Summary
Inflammation is paramount in pancreatic oncogenesis. We identified a uniquely-activated γδT cell population which constituted ∼40% of tumor-infiltrating T cells in human pancreatic ductal adenocarcinoma (PDA). Recruitment and activation of γδT cells was contingent on diverse chemokine signals. Deletion, depletion, or blockade of γδT cell recruitment was protective against PDA and resulted in increased infiltration, activation, and Th1-polarization of αβT cells. Whereas αβT cells were dispensable to outcome in PDA, they became indispensable mediators of tumor-protection upon γδT cell ablation. PDA-infiltrating γδT cells expressed high levels of exhaustion ligands and thereby negated adaptive anti-tumor immunity. Blockade of PD-L1 in γδT cells enhanced CD4+ and CD8+ T cell infiltration and immunogenicity and induced tumor-protection suggesting that γδT cells are critical sources of immune-suppressive checkpoint ligands in PDA. We describe γδT cells as central regulators of effector T cell activation in cancer via novel cross-talk.
Keywords: Cancer, Kras, Checkpoint ligands
Graphical Abstract
Blurb
γδT cells are key regulators of effector T cell activation in pancreatic cancer and a new target for cancer immunotherapy
Introduction
Pancreatic ductal adenocarcinoma (PDA) is a devastating disease in which the mortality rate approaches the incidence rate (Yadav and Lowenfels, 2013). PDA is almost invariably associated with a robust inflammatory cell infiltrate which has considerable influence on disease progression (Andren-Sandberg et al., 1997; Clark et al., 2007). Peri-pancreatic leukocytic subsets can have divergent effects on tumorigenesis by either combating cancer growth via antigen-restricted tumoricidal immune responses or by promoting tumor progression via induction of immune suppression (Zheng et al., 2013). For example, CD8+ T cells and Th1-polarized CD4+ T cells mediate tumor-protection in murine models of PDA and are associated with prolonged survival in human disease (De Monte et al., 2011; Fukunaga et al., 2004). Similarly, we found that negating cytotoxic CD8+ anti-tumor responses by myeloid-derived suppressor cells (MDSC) markedly accelerates PDA growth (Pylayeva-Gupta et al., 2012). Conversely, we recently reported that antigen-restricted Th2-deviated CD4+ T cells strongly promote PDA progression in mice (Ochi et al., 2012b). Accordingly, intra-tumoral CD4+ Th2 cell infiltrates correlate with reduced survival in human PDA (De Monte et al., 2011; Fukunaga et al., 2004).
γδT cells are a non-MHC-restricted lymphocyte subset closely aligned with innate immunity. In vitro activated PBMC-derived γδT cells have cytolytic efficacy against PDA (Oberg et al., 2014). Conversely, a recent study showed that γδT cells produce high levels of tumor-promoting IL-17 in PDA (McAllister et al., 2014). Nevertheless, intra-pancreatic γδT cells have not been well characterized. We found that a novel population of γδT cells with a uniquely activated phenotype infiltrates the pre-neoplastic pancreas and invasive PDA in mice. In human PDA, γδT cells are a dominant T cell population comprising up to 75% of all T lymphocytes. Deletion of intra-pancreatic γδT cells markedly protects against oncogenesis in vivo and results in an influx of immunogenic Th1 cells and CD8+ T cells to the TME. Based on these observations, we postulated that pancreas-infiltrating γδT cells promote PDA progression by inducing adaptive immune suppression. We discovered novel γδT cells cross-talk with CD4+ and CD8+ T cells implicating γδT cells as primary regulators of αβT cell activation in PDA.
Results
Activated γδT cells are ubiquitous in human PDA
Immunohistochemical analysis revealed that γδT cells are widely distributed within the human PDA tumor stroma but absent in normal pancreas (Figure 1a). Moreover, up to 75% of human PDA-infiltrating T cells were TCRγ/δ+ compared with a much lower fraction in PBMC (Figure 1b). On average, γδT cells had a similar prevalence to select myeloid-derived cellular subsets within the PDA TME (Figure 1c) and comprised a significantly higher percentage of tumor-infiltrating lymphocytes compared with CD8+ T cells (Figure 1d). Human T cell subsets, including γδT cells, can be broadly classified as central memory (TCM) or effector memory (TEM) based on their co-expression of CD45RA and CD27 (Sallusto et al., 2004). We found γδT cells in PBMC were predominantly TCM whereas PDA-infiltrating γδT cells were mostly TEM cells, indicative of a distinctly activated phenotype (Figure 1e). Accordingly, tumor-infiltrating γδT cells down-regulated CD62L compared with their counterparts in PBMC (Figure 1f). However, Vγ9+ γδT cells – associated with tumoricidal function (Izumi et al., 2013; Kunzmann et al., 2012) – were notably absent in PDA, suggestive of tumor-permissive properties (Figure 1g).
Figure 1. γδT cells are ubiquitous and activated in human PDA.
(a) Frozen sections of human PDA and normal pancreas were stained using a mAb specific for TCRγ/δ or isotype control. Representative images and quantitative data are shown. (b) Single cell suspensions from human PDA tumors and PBMC were co-stained for CD45, CD3, and TCRγ/δ. The percentage of γδT cells among CD3+ cells was calculated. Representative contour plots and summary data are shown. Each dot represents a different patient sample. (c) The percentage of PDA-infiltrating γδT cells among CD45+ cells was compared with tumor-infiltrating cells expressing select myeloid differentiation markers. (d) The percentage of PDA-infiltrating and PBMC γδT cells among CD3+ cells was compared with that of CD4+ and CD8+ αβT cell subsets in each respective compartment. (e) PBMC and PDA-infiltrating CD3+TCRγ/δ+ cells from PDA patients were gated and co-stained using mAbs specific for CD45RA and CD27. The gating paradigms for Tnaive, TCM, TEM, and TEM-RA populations are shown. Representative contour plots and quantitative data indicating the fraction of TEM γδT cells in each compartment are indicated. (f) PDA-infiltrating and PBMC γδT cells from PDA patients were stained using mAbs specific for CD62L and (g) Vγ9. Representative histograms and quantitative data are shown. Human data are based on tumor tissue or PBMC analyzed from 9–13 PDA patients (*p<0.05, **p<0.01, ***p<0.001).
A distinctly activated γδT cell population is prominent in invasive and pre-invasive murine PDA
In vivo imaging of pancreata from C57BL/6-Trdctm1Mal mice harboring orthotopically implanted Pdx1Cre;KrasG12D;Tp53R172H (KPC)-derived invasive PDA suggested that γδT cells were highly prevalent in the interstitial space of murine PDA (Figure 2a). Flow cytometry suggested a higher frequency of γδT cells infiltrating orthotopic KPC tumors compared with the spleen of tumor-bearing mice (Figure 2b). Similar to human disease, the population of PDA-infiltrating γδT cells in mice were distinctly activated expressing higher FasL, NK1.1, CD39, CD44, JAML, and OX40 compared with spleen γδT cells (Figure 2c). Further, in contrast to spleen, PDA-infiltrating γδT cells contained a prominent Vγ4+ subset whereas Vγ1+ cells were rare (Figure 2c). Tumor-infiltrating γδT cells also expressed elevated levels of IL-10 and IL-17 (Figure 2d, e). Similarly, Th1- (TNFα, IFNγ), and additional Th2- (IL-13) cytokines were highly expressed in PDA-infiltrating γδT cells (not shown). Moreover, PDA-infiltrating γδT cells exhibited a substantial FoxP3+ fraction which has been associated with immune suppressive function (Kang et al., 2009), compared with absent expression of FoxP3+ in spleen γδT cells (Figure S1a). Conversely, T-bet was equally expressed in γδT cells in both compartments (Figure S1b). Further, PDA-infiltrating γδT cells expressed high levels of the NKG2D receptor (Figure 2f) as well as elevated TLR4, TLR7 and TLR9 (Figure 2g) which are potential avenues for cellular activation in PDA (Zambirinis et al., 2015). CCR2, CCR5, and CCR6 were also upregulated in PDA-infiltrating γδT cells (Figure 2h).
Figure 2. γδT cells are highly prevalent and exhibit a uniquely activated phenotype in murine invasive PDA.
(a) C57BL/6-Trdctm1Mal mice whose γδT cells express GFP were orthotopically implanted with KPC-derived tumor and imaged by intra-vital two-photon laser-scanning microscopy at 21 days. (b) WT mice were orthotopically implanted with KPC-derived tumor cells. On day 21, single cell suspensions of digested PDA tumors and splenocytes were co-stained for CD45, CD3, TCRγ/δ, CD4, and CD8 and analyzed by flow cytometry. Representative contour plots and quantitative data are shown. (c) WT mice were orthotopically implanted with KPC-derived tumor cells. On day 21 spleen (blue histograms) and PDA-infiltrating (red histograms) γδT cells were gated and tested for co-expression of select surface activation markers and Vγ chains. Representative histogram overlays and summary data from 5 mice are shown. (d) Spleen and PDA-infiltrating γδT cells from the same mice were tested for expression of IL-10, (e) IL-17, (f) NKG2D, (g) TLR4, TLR7, TLR9, and (h) CCR2, CCR5, and CCR6. Each experiment was repeated at least 3 times using 3–5 mice per data point (*p<0.05, **p<0.01).
