Abstract
Background: Hepatitis C virus (HCV) infection is associated with hepatocellular carcinoma and non-Hodgkin’s lymphoma. In 2009, MD Anderson established the first US clinic for treating HCV-infected cancer patients, where we observed an unexpectedly large number of patients with head and neck cancers (HNCs). We sought to determine whether HCV is associated with HNCs.
Methods: In this case-control study, medical records of cancer patients tested for HCV antibodies at our center from 2004 through 2014 were identified. Case subjects had new-onset primary oropharyngeal or nonoropharyngeal (oral cavity, nasopharynx, hypopharynx, or larynx) HNCs. Control subjects had smoking-associated (lung, esophagus, or urinary bladder) cancers. Biopsy reports of oropharyngeal cancers tested for human papillomavirus (HPV) were reviewed. Patients with lymphoma were excluded. Multivariable logistic regression models were constructed. All statistical tests were two-sided.
Results: Of 34 545 cancer patients tested for HCV antibodies, 409 case subjects (164 oropharyngeal and 245 nonoropharyngeal) and 694 control subjects (378 lung, 168 esophagus, and 148 urinary bladder) were studied. The prevalence of HCV seropositivity was higher in oropharyngeal cancer patients (14.0%, 95% confidence interval [CI] = 8.7% to 19.4%, vs 6.5%, 95% CI = 4.6% to 8.3%), particularly HPV-positive oropharyngeal cancer patients (16.9%, 95% CI = 8.7% to 24.9%, vs 6.5%, 95% CI = 4.6% to 8.3%), and nonoropharyngeal HNC patients (20.0%, 95% CI = 14.9% to 25.0%, vs 6.5%, 95% CI = 4.6% to 8.3%) than in control subjects. Adjusted models showed a statistically significant association of HCV seropositivity with nonoropharyngeal (except nasopharyngeal) HNCs (odds ratio [OR] = 2.85, 95% CI = 1.38 to 5.88) and HPV-positive oropharyngeal cancers (OR = 2.97, 95% CI = 1.31 to 6.76).
Conclusions: HCV is associated with nonoropharyngeal (except nasopharyngeal) and HPV-positive oropharyngeal HNCs. Further studies are required to explore the possible interaction between HCV and HPV, and the association between HCV and other HPV-related malignancies.
Approximately 2.7 to 3.9 million individuals in the United States (up to 1.5% of the population) are chronically infected with hepatitis C virus (HCV) (1). HCV is carcinogenic, and HCV-seropositive individuals have 15 to 20 times the risk of developing hepatocellular carcinoma that HCV-seronegative individuals have (2). Chronic HCV infection causes not only liver cancer but also lymphoproliferative disorders (3–5).
Clinical and epidemiological evidence supports a statistically significant association between HCV infection and development of several types of B-cell non-Hodgkin’s lymphomas (6–8). Additionally, a recent analysis of data from the Chronic Hepatitis C Cohort Study showed that HCV-infected individuals also have increased incidences of other non-liver-related malignancies, including pancreas, lung, renal, and rectal cancers, along with increased cancer-associated mortality (9). Likewise, a prospective study from Taiwan showed increased mortality from extrahepatic diseases in HCV-seropositive individuals (10).
In 2009, we established the first US clinic devoted to managing HCV infection in cancer patients (11), where we observed an unexpectedly large number of patients with head and neck cancers (HNCs). In this study, we sought to determine whether HCV seropositivity is associated with oropharyngeal and nonoropharyngeal HNCs.
Methods
Study Design and Patient Population
We conducted a case-control study. After obtaining approval from our institution’s institutional review “board” (IRB), we identified medical records of all cancer patients age 18 years old and older at cancer diagnosis seen at our institution and tested for HCV antibodies between June 1, 2004, and May 31, 2014. A waiver for the written informed consent was obtained from the IRB because this study was a retrospective evaluation of the patients' information and did not involve more than minimal risk to the subjects. Using the Clinical Informatics database, we obtained data on the sites and histopathological diagnoses of cancers in these patients. We selected patients with HNCs as case subjects and divided them into two groups with different cancer etiologies: patients with oropharyngeal and patients with nonoropharyngeal (oral cavity, nasopharynx, hypopharynx, and larynx) cancers (Figure 1). The tumor sites and International Classification of Diseases for Oncology topographical codes for the case subjects are presented in Supplementary Table 1 (available online) (12). As smoking is a major risk factor for HNCs (13), we selected as the control group patients with three major smoking-associated cancers—lung, esophagus, and urinary bladder (14)—to balance the distribution of smoking-related risk factors between case and control groups (Figure 1). All cancer diagnoses were histopathologically confirmed at our center. Because HCV infection is associated with B-cell non-Hodgkin’s lymphoma, we excluded patients with lymphoma.
Figure 1.
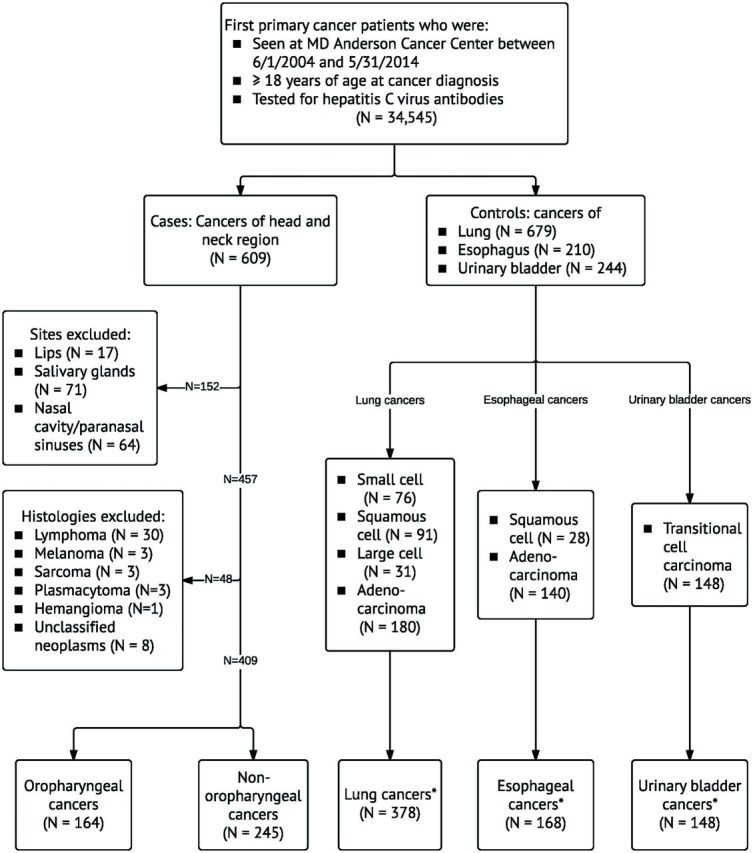
Selection of case and control groups. Asterisk indicates that histologies that are associated with smoking were included in the study population.
