Abstract
Background: While clinical outcomes from colorectal cancer (CRC) are influenced by stage at diagnosis and treatment, mounting evidence suggests that an enhanced lymphocytic reaction to a tumor may also be an informative prognostic indicator.
Methods: The roles of intratumoral T lymphocyte infiltration (TIL), peritumoral Crohn’s-like lymphoid reaction (CLR), microsatellite instability (MSI), and clinicopathological characteristics in survival from CRC were examined using 2369 incident CRCs from a population-based case-control study in northern Israel. Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for CRC-specific and all-cause mortality in multivariable models adjusted for age, sex, ethnicity, grade, stage, and MSI. All statistical tests were two-sided.
Results: Tumors with TIL/high-powered field (HPF) of 2 or greater were associated with a statistically significant increase in CRC-specific (P < .001) and overall survival (P < .001) compared with tumors with TIL/HPF of less than 2. Similarly, tumors with a prominent CLR experienced better CRC-specific (P < .001) and overall survival (P < .001) as compared with those with no response. High TILs (HR = 0.76, 95% CI = 0.64 to 0.89, P < .001) and a prominent CLR (HR = 0.71, 95% CI = 0.62 to 0.80, P < .001), but not MSI, were associated with a statistically significant reduction in all-cause mortality after adjustment for established prognostic factors.
Conclusions: TILs and CLR are both prognostic indicators for CRC after adjusting for traditional prognostic indicators.
Colorectal cancer (CRC) is the fourth leading cause of cancer deaths worldwide (1). While clinical outcomes are largely dependent on stage at diagnosis and treatment, mounting evidence suggests that microsatellite instability (MSI) and host immune infiltration may also be highly informative prognostic indicators (2–6). Molecular genetic studies of colorectal cancer (CRC) have identified high levels of MSI (MSI-H) in approximately 15% of CRCs. Histologic differences between MSI-H and microsatellite stable (MSS)/microsatellite-low (MSI-L) tumors have been well described (7–10), and the MSI-H phenotype has been associated with a better prognosis than tumors with an MSS or MSI-L phenotype (2,3,6,11). While the underlying drivers for the MSI-H survival advantage are not fully understood, the prognostic benefit has been at least partially attributed to the pronounced lymphocytic infiltration in this subset of cancers (12,13).
With the identification of the MSI phenotype and the corresponding prognostic advantage, the host immune response, notably an enhanced lymphocytic reaction, has become a recent focus of investigation. Tumor infiltrating lymphocytes (TILs) can trigger preferential lysis of cancer cells by recognizing enhanced expression of abnormally expressed antigens presented in the context of HLA molecules. The presence of TILs is more common in MSI-H than microsatellite-stable (MSS) tumors (21% vs 3%) (14), likely because of DNA mismatch repair deficiencies causing frameshift mutations that lead to the introduction of potentially immunogenic neoantigens (15). It has been understood for years that individuals with CRCs containing many TILs have a survival advantage over those that do not (16–19). In addition, the number of TILs discriminates between MSI-H and MSS CRCs in our sample of Israeli CRCs (9). Further, a Crohn’s disease-like lymphoid reaction (CLR) characterized by peritumoral lymphoid aggregates is a common feature of MSI-H tumors (8,10) and has also been associated with improved prognosis (20–23).
A pronounced host immune reaction is not unique to MSI-high cancers (18,24), and the independent contributions of intratumoral and peritumoral lymphocytic responses to survival have not been fully characterized in the context of established prognostic indicators. The present study evaluated the importance of TILs and the CLR as prognostic factors for CRC, in addition to age, sex, MSI, stage, and grade, in a large population-based case-control study in northern Israel.
Methods
Study Population
The Molecular Epidemiology of Colorectal Cancer (MECC) study is a population-based, case-control study of incident CRC patients and their corresponding age-, sex-, and ethnicity-matched control subjects. The MECC study participants and response rates have previously been described (25). Eligible patients included any person newly diagnosed with CRC between February 1998 and March 2006 in northern Israel for whom a tissue sample was available. Individuals previously diagnosed with cancer of the colorectum were not eligible to participate. Eligible patients were invited to participate and interviewed. All participants provided extensive information about their personal characteristics, detailed cancer family history, personal medical history, and exposure to epidemiologic risk factors. Each provided a venous blood sample and gave permission to retrieve their paraffin-embedded tumor tissue. Follow-up information on survival was collected from the Population Registry and/or medical records. The study was approved by Institutional Review Boards at the University of Michigan, University of Southern California, and Carmel Medical Center in Haifa, Israel. Written informed consent was required for participation.
Pathologic Analysis
All tumors were reviewed blindly by a single expert gastrointestinal pathologist (JKG). One or two representative blocks of normal and tumor were sent from Israel to The University of Michigan Department of Pathology, where one hematoxylin and eosin–stained (H&E) section and ten 5 micron unstained nonheated sections from each block were prepared. The coded H&E stained sections were reviewed, and the following histologic criteria were used to evaluate the tumors. In the majority of cases, the tumor block contained the advancing edge of the neoplasm as the contributing pathologists were instructed to include this area in the blocks they sent for review. Only resection specimens showing invasive adenocarcinoma of the colon and rectum were accepted into this analysis. Adenomas with “intramucosal carcinoma or carcinoma in-situ” were not included.