To determine whether γδT cells were similarly prominent in a slowly progressive model of PDA, we interrogated pancreata of 6 month-old p48Cre;KrasG12D (KC) mice harboring preinvasive tumor. γδT cells represented ∼6–8% of CD3+ T cells in the pancreas of KC mice compared with ∼2% in the spleen and tumor-draining lymph nodes (Figure S2a). Further, similar to mice with invasive PDA, γδT cells expressed high levels of chemokine receptors (Figure S2b), TLRs (Figure S2c), and activation markers, and included a prominent Vγ4+ fraction (Figure S2d).
γδT cell recruitment and activation in PDA is contingent on diverse chemokine signaling
Since we found that PDA-infiltrating γδT cells express high CCR2, CCR5, and CCR6, we postulated that ligation of these receptors is critical in their recruitment to the TME. To test this, we challenged CCR2−/−, CCL2−/−, CCR5−/−, and CCR6−/− mice with orthotopic KPC-derived tumor and measured γδT cell infiltration on day 21. Deletion of CCR2, CCL2, or CCR6 significantly reduced γδT cell infiltration to the TME (Figure S3a). Moreover, selective CCR2, CCR5, CCR6 or CCL2 deletion mitigated TNF-α and IL-13 expression from PDA-infiltrating γδT cells whereas γδT cell expression of IL-17 and IFN-γ were not affected (Figure S3b–e). γδT cell expression of FoxP3 or IL-10 were similarly not perturbed by blockade of chemokine signaling (not shown).
γδT cells promote pancreatic oncogenesis
Since γδT cells are a prominent lymphocytic subset within the pancreatic TME, we postulated that they play a critical role in oncogenesis. To test this, we crossed KC mice with Tcrδ−/− mice. Pancreata of KC;Tcrδ−/− mice were protected from progressive oncogenesis exhibiting a diminished rate of acinar replacement by dysplastic ducts and substantially slower PanIN progression at multiple time-points (Figure 3a). Analysis of pancreas weights confirmed the protective effects of γδT cell deletion (Figure 3b). γδT cell ablation was also associated with reduced peri-tumoral fibrosis (Figure 3c). Moreover, Kaplan-Meier analysis revealed a nearly 1 year increase in the median survival of γδT cell-deficient KC mice compared with controls (Figure 3d).
Figure 3. Ablation of γδT cells protects against pancreatic oncogenesis in a slowly progressive model of PDA.
(a) KC;Tcrδ+/+ and KC;Tcrδ−/− mice were sacrificed at 3, 6, or 9 months of life (n=10–12 mice/cohort). Representative H&E-stained frozen sections are shown. The percentage of pancreatic area occupied by intact acinar structures, and the fractions of ductal structures exhibiting normal morphology, ADM, or graded PanIN I-III lesions were calculated. (b) Weights of pancreata were compared in 3 month-old KC;Tcrδ+/+ and KC;Tcrδ−/− mice. (c) Pancreata from 9 month-old KC;Tcrδ+/+ and KC;Tcrδ−/− mice were assayed for peri-tumoral fibrosis using trichrome staining. (d) Kaplan-Meier survival analysis was performed for KC;Tcrδ+/+ (n=29) and KC;Tcrδ−/− (n=44) mice (p<0.0001). (e, f) KC;Tcrδ+/+ mice were treated with UC3-10A6 or isotype control for 8 weeks beginning at 6 weeks of life. (e) Representative H&E stained pancreatic sections are shown. The percentage of pancreatic area occupied by intact acinar structures, and the fractions of ductal structures exhibiting normal morphology, ADM, or graded PanIN I-III lesions were calculated. (f) Tumor weight was recorded (n=5/group; *p<0.05, **p<0.01).
Since genetic deletion of γδT cells has limited translational applicability to human disease, we tested whether in vivo depletion of Vγ4+ γδT cells using a neutralizing mAb would offer similar protection (Figure S3f). We treated 6 week-old KC mice for 8 weeks with UC3-10A6 or isotype control and assessed their effects on tumorigenesis. γδT cell depletion protected against oncogenic progression based on histological analysis of ductal transformation (Figure 3e) and tumor mass (Figure 3f). To determine whether the presence of γδT cells are similarly associated with accelerated tumorigenesis in an invasive model of PDA, we orthotopically implanted KPC-derived tumor cells into the pancreatic body of WT and Tcrδ−/− mice. Consistent with our data in KC mice, deletion of γδT cells impressively protected against tumor growth and extended survival in the orthotopic KPC model (Figure S3g, h). γδT cell depletion similarly extended survival in invasive PDA (Figure S3g). Moreover, blocking γδT cell recruitment and activation using mice deficient in selective chemokine signaling was also protective (Figure S3i). Notably, disease phenotype in caerulein-induced pancreatitis was not mitigated in Tcrδ−/− mice suggesting that the ability of γδT cells to modulate pancreatic disease is specific to PDA (Figure S4a–g).
PDA-infiltrating γδT cells do not have direct pro-tumorigenic effects on epithelial cells
We hypothesized that γδT cells may have direct oncogenic effects on transformed epithelial cells. To test this, we co-cultured tumor cells derived from KPC mice with FACS-sorted PDA-infiltrating γδT cells. However, γδT cells failed to enhance proliferation (Figure S4h) or deregulate expression of oncogenic or tumor suppressor genes (Figure S4i) in transformed epithelial cells. Similarly, γδT cell co-culture did not elicit pro-inflammatory or regulatory cytokine production from tumor cells suggesting that PDA-infiltrating γδT cells do not promote tumorigenesis via direct engagement of cancer cells (Figure S4j).
γδT cells support an immune suppressive pancreas tumor microenvironment in invasive and preinvasive PDA
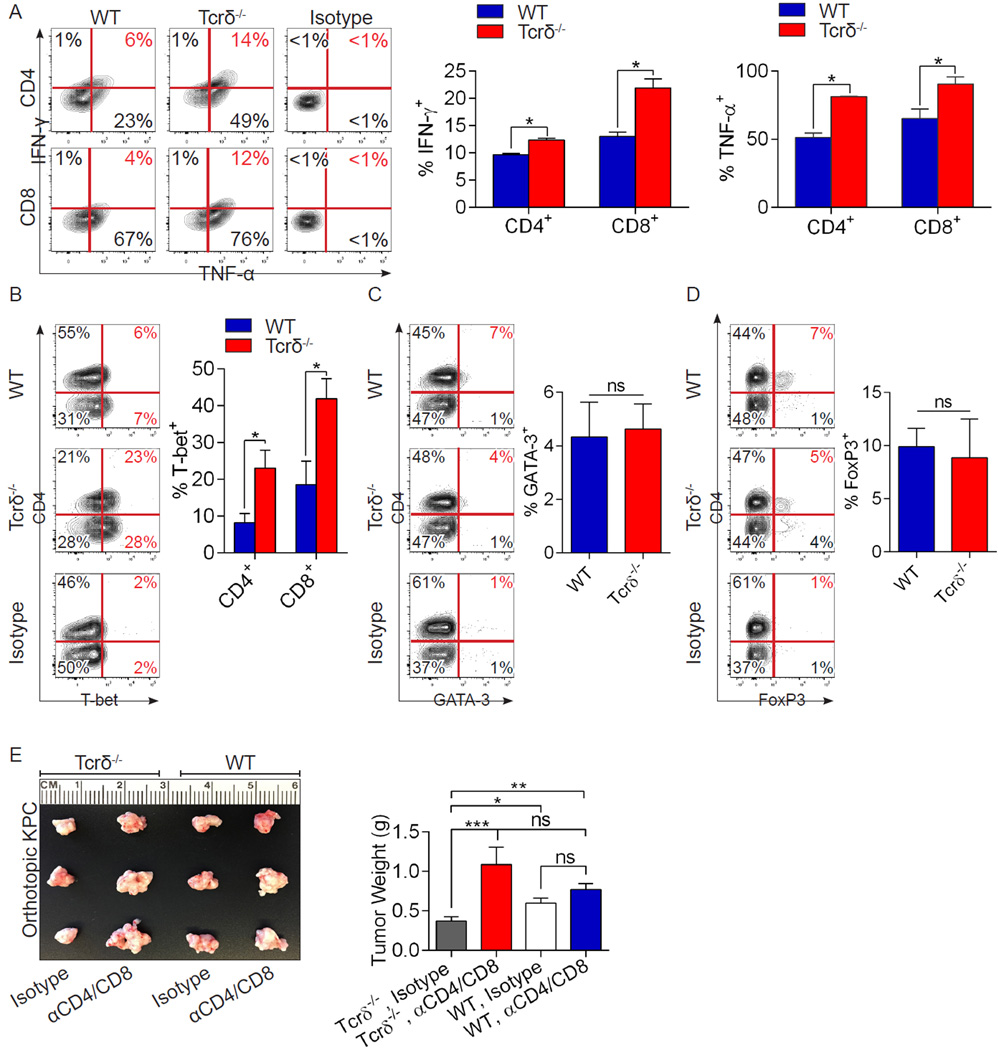
We postulated that intra-pancreatic γδT cells may promote tumorigenesis by engendering an immune-suppressive pancreatic TME. We found that whereas CD4+ and CD8+ T cells were scarce in invasive PDA tumors, tumor-infiltrating CD4+ and CD8+ T cells increased ∼10-fold in absence of γδT cells (Figure 4a, b). Moreover, besides expanding in number, PDA-infiltrating αβT cells were markedly activated in Tcrδ−/− hosts. CD8+ T cells infiltrating γδT cell-deficient tumors expressed higher CD44 (Figure 4c), ICOS (Figure 4d), CTLA4 (Figure 4e), and Granzyme B (Figure 4f), each indicative of higher cytotoxic T cell activation. Similarly, CD4+ T cells infiltrating γδT cell-deficient tumors expressed higher CD44 (Figure 4g), OX40 (Figure 4h), and PD-1 (Figure 4i), and lower CD62L (Figure 4j). Further, both CD4+ and CD8+ T cells expressed elevated TNF-α and IFN-γ in γδT cell-deleted tumors, indicative of enhanced Th1-differentation and higher CD8+ T cell cytotoxicity (Figure 5a). Accordingly, PDA-infiltrating CD4+ and CD8+ T cells each sharply upregulated T-bet expression in the context of γδT cell deletion (Figure 5b). GATA-3 and FoxP3 expression in CD4+ T cells were not affected by γδT cell deletion (Figure 5c, d). Collectively, these data suggest immunogenic reprogramming of adaptive αβT lymphocytes in PDA in the absence of γδT cells.