Ascertainment of Exposures and Covariates
Patients with HCV were identified using International Classification of Diseases (ninth revision) codes for HCV infection (070.41, 070.44, 070.51, 070.54, 070.70, 070.71, or V02.62), and the diagnosis was verified by medical records review. HCV seropositivity was defined as detectable HCV antibody in serum. Patients were tested for HCV antibodies using the ORTHO HCV Version 3.0 ensyme-linked immunosorbent assay (ELISA; Ortho-Clinical Diagnostics, Raritan, NJ) from 2004 to 2007 and the ABBOTT PRISM HCV assay (Abbott Park, IL) from 2007 to 2014 (screening tests were changed in 2007 by the institution). Both tests are US Food and Drug Administration–approved, commercially available assays with specificity greater than 99% (15,16). HCV infection was defined as detectable HCV RNA in serum. HCV RNA was tested by Cobas TaqMan HCV Monitor version 1.0 or 2.0 assay (Roche Molecular Systems, Branchburg, NJ). Data on co-infections with hepatitis B virus (HBV) and HIV were also obtained. HBV exposure was defined as positivity for HBV core antibody, and HIV infection was defined as positivity for HIV antibody.
From the standardized questionnaire administered to all patients at their first visit at our institution, we extracted data on demographics, including birth year, highest education level (surrogate for socioeconomic status), cigarette smoking, and alcohol consumption. Smoking-related information collected included smoking status (never, former, or current smoker), age at starting/quitting smoking, number of years of smoking, number of cigarettes smoked per day, and duration of smoking in pack-years (number of cigarette packs smoked per day multiplied by number of years smoked). Never smokers were those who smoked fewer than 100 cigarettes in their lifetime. Former smokers were those who had quit at least one year before cancer diagnosis. Alcohol-related information collected included drinking status (never, former, or current drinker), age at starting/quitting alcohol drinking, number of years of drinking, and number of alcoholic drinks consumed per week, with a standard drink defined as 4 ounces for wine, 12 ounces for beer, and 1.5 ounces for hard liquor (17).
Pathology reports of all case subjects were reviewed in our center to determine whether tumors were positive for human papillomavirus (HPV) or Epstein-Barr virus (EBV). HPV p16 protein expression was examined using immunohistochemistry, and the presence of high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 66) was examined using in situ hybridization (18). Oropharyngeal cancers were considered HPV positive if high-risk HPV tumors were positive for p16. Tumors were tested for EBV-encoded RNA using in situ hybridization (19).
Statistical Analyses
We conducted different analyses to determine whether HCV seropositivity was associated with oropharyngeal or nonoropharyngeal HNCs. We separately compared characteristics of the two case groups and the control group. Categorical variables were compared using Pearson’s chi-squared test or Fisher’s exact test, and continuous variables were compared using Wilcoxon rank-sum test (for non-normal distributions).
Univariate logistic regression analysis was conducted to examine the association between HCV seropositivity and the case group. Potential confounders were assessed by introducing them in the model, and we included variables that changed the estimates by 10% or more in the adjusted model. Final adjusted multivariable logistic regression models were constructed and the most parsimonious models selected using Akaike information criterion. We reported the effect sizes as odds ratios (ORs) and corresponding 95% confidence intervals (CIs) and P values.
To account for different pathogenesis of cancers, we conducted subgroup analyses to determine the associations between HCV seropositivity and oropharyngeal cancers, according to HPV status, and nasopharyngeal cancers. We performed sensitivity analyses and evaluated the associations by including only patients with HCV infection. Finally, in an exploratory analysis, we fit separate unconditional logistic regression models for each HNC subsite and adjusted for potential confounders. We corrected the P values for multiple comparisons using a false-discovery rate of 10% according to the Benjamini and Hochberg method (20).
All statistical tests were two-sided, and P values of less than .05 were considered statistically significant. Statistical analyses were conducted using Stata/IC software, version 13.0 (StataCorp LP, College Station, TX).
Results
Study Population
We identified 34 545 cancer patients tested for HCV antibodies during the study period. Of these, 1103 were included as case subjects (n = 409: 164 oropharyngeal and 245 nonoropharyngeal) or control subjects (n = 694: 378 lung, 168 esophagus, and 148 urinary bladder) (Figure 1). Patient characteristics are summarized in Table 1. Most patients were male, white, and born between 1945 and 1965; the median age at cancer diagnosis was 62 years. Approximately 41.1% of patients had a bachelor’s degree or beyond, 49.0% were former smokers, and 52.3% current alcohol drinkers at cancer diagnosis. In the overall sample of case and control subjects, the prevalence of HCV seropositivity was 10.6% (n = 117, 95% CI = 8.9% to 12.5%), and 86.4% (n = 76 of 88, 95% CI = 79.2% to 93.5%) of those tested for HCV RNA had HCV infection. Of the 145 patients whose cancers were tested for HPV, 70 had high-risk HPV and 83 had p16-positive tumors.
Table 1.