Tumor Grading
Tumors were given a single grade of differentiation (well, moderate, or poor) based on the criteria of Jass and colleagues with minor modification (26). The worst grade of tumor seen was used for the overall grade, unless the worst area was a small focus (<10%) at the advancing margin of the tumor (the presence of tumor budding was not counted as poor differentiation and did not impact the overall grade given to any tumor).
Prominent Crohn’s-Like Lymphoid Reaction
The advancing edge of the tumor was assessed for the presence of a prominent inflammatory reaction. For the reaction to be considered prominent, a minimum of three lymphoid aggregates was required per section. If the advancing edge of the tumor was not present, this field was graded as unknown.
Tumor-Infiltrating Lymphocytes
Tumor-infiltrating lymphocytes (TILs) were identified on H&E-stained sections as small blue mononuclear cells that typically had a halo around them. Only lymphocytes infiltrating between tumor cells were counted. Care was taken not to count apoptotic cells. The tumor was scanned at low power to look for the area with the most TILs (which was often the more superficial region of a deeply invasive carcinoma). Once this area was identified, five consecutive 40x fields of an Olympus BX40 microscope with UPlanF1 objective (Olympus America Inc, Melville, NY) were counted (total area equal to 0.94 mm2). The mean TIL/high-powered field (HPF) for each tumor was calculated by dividing the total number of TILs by five. Tumors were then separated into two groups (TIL/HPF ≥ 2 or TIL/HPF < 2) according to a value previously established to accurately predict the likelihood of MSI (10).
DNA Extraction From Tumor
DNA was extracted from tumor slides as previously described (10). Briefly, tumor DNA was microdissected from unstained, recut slides of formalin-fixed, paraffin-embedded tumors. Areas for microdissection were circled by one pathologist (JKG), and the H&E-stained slide was used as a template. Following dissection from slides, xylene was added to remove paraffin and the DNA was precipitated with ethanol. Following centrifugation, the supernatant was discarded, and the pellet was lyophilized. The pellet was resuspended in 100 μL Proteinase K buffer (50 mM tris and 200 ng/μL Proteinase K). The samples were incubated overnight at 37 °C and then denatured at 95 °C.
Microsatellite Instability Analyses
Microsatellite instability (MSI) testing was performed in colorectal tumor tissue from the first primary CRC that was case-defining by comparing at least three informative and up to ten microsatellite markers (BAT25, BAT26, BAT40, B-CATENIN, TGFBIIR, D2S123, D5S346, D17S250, D10S196, D18S58) between normal and tumor tissue from the same individual. Differences in the length of the microsatellites between healthy and tumor tissues indicate dysfunction of one or more of the mismatch repair genes. The criteria for testing MSI and determining MSI-high (MSI-H) status have been described previously in detail (27,28). Briefly, tumors considered for MSI status determination were those with at least one mononucleotide marker and a minimum of three markers in total with high-quality results. MSI-H status was called if 30% or more markers with good quality were unstable. Patients who subsequently developed a second primary cancer were followed as part of the cohort using date of diagnosis of the first primary cancer as the time of entry into the cohort, and the molecular features of the first primary cancer were used as the molecular signature for the patient.
Statistical Analysis
Chi-square analyses, Fisher’s exact test, and unconditional logistic regression were used to examine the associations between demographic, clinical, and tumor characteristics. Overall survival was defined as the length of time from the date of diagnosis of the cancer to the recorded date of death (from any cause) or to the last date of follow-up for participants who were alive on September 17, 2014. CRC-specific survival was calculated from the date of diagnosis to the date of death from CRC or to the last date of follow-up (deaths from another cause were censored). Kaplan-Meier curves and log-rank tests were used to compare survival across groups of two or more TILs vs fewer than two TILs, CLR vs no CLR, and MSI-H vs MSS/MSI-L as well as to visualize survival stratified by MSI and stage. Cox proportional hazards regression was performed to evaluate tumor characteristics associated with CRC-specific and overall survival in a multivariable setting. The assumption of proportional hazards was tested using the cox.zph function in the R ‘survival’ package, and as expected, we appreciated some evidence of departure because of our large sample size (29). All models were adjusted for the study’s matching factors: age, sex, and Jewish vs non-Jewish ethnicity. To assess the independent contribution of TILs and CLR, further analyses were additionally adjusted for known prognostic indicators including MSI, stage, and grade. We also conducted Cox regression treating TIL/HPF as a continuous variable. Among the 2369 with TIL and/or CLR data available, 1484 (1337) patients were included in the fully adjusted overall (CRC-specific) survival analyses because they had complete data on mortality and all covariates. The distributions of TIL and CLR were not statistically significantly different between included and excluded participants. Prediction models for CRC-specific and overall mortality hazard ratios are detailed in the Supplementary Material (available online). All analyses used R version 3.1.0. P values reported for tests of statistical significance are two-sided.