Figure 4. γδT cell deletion results in massive CD4+ and CD8+ T cell infiltration and activation in invasive PDA.
(a, b) WT and Tcrδ−/− mice were implanted with KPC-derived tumor cells. On day 21 mice were sacrificed. Frozen pancreatic sections were tested for (a) CD8+ and (b) CD4+ T cell infiltration by IHC (n=5/group). (c) CD8+ T cells infiltrating orthotopically-implanted KPC-derived tumors in WT and Tcrδ−/− mice were tested for expression of CD44, (d) ICOS, (e) CTLA-4, and (f) Granzyme B. (g) Similarly, CD4+ T cells infiltrating orthotopically-implanted KPC tumors in WT and Tcrδ−/− mice were tested for expression of CD44, (h) OX40, (i) PD-1, and (j) CD62L. Experiments were repeated more than 3 times with similar results using 5 mice per group (*p<0.05, **p<0.01, ***p<0.001).
Figure 5. γδT cell deletion results in CD4+ T cell Th1 differentiation, CD8+ T cell activation, and αβT cell-dependent tumor protection in invasive PDA.
(a–d) WT and Tcrδ−/− mice were orthotopically implanted with KPC-derived tumor cells. On day 21, tumor-infiltrating CD4+ and CD8+ T cells were interrogated for (a) co-expression of TNF-α and IFN-γ, (b) expression of T-bet, (c) GATA-3, and (d) FoxP3. Representative contour plots and quantitative data are shown. Experiments were repeated twice with similar results (n=5/group; *p<0.05). (e) WT and Tcrδ−/− pancreata were orthotopically implanted with KPC-derived tumor cells and serially treated with α-CD4 and α-CD8 neutralizing mAbs or isotype controls. Pancreatic tumors were harvested at 3 weeks. Representative images and tumor weights are shown (n=5/group; *p<0.05, **p<0.01, ***p<0.001).
To determine whether γδT cells similarly delimit αβT cell expansion and activation in a slowly progressive model of PDA, we compared CD4+ and CD8+ T cell phenotype in KC;Tcrδ+/+ versus KC;Tcrδ−/− pancreata. We found that while CD4+ and CD8+ T cells were scarce in KC;Tcrδ+/+ controls, both lymphocyte populations were markedly expanded in KC;Tcrδ pancreata (Figure S5a, b). Further, both CD4+ and CD8+ T cells in PDA-draining lymph nodes expressed higher CD44 (Figure S5c) and PD-1 (Figure S5d) in KC;Tcrδ−/− animals compared with KC;Tcrδ+/+. Similarly, ICOS and Granzyme B expression were increased in CD8+ T cells in KC;Tcrδ−/− hosts (Figure S5e). Moreover, similar to the orthotopic KPC model, pancreas-draining CD4+ and CD8+ T cells in KC mice upregulated IFN-γ (Figure S5f) and T-bet (Figure S5g) in the context of γδT cell deletion whereas CD4+ T cell expression of GATA-3 and FoxP3 were unaffected by γδT cell deletion (Figure S5h, i).
To definitively test whether enhanced αβT cell immunogenicity accounts for the protection against PDA observed in γδT cell-deficient animals, we depleted CD4+ and CD8+ T cells in Tcrδ−/− mice and WT controls coincident with KPC-derived orthotopic tumor challenge. Ablation of αβT cell populations did not accelerate tumor growth in WT hosts but completely reversed the tumor-protective effects of γδT cell deletion. These data suggest that tumor-protection in PDA-bearing Tcrδ−/− mice is mediated by αβT cells (Figure 5e). To test whether PDA-infiltrating γδT cell inhibition of CD4+ and CD8+ T cells requires direct cellular interaction, we activated spleen αβT cells in vitro using CD3/CD28 co-ligation alone or in the context of either co-culture with PDA-derived γδT cells or admixture with γδT cell-conditioned media. Direct γδT cell coculture prevented CD4+ and CD8+ T cells from adopting an activated CD44+CD62L− phenotype (Figure S6a, b) and expressing immune-modulatory cytokines (Figure S6c–e); however, γδT cell-conditioned media was non-inhibitory. These data suggest that γδT cells do not inhibit αβT cells via secreted factors but require direct cellular interaction.
Pancreas-infiltrating γδT cells express high T cell exhaustion ligands
We postulated that γδT cells may directly inhibit CD4+ and CD8+ T cell activation. We discovered that PDA-infiltrating γδT cells in KC mice expressed high PD-L1 (Figure 6a) and Galectin-9 (Figure 6b) compared with absent expression of these ligands in spleen γδT cells. Similarly, γδT cells in orthotopic KPC tumors also expressed elevated PD-L1 and Galectin-9 (Figure 6c). Expression levels of PD-L1 and Galectin-9 in PDA-infiltrating γδT cells were markedly higher than in cancer cells and comparable with that of tumor-infiltrating myeloid cell populations (Figure 6c). By contrast, PDA-infiltrating γδT cells expressed elevated B7-1 but low levels of other activating ligands including B7-2, ICOSL, and OX40L in orthotopic KPC (Figure 6d) and KC (not shown) tumors. Exhaustion ligand expression in myeloid or tumor cells in PDA was not affected by γδT cell deletion (Figure 6e). Notably, besides regulating γδT cell expansion and activation, CCR2, CCR5, and CCR6 signaling were necessary for γδT cell expression of PD-L1 or Galectin-9 (Figure 6f, g). To determine whether these findings translated to human disease, we tested PD-L1 expression in human PDA. Remarkably, PBMC γδT cells in PDA patients expressed elevated PD-L1 compared with absent PD-L1 expression in PBMC γδT cells from healthy subjects (Figure 6h). Moreover, PD-L1 was expressed in ∼50% of tumor-infiltrating γδT cells in human PDA (Figure 6i). Similarly, Galectin-9 was upregulated in human PDA-infiltrating γδT cells (Figure 6j).
Figure 6. PDA-associated γδT cells express high levels of T cell exhaustion ligands in multiple murine tumor models and in human disease.
(a) Expression of PD-L1 and (b) Galectin-9 were compared in pancreas and spleen γδT cells of 3-month-old KC mice by flow cytometry. Representative contour plots and quantitative data are shown (n=5/group). (c) WT mice were orthotopically implanted with KPC-derived tumor cells. Expression of PD-L1 and Galectin-9 were compared in PDA tumor cells, TAMs (Mφ), MDSC, and γδT cells on day 21 (n=5/group). (d) WT mice were orthotopically implanted with KPC-derived tumor cells. On day 21, spleen and PDA-infiltrating γδT cells were tested for expression of select activating ligands. Representative histograms and quantitative data are shown (n=5/group). (e) Orthotopic PDA-bearing WT and Tcrδ−/− mice were tested for expression of PD-L1 in tumor cells, TAMs, and MDSC (n=5/group). (f) WT, CCR2−/−, CCR5−/−, and CCR6−/− mice were orthotopically implanted with KPC-derived PDA cells (n=5/group). Animals were sacrificed at 3 weeks, and the fraction of tumor-infiltrating γδT cells expressing PD-L1 and (g) Galectin-9 were determined by flow cytometry. (h, i) PBMC γδT cells from healthy volunteers and PDA patients, and PDA-infiltrating γδT cells and were tested for expression of (h) PD-L1 and (i) Galectin-9. Representative histograms and quantitative data are shown (n=11 patients; *p<0.05, **p<0.01, ***p<0.001).