Characteristics of the total study population (n = 1103)
| Characteristic | Value |
|---|---|
| Case subjects, No. (%) | 409 (37.1) |
| Oropharyngeal cancers | 164 (40.1) |
| Nonoropharyngeal (except nasopharyngeal) cancers | 213 (52.1) |
| Nasopharyngeal cancers | 32 (7.8) |
| Control subjects, No. (%) | 694 (62.9) |
| Lung cancers | 378 (54.5) |
| Esophagus cancers | 168 (24.2) |
| Urinary bladder cancers | 148 (21.3) |
| Hepatitis C virus antibody, No. (%) | |
| Positive* | 117 (10.6) |
| Negative | 986 (89.4) |
| Race/ethnicity, No. (%) | |
| White | 880 (79.2) |
| African American | 84 (7.6) |
| Hispanic | 85 (7.5) |
| Asian/Pacific Islander | 44 (3.8) |
| Middle Eastern | 9 (0.8) |
| Native American | 1 (0.1) |
| Sex, No. (%) | |
| Female | 305 (27.6) |
| Male | 798 (72.4) |
| Age at cancer diagnosis, median (IQR), y | 62 (54–68) |
| Birth cohort | |
| Born before 1945 | 386 (35) |
| Born between 1945 and 1965 | 648 (58.7) |
| Born after 1965 | 69 (6.3) |
| Highest education level, No. (%) | |
| Available | 942 (85.4) |
| Less than 8th grade | 34 (3.6) |
| 9th–11th grade | 51 (5.4) |
| High school graduate/GED | 244 (25.9) |
| Vocational/technical school | 78 (8.3) |
| Associate degree/some college | 148 (15.7) |
| Bachelor’s degree | 230 (24.4) |
| Advanced degree | 157 (16.7) |
| Cigarette smoking status | |
| Never smoker, No. (%) | 255 (23.1) |
| Former smoker, No. (%) | 540 (49.0) |
| Number of years of smoking, median (IQR) | 29 (15–39) |
| Pack-years of smoking, median (IQR) | 27 (15–45) |
| Age when quit smoking, median (IQR), y | 50 (37–58) |
| Current smoker, No. (%) | 308 (27.9) |
| Number of years of smoking, median (IQR) | 40 (32–46) |
| Pack-years of smoking, median (IQR) | 40 (27–63) |
| Age when quit smoking, median (IQR), y | 63 (50–68) |
| Pack-years of cigarette smoking, No. (%) | |
| 0 | 255 (23.1) |
| 1–25 | 316 (28.6) |
| 26–50 | 276 (25.0) |
| 51–100 | 177 (16.1) |
| >100 | 79 (7.2) |
| Alcohol consumption | |
| Never drinker, No. (%) | 247 (22.4) |
| Former drinker, No. (%) | 279 (25.3) |
| Current drinker, No. (%) | 577 (52.3) |
| Number of drinks/wk, median (IQR) | 6 (1–17) |
| Number of alcoholic drinks/wk, No. (%) | |
| 0 | 247 (22.4) |
| ≤1 | 239 (21.7) |
| 2–14 | 309 (28.0) |
| 15–28 | 109 (9.9) |
| >28 | 199 (18.0) |
| Duration of alcohol consumption, median (IQR), y | 30 (15–40) |
| HCV genotype, No./tested (%) | |
| 1 | 32/42 (76.2) |
| 2 | 7/42 (16.7) |
| 3 | 3/42 (7.1) |
| HCV RNA, No. positive/tested (%) | 76/88 (86.4) |
| Hepatitis B exposure, No. positive/tested (%)† | 109/950 (11.5) |
| HIV infection, No. positive/tested (%)‡ | 16/652 (2.5) |
| EBV-encoded small nuclear RNA, No. positive/tested (%) | 20/39 (51.3) |
| HPV p16 status (oropharyngeal cancers only), No./tested (%)§ | |
| p16 positive§ | 83/145 (57.2) |
| Tumor positive for high-risk HPV‖ | 62/83 (74.7) |
| Tumor negative for high-risk HPV‖ | 20/83 (24.1) |
| High-risk HPV status unknown | 1/83 (1.2) |
| p16 negative§ | 43/145 (29.7) |
| Tumor positive for high-risk HPV‖ | 0/43 (0.0) |
| Tumor negative for high-risk HPV‖ | 42/43 (97.7) |
| High-risk HPV status unknown | 1/43 (2.3) |
| p16 unknown | 19/145 (13.1) |
| Tumor positive for high-risk HPV‖ | 8/19 (42.1) |
| Tumor negative for high-risk HPV‖ | 11/19 (57.9) |
*HCV RNA was tested in 88 of 117 (75.2%) patients with positive HCV antibody; 76 (86.4%) of those tested had detectable HCV RNA. EBV = Epstein-Barr virus; GED = general equivalency diploma; HPV = human papillomavirus; IQR = interquartile range.
†Positive for hepatitis B core antibody.
‡Positive for HIV antibody.
§Cancers tested for p16 using immunohistochemistry.
‖Cancers tested for high-risk HPV using in situ hybridization.
Oropharyngeal Cancers
Compared with control subjects, oropharyngeal cancer patients were more likely to be male, born between 1945 and 1965, diagnosed with cancer at younger ages, and better educated; to have smoked fewer cigarettes; and to consume more alcohol (Table 2). There was no statistically significant difference between groups with respect to HBV exposure or HIV.
Table 2.