Results
This analysis included 2369 tumors from incident CRC patients with data on clinicopathological characteristics, MSI status, and survival. Representative images of TIL and CLR assay results are shown in Supplementary Figure 1 (available online). The range of TIL quantity observed in our study was 0-85 TIL/HPF, with a mean of 2.3 (SD = 5.2) and a median of 0.6. Six hundred twenty-one (26.2%) CRCs had TIL/HPF of 2 or more, 784 (33.1%) had a prominent CLR, and 346 (14.6%) had an MSI-H phenotype. Demographic characteristics of the study sample are described in Table 1. The average age of the patients was 70.1 years, with a range from 19 to 100 years (SD = 11.9). The median survival was 76.2 months (SD = 54.9, range = 0–196.9 months), and 57.2% of the participants in this analysis were alive at 60 months.
Table 1.
Demographic and tumor characteristics of Molecular Epidemiology of Colorectal Cancer Study participants (n = 2369)
| Demographic and tumor characteristics | Frequency |
|---|---|
| Age, mean (SD), y | 70.1 (11.9) |
| Survival, mean (SD), mo | 79.9 (54.9) |
| Sex, No. (%) | |
| Male | 1202 (50.7) |
| Female | 1167 (49.3) |
| Ethnicity, No. (%) | |
| Jew | 2053 (86.7) |
| Non-Jew | 312 (13.2) |
| Family history of CRC (first-degree relative), No. (%) | 151 (6.4) |
| Site, No. (%) | |
| Colon | 1902 (80.3) |
| Rectum | 442 (18.7) |
| Other or missing | 25 (1.1) |
| TIL/HPF*, No. (%) | |
| ≥2 | 621 (26.2) |
| <2 | 1647 (69.5) |
| Crohn’s-like lymphoid reaction†, No. (%) | |
| Yes | 784 (33.1) |
| No | 879 (37.1) |
*T lymphocyte infiltration/high-powered field (TIL/HPF) was missing for 101 samples. CRC = colorectal cancer.
†Crohn’s-like lymphoid reaction was missing for 706 samples where the advancing edge of the tumor was not present.
Table 2 demonstrates that TIL status was highly associated with stage (χ2 = 78.9, P < .001), grade (χ2 = 176.3, P < .001), and MSI-H status (odds ratio [OR] = 4.41, 95% confidence interval [CI] = 3.42 to 5.69, P < .001). TILs were observed at a higher frequency in tumors with a CLR (OR = 1.70, 95% CI = 1.35 to 2.14, P < .001) and in colon tumors on the right side (OR = 2.10, 95% CI = 1.69 to 2.61, P < .001). There were no statistically significant differences in TIL status by age, sex, ethnicity, family history, or tumor site (data not shown). Table 3 provides a detailed cross-tabulation of TIL and CLR by MSI status. TIL and CLR are statistically significantly associated among MSS/MSI-L cancers but not among MSI-H cancers, likely because of the small sample size in this subset. The statistical interaction is not significant (P = .86), indicating that the association between CLR and TIL does not differ by MSI status.
Table 2.
Tumor characteristics stratified by T lymphocyte infiltration status (n = 2268)
| Tumor characteristics | Frequency (%) |
OR (95% CI) | P* | |
|---|---|---|---|---|
| TIL/HPF ≥ 2 (n = 621) | TIL/HPF < 2 (n = 1647) | |||
| Side† | ||||
| Left | 205 (33.0) | 771 (46.8) | – | |
| Right | 284 (45.7) | 508 (30.8) | 2.10 (1.69 to 2.61) | <.001 |
| Stage | ||||
| I | 113 (18.2) | 135 (10.7) | – | <.001 |
| II | 233 (37.5) | 511 (40.4) | 0.54 (0.41 to 0.73) | |
| III | 99 (15.9) | 383 (30.3) | 0.31 (0.22 to 0.43) | |
| IV | 42 (6.8) | 237 (18.7) | 0.21 (0.14 to 0.32) | |
| Grade | ||||
| Well-differentiated | 175 (28.2) | 161 (9.8) | – | <.001 |
| Moderately differentiated | 346 (55.7) | 1357 (82.4) | 0.23 (0.18 to 0.30) | |
| Poorly differentiated | 97 (15.6) | 124 (7.5) | 0.72 (0.51 to 1.01) | |
| Microsatellite instability | ||||
| MSS/MSI-L | 414 (66.7) | 1417 (86.0) | – | |
| MSI-H | 179 (28.8) | 139 (8.4) | 4.41 (3.42 to 5.69) | <.001 |
| Crohn’s-like lymphoid reaction | ||||
| No | 186 (30.0) | 637 (38.7) | – | |
| Yes | 245 (39.5) | 494 (30.0) | 1.70 (1.35 to 2.14) | <.001 |
*Chi-square tests for independence. P values are two-sided. CI = confidence interval; MSI = microsatellite instability; MSS/MSI-H = microsatellite-stable/microsatellite-high; MSS/MSI-L = microsatellite-stable/microsatellite-low; OR = odds ratio; TIL/HPF = tumor infiltrating lymphocytes per high powered field.
†For colon cancers. Four hundred forty-two were rectal cancers, 53 were colon cancers with location not otherwise specified, one was a case with both right- and left-sided cancers (excluded from subsequent side-specific analyses), and 25 were missing location.