γδT cells inhibit αβT cell activation via checkpoint receptor ligation
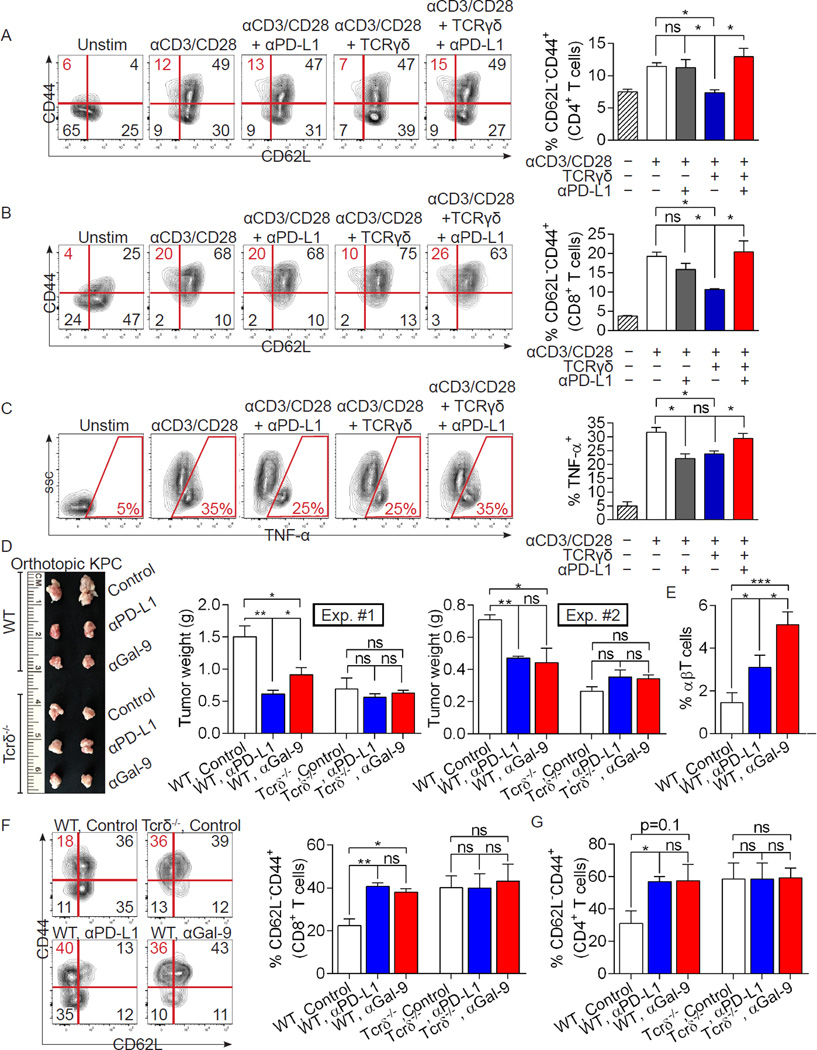
Previous reports have shown that low PD-L1 expression is associated with improved survival in human PDA and that PD-L1 blockade in murine PDA protects mice longitudinally (Nomi et al., 2007). We postulated that γδT cells promote PDA progression by preventing αβT cell activation via checkpoint receptor ligation. To test this, we again activated spleen CD4+ and CD8+ T cells in vitro using CD3/CD28 co-ligation alone or in the context of co-culture with PDA-derived γδT cells. Similar to our previous experiments, γδT cells prevented CD4+ (Figure 7a) and CD8+ (Figure 7b) T cells from adopting an activated CD44+CD62L− phenotype; however, γδT cell-mediated suppression was reversed with PD-L1 blockade. Further, whereas PDA-infiltrating γδT cells prevented αβT cell expression of TNF-α in vitro, this was again reversed by PD-L1 blockade (Figure 7c).
Figure 7. Exhaustion ligand blockade reverses the direct suppressive effects of γδT cells on αβT cells and on pancreatic tumorigenesis.
(a) Splenic CD4+ or (b) CD8+ T cells from untreated WT mice were either unstimulated, or stimulated with αCD3/αCD28 alone or in co-culture with PDA-infiltrating γδT cells (5:1 ratio). αPD-L1 (10µg/ml) was selectively added to each group. The fraction of CD62L−CD44+ cells were determined at 72h by flow cytometry. Representative contour plots and quantitative data are shown. (c) Similarly, CD4+ and CD8+ T cell expression of TNF-α was measured. Experiments were performed in quadruplicate and repeated 3 times. (d) WT and Tcrδ−/− mice were orthotopically implanted with KPC-derived tumor cells and serially treated with αPD-L1 or αGalectin-9 neutralizing mAbs, or respective isotype controls. Pancreatic tumors were harvested at 3 weeks. Representative gross images are shown (Experiment #1) as are quantitative data on tumor weights from 2 separate experiments using different stocks of KPC-derived tumor cells (n=5/group for each experiment). (e–g) WT and Tcrδ−/− pancreata were again orthotopically implanted with KPC-derived tumor cells and serially treated with αPD-L1 or αGalectin-9 neutralizing mAbs or the respective isotype controls. Pancreatic tumors were harvested at 3 weeks. (e) The fraction of PDA-infiltrating αβT cells among CD45+ leukocytes, and (f) CD8+ and (g) CD4+ T cell adoption of an activated CD62L−CD44+ phenotype, were determined by flow cytometry (n=5/group; *p<0.05, **p<0.01, ***p<0.01).
To definitively test whether γδT cells promote PDA progression in vivo via checkpoint ligand-dependent immune-suppression, we serially blocked PD-L1 or Galectin-9 using neutralizing mAbs in cohorts of WT and Tcrδ−/− mice challenged with orthotopic KPC-derived tumor. Consistent with our hypothesis, PD-L1 or Galectin-9 blockade protected WT mice but were ineffective at further inducing tumor-protection in Tcrδ−/− animals (Figures 7d). Moreover, αPD-L1 and αGalectin-9 each substantially increased αβT cell infiltration of PDA in WT mice (Figure 7e) but failed to enhance αβT cell infiltration in Tcrδ−/− hosts (not shown). Similarly, both PD-L1 and Galectin-9 blockade in vivo induced an activated CD4+ and CD8+ T cell phenotype in orthotopic PDA in WT mice but did not enhance αβT cell activation or Th1-polarization in PDA in Tcrδ−/− hosts (Figure 7f, g and S6f, g). To determine whether checkpoint ligand antagonism was also only efficacious in γδT cell-competent hosts in a slowly progressive model of PDA, we serially treated cohorts of 6 week old KC;Tcrδ+/+ and KC;Tcrδ−/− mice for 8 weeks with an αPD-L1 mAb. Again, PD-L1 inhibition protected KC pancreata from oncogenic progression but offered no benefit in KC;Tcrδ−/− mice (Figure S6h). Moreover, adoptive transfer of PDA-entrained γδT cells to Tcrδ−/− mice coincident with orthotopic tumor challenge resulted in tumor growth rates comparable to WT mice (Figure S6i). However, ex-vivo blockade of PD-L1 in γδT cells prior to adoptive transfer failed to accelerate tumor growth (Figure S6j). To determine whether PDA-infiltrating γδT cells abrogate antigen-restricted anti-tumor immunity in a PD-L1 dependent manner, we directly inoculated PDA-infiltrating γδT cells into established subcutaneous PDA tumors engineered to express OVA in Tcrδ−/− hosts. γδT cell administration again accelerated tumor growth and concomitantly diminished OVA-specific CD8+ T cell proliferation and activation. However, ex-vivo blockade of PD-L1 blockade in γδT cells abrogated their tumor-promoting and immune-suppressive effects (Figure S6k–m). Collectively, these data imply γδT cells are important mediators of checkpoint receptor dependent immune-suppression in PDA.
Notably, whereas γδT cell deletion augmented αβT cell infiltration and activation in PDA, it did not alter the fraction of PDA-infiltrating MDSCs or tumor-associated macrophages (TAMs) (Figure S7a). Similarly, γδT cell deletion did not affect the capacity of MDSCs or TAMs to mitigate T cell proliferation in PDA (Figure S7b, c). Further, in contrast to the exhaustion ligand-dependent immune-suppressive effects of γδT cells, PDA-infiltrating MDSC inhibition of αβT cell activation was independent of PD-L1 and macrophage-mediated inhibition was only partially mitigated by PD-L1 blockade based on αCD3/αCD28-mediated αβT cell proliferation (Figure S7b, c), expression of TNF-α (Figure S7d, e) and adoption of a CD44+CD62L− phenotype (not shown). Moreover, whereas αβT cells were in intimate proximity with γδT cells in the PDA TME, myeloid cells were separated by great distances from αβT cells in situ in human PDA (Figure S7f), in invasive murine PDA (Figure S7g) and in pre-invasive disease (not shown) suggesting enhanced opportunity for direct γδT cell–αβT cell interaction and limited opportunity for direct cross-talk between macrophages and αβT cells. Similarly, whereas αβT cells were in direct contact with PD-L1+ γδT cells (Figure S7h), αβT cells were not in close proximity of PD-L1+ epithelial cells (Figure S7i).
Discussion
Immune suppressive inflammation is paramount for PDA progression. Murine modeling of PDA using animals that endogenously express pancreas-specific oncogenic Kras revealed that pancreatic dysplasia is preceded by and accompanied by vigorous pancreatitis (Hingorani et al., 2003). Moreover, a driving oncogenic mutation alone is insufficient for disease progression and concomitant pancreatitis is necessary for PDA development (Guerra et al., 2007). The peri-pancreatic immune infiltrate is rife with immune-suppressive elements that support oncogenesis. In particular, innate immune cells within the TME are apt at educating adaptive immune effectors towards a tumor-permissive phenotype. APC populations, including M2-polarized TAMs and myeloid dendritic cells, induce the generation of PDA-promoting Th2 cells over Th1 cells that facilitate cytotoxic T lymphocytes (CTL) (Ochi et al., 2012c; Zhu et al., 2014). Similarly, we and others have shown that GM-CSF-recruited MDSC negate anti-tumor CD8+ CTL responses in PDA and promote metastatic progression (Bayne et al., 2012; Connolly et al., 2010; Pylayeva-Gupta et al., 2012). Effector T cells are also thought to be excluded from the PDA TME by CXCL12 produced by a subset of carcinoma-associated fibroblasts which express fibroblast activation protein (FAP) (Feig et al., 2013). However, a comprehensive understanding of the basis for T cell scarcity and poor immunogenicity in PDA is lacking.