Comparison of characteristics of control subjects and patients with oropharyngeal and nonoropharyngeal head and neck cancer
| Characteristic | Control subjects (n = 694) | Patients with oropharyngeal cancers (n = 164) | Univariate P* | Patients with nonoropharyngeal cancers (n = 245) | Univariate P* |
|---|---|---|---|---|---|
| Hepatitis C virus antibody, No. (%) | .001 | <.001 | |||
| Negative | 649 (93.5) | 141 (86.0) | 196 (80.0) | ||
| Positive | 45 (6.5) | 23 (14.0) | 49 (20.0) | ||
| Hepatitis C virus RNA, No. (%)† | <.001 | <.001 | |||
| Negative | 649 (96.6) | 141 (89.3) | 196 (84.5) | ||
| Positive | 23 (3.4) | 17 (10.7) | 36 (15.5) | ||
| Race/ethnicity, No. (%) | .41 | <.001 | |||
| White | 567 (81.7) | 141 (86.0) | 172 (70.2) | ||
| African American | 55 (7.9) | 6 (3.7) | 23 (9.4) | ||
| Hispanic | 41 (5.9) | 14 (8.5) | 30 (12.2) | ||
| Asian/Pacific Islander | 25 (3.6) | 1 (0.6) | 18 (7.4) | ||
| Middle Eastern | 5 (0.7) | 2 (1.2) | 2 (0.8) | ||
| Native American | 1 (0.1) | 0 (0.0) | 0 (0.0) | ||
| Sex, No. (%) | .001 | .01 | |||
| Female | 219 (31.6) | 30 (18.3) | 56 (22.9) | ||
| Male | 475 (68.4) | 134 (81.7) | 189 (77.1) | ||
| Age at cancer diagnosis | |||||
| Median (IQR), y | 63 (56–70) | 59 (51–65) | <.001 | 59 (51–66) | <.001 |
| ≤60 years, No. (%) | 264 (38.0) | 90 (54.9) | <.001 | 142 (57.9) | <.001 |
| >60 years, No. (%) | 430 (62.0) | 74 (45.1) | 103 (42.1) | ||
| Born between 1945 and 1965, No. (%) | <.001 | .17 | |||
| No | 313 (45.1) | 44 (26.8) | 98 (40.0) | ||
| Yes | 381 (54.9) | 120 (73.2) | 147 (60.0) | ||
| Highest education level, No. (%)† | |||||
| ≤High school graduate/GED | 235 (37.0) | 28 (21.9) | <.001 | 66 (36.9) | .62 |
| Vocational/technical school, associate degree, or some college | 160 (25.2) | 27 (21.1) | 39 (21.8) | ||
| ≥Bachelor’s degree | 240 (37.8) | 73 (57.0) | 74 (41.3) | ||
| Cigarette smoking status | .02 | .92 | |||
| Never smoker, No. (%) | 142 (20.5) | 50 (30.5) | 63 (25.7) | ||
| Former smoker, No. (%) | 361 (52.0) | 76 (46.3) | 103 (42.1) | ||
| Current smoker, No. (%) | 191 (27.5) | 38 (23.2) | 79 (32.2) | ||
| Pack-years of smoking, median (IQR) | 36 (20–54) | 23.9 (12.5–40) | <.001 | 30 (15–54) | .19 |
| Pack-years of smoking, No. (%) | |||||
| 0 | 142 (20.5) | 50 (30.5) | .002 | 63 (25.7) | .72 |
| 1–25 | 186 (26.8) | 54 (32.9) | 76 (31.0) | ||
| 26–50 | 199 (28.7) | 33 (20.1) | 44 (18.0) | ||
| 51–100 | 124 (17.9) | 15 (9.2) | 38 (15.5) | ||
| >100 | 43 (6.2) | 12 (7.3) | 24 (9.8) | ||
| Alcohol consumption No. (%) | .03 | .47 | |||
| Never drinker | 167 (24.1) | 30 (18.3) | 50 (20.4) | ||
| Former drinker | 175 (25.2) | 35 (21.3) | 69 (28.2) | ||
| Current drinker | 352 (50.7) | 99 (60.4) | 126 (51.4) | ||
| Number of alcoholic drinks/wk | .002 | <.001 | |||
| 0 | 167 (24.1) | 30 (18.3) | 50 (20.4) | ||
| ≤1 | 158 (22.8) | 35 (21.3) | 46 (18.8) | ||
| 2–14 | 212 (30.6) | 41 (25.0) | 56 (22.9) | ||
| 15–28 | 67 (9.6) | 20 (12.2) | 22 (9.0) | ||
| >28 | 90 (12.9) | 38 (23.2) | 71 (29.0) | ||
| Diabetes mellitus, No. (%)† | .18 | .97 | |||
| No | 580 (83.6) | 144 (87.8) | 205 (83.7) | ||
| Yes | 114 (16.4) | 20 (12.2) | 40 (16.3) | ||
| Hepatitis B exposure, No. (%)†,‡ | .26 | .19 | |||
| No | 484 (88.2) | 129 (91.5) | 175 (84.5) | ||
| Yes | 65 (11.8) | 12 (8.5) | 32 (15.5) | ||
| HIV infection, No. (%)†,§ | .11 | .05 | |||
| No | 389 (98.7) | 106 (96.4) | 143 (96.0) | ||
| Yes | 5 (1.3) | 4 (3.6) | 6 (4.0) |
*Categorical variables were compared using Pearson’s chi-squared test or Fisher’s exact test (for variables with cell numbers < 5), and continuous variables were compared using Wilcoxon rank-sum test. All statistical tests were two-sided. GED = general equivalency diploma; IQR = interquartile range.
†For those with available data.
‡Positive for hepatitis B core antibody.
§Positive for HIV antibody.
Compared with control subjects, HPV-positive oropharyngeal cancer patients were more likely to be white, male, born between 1945 and 1965, diagnosed with cancer at younger ages, and better educated; and to have smoked fewer cigarettes (Table 3).
Table 3.