Table 3.
Cross-tabulation of T lymphocyte infiltration and Crohn’s-like lymphoid reaction by microsatellite instability status
| CLR status | MSI-H (n = 346) |
MSS/MSI-L (n = 1902) |
||||||
|---|---|---|---|---|---|---|---|---|
| TIL/HPF ≥ 2 | TIL/HPF < 2 | OR (95% CI) | P* | TIL/HPF ≥ 2 | TIL/HPF < 2 | OR (95% CI) | P* | |
| CLR | 71 | 40 | 1.52 (0.81 to 2.86) | .21 | 157 | 431 | 1.43 (1.10 to 1.87) | .007 |
| No CLR | 42 | 36 | 144 | 567 | ||||
*Chi-square tests for independence. P values are two-sided. CI = confidence interval; CLR = Crohn’s-like lymphoid reaction; MSI = microsatellite instability; MSS/MSI-H = microsatellite-stable/microsatellite-high; MSS/MSI-L = microsatellite-stable/microsatellite-low; OR = odds ratio; TIL/HPF = tumor infiltrating lymphocytes per high powered field.
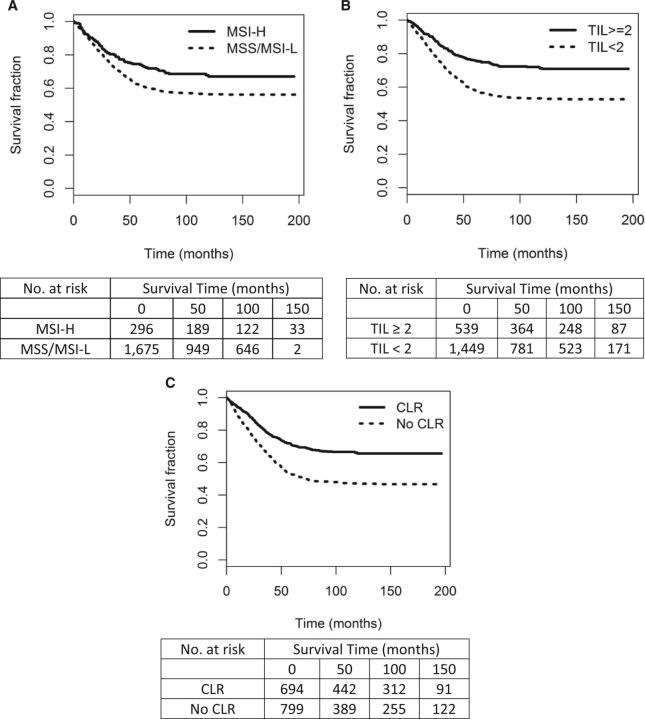
In a univariate analysis, tumors with TIL/HPF of 2 or greater were associated with a statistically significant increase in CRC-specific (HR = 0.53, 95% CI = 0.44 to 0.64, P < .001) and overall survival (HR = 0.75, 95% CI = 0.66, 0.84, P < .001) over tumors with TIL/HPF of less than 2. Patients with a prominent CLR experienced better CRC-specific (HR = 0.55, 95% CI = 0.47 to 0.65, P < .001) and overall survival (HR = 0.69, 95% CI = 0.61 to 0.78, P < .001) as compared with those without prominent peritumoral lymphoid aggregates. The MSI-H phenotype was associated with better CRC-specific (HR = 0.69, 95% CI = 0.55 to 0.86, P = .001 but not overall survival (HR = 0.90, 95% CI = 0.77 to 1.04, P = .14) as compared with MSS/MSI-L. Corresponding Kaplan-Meier curves for CRC-specific survival are presented in Figure 1.
Figure 1.
Kaplan-Meier colorectal cancer (CRC)–specific survival curves by (A) microsatellite instability (MSI) status (Nsolid = 296, events = 85; Ndashed = 1675, events = 664), (B) T lymphocyte infiltration (TIL) status (Nsolid = 539, events = 139; Ndashed = 1449, events = 619), and (C) Crohn’s-like lymphoid reaction (CLR) (Nsolid = 692, events = 215; Ndashed = 799, events = 385). MSI log-rank χ2 = 10.9, P < .001. TIL log-rank χ2 = 46.9, P < .001. CLR log-rank χ2 = 50.8, P < .001. All statistical tests were two-sided. CLR = Crohn’s-like reaction; MSI-H = microsatellite instability–high; MSS/MSI-L = microsatellite-stable/microsatellite-low; TIL = tumor infiltrating lymphocytes per high powered field.
Kaplan-Meier plots of TIL of 2 or greater vs TIL of less than 2 and CLR vs no CLR stratified by MSI status indicate that these host immune factors are associated with a survival advantage in patients with both MSI-H and MSS/MSI-L tumors (Figure 2, A and B). Further, Kaplan-Meier plots of TIL of 2 or greater vs TIL of less than 2 and CLR vs no CLR stratified by stage demonstrate that TILs and host response are important prognostic indicators for every stage at diagnosis, with the exception of TILs in stage IV cancers (Figure 2, C and D).
Figure 2.