γδT cells have not been well-characterized in PDA and their role in the programming of the TME remains ill-defined. We found that γδT cells are pervasive in human and murine PDA and tumor infiltration with γδT cells promotes oncogenic progression whereas genetic deletion, therapeutic depletion, and blockade of recruitment of γδT cells markedly delays morphologic transformation of the pancreas and increases median animal survival by nearly one year in a slowly progressive model of PDA. In contrast to our findings, γδT cells have long been considered potent anti-tumor entities in diverse tumor subtypes (Cordova et al., 2012; Todaro et al., 2009). In melanoma, renal cell cancer, and colon cancer the putative protective effects of γδT cells have led to strategies employing exogenous activation of γδT cells to maximize their tumoricidal activity in vivo (Gao et al., 2003; Girardi et al., 2001; Lanca and Silva-Santos, 2012). While our findings are ostensibly paradoxical to the described function of γδT cells in these cancer models, we demonstrate that the γδT cells in PDA exhibit a unique phenotype. Most interestingly, PDA-infiltrating γδT cells express substantial FoxP3 which is absent in spleen γδT cells from the same animals. Endogenous FoxP3 expression in γδT cells has not been previously reported. However, FoxP3 can be induced in γδT cells upon in vitro stimulation with TGF-β in combination with TCRγδ ligation (Kang et al., 2009). Resultant FoxP3+ γδT cells are potently suppressive to T cell activation and proliferation. We found that chemokine signaling does not influence γδT cell expression of FoxP3 in PDA. However, we and others have shown that the PDA TME is rife with TGF-β which can possibly induce FoxP3 expression (Goggins et al., 1998; Greco et al., 2015). Also consistent with a tumor-permissive phenotype, human PDA-infiltrating γδT cells do not express the Vγ9 TCR whose ligation has been implicated in the direct tumoricidal activity of γδT cells in melanoma and colon cancer (Izumi et al., 2013; Kunzmann et al., 2012).
While most early reports suggested that γδT cells were notable for their anti-cancer properties, emerging data suggest that γδT cells can have pro-tumorigenic effects. Select subsets of tumor infiltrating γδT cells in breast cancer block the maturation of TLR8-sensitive dendritic cells and their capacity to prime αβT cells (Peng et al., 2007). In murine B16 melanoma, Vγ4+ and Vγ1+ subsets of γδT cells reportedly have opposing roles in tumorigenesis with Vγ4+ cells mediating protective anti-tumor immunity via IFNγ and perforin, whereas Vγ1+ cells produce tumor-permissive Th2-family cytokines (Hao et al., 2011). By contrast, in PDA we found that tumor-promoting γδT cells are almost exclusively Vγ4+Vγ1−. Coffelt et al. showed in breast cancer models that IL-17 expression from γδT cells results in G-CSF-dependent expansion of neutrophils which acquire the ability to suppress anti-tumor CTL activity (Coffelt et al., 2015). Similarly, IL-17 production by γδT cells in murine hepatocellular carcinoma and colorectal cancer models mediates MDSC infiltration and their subsequent inhibition of cytotoxic CD8+ T cells (Ma et al., 2014; Wu et al., 2014). By contrast, we demonstrate that deletion of γδT cells in PDA does not influence the fraction of myeloid cells in the TME nor does it affect their functional capacity to suppress T cell proliferation. Consistent with recent reports, we show that PDA-infiltrating γδT cells express high IL-17 which can directly promote pancreatic oncogenesis via ligation of IL-17R on transformed epithelial cells (McAllister et al., 2014; Wu et al., 2015). However, our cumulative data suggest that IL-17 may not be critical to the pro-tumorigenic effects of PDA-infiltrating γδT cells since blockade of select chemokine signaling mitigated γδT cell infiltration, activation, and exhaustion ligand expression and was protective against PDA despite IL-17 expression being unaffected. Further, our in vitro correlative studies suggested that secreted factors in γδT cell conditioned media were non-inhibitory to CD4+ and CD8+ T cell activation.
We demonstrate that γδT cells create an immune-suppressive adaptive TME through checkpoint receptor ligation in tumor-infiltrating αβT cells. Deletion of γδT cells in PDA results in a robust influx of CD4+ and CD8+ T cells. Furthermore, in the absence of γδT cells, CD4+ T cells exhibit accentuated Th1-differentiation and CD8+ T cells exhibit a heightened cytotoxic phenotype. Moreover, whereas deletion of CD4+ and CD8+ T cells did not accelerate tumor progression in γδT cell-competent hosts, in Tcrδ−/− mice αβT cell deletion nearly tripled the rate of PDA growth. This observation supports the notion that αβT cells are entirely dispensable in PDA, but are reprogramed into powerful anti-tumor entities in the absence of γδT cells.
The volume of T cell exhaustion ligand levels in carcinomas, including in PDA, has been largely attributed to expression from tumor cells and macrophages (Nomi et al., 2007; Sharma and Allison, 2015). However, we show that γδT cells express considerably higher levels of PD-L1 and Galectin-9 in PDA than cancer cells. More importantly, we demonstrate that γδT cells are important contributors to PD-L1 and Galectin-9 induced T cell exhaustion in the TME based on our observation that inhibition of PD-L1 and Galectin-9 in PDA is protective in vivo in the presence of γδT cells, whereas in absence of γδT cells PD-L1 or Galectin-9 blockade offers no additional tumor-protective benefit. Even more, PD-L1 or Galectin-9 blockade expand and potently activate PDA-infiltrating CD4+ and CD8+ T cells in γδT cell-competent hosts but do not enhance αβT cell immunogenicity in the absence of γδT cells. It is perhaps surprising that checkpoint receptor blockade would not have potency in Tcrδ−/− mice considering the substantial myeloid cell infiltrate in PDA (Liou et al., 2015; Pylayeva-Gupta et al., 2012). Indeed we found that myeloid cells from Tcrδ−/− mice have equivalent T cell inhibitory capacity to their cellular counterparts in WT mice. However, we found that whereas αβT cells are in intimate proximity to γδT cells in the PDA TME, myeloid cells are separated by great distances from αβT cells in situ in human PDA, invasive murine PDA, and pre-invasive disease suggesting enhanced opportunity for γδT cell-αβT cell interaction and limited opportunity for direct cross-talk between macrophages and αβT cells. Further, tumor cell expression of exhaustion ligands is also possibly of lesser significance as T cells are excluded from direct contact with tumor cells via CXCL12 produced from FAP-expressing carcinoma-associated fibroblasts in PDA (Feig et al., 2013; Joyce and Fearon, 2015). Collectively, these data may suggest that αβT cells are prevented from having immunogenic relevance in PDA via a “double-hit”: fibroblast-mediated chemokine signaling excludes αβT cells from the direct tumor environs where γδT cells serve to check their activation via ligation of inhibitory receptors.
In summary, we show that PDA-infiltrating γδT cells are a highly influential lymphocyte subset in human and murine PDA which promote pancreatic oncogenesis and reduce survival via novel cross-talk with the adaptive immune compartment. These data implicate γδT cells as high-yield targets for the development of experimental therapeutics in PDA and has potential implications for the mechanistic progression of oncogenesis in other cancer subtypes. Finally, γδT cells may have prognostic significance in PDA, and may be predictive of response to immunotherapeutic regimens.
Methods and Resources
Contact for Reagent and Resource Sharing, George Miller, MD, Departments of Surgery and Cell Biology, New York University School of Medicine, 430 East 29th Street, New York, NY 10016, Tel: (646) 501-2208, george.miller@nyumc.org
Experimental Model and Subject Details
Animals and In Vivo Procedures
C57BL/6 (H-2Kb), C57BL/6-Trdctm1Mal, CCR2−/−, CCR5−/−, CCR6−/−, CCL2−/−, and B6.129P2-Tcrdtm1Mom/J (Tcrδ−/−) mice were purchased from Jackson Labs (Bar Harbor, ME). KC mice (gift of D. Bar-Sagi), which develop pancreatic neoplasia endogenously by expressing mutant Kras, were generated by crossing LSL-KrasG12D and p48Cre mice (Hingorani et al., 2003). Tcrδ−/− mice were crossed with KC mice to generate KC;Tcrδ−/− animals. Both male and female mice were used but animals were sex- and age-matched in each experiment. Randomization was not performed. There were no specific inclusion or exclusion criteria. Sample sizes for experiments were determined without formal power calculations. Data for control KC mice have been previously reported (Seifert et al., 2016). For orthotopic tumor challenge, mice were administered intra-pancreatic injections of tumor cells derived from KPC mice (1×105 cells in Matrigel) and sacrificed at 3 weeks as described (Zambirinis et al., 2015). For subcutaneous tumor challenge, KPC-derived tumor cells (1×106) engineered to express OVA using pCI-neo-cOVA (gift of Maria Castro; Addgene plasmid # 25097) were administered to the flanks of mice (Yang et al., 2010). In select experiments, FACS-sorted PDA-infiltrating γδT cells were orthotopicaly transferred (8×105) together with tumor cells or directly inoculated into subcutaneous tumors (3×105). In other experiments, animals were treated twice weekly with i.p. injection of neutralizing mAbs directed against TCR Vγ4 (UC3–10A6, 8mg/kg), PD-L1 (10F.9G2, 5mg/kg), or Galectin-9 (RG9-1, 6mg/kg; all BioXCell, West Lebanon, NH). In select experiments CD4 (GK1.5; BioXCell) or CD8 (53-6.72; Monoclonal Antibody Core Facility, Sloan Kettering Institute, New York, NY) T cells were depleted using previously described regimens (Bedrosian et al., 2011). Acute pancreatitis was induced using a regimen of seven hourly i.p. injections of caerulein (50 µg/kg; Sigma, St. Louis, MO) for two consecutive days as we have described (Bedrosian et al., 2011). Serum amylase and lipase levels were measured using commercial kits (Sigma) according to the manufacturer’s instructions. Animal procedures were approved by the NYU School of Medicine IACUC.