Comparison of characteristics of control subjects and patients with HPV-positive and HPV-negative oropharyngeal cancers
| Characteristic | Control subjects (n = 694) | Patients with HPV-positive oropharyngeal cancers (n = 83) | Univariate P* | Patients with HPV-negative oropharyngeal cancers (n = 43) | Univariate P* |
|---|---|---|---|---|---|
| Hepatitis C virus antibody, No. (%) | .001 | .52 | |||
| Negative | 649 (93.5) | 69 (83.1) | 39 (90.7) | ||
| Positive | 45 (6.5) | 14 (16.9) | 4 (9.3) | ||
| Hepatitis C virus RNA, No. (%)† | <.001 | .65 | |||
| Negative | 649 (96.6) | 69 (87.3) | 39 (95.1) | ||
| Positive | 23 (3.4) | 10 (12.7) | 2 (4.9) | ||
| Race/ethnicity, No. (%) | .03 | .56 | |||
| White | 567 (81.7) | 79 (95.2) | 31 (72.1) | ||
| African American | 55 (7.9) | 0 (0.0) | 6 (13.9) | ||
| Hispanic | 41 (5.9) | 3 (3.6) | 3 (7.0) | ||
| Asian/Pacific Islander | 25 (3.6) | 0 (0.0) | 3 (7.0) | ||
| Middle Eastern | 5 (0.7) | 1 (1.2) | 0 (0.0) | ||
| Native American | 1 (0.1) | 0 (0.0) | 0 (0.0) | ||
| Sex, No. (%) | <.001 | .01 | |||
| Female | 219 (31.6) | 10 (12.1) | 13 (30.2) | ||
| Male | 475 (68.4) | 73 (87.9) | 30 (69.8) | ||
| Age at cancer diagnosis | |||||
| Median (IQR), y | 63 (56–70) | 58 (51–65) | <.001 | 56 (48–66) | <.001 |
| ≤60 years, No. (%) | 264 (38.0) | 49 (59.0) | <.001 | 27 (62.8) | .001 |
| >60 years, No. (%) | 430 (62.0) | 34 (41.0) | 16 (37.2) | ||
| Born between 1945 and 1965 | <.001 | .91 | |||
| No | 313 (45.1) | 15 (18.1) | 19 (44.2) | ||
| Yes | 381 (54.9) | 68 (81.9) | 24 (55.8) | ||
| Highest education level, No. (%)† | <.001 | .64 | |||
| ≤High school graduate/GED | 235 (37.0) | 12 (18.2) | 9 (33.3) | ||
| Vocational/technical school, associate degree, or some college | 160 (25.2) | 9 (13.6) | 9 (33.3) | ||
| ≥Bachelor’s degree | 240 (37.8) | 45 (68.2) | 9 (33.3) | ||
| Cigarette smoking status | .11 | .61 | |||
| Never smoker, No. (%) | 142 (20.5) | 25 (30.1) | 10 (23.3) | ||
| Former smoker, No. (%) | 361 (52.0) | 40 (48.2) | 19 (44.2) | ||
| Current smoker, No. (%) | 191 (27.5) | 18 (22.7) | 14 (32.6) | ||
| Pack-years of smoking, median (IQR) | 36 (20–54) | 20 (4–38) | <.001 | 30 (20–60) | .74 |
| Pack-years of smoking, No. (%) | |||||
| 0 | 142 (20.5) | 25 (30.1) | .002 | 10 (23.2) | .21 |
| 1–25 | 186 (26.8) | 33 (39.8) | 13 (30.2) | ||
| 26–50 | 199 (28.7) | 13 (15.7) | 8 (18.6) | ||
| 51–100 | 124 (17.9) | 6 (7.2) | 6 (14.0) | ||
| >100 | 43 (6.2) | 6 (7.2) | 6 (14.0) | ||
| Alcohol consumption | .40 | .71 | |||
| Never drinker, No. (%) | 167 (24.1) | 19 (22.9) | 8 (18.6) | ||
| Former drinker, No. (%) | 175 (25.2) | 16 (19.3) | 12 (27.9) | ||
| Current drinker, No. (%) | 352 (50.7) | 48 (57.8) | 23 (53.5) | ||
| No. of alcoholic drinks/wk, No. (%) | .86 | .001 | |||
| 0 | 167 (24.1) | 19 (22.9) | 8 (18.6) | ||
| ≤ 1 | 158 (22.8) | 16 (19.3) | 11 (25.6) | ||
| 2–14 | 212 (30.6) | 25 (30.1) | 4 (9.3) | ||
| 15–28 | 67 (9.6) | 10 (12.0) | 6 (13.9) | ||
| >28 | 90 (12.9) | 13 (15.7) | 14 (32.6) | ||
| Diabetes mellitus, No. (%) | .30 | .97 | |||
| No | 580 (83.6) | 73 (88.0) | 36 (83.7) | ||
| Yes | 114 (16.4) | 10 (12.0) | 7 (16.3) | ||
| Hepatitis B exposure, No. (%)†,‡ | .83 | .79 | |||
| No | 484 (88.2) | 65 (89.0) | 30 (90.9) | ||
| Yes | 65 (11.8) | 8 (11.0) | 3 (9.1) | ||
| HIV infection, No. (%)†,§ | .25 | .32 | |||
| No | 389 (98.7) | 61 (96.8) | 25 (96.2) | ||
| Yes | 5 (1.3) | 2 (3.2) | 1 (3.8) |
*Categorical variables were compared using Pearson’s chi-squared test or Fisher’s exact test (for variables with cell numbers < 5), and continuous variables were compared using Wilcoxon rank-sum test. All statistical tests were two-sided. GED = general equivalency diploma; IQR = interquartile range.
†For those with available data.
‡Positive for hepatitis B core antibody.
§Positive for HIV antibody.
The prevalence of HCV seropositivity was higher in oropharyngeal cancer patients than control subjects (14.0%, 95% CI = 8.7 to 19.4%, vs 6.5%, 95% CI = 4.6 to 8.3%), particularly HPV-positive oropharyngeal cancer patients (16.9%, 95% CI = 8.7 to 24.9%, vs 6.5%, 95% CI = 4.6 to 8.3%). After adjustment for age at cancer diagnosis, birth between 1945 and 1965, sex, highest education level, smoking history, and alcohol consumption, HCV seropositivity was statistically significantly associated with oropharyngeal cancer (OR = 2.04, 95% CI = 1.04 to 4.01) (Table 4). Because they have different pathogenesis, we conducted separate analyses for HPV-positive and HPV-negative oropharyngeal cancers. Final adjusted models showed a statistically significant association between HCV seropositivity and HPV-positive (OR = 2.97, 95% CI = 1.31 to 6.76) but not HPV-negative oropharyngeal cancers (OR = 1.44, 95% CI = 0.39 to 5.30) (Table 4). Similar results were obtained with sensitivity analysis for HCV infection (Table 4).
Table 4.