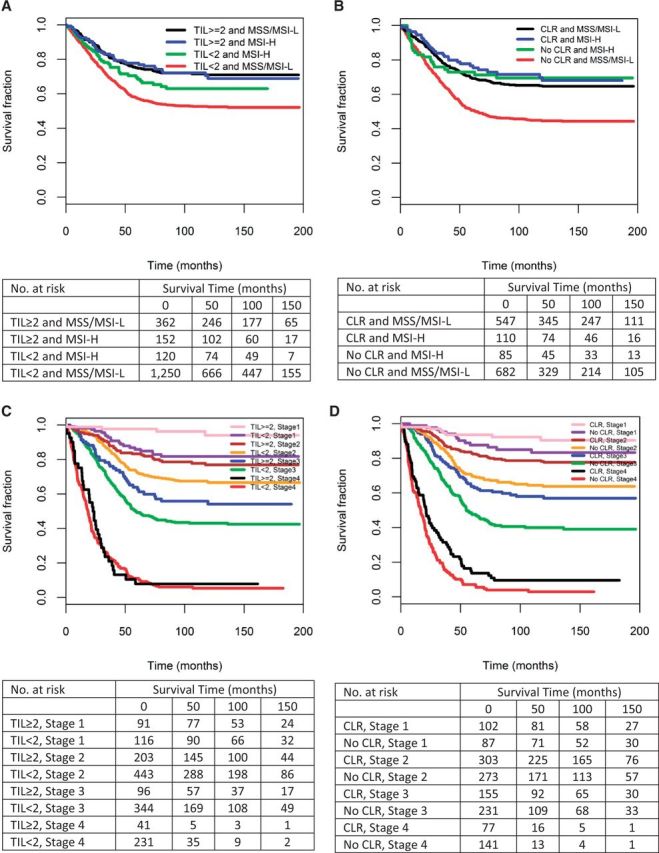
Kaplan-Meier colorectal cancer (CRC)–specific survival curves by (A) T lymphocyte infiltration (TIL) and microsatellite instability (MSI) status (Nblack = 362, events = 95; Nblue = 152, events = 39; Ngreen = 120, events = 40; Nred = 1250, events = 541); (B) Crohn’s-like lymphoid reaction (CLR) and MSI status (Nblack = 547, events = 175; Nblue = 110, events = 30; Ngreen = 85, events = 23; Nred = 682, events = 346); (C) TIL status and stage (Npink = 91, events = 4; Npurple = 116, events = 19; Nmaroon = 203, events = 40; Norange = 443, events = 129; Nblue = 96, events = 39; Ngreen = 344, events = 180; Nblack = 41, events = 36; Nred = 231, events = 208); and (D) CLR and stage (Npink = 102, events = 8; Npurple = 87, events = 13; Nmaroon = 303, events = 61; Norange = 273, events = 86; Nblue = 155, events = 59; Ngreen = 231, events = 127; Nblack = 77, events = 67; Nred = 141, events = 131). All statistical tests were two-sided. CLR = Crohn’s-like reaction; MSI-H = microsatellite instability–high; MSS/MSI-L = microsatellite-stable/microsatellite-low; TIL = tumor infiltrating lymphocytes per high powered field.
A Cox proportional hazards model including dichotomous TIL status, CLR, MSI status, stage, grade, age, sex, and ethnicity indicates that TILs, CLR, and stage are all associated with better CRC-specific and overall survival (Table 4). The fitted model for predicting mortality log-HR is described in detail in the Supplementary Methods (available online). Also, Supplementary Table 1 (available online) summarizes the distribution of key variables for participants included and excluded in the fully adjusted overall survival model. High TILs (HR = 0.76, 95% CI = 0.64 to 0.89, P < .001) and a prominent CLR (HR = 0.71, 95% CI = 0.62 to 0.80, P < .001) but not MSI were associated with a statistically significant reduction in all-cause mortality after adjustment for established prognostic factors. Diagnosis at stage III or stage IV (HRstageIII = 1.33, 95% CI = 1.10 to 1.62, P = .004; HRstageIV = 5.31, 95% CI = 4.28 to 6.58, P < .001), moderate differentiation (HR = 1.94, 95% CI = 1.50 to 2.52, P < .001), and increasing age (HR = 1.04, 95% CI = 1.04 to 1.05, P < .001) were associated with increased all-cause mortality. MSI status was not statistically significantly associated with CRC-specific or overall survival after adjustment for dichotomous TIL status, host response status, and other established prognostic indicators. Notably, when considering TIL quantity as a continuous rather than a dichotomous predictor in the context of CLR, MSI, stage, grade, age, sex, and Jewish ethnicity, the HR associated with a one-unit increase in TIL/HPF for overall survival was 0.97 (95% CI = 0.95 to 0.99, P < .001) and for CRC-specific survival was 0.96 (95% CI = 0.93 to 0.98, P = .002). Thus, each TIL/HPF is associated with approximately a 3% to 4% reduction in the risk of mortality, and the association remains highly statistically significant (Supplementary Table 2, available online).
Table 4.