Human and Murine Cellular Isolation
Pancreatic leukocytes were harvested from mouse PDA as described previously (Ochi et al., 2012a). Briefly, pancreata were resected in total and placed in ice-cold PBS with 1% FBS with Collagenase IV (1 mg/mL; Worthington Biochemical, Lakewood, NJ) and DNAse I (2 U/mL; Promega, Madison, WI). After mincing, tissues were incubated in the same solution at 37°C for 30 minutes with gentle shaking. Specimens were passed through a 70 urn mesh, and centrifuged at 350g for 5 minutes. Human pancreatic tissues and PBMC were collected under an IRB approved protocol and donors of de-identified specimens gave informed consent. Sample sizes for human experiments were not determined based on formal power calculations. Human pancreatic leukocytes were prepared in a similar manner to mice. PBMC were isolated by overlaying whole blood diluted 3:1 in PBS over an equal amount of Ficoll (GE Healthcare, Princeton, NJ). Cells were then centrifuged at 2100 RPM and the buffy coat harvested as we have described (Rehman et al., 2013).
Method Details
Flow Cytometry and FACS sorting
Cells were suspended in ice-cold PBS with 1% FBS. After blocking FcyRIII/II with an anti-CD16/CD32 mAb (eBioscience, San Diego, CA), cell labeling was performed by incubating 106 cells with 1 µg of fluorescently conjugated antibodies directed against murine CD45 (30-F11), CD3 (17A2), CD4 (RM4–5), CD8 (53–6.7), TCR γδ (GL3), CD62L (MEL-14), FasL (MFL3), Vγ1 (2.11), Vγ4 (UC3–10A6), NK1.1 (PK136), CD39 (Duha59), CCR2 (SA203G11), CCR5 (HM-CCR5), CCR6 (29-2L17), CD44 (FM7), JAML (4E10), NKG2D (CX5), CD11b (Ml/70), Grl (RB6–8C5), PD-1 (29F.1A12), ICOS (15F9), TCR α/β (H57–597), TLR4 (SA15–21), TNF-α (MP6-XT22), IL-17 (TC11–18H10.1), IL-10 (JES5–16E3), INF-γ (XMG1.2), PD-L1 (10F.9G2), Galectin-9 (RG9–35), B7-1 (16-10A1), B7-2 (P03), ICOSL (HK5.3), OX-40L (RM134L), CD107A (1D4B), CTLA4 (UC10-4B9), Ly6C (HK1.4), Ly6G (1A8), OX-40 (OX-86; all Biolegend, San Diego, CA), TLR7 (FMG-581A), TLR9 (26C593.2; both Imgenex, San Diego, CA), T-bet (eBio4B10), IL-13 (eBiol3A), Granzyme B (NGZB), GATA-3 (TWAJ), and FoxP3 (FJK-16s; all eBioscience). OVA-restricted CD8+ T cell proliferation was determined using an H-2kb SIINFEKL OVA Pentamer (Prolmmune, Oxford, United Kingdom). Intracellular staining was performed using the FoxP3 Fixation/Permeabilization Solution Kit (eBiosciences). Analysis of human cells was performed using fluorescently conjugated antibodies directed against CD45 (HI30), CD3 (SK7), CD45RA (HI100), CD27 (0323), CD62L (DREG-56), CD14 (HCD14), PD-L1 (29E.2A3), Galectin 9 (9M1–3), CD15 (W6D3), CD11c (3.9), Vγ9 (B3; all Biolegend), Tcrγ/δ (B1.1; eBioscience). Flow cytometry was performed on the LSR-II (BD Biosciences, Franklin Lakes, NJ). Cytokine levels in cell culture supernatant were measured using a cytometric bead array (BD Biosciences). FACS-sorting was performed on the SY3200 (Sony, Tokyo, Japan). Data were analyzed using FlowJo (Treestar, Ashland, OR).
Western Blotting
Cells or tissues were lysed in ice-cold RIPA buffer. Total protein was quantified using the BioRad DC Protein Assay according to the manufacturer’s instructions (BioRad, Hercules, CA). Western blotting was performed as described previously with minor modifications (Ochi et al., 2012a). Briefly, 10% Bis-Tris polyacrylamide gels (NuPage; Invitrogen, Carlsbad, CA) were equiloaded with 10–30µg protein, electrophoresed at 200 V and electrotransferred to PVDF membranes. After blocking with 5% BSA, membranes were probed with primary antibodies to Bcl-XL (54H6), Rb (D20), c-Myc (D84C12), PTEN (26H9), p53 (1C12), and β-actin (8H10D10), all Cell Signaling, Beverly, MA. Blots were developed by ECL (Thermo Scientific, Asheville, NC).
Histology, Immunohistochemistry, and Microscopy
For histological analysis, pancreatic specimens were frozen in OCT medium or fixed with 10% buffered formalin, dehydrated in ethanol, embedded with paraffin, and stained with H&E or Gomori’s Trichrome. The fraction of preserved acinar area was calculated as previously described (Ochi et al., 2012a). The fraction and number of ducts containing all grades of PanIN lesions was measured by examining 10 high-power fields (HPFs; 40X) per slide. PanFNs were graded according to established criteria (Hruban et al., 2001): In PanIN I ducts, the normal cuboidal pancreatic epithelial cells transition to columnar architecture (PanIN Ia) and gain polyploid morphology (PanIN Ib). PanIN II lesions are associated with loss of polarity. PanIN III lesions, or in-situ carcinoma, show cribriforming, budding off of cells, and luminal necrosis with marked cytological abnormalities, without invasion beyond the basement membrane. Slides were evaluated by an expert pancreas pathologist (CH). Immunohistochemistry (IHC) was performed using antibodies directed against CD4 (RM4–5; BD Bioscience), CD8 (YTS169.4; Abcam), GFP (D5.1; Cell Signalling) and TCRγ/δ (B1; Biolegend). Quantifications were performed by assessing 10 HPF per slide. For immunofluorescent staining, frozen specimens were probed with antibodies directed against TCRγ/δ (GL3; Biolegend), TCRaP (H57–597; Biolegend), PD-L1 (Polyclonal, Abcam), CK19 (Troma-III; University of Iowa) or CD11b (M1/70; Biolegend). For analysis of human tissues, frozen sections of human pancreatic cancer specimens were probed with antibodies directed against TCRγ/δ (B1.1; eBioscience), TCRαβ (IP26; Biolegend), or CD11b (M1/70; Biolegend). Images were acquired using the Zeiss LSM700 confocal microscope along with ZEN software (Carl Zeiss, Thornwood, New York). The proximity of αβT cells to γδT cells or CD1 1b+ cells, respectively, was determined by measuring the distance between each αβT cell and its spatially closest counterpart. Distances were measured in micrometers on low power fields (20X). The averages distances were calculated for 10 low power fields per pancreas.
Intravital Imaging
Orthotopic pancreas tumor-bearing C57BL/6-Trdctm1Mal mice were anesthetized and a left subcostal laparotomy incision was made. The spleen and pancreatic tumor were externalized. The mouse was then placed prone on a heated (37°C) stage mounted with a coverslip which was in contact with the pancreatic tumor. To visualize the pancreatic vasculature, mice were injected i.v. with 25 µg Evan Blue (Sigma) 10 min before imaging. Images were acquired with a LSM 710 inverted microscope (Zeiss) with a MaiTai Ti:Sapphire laser (Spectra-Physics, Santa Clara, CA) tuned to 910–930 nm. Emitted fluorescence was detected through 420/40, 465/30, 520/30, 575/70, and 660/50 nm band-pass filters and nondescanned detectors to generate second harmonic signals (collagen fibers) and 4-color images. All the images were acquired at least 50 µm below the tumor capsule. ZEN software was used for analysis.
In vitro T cell activation assays
For T cell activation assays, spleen CD4+ or CD8+ T cells (5xl04) were labeled with CFSE (eBioscience) and plated alone or with PDA-infiltrating γδT cells, MDSC, or TAMs (5:1 ratio) in 96 well plates coated with anti-CD3 (145-2C11, 10µg/ml) and anti-CD28 (37.51; 10µg/ml, both Biolegend). After 72 hours, αβT cells were harvested and analyzed by flow cytometry. In selected experiments, cells were treated with a neutralizing mAb directed against PD-L1 (10F.9G2, 10µg/ml; BioXCell).