Association between HCV and head and neck cancers
| Cancer sites | Univariate analysis |
Multivariable analysis* |
||
|---|---|---|---|---|
| OR (95% CI) | P† | OR (95% CI) | P† | |
| HCV seropositivity‡ | ||||
| Control sites | 1.00 (Reference) | 1.00 (Reference) | ||
| Oropharyngeal | 2.35 (1.38 to 4.01) | .002 | 2.04 (1.04 to 4.01)§ | .04§ |
| HPV-positive oropharyngeal | 3.61 (1.86 to 6.97) | <.001 | 2.97 (1.31 to 6.76)§ | .009§ |
| HPV-negative oropharyngeal | 1.29 (0.53 to 3.14) | .57 | 1.44 (0.39 to 5.30)§ | .59§ |
| Nonoropharyngeal | 3.61 (2.33 to 5.57) | <.001 | 2.85 (1.38 to 5.88)‖ | .005‖ |
| Non-nasopharyngeal | 3.97 (2.55 to 6.20) | <.001 | 3.17 (1.49 to 6.73)‖ | .003‖ |
| Nasopharyngeal | 1.49 (0.44 to 5.09) | .52 | 1.30 (0.22 to 7.64)‖ | .77‖ |
| HCV infection‡ (sensitivity analyses) | ||||
| Control sites | 1.00 (Reference) | 1.00 (Reference) | ||
| Oropharyngeal | 3.40 (1.77 to 6.54) | <.001 | 2.63 (1.25 to 5.55)§ | .01§ |
| HPV-positive oropharyngeal | 5.04 (2.28 to 11.11) | <.001 | 3.58 (1.49 to 8.61)§ | .004§ |
| HPV-negative oropharyngeal | 1.26 (0.37 to 4.32) | .71 | 1.86 (0.38 to 9.05)§ | .89§ |
| Nonoropharyngeal | 5.18 (2.99 to 8.96) | <.001 | 3.77 (1.58 to 8.96)‖ | .004‖ |
| Non-nasopharyngeal | 5.74 (3.30 to 10.02) | <.001 | 4.30 (1.77 to 10.45)‖ | .001‖ |
| Nasopharyngeal | 1.95 (0.44 to 8.65) | .38 | 1.56 (0.16 to 15.21)‖ | .70‖ |
*Final parsimonious models were deduced using Akaike Information Criterion, confounders were assessed according to whether their inclusion changed the estimates by ≥ 10%, and goodness of fit was assessed by Hosmer-Lemeshow test. There was no multicollinearity between smoking and alcohol variables as checked by variance inflation factor and tolerance. CI = confidence interval; HCV = hepatitis C virus; HPV = human papillomavirus; OR = odds ratio.
†Unconditional logistic regression P values are reported. All statistical tests were two-sided.
‡HCV seropositivity was defined as positive HCV antibodies in serum; HCV infection was defined as having detectable HCV RNA in serum.
§Adjusted for age at cancer diagnosis, birth between 1945 and 1965 (yes/no), sex, highest education level, smoking (pack-years), and alcohol consumption (drinks/week).
‖Adjusted for age at cancer diagnosis, birth between 1945 and 1965 (yes/no), sex, race/ethnicity, highest education level, smoking (pack-years), alcohol consumption (drinks/week), and HIV infection.
Nonoropharyngeal HNCs
Compared with control subjects, nonoropharyngeal HNC patients were more likely to be male, be diagnosed with cancer at younger ages, consume more alcohol, and have HIV co-infection (Table 2). There was no statistically significant difference between groups with respect to cigarette smoking or HBV exposure.
The prevalence of HCV seropositivity was higher in nonoropharyngeal HNC patients than control subjects (20.0%, 95% CI = 14.9 to 25.0%, vs 6.5%, 95% CI = 4.6 to 8.3%). After adjustment for age at cancer diagnosis, birth between 1945 and 1965, sex, race, highest education level, smoking history, alcohol consumption, and HIV infection, HCV seropositivity was statistically significantly associated with nonoropharyngeal HNC (OR = 2.85, 95% CI = 1.38 to 5.88) (Table 4). Within nonoropharyngeal HNCs, we conducted separate analyses for non-nasopharyngeal and nasopharyngeal cancer patients. Final adjusted models showed a statistically significant association between HCV seropositivity and non-nasopharyngeal (OR = 3.17, 95% CI = 1.49 to 6.73) but not nasopharyngeal cancers (OR = 1.30, 95% CI = 0.22 to 7.64) (Table 4). Similar results were obtained with sensitivity analysis for HCV infection (Table 4). Associations by EBV status could not be evaluated because of the small number of EBV-positive patients in each cancer group.
Case-Case Analyses
We performed case-case analyses by comparing HPV-positive to HPV-negative oropharyngeal cancers and, within nonoropharyngeal HNCs, non-nasopharyngeal to nasopharyngeal cancers to assess etiologic heterogeneity. We found no statistically significant association of HCV seropositivity with HPV-positive compared with HPV-negative oropharyngeal cancers (OR = 1.56, 95% CI = 0.32 to 7.52, P = .58) and no statistically significant association of HCV seropositivity with non-nasopharyngeal compared with nasopharyngeal cancers (OR = 1.25, 95% CI = 0.19 to 8.33, P = .82) after adjusting for the confounders mentioned above.
Exploratory Analyses
After adjustment for age at cancer diagnosis, birth between 1945 and 1965, sex, highest education level, smoking, and alcohol consumption, HCV seropositivity was statistically significantly associated with oral cavity, oropharyngeal, and laryngeal cancers after corrections for multiple comparisons (Table 5). Unfortunately, the numbers of patients with tumors of the nasopharynx, hypopharynx, and larynx tested for HCV RNA were too small to permit analysis of any association with HCV infection.
Table 5.
Association between HCV seropositivity and head and neck cancers by subsite
| Cancer site | No. of cancer patients | OR* (95% CI) | P† |
|---|---|---|---|
| Control sites | 694 | ||
| Oral cavity | 110 | 2.43 (1.17 to 5.08) | .02‡ |
| Oropharynx | 164 | 2.04 (1.04 to 4.01) | .04‡ |
| Nasopharynx | 32 | 1.64 (0.42 to 6.50) | .48 |
| Hypopharynx | 21 | 1.08 (0.13 to 9.29) | .94 |
| Larynx | 82 | 4.96 (2.36 to 10.43) | <.001‡ |
*Adjusted for age at cancer diagnosis, birth between 1945 through 1965 (yes/no), sex, highest education level, smoking (pack-years), and alcohol consumption (drinks/week). CI = confidence interval; OR = odds ratio.