Adjusted hazard ratio estimates for overall and colorectal cancer–specific mortality from multivariable Cox proportional hazards regression in the Molecular Epidemiology of Colorectal Cancer Study
| Variable | Overall survival (n = 1484) |
CRC-specific survival (n = 1337) |
||
|---|---|---|---|---|
| HR (95% CI) | P† | HR (95% CI) | P† | |
| TIL/HPF (≥2 vs < 2) | 0.76 (0.64 to 0.89) | <.001 | 0.66 (0.52 to 0.84) | <.001 |
| Crohn’s-like lymphoid reaction(yes vs no) | 0.71 (0.62 to 0.80) | <.001 | 0.65 (0.54 to 0.78) | <.001 |
| MSI (MSI-H vs MSS/MSI-L) | 0.91 (0.73 to 1.13) | .41 | 0.77 (0.56 to 1.06) | .10 |
| Stage II* | 0.99 (0.83 to 1.19) | .95 | 1.12 (0.83 to 1.53) | .46 |
| Stage III* | 1.33 (1.10 to 1.62) | .004 | 2.10 (1.56 to 2.84) | <.001 |
| Stage IV* | 5.31 (4.28 to 6.58) | <.001 | 9.24 (6.79 to 12.58) | <.001 |
| Grade 2 (moderately differentiated)* | 1.94 (1.50 to 2.52) | <.001 | 2.67 (1.87 to 3.81) | <.001 |
| Grade 3 (poorly differentiated)* | 0.96 (0.79 to 1.17) | .67 | 1.11 (0.82 to 1.52) | .49 |
| Age | 1.04 (1.04 to 1.05) | <.001 | 1.02 (1.01 to 1.03) | <.001 |
| Female sex* | 0.95 (0.84 to 1.08) | .46 | 1.10 (0.93 to 1.30) | .28 |
| Jewish ethnicity* | 0.87 (0.71 to 1.07) | .18 | 0.83 (0.65 to 1.07) | .15 |
*Reference categories: stage I, grade 1 (well-differentiated), male sex, non-Jewish ethnicity. CI = confidence interval; HPF = high-powered field; HR = hazard ratio; MSI-H = microsatellite instability–high; MSS/MSI-L = microsatellite-stable/microsatellite-low; TIL = tumor infiltrating lymphocyte.
†P value from multivariable Cox regression adjusted for all other variables in the table. P values are two-sided.
We also estimated disease-specific and overall mortality hazard ratios upon stratification by stage, by tumor site (colon vs rectum), and by side for colon cancers (30). The inverse association between TIL and CLR and survival was evident across all stages (Supplementary Table 3, available online). The direction of HR estimates for TIL/HPF and CLR was consistent with CRC results for both colon and rectal cancers, and there was no statistically significant interaction between either immune-related variable and site (Supplementary Table 4, available online). The same was true for left- and right-sided colon cancers (Supplementary Table 5, available online). MSI was a statistically significant prognostic factor for only right-sided colon cancers (Pinteraction = .01).
Discussion
This study examined the role of intratumoral lymphocytic infiltration, Crohn’s-like host immune response at the tumor’s advancing edge, MSI, and clinical characteristics in disease-specific and overall survival following CRC diagnosis. The observations from this large collection of CRCs indicate that TILs and a CLR are both prognostic indicators after adjusting for age, sex, ethnicity, stage, grade, and MSI status. Further, our findings suggest that the survival benefit of MSI is potentially attributable to an enhanced immune response, which is not limited to MSI-H tumors. Studies that stratify by MSI status are often limited by sample size as MSI-H tumors account for only 10% to 15% percent of all CRCs. Here, we were able to take advantage of a well-characterized, large population-based sample of CRCs that provided the ability to comprehensively evaluate the phenotype.
It is not entirely unexpected to find that TILs and CLR are both prognostic indicators for CRC. The host immune response has generally been correlated with the survival-associated MSI phenotype (12,13), and several groups have established the relationship between TIL and MSI (14,31–35). Several studies have indicated that TILs may be the best prognostic indicator overall for CRC. Galon et al. (18) showed that immune cell characteristics in CRCs have a better prognostic value than Union for International Cancer Control Tumor, Node, and Metastases (UICC-TNM) staging, but the cancers were not stratified by MSI status. Further, Chang et al. (24) showed in 150 CRCs that lymphocytic infiltrate was associated with a survival benefit regardless of MSI status but did not note a survival difference between MSI-H tumors with a host immune response and MSS tumors with a host immune response. High overall and subtype-specific lymphocyte infiltration have been suggested as independent prognostic factors for overall survival in a variety of other cancers as well (36). Here we showed that the presence of TILs is strongly associated with improved prognosis and then quantified the relationship to illustrate that each TIL/HPF is associated with approximately a 3% to 4% reduction in the risk of mortality.
With respect to Crohn’s-like lymphoid aggregation, previous evidence has suggested its association with improved survival (20–23). A recent study identified that high CLR density was associated with intratumoral density of T-cells as well as survival independent of stage, peritumoral inflammation, and TIL quantity (21). Another study by Ogino and colleagues demonstrated that lymphocytic reaction score (comprised of Crohn’s-like reaction, peritumoral reaction, intratumoral periglandular reaction, and TILs) was associated with a statistically significant improvement in CRC-specific and overall survival (20). However, when considering each component of the score separately, they observed a statistically significant survival advantage for Crohn’s-like reaction but not TILs (20).
