.1052. [DOI] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, Yeun Lee S, Marshel JH, Kim CK, Mallory CS, Lo M, Pak S, Mattis J, Kook Lim B, Malenka RC, Warden MR, Neve R, Tye KM, Deisseroth K. Diverging neural pathways assemble a behavioral state from separable features in anxiety. Nature. 2013;496:219–223. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid AE, Wilson CJ. Corticostriatal innervation of the patch and matrix in the rat neostriatum. J Comp Neurol. 1996;374:578–592. doi: 10.1002/(SICI)1096-9861(19961028)374:4<578::AID-CNE7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Morigaki R, Okita S, Nagahiro S, Kajo R, Nakagawa M, Goto S. Response of striosomal opioid signaling to dopamine depletion in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease: a potential compensatory role. Front Cellular Neurosci. 2013;7:1–10. doi: 10.3389/fncel.2013.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimizu Y, Wu SX, Unzai T, Hioki H, Sonomura T, Nakamura KC, Fujiyama F, Kaneko T. Paucity of enkephalin production in neo- striatal striosomal neurons: analysis with preproenkephalin-green fluorescent protein transgenic mice. Eur J Neurosci. 2008;28:2053–2064. doi: 10.1111/j.1460-9568.2008.06502.x. [DOI] [PubMed] [Google Scholar]
- Kress GJ, Yamawaki N, Wokosin DL, Wickersham IR, Sheperd GMG, Surmeier DJ. Convergent cortical innervation of striatal projection neurons. Nat Neurosci. 2013;16:665–667. doi: 10.1038/nn.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Uchigashima M, Miyazaki T, Konno K, Yamasaki M, Yanagawa Y, Minami M, Watanabe M. Three types of neurochemical projection from the bed nucleus of the stria terminalis to the ventral tegmental area in adult mice. J Neurosci. 2012;32:18035–18046. doi: 10.1523/JNEUROSCI.4057-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawhorn C, Smith DM, Brown LL. Striosome-matrix pathology and motor deficits in the YAC128 mouse model of Huntington’s disease. Neurobiol Dis. 2008;32:471–478. doi: 10.1016/j.nbd.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Lawhorn C, Smith DM, Brown LL. Partial ablation of mu-opioid receptor rich striosomes produces deficits on a motor-skill learning task. Neuroscience. 2009;163:109–119. doi: 10.1016/j.neuroscience.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner TN, Shilyansky C, Davidson TJ, Evans KE, Beier KT, Zalocusky KA, Crow AK, Malenka RC, Luo L, Tomer R, Deisseroth K. Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits. Cell. 2015;162:635–647. doi: 10.1016/j.cell.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason HA, Rakowiecki SM, Raftopoulou M, Nery S, Huang Y, Gridly T, Fishell G. Notch signaling coordinates the patterning of striatal compartments. Development. 2005;132:4247–4258. doi: 10.1242/dev.02008. [DOI] [PubMed] [Google Scholar]
- Miura M, Saino-Saito S, Masuda M, Kobayashi K, Aosaki T. Compartment-specific modulation of GABAergic synaptic transmission by mu-opioid receptor in the mouse striatum with green fluorescent protein-expressing dopamine islands. J Neurosci. 2007;27:9721–9728. doi: 10.1523/JNEUROSCI.2993-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WX, Brown J, Dudman JT. Neural signals of extinction in the inhibitory microcircuit of the ventral midbrain. Nat Neurosci. 2013;16:71–78. doi: 10.1038/nn.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pert CB, Kuhar MJ, Snyder SH. Opiate receptor: autoradiographic localization in rat brain. Proc Natl Acad Sci USA. 1976;73:3729–3733. doi: 10.1073/pnas.73.10.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale CW, Graybiel AM. The fronto-striatal projection in the cat and monkey and its relatoinship to inhomogeneities established by acetylcholinesterase histochemistry. Brain Res. 1981;208:259–266. doi: 10.1016/0006-8993(81)90556-4. [DOI] [PubMed] [Google Scholar]
- Ragsdale CW, Graybiel AM. Fibers from the basolateral nucleus of the amygdala selectively innervate striosomes in the caudate nucleus of the cat. J Comp Neurol. 1988;269:506–522. doi: 10.1002/cne.902690404. [DOI] [PubMed] [Google Scholar]
- Reiner A, Hart NM, Lei W, Deng Y. Corticostriatal projection neurons - dichotomous types and dichotomous functions. Front Neuroanat. 2010;4:142. doi: 10.3389/fnana.2010.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Sparta DR, Jennings JH, McElligott ZA, Decot H, Stuber GD. Amygdala and bed nucleus of the stria terminalis circuitry: implications for addiction-related behaviors. Neuropharmacology. 2014;76:320–328. doi: 10.1016/j.neuropharm.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Gene Expression Nervous System Atlas (GENSAT) Project, NINDS Contracts N01NS02331 & HHSN271200723701C to The Rockefeller University (New York, NY).
- Turner RS, DeLong MR. Corticostriatal activity in primary motor cortex of the macaque. J Neurosci. 2000;20:7096–7108. doi: 10.1523/JNEUROSCI.20-18-07096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall NR, De La Parra M, Callaway EM, Kreitzer AC. Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron. 2013;79:347–360. doi: 10.1016/j.neuron.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall NR, Wickersham IR, Cetin A, De La Parra M, Callaway EM. Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. 2010;107:21848–21853. doi: 10.1073/pnas.1011756107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- White NM, Hiroi N. Preferential localization of self-stimulation sites in striosomes/patches in the rat striatum. Proc Natl Acad Sci USA. 1998;95:6486–6491. doi: 10.1073/pnas.95.11.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham IR, Finke S, Conzelmann KK, Callaway EM. Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat Methods. 2007a;4:47–49. doi: 10.1038/NMETH999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham IR, Lyon DC, Barnard RJ, Mori T, Finke S, Conzelmann KK, Young JA, Callaway EM. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007b;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nature Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.