†Unconditional logistic regression P values are reported. All statistical tests were two-sided.
‡Cancers statistically significantly associated with hepatitis C virus seropositivity after correction for multiple testing using false-discovery rate of 10% according to Benjami and Hochberg method (20).
Discussion
This case-control study conducted at a National Cancer Institute–designated cancer center is the first to show that HCV is statistically significantly associated not only with nonoropharyngeal (except nasopharyngeal) HNCs but also with HPV-positive oropharyngeal cancers. Our results add to the growing body of epidemiological evidence that HCV infection has extrahepatic manifestations and may be associated with non-liver-related cancers (9,21).
Our findings are clinically significant. HCV infection is associated with several types of B-cell non-Hodgkin’s lymphoma, especially the diffuse large B-cell, marginal zone, follicular, and lymphoplasmacytic types (6–8,22). The reported odds ratios for these associations range from 2.47 to 5.2 (6,22). We found a similar strength of association between HCV and nonoropharyngeal HNCs and between HCV and HPV-positive oropharyngeal cancers. Thus, clinicians should be aware that nonliver cancers besides non-Hodgkin’s lymphomas can develop in patients chronically infected with HCV. Additionally, oncologists treating patients with HNCs should consider testing patients for HCV to enable early identification and linkage of care for this infection and to prevent progression of underlying liver disease. It remains unknown whether early HCV treatment may prevent development of HCV-associated HNCs or improve the oncologic outcome of patients in whom such cancers develop as has been reported in patients with HCV-associated non-Hodgkin’s lymphoma (6–8,23).
HNCs are a heterogeneous group of cancers accounting for approximately 3% of all cancers diagnosed in the United States (24). Risk factors for these cancers include smoking, alcohol consumption, chewing betel nut, African American race, low socioeconomic status, HPV infection (for oropharyngeal cancers), and EBV infection (for nasopharyngeal cancers) (13,25,26).
Little has previously been published about the relationship between HCV infection and HNCs. A few articles suggest that HCV infection may be associated with squamous cell carcinomas of the oral cavity (27,28). A US study showed a higher HCV prevalence in patients with squamous cell carcinoma of the head and neck than in noncancer patients (21.2% vs 9.9%, P < .004) (27). A population-based study from Taiwan found that the incidence of oral cavity cancers was 2.28 times as high in HCV-infected individuals as in patients with nonviral hepatitis (6.15 vs 2.69 per 100 000 person-years) and that HCV alone was a statistically significant risk factor for oral cavity cancers (HR = 1.9) (28). Recently, the US population-wide Chronic Hepatitis C Cohort Study analyzed data on 12 126 patients with chronic HCV infection and found that such patients had an increased incidence of oral cavity cancers (standardized rate ratio = 2.5) and mortality from oral cavity cancers (relative risk = 5.2) compared with noninfected patients (9). In our present study, HCV seropositivity was associated not only with nonoropharyngeal HNCs but also with HPV-positive oropharyngeal cancers. Our results also confirmed that HCV seropositivity was associated with oral cavity cancers, as previously reported (27,28).
The mechanisms by which HCV may be associated with HNCs remain unclear and need further investigation. HCV has been detected in saliva and salivary glands in patients with chronic sialadenitis and salivary gland tumors (29). Furthermore, an extrahepatic manifestation of chronic HCV infection is oral lichen planus, which is a premalignant condition associated with development of squamous cell carcinoma of the oral cavity (30). Case reports also have described progression of lichen planus to squamous cell carcinoma of the oral cavity in HCV-infected patients (30,31). Enhanced replication of HCV in oropharyngeal tissues may contribute to chronic inflammation predisposing to cancer development.
A novel finding from our study was that HCV seropositivity was associated with HPV-positive but not HPV-negative oropharyngeal cancers. HPV persists as a chronic infection, in part, by evading antiviral immune responses (32). High-risk HPV types have been shown to downregulate interferon-α-inducible gene expression (32). HPV oncoproteins E6 and E7 directly interact with and inhibit type 1 interferon signaling (32), which plays a central role in controlling intracellular replication of HCV (32). Thus, HPV may facilitate proliferation of HCV in the oropharyngeal cells, thereby facilitating its oncogenic action.
The role of HCV in oropharyngeal carcinogenesis may also relate to direct involvement of HCV proteins, including HCV nonstructural proteins NS5B and NS3 and HCV core proteins, in disrupting cell cycle regulation. NS5B binds the retinoblastoma tumor suppressor protein (Rb) in the cytoplasm of infected cells and recruits E6-associated protein, leading to polyubiquitination and proteasomal degradation of Rb (33). Because Rb is critical in regulating G1-S cell cycle transition, DNA damage response, mitotic spindle checkpoints, and apoptosis, loss of Rb may lead to uncontrolled cell proliferation (33). Notably, HPV protein E6 also exerts a pro-oncogenic impact by targeting tumor suppressor proteins through E6-associated protein, leading to degradation of cellular p53 and enhanced phosphorylation of Rb (34). Various interactions of HCV core and NS3 proteins with p53 have also been documented (33). The loss of p53 induced by HPV E6 protein and the degradation of Rb caused by HCV NS5B protein may play a synergistic role in the development of oropharyngeal cancers and merits further investigation. Additional studies are also warranted to examine the association between HCV and HPV-related anogenital (cervix, vagina, vulva, penis, and anus) cancers.
Another novel finding of our study was that HCV seropositivity was associated with laryngeal cancers. Risk factors previously identified for laryngeal cancers include smoking, alcohol intake, injection drug use, gastroesophageal reflux, and occupational exposures like asbestos (35,36). Of interest, recent studies have shown an association between HPV infection and laryngeal cancers (37–39). The association of HCV with laryngeal cancers may be because of mechanisms similar to those described above for HPV-positive oropharyngeal cancers. Correlation between smoking and/or drug use could partially explain the association of HCV with laryngeal cancers. However, detailed evaluation of risk factors for laryngeal cancers, including testing of cancers for HPV, was not possible because of lack of data.