The American Joint Committee on Cancer (AJCC) staging system has not yet incorporated TILs or CLR (37,38). However, results from our large sample suggest that these immune-related factors are both prognostic predictors beyond MSI status and the standard stage and grade, and thus, provide evidence in support of their consideration for inclusion. While high TIL/HPF and CLR were clear prognostic indicators in this study, the standard methods for quantifying and characterizing them are highly labor intensive and pathologist-dependent. Advanced techniques for measuring and characterizing TILs and lymphoid aggregates in a standardizable way will be necessary for widespread clinical utility.
Despite our study’s considerable strengths, including a population-based sampling frame, large sample size, and comprehensive molecular characterization, it is not without limitations. First, because these data are not derived from an RCT, there are limited data available for treatment and uniform patient follow-up for recurrence. This limitation is attenuated by the representative population-based sampling procedures that minimize selection bias; nonetheless, this is an observational study. Second, immunohistochemistry data for CD4+, CD8+ and FoxP3+ were not available, and thus, we could not examine with more granularity than overall quantity the types of infiltrating T-cells that are important for survival. Third, we did not assess treatment patterns or all classical epidemiologic factors that have previously been noted as potential prognostic factors. Evidence supports potential roles for BMI (39), smoking (40), aspirin (41,42), metformin (43), and racial/ethnic differences partially attributable to variable KRAS/BRAF mutation rates (11,44), among other factors. Finally, it is important to note that the standard of care has changed considerably since the inception of the study.
In summary, this large study of incident CRCs identified TILs and CLR as prognostic factors above and beyond the influences of stage, grade, and MSI, and it provides strong evidence in support of their consideration in staging guidelines.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (R01 CA81488, R01 CA197350, U19 CA148107, and P30 CA014089 to SBG), the National Institute of Environmental Health Sciences at the National Institutes of Health (T32 ES013678 to SLS), and the Anton B. Burg Foundation.
Notes
The study sponsor(s) had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
All authors contributed to the data analysis, interpretation of the results, and preparation of the manuscript. JKG individually reviewed all pathologic specimens and scored TILs and Crohn’s-like host response. LPT performed all microsatellite instability analyses. HSR prepared the data for analysis. GR and SBG provided overall supervision of the study and, specifically, contributed to the study design, interpretation of results, and critical review of the manuscript. All authors reviewed the manuscript and contributed to the final draft. SBG had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors declare no conflicts of interest.
Supplementary Material
References
- 1.Ferlay J, Soerjomataram II, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.Samowitz WS, Curtin K, Ma KN, et al. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev. 2001;10(9):917–923. [PubMed] [Google Scholar]
- 3.Elsaleh H, Iacopetta B. Microsatellite instability is a predictive marker for survival benefit from adjuvant chemotherapy in a population-based series of stage III colorectal carcinoma. Clin Colorectal Cancer. 2001;1(2):104–109. [DOI] [PubMed] [Google Scholar]
- 4.Mei Z, Liu Y, Liu C, et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. 2014;110(6):1595–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galon J, Pages F, Marincola FM, et al. The immune score as a new possible approach for the classification of cancer. J Transl Med. 2012;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elsaleh H, Joseph D, Grieu F, et al. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet. 2000;355(9217):1745–1750. [DOI] [PubMed] [Google Scholar]
- 7.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50(1):113–130. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins MA, Hayashi S, O'Shea AM, et al. Pathology features in Bethesda guidelines predict colorectal cancer microsatellite instability: a population-based study. Gastroenterology. 2007;133(1):48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenson JK, Bonner JD, Ben Yzhak O, et al. Phenotype of microsatellite unstable colorectal carcinomas: well-differentiated and focally mucinous tumors and the absence of dirty necrosis correlate with microsatellite instability. Am J Surg Pathol. 2003;27(5):563–570. [DOI] [PubMed] [Google Scholar]
- 10.Greenson JK, Huang SC, Herron C, et al. Pathologic predictors of microsatellite instability in colorectal cancer. Am J Surg Pathol. 2009;33(1):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rennert G LF, Rennert HS, Raskin L, Cohen I, Friedman V, et al. Molecularly driven survival patterns in colorectal cancer. Under review. [Google Scholar]
- 12.Drescher KM, Sharma P, Watson P, et al. Lymphocyte recruitment into the tumor site is altered in patients with MSI-H colon cancer. Fam Cancer. 2009;8(3):231–239. [DOI] [PubMed] [Google Scholar]
- 13.Banerjea A, Ahmed S, Hands RE, et al. Colorectal cancers with microsatellite instability display mRNA expression signatures characteristic of increased immunogenicity. Mol Cancer. 2004;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smyrk TC, Watson P, Kaul K, et al. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001;91(12):2417–2422. [PubMed] [Google Scholar]