Quantification and Statistical Analysis
Statistical Analysis
Data is presented as mean +/− standard error. Survival was measured according to the Kaplan-Meier method. The sample size for each experiment, n, is included in the results section and the associated figure legend. Statistical significance was determined by the Student’s t test and the Wilcoxon test using GraphPad Prism 6 (GraphPad Software, La Jolla, CA). P-values <0.05 were considered significant. P values for each experiment are also included in the associated figure legends.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| InVivoMAb Anti-mouse TCR Vγ2 | BioXCell | Clone:UC3-106 Cat#: BE0168 |
| InVivoMAb Anti-mouse PD-L1 | BioXCell | Clone: 10F.9G2 Cat#: BE0101 |
| InVivoMAb Anti-mouse Galectin-9 | BioXCell | Clone: RG9-1 Cat#: BE0218 |
| InVivoMAb Anti-mouse CD4 | BioXCell | Clone: GK1.5 Cat#: BE0003-1 |
| InVivoMAb Anti-Mouse CD8 | Monoclonal Antibody Core Facility, Sloan Kettering Institute |
Clone: 53-6.72 |
| WB: Rabbit mAb Bcl-XL | Cell Signalling | Clone: 54H6 |
| WB: Rabbit mAb Rb | Cell Signalling | Clone: D20 |
| WB: Rabbit mAb c-Myc | Cell Signalling | Clone: D84C12 |
| WB: Mouse mAb PTEN | Cell Signalling | Clone: 26H9 |
| WB: Mouse mAb p53 | Cell Signalling | Clone: 1C12 |
| WB: Mouse mAb β-actin | Cell Signalling | Clone: 8H10D10 |
| IHC: Anti-mouse CD4 | BD Biosciences | Clone: RM4-5 Cat#: 550280 |
| IHC: Anti-mouse CD8 | Abcam | Clone: YTS169.4 Cat#: ab22378 |
| IHC: Anti-mouse GFP | Cell Signalling | Clone: D5.1 Cat#: 2956 |
| IHC: Anti-human γ/δ TCR | Biolegend | Clone: B1 |
| Purified anti-mouse CD3ε | Biolegend | Clone: 145-2C11 |
| Purified anti-mouse CD28 | Biolegend | Clone: 37.51 |
| FC: anti-mouse CD3 | Biolegend | Clone: 17A2 |
| FC: anti-mouse CD4 | Biolegend | Clone: RM4-5 |
| FC: anti-mouse CD8 | Biolegend | Clone: 53-6.7 |
| FC/IF: anti-mouse γ/δ TCR | Biolegend | Clone: GL3 |
| FC: anti-mouse CD62L | Biolegend | Clone: MEL-14 |
| FC: anti-mouse FasL | Biolegend | Clone: MFL3 |
| FC: anti-mouse Vγ1 | Biolegend | Clone: 2.11 |
| FC: anti-mouse Vγ2(4) | Biolegend | Clone: UC-10A6 |
| FC: anti-mouse NK1.1 | Biolegend | Clone: PK136 |
| FC: anti-mouse CD39 | Biolegend | Clone: Duha59 |
| FC: anti-mouse CCR2 | Biolegend | Clone: SA203G11 |
| FC: anti-mouse CCR5 | Biolegend | Clone: HM-CCR5 |
| FC: anti-mouse CCR6 | Biolegend | Clone: 29-2L17 |
| FC: anti-mouse CD44 | Biolegend | Clone: IM7 |
| FC: anti-mouse JAML | Biolegend | Clone: 4E10 |
| FC: anti-mouse NKG2D | Biolegend | Clone: CX5 |
| FC/IF: anti-mouse/human CD11b | Biolegend | Clone: M1/70 |
| FC: anti-mouse Gr1 | Biolegend | Clone: RB6-8C5 |
| FC: anti-mouse PD-1 | Biolegend | Clone: 29F.1A12 |
| FC: anti-mouse ICOS | Biolegend | Clone: 15F9 |
| FC/IF: anti-mouse αβ TCR | Biolegend | Clone: H57-597 |
| FC: anti-mouse TLR4 | Biolegend | Clone: SA15-21 |
| FC: anti-mouse TNF-α | Biolegend | Clone: MP6-XT22 |
| FC: anti-mouse IL-17 | Biolegend | Clone: TC11-1810.1 |
| FC: anti-mouse IL-10 | Biolegend | Clone: JES5-16E3 |
| FC: anti-mouse INF-γ | Biolegend | Clone: XMG1.2 |
| FC: anti-mouse PD-L1 | Biolegend | Clone: 10F.9G2 |
| FC: anti-mouse Galectin 9 | Biolegend | Clone: RG9-35 |
| FC: anti-mouse B7-1 | Biolegend | Clone: 16-10A1 |
| FC: anti-mouse B7-2 | Biolegend | Clone: PO3 |
| FC: anti-mouse ICOSL | Biolegend | Clone: HK5.3 |
| FC: anti-mouse OX-40L | Biolegend | Clone: RM134L |
| FC: anti-mouse CD107a | Biolegend | Clone: 1D4B |
| FC: anti-mouse OX40 | Biolegend | Clone: OX-86 |
| FC: anti-mouse CTLA4 | Biolegend | Clone: UC10-4B9 |
| FC: anti-mouse Ly6C | Biolegend | Clone: HK1.4 |
| FC: anti-mouse Ly6G | Biolegend | Clone: 1A8 |
| FC: anti-mouse TLR7 | Imgenex | Clone: IMG-581A |
| FC: anti-mouse TLR9 | Imgenex | Clone: 26C593.2 |
| FC: anti-mouse Tbet | eBioscience | Clone: eBio4B10 |
| FC: anti-mouse IL-13 | eBioscience | Clone: eBio13A |
| FC: anti-mouse Granzyme B | eBioscience | Clone: NGZB |
| FC: anti-mouse GATA-3 | eBioscience | Clone: TWAJ |
| FC: anti-mouse FoxP3 | eBioscience | Clone: FJK-16s |
| FC: H-2kb SINFEKL OVA Pentamer | ProImmune | Cat#: F093 |
| FC: anti-human CD45 | Biolegend | Clone: HI30 |
| FC: anti-human CD3 | Biolegend | Clone: SK7 |
| FC: anti-human CD45RA | Biolegend | Clone: HI100 |
| FC: anti-human CD27 | Biolegend | Clone: O323 |
| FC: anti-human CD62L | Biolegend | Clone: DREG-56 |
| FC: anti-human CD14 | Biolegend | Clone: HCD14 |
| FC: anti-human CD15 | Biolegend | Clone: W6D3 |
| FC: anti-human CD11c | Biolegend | Clone: 3.9 |
| FC: anti-human Vγ9 | Biolegend | Clone: B3 |
| FC: anti-human PD-L1 | Biolegend | Clone: 29E.2A3 |
| FC: anti-human Galectin 9 | Biolegend | Clone: 9M1-3 |
| FC/IF: anti-human γ/δ TCR | eBioscience | Clone: B1.1 |
| IF: anti-human αβ TCR | Biolegend | Clone: IP26 |
| IF: anti-mouse PD-L1 | Abcam | Cat# ab58810 |
| IF: anti-mouse CK-19 | Developmental Studies Hybridoma Bank, University of Iowa |
Clone: TROMA-III |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Caerulein | Sigma-Aldrich | Cat#: C9026 |
| CFSE | eBioscience | Cat#: 65-0850-84 |
| Evans Blue | Sigma-Aldrich | Cat#: E2129 |
| Critical Commercial Assays | ||
| Lipase Basic Kit | Sigma-Aldrich | Cat#: 626327 |
| Amylase Activity Kit | Sigma-Aldrich | Cat#: MAK009 |
| Cytometric Bead Array Mouse Inflammation Kit | BD Biosciences | Cat#: 52364 |
| Cytometric Bead Array Mouse Th1/Th2 Cytokine Kit | BD Biosciences | Cat#: 551287 |
| Deposited Data | ||
| Experimental Models: Cell Lines | ||
| Mouse: KPC Pancreatic Cancer Cell line | Laboratory of Dave Tuveson | Cell line: FC1242 |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6 (H-2Kb) | The Jackson Laboratory | JAX: 000664 |
| Mouse: C57BL/6-Trdctm1Mal | The Jackson Laboratory | JAX: 016941 |
| Mouse: CCR2−/− | The Jackson Laboratory | JAX: 004999 |
| Mouse: CCR5−/− | The Jackson Laboratory | JAX: 005427 |
| Mouse: CCL2−/− | The Jackson Laboratory | JAX: 004434 |
| Mouse: CCR6−/− | The Jackson Laboratory | JAX: 005793 |
| Mouse: B6.129P2-Tcrdtm1Mom/J (Tcrδ−/−) | The Jackson Laboratory | JAX: 002120 |
| Mouse: B6. LSL-KrasG12D and p48Cre (KC ) | Laboratory of Dafna Bar- Sagi |
|
| Recombinant DNA | ||
| Plasmid: pCI-neo-cOVA | Addgene | Plasmid # 25097 |
| Sequence-Based Reagents | ||
| Software and Algorithms | ||
| Other | ||
Highlights.