- 15.Tougeron D, Fauquembergue E, Rouquette A, et al. Tumor-infiltrating lymphocytes in colorectal cancers with microsatellite instability are correlated with the number and spectrum of frameshift mutations. Mod Pathol. 2009;22(9):1186–1195. [DOI] [PubMed] [Google Scholar]
- 16.Svennevig JL, Lunde OC, Holter J, et al. Lymphoid infiltration and prognosis in colorectal carcinoma. Br J Cancer. 1984;49(3):375–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ropponen KM, Eskelinen MJ, Lipponen PK, et al. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997;182(3):318–324. [DOI] [PubMed] [Google Scholar]
- 18.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. [DOI] [PubMed] [Google Scholar]
- 19.Nosho K, Baba Y, Tanaka N, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222(4):350–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogino S, Nosho K, Irahara N, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res. 2009;15(20):6412–6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vayrynen JP, Sajanti SA, Klintrup K, et al. Characteristics and significance of colorectal cancer associated lymphoid reaction. Int J Cancer. 2014;134(9):2126–2135. [DOI] [PubMed] [Google Scholar]
- 22.Graham DM, Appelman HD. Crohn's-like lymphoid reaction and colorectal carcinoma: a potential histologic prognosticator. Mod Pathol. 1990;3(3):332–335. [PubMed] [Google Scholar]
- 23.Harrison JC, Dean PJ, el-Zeky F, et al. Impact of the Crohn's-like lymphoid reaction on staging of right-sided colon cancer: results of multivariate analysis. Hum Pathol. 1995;26(1):31–38. [DOI] [PubMed] [Google Scholar]
- 24.Chang EY, Dorsey PB, Frankhouse J, et al. Combination of microsatellite instability and lymphocytic infiltrate as a prognostic indicator in colon cancer. Arch Surg. 2009;144(6):511–515. [DOI] [PubMed] [Google Scholar]
- 25.Poynter JN, Gruber SB, Higgins PD, et al. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352(21):2184–2192. [DOI] [PubMed] [Google Scholar]
- 26.Jass JR, Atkin WS, Cuzick J, et al. The grading of rectal cancer: historical perspectives and a multivariate analysis of 447 cases. Histopathology. 1986;10(5):437–459. [DOI] [PubMed] [Google Scholar]
- 27.Raskin L, Dakubo JC, Palaski N, et al. Distinct molecular features of colorectal cancer in Ghana. Cancer Epidemiol. 2013;37(5):556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vilar E, Bartnik CM, Stenzel SL, et al. MRE11 deficiency increases sensitivity to poly(ADP-ribose) polymerase inhibition in microsatellite unstable colorectal cancers. Cancer Res. 2011;71(7):2632–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 30.Loupakis F, Yang D, Yau L, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst. 2015;107(3):dju427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takemoto N, Konishi F, Yamashita K, et al. The correlation of microsatellite instability and tumor-infiltrating lymphocytes in hereditary non-polyposis colorectal cancer (HNPCC) and sporadic colorectal cancers: the significance of different types of lymphocyte infiltration. Jpn J Clin Oncol. 2004;34(2):90–98. [DOI] [PubMed] [Google Scholar]
- 32.Dolcetti R, Viel A, Doglioni C, et al. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol. 1999;154(6):1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernal M, Concha A, Saenz-Lopez P, et al. Leukocyte infiltrate in gastrointestinal adenocarcinomas is strongly associated with tumor microsatellite instability but not with tumor immunogenicity. Cancer Immunol Immunother. 2011;60(6):869–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexander J, Watanabe T, Wu TT, et al. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001;158(2):527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips SM, Banerjea A, Feakins R, et al. Tumour-infiltrating lymphocytes in colorectal cancer with microsatellite instability are activated and cytotoxic. Br J Surg. 2004;91(4):469–475. [DOI] [PubMed] [Google Scholar]
- 36.Gooden MJ, de Bock GH, Leffers N, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105(1):93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walther A, Johnstone E, Swanton C, et al. Genetic prognostic and predictive markers in colorectal cancer. Nat Rev Cancer. 2009;9(7):489–499. [DOI] [PubMed] [Google Scholar]
- 38.Phipps AI, Limburg PJ, Baron JA, et al. Association Between Molecular Subtypes of Colorectal Cancer and Patient Survival. Gastroenterology. 2014;148(1):77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinicrope FA, Foster NR, Yothers G, et al. Body mass index at diagnosis and survival among colon cancer patients enrolled in clinical trials of adjuvant chemotherapy. Cancer. 2013;119(8):1528–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parajuli R, Bjerkaas E, Tverdal A, et al. Cigarette smoking and colorectal cancer mortality among 602,242 Norwegian males and females. Clin Epidemiol. 2014;6:137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302(6):649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao X, Lochhead P, Nishihara R, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367(17):1596–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JH, Kim TI, Jeon SM, et al. The effects of metformin on the survival of colorectal cancer patients with diabetes mellitus. Int J Cancer. 2012;131(3):752–759. [DOI] [PubMed] [Google Scholar]
- 44.HH Y, Q S, SR A, et al. Racial Differences in KRAS/BRAF mutation rates and survival in colon cancer (NCCTG N0147 [Alliance]). In. American Society of Clinical Oncology Annual Meeting. Chicago, IL; 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.