γδT cells are highly prevalent in human pancreatic carcinoma
Deletion or interruption of γδT cell recruitment is protective in pancreatic cancer
Pancreatic cancer infiltrating γδT cells express high levels of checkpoint ligands
γδT cells disable αβT cell activation via checkpoint receptor ligation
Acknowledgments
This work was supported by grants for the German Research Foundation (LT), the National Pancreas Foundation (CPZ), the Pancreatic Cancer Action Network (GM), the Lustgarten Foundation (GM), and National Institute of Health Awards CA155649 (GM), CA168611 (GM), and CA193111 (GM, ATH). We thank the New York University Langone Medical Center (NYU LMC) Histopathology Core Facility, the NYU LMC Flow Cytometry Core Facility, the NYU LMC Microscopy Core Facility, and the NYU LMC BioRepository Center, each supported in part by the Cancer Center Support Grant P30CA016087 and by grant UL1 TR000038 from the National Center for the Advancement of Translational Science (NCATS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
DD (project leadership, data collection and analysis, manuscript preparation), CZ (project conception, data collection), LS (data analysis, manuscript preparation), NA and NM (IHC, flow cytometry, in vivo experiments), GW (manuscript preparation), AA (In vivo experiments, tissue culture), RB (technical assistance), DT (mouse breeding and genotyping), RN (Data analysis), ATH, MH and VRJM (technical assistance), JEJ (intravital imaging), EN (human tissue procurement), VGP (IHC), MLD (intravital imaging), DBS (intravital imaging), CH (human tissue procurement, pathologic analysis), GM (project design, data analysis, manuscript preparation).
References
- Andren-Sandberg A, Dervenis C, Lowenfels B. Etiologic links between chronic pancreatitis and pancreatic cancer. Scand J Gastroenterol. 1997;32:97–103. doi: 10.3109/00365529709000177. [DOI] [PubMed] [Google Scholar]
- Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer cell. 2012;21:822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrosian AS, Nguyen AH, Hackman M, Connolly MK, Malhotra A, Ibrahim J, Cieza-Rubio NE, Henning JR, Barilla R, Rehman A, et al. Dendritic cells promote pancreatic viability in mice with acute pancreatitis. Gastroenterology. 2011;141:1915–1926. e1911–e1914. doi: 10.1053/j.gastro.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer research. 2007;67:9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstegen NJ, Ciampricotti M, Hawinkels LJ, Jonkers J, et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015 doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly MK, Mallen-St Clair J, Bedrosian AS, Malhotra A, Vera V, Ibrahim J, Henning J, Pachter HL, Bar-Sagi D, Frey AB, et al. Distinct populations of metastases-enabling myeloid cells expand in the liver of mice harboring invasive and preinvasive intra-abdominal tumor. Journal of leukocyte biology. 2010;87:713–725. doi: 10.1189/jlb.0909607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova A, Toia F, La Mendola C, Orlando V, Meraviglia S, Rinaldi G, Todaro M, Cicero G, Zichichi L, Donni PL, et al. Characterization of human gammadelta T lymphocytes infiltrating primary malignant melanomas. PLoS One. 2012;7:e49878. doi: 10.1371/journal.pone.0049878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26–e31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, Craft J, Yin Z. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. The Journal of experimental medicine. 2003;198:433–442. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- Goggins M, Shekher M, Turnacioglu K, Yeo CJ, Hruban RH, Kern SE. Genetic alterations of the transforming growth factor beta receptor genes in pancreatic and biliary adenocarcinomas. Cancer Res. 1998;58:5329–5332. [PubMed] [Google Scholar]
- Greco SH, Tomkotter L, Vahle AK, Rokosh R, Avanzi A, Mahmood SK, Deutsch M, Alothman S, Alqunaibit D, Ochi A, et al. TGF-beta Blockade Reduces Mortality and Metabolic Changes in a Validated Murine Model of Pancreatic Cancer Cachexia. PLoS One. 2015;10:e0132786. doi: 10.1371/journal.pone.0132786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Hao J, Dong S, Xia S, He W, Jia H, Zhang S, Wei J, O’Brien RL, Born WK, Wu Z, et al. Regulatory role of Vgamma1 gammadelta T cells in tumor immunity through IL-4 production. Journal of immunology. 2011;187:4979–4986. doi: 10.4049/jimmunol.1101389. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Kloppel G, Longnecker DS, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. The American journal of surgical pathology. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Izumi T, Kondo M, Takahashi T, Fujieda N, Kondo A, Tamura N, Murakawa T, Nakajima J, Matsushita H, Kakimi K. Ex vivo characterization of gammadelta T-cell repertoire in patients after adoptive transfer of Vgamma9Vdelta2 T cells expressing the interleukin-2 receptor beta-chain and the common gamma-chain. Cytotherapy. 2013;15:481–491. doi: 10.1016/j.jcyt.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- Kang N, Tang L, Li X, Wu D, Li W, Chen X, Cui L, Ba D, He W. Identification and characterization of Foxp3(+) gammadelta T cells in mouse and human. Immunology letters. 2009;125:105–113. doi: 10.1016/j.imlet.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Kunzmann V, Smetak M, Kimmel B, Weigang-Koehler K, Goebeler M, Birkmann J, Becker J, Schmidt-Wolf IG, Einsele H, Wilhelm M. Tumor-promoting versus tumor-antagonizing roles of gammadelta T cells in cancer immunotherapy: results from a prospective phase I/II trial. Journal of immunotherapy. 2012;35:205–213. doi: 10.1097/CJI.0b013e318245bb1e. [DOI] [PubMed] [Google Scholar]
- Lanca T, Silva-Santos B. The split nature of tumor-infiltrating leukocytes: Implications for cancer surveillance and immunotherapy. Oncoimmunology. 2012;1:717–725. doi: 10.4161/onci.20068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou GY, Doppler H, Necela B, Edenfield B, Zhang L, Dawson DW, Storz P. Mutant KRAS-induced expression of ICAM-1 in pancreatic acinar cells causes attraction of macrophages to expedite the formation of precancerous lesions. Cancer discovery. 2015;5:52–63. doi: 10.1158/2159-8290.CD-14-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Cheng Q, Cai Y, Gong H, Wu Y, Yu X, Shi L, Wu D, Dong C, Liu H. IL-17A produced by gammadelta T cells promotes tumor growth in hepatocellular carcinoma. Cancer research. 2014;74:1969–1982. doi: 10.1158/0008-5472.CAN-13-2534. [DOI] [PubMed] [Google Scholar]
- McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, Rattigan Y, Roeser JC, Lankapalli RH, Zhang H, et al. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell. 2014;25:621–637. doi: 10.1016/j.ccr.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- Oberg HH, Peipp M, Kellner C, Sebens S, Krause S, Petrick D, Adam-Klages S, Rocken C, Becker T, Vogel I, et al. Novel bispecific antibodies increase gammadelta T-cell cytotoxicity against pancreatic cancer cells. Cancer Res. 2014 doi: 10.1158/0008-5472.CAN-13-0675. [DOI] [PubMed] [Google Scholar]
- Ochi A, Graffeo CS, Zambirinis CP, Rehman A, Hackman M, Fallon N, Barilla RM, Henning JR, Jamal M, Rao R, et al. Toll-like receptor 7 regulates pancreatic carcinogenesis in mice and humans. The Journal of clinical investigation. 2012a;122:4118–4129. doi: 10.1172/JCI63606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi A, Nguyen AH, Bedrosian AS, Mushlin HM, Zarbakhsh S, Barilla R, Zambirinis CP, Fallon NC, Rehman A, Pylayeva-Gupta Y, et al. MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J Exp Med. 2012b;209:1671–1687. doi: 10.1084/jem.20111706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi A, Nguyen AH, Bedrosian AS, Mushlin HM, Zarbakhsh S, Barilla R, Zambirinis CP, Fallon NC, Rehman A, Pylayeva-Gupta Y, et al. MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. The Journal of experimental medicine. 2012c;209:1671–1687. doi: 10.1084/jem.20111706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27:334–348. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836–847. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman A, Hemmert KC, Ochi A, Jamal M, Henning JR, Barilla R, Quesada JP, Zambirinis CP, Tang K, Ego-Osuala M, et al. Role of fatty-acid synthesis in dendritic cell generation and function. J Immunol. 2013;190:4640–4649. doi: 10.4049/jimmunol.1202312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annual review of immunology. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Seifert L, Werba G, Tiwari S, Giao Ly NN, Alothman S, Alqunaibit D, Avanzi A, Barilla R, Daley D, Greco SH, et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature. 2016;532:245–249. doi: 10.1038/nature17403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- Todaro M, D’Asaro M, Caccamo N, Iovino F, Francipane MG, Meraviglia S, Orlando V, La Mendola C, Gulotta G, Salerno A, et al. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol. 2009;182:7287–7296. doi: 10.4049/jimmunol.0804288. [DOI] [PubMed] [Google Scholar]
- Wu HH, Hwang-Verslues WW, Lee WH, Huang CK, Wei PC, Chen CL, Shew JY, Lee EY, Jeng YM, Tien YW, et al. Targeting IL-17B–IL-17RB signaling with an anti-IL-17RB antibody blocks pancreatic cancer metastasis by silencing multiple chemokines. J Exp Med. 2015;212:333–349. doi: 10.1084/jem.20141702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, Wang Z, Wang C, Zhang Z, Xia W, et al. gammadeltaT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40:785–800. doi: 10.1016/j.immuni.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Sanderson NS, Wawrowsky K, Puntel M, Castro MG, Lowenstein PR. Kupfer-type immunological synapse characteristics do not predict anti-brain tumor cytolytic T-cell function in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4716–4721. doi: 10.1073/pnas.0911587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambirinis CP, Levie E, Nguy S, Avanzi A, Barilla R, Xu Y, Seifert L, Daley D, Greco SH, Deutsch M, et al. TLR9 ligation in pancreatic stellate cells promotes tumorigenesis. The Journal of experimental medicine. 2015;212:2077–2094. doi: 10.1084/jem.20142162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology. 2013;144:1230–1240. doi: 10.1053/j.gastro.2012.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.