Our study had several strengths. We evaluated the largest series of oropharyngeal and nonoropharyngeal HNCs for evaluating associations with HCV as separate groups because their risk factors differ. All cancers in our study population were histopathologically confirmed. The sensitivity analyses that included only subjects who had HCV infection (detectable HCV RNA) improved the validity of our findings. We evaluated smoking and alcohol consumption in detail and adjusted for them in final analyses, an approach not previously taken and often difficult through population-based databases and cancer registries. We also utilized existing data on HPV status of oropharyngeal cancers and found that HCV was associated with HPV-positive oropharyngeal cancers, suggesting oncogenic synergy between the two chronic viral infections.
Our study also had several limitations. First, the control group was not cancer free; rather, control subjects were patients with any of the three major smoking-associated cancers. In this retrospective study, we did not have access to a cancer-free population. We selected this control group so that case and control subjects were similar with respect to major risk factors—smoking, alcohol, and socioeconomic status—and adjusted for them in the final analyses. Second, since we used hospital-based control subjects, there was a possibility of Berkson’s bias, which pertains to different exposure rates in the control group and the general population (40). Notably, the prevalence of HCV infection in our control group was 6%, higher than the prevalence of approximately 1.5% in the general US population (1). Thus, our results may actually underestimate the true effect size of the association between HCV seropositivity and HNCs. An alternate explanation is that the HCV seropositivity rate is higher in cancer patients than in the general population. Third, there is the possibility of selection bias as patients with solid tumors are not routinely tested for HCV antibodies in our center. However, the proportions of cancer patients seen at our institution during the study period who were tested for HCV antibodies did not differ among the case and control groups (Supplementary Table 2, available online). Nevertheless, bias resulting from different reasons for HCV screening in case and control groups may be possible. Fourth, the association between HCV and HPV-positive oropharyngeal cancers may reflect a correlation between HCV and HPV infection because of lifestyle behaviors. Both HPV-positive oropharyngeal cancer patients and HCV-infected individuals tend to have high numbers of lifetime sex partners (41,42). However, whereas HPV is mostly transmitted sexually, sexual transmission of HCV infection is extremely rare, with the incidence being one case per 190 000 sexual contacts among heterosexual couples (43); the most frequent route of HCV transmission is injection drug use (44). Thus, this presumed correlation between HCV and HPV infection may reflect correlation between the two high-risk behaviors. Because of the retrospective nature of our study and the nonavailability of reliable assessments of sexual and drug use history, evaluation of such behaviors was not possible. Fifth, we did not have data on HCV treatment in our study subjects. Because population-based studies have shown that most HCV-infected patients are not aware of their infection and healthcare utilization rates are low for patients with HCV infection (42), we believe that lack of HCV treatment data is unlikely to affect our results. Sixth, more than 50% of the patients in our study were born between 1945 and 1965; individuals born during that period have a high prevalence of HCV infection (1). However, because the recommendations for screening such individuals were made in 2012 (1) and our study included patients tested between 2004 and 2014, we believe that birth between 1945 and 1965 is unlikely to have been the reason for HCV testing for the majority of patients in our study population. Nevertheless, our results were adjusted for the birth cohort effect in our multivariable analyses. Seventh, even though we tried to adjust for smoking, alcohol intake, and socioeconomic status, data were obtained from self-reported questionnaires and underreporting or misreporting of information along with residual confounding is possible. Eighth, the case-case analyses showed no etiologic heterogeneity between the case groups compared with respect to HCV seropositivity; however, the number of patients in comparison groups was small to determine differences. Finally, we were unable to evaluate the association between HCV and nasopharyngeal cancers based on EBV status because of small sample size.
In conclusion, HCV seems to be associated with HNCs, particularly nonoropharyngeal and HPV-positive oropharyngeal cancers. Validation of these associations by analysis of population-based datasets and cancer-free control group is essential. Further studies are also required to explore the possible interaction between HCV and HPV and the association between HCV and other HPV-related malignancies.
Notes
This study was supported by the NIH/NCI under award number P30CA016672. This study was presented in part at the 2015 Annual Meeting of the American Society of Clinical Oncology, May 29 through June 2, 2015, Chicago, IL, and was the recipient of the 2015 Conquer Cancer Foundation of American Society of Clinical Oncology Merit Award.
Harrys A. Torres is a consultant for Gilead Sciences, Janssen Pharmaceuticals, Inc., Merck & Co., Inc., Vertex Pharmaceuticals, Novartis, Genentech, Astellas, Pfizer, and Theravance, Inc., and received research grants from Gilead Sciences, Merck & Co., Inc., and Vertex Pharmaceuticals. David J. Tweardy has ownership interests in StemMed, Ltd. All other authors have no conflicts of interest to disclose.
We thank Ms. Stephanie Deming at The University of Texas MD Anderson Cancer Center for editorial assistance.
Supplementary Material
References
- 1.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(Rr-4):1–32. [PubMed] [Google Scholar]
- 2.El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology. 2014;60(5):1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Serag HB, Hampel H, Yeh C, Rabeneck L. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36(6):1439–1445. [DOI] [PubMed] [Google Scholar]
- 4.Gumber SC, Chopra S. Hepatitis C: a multifaceted disease. Review of extrahepatic manifestations. Ann Intern Med. 1995;123(8):615–620. [DOI] [PubMed] [Google Scholar]
- 5.Pawlotsky JM, Ben Yahia M, Andre C, et al. Immunological disorders in C virus chronic active hepatitis: a prospective case-control study. Hepatology. 1994;19(4):841–848. [PubMed] [Google Scholar]
- 6.Hartridge-Lambert SK, Stein EM, Markowitz AJ, Portlock CS. Hepatitis C and non-Hodgkin lymphoma: the clinical perspective. Hepatology. 2012;55(2):634–641. [DOI] [PubMed] [Google Scholar]
- 7.Marcucci F, Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood. 2011;117(6):1792–1798. [DOI] [PubMed] [Google Scholar]
- 8.Peveling-Oberhag J, Arcaini L, Hansmann ML, Zeuzem S. Hepatitis C-associated B-cell non-Hodgkin lymphomas. Epidemiology, molecular signature and clinical management. J Hepatol. 2013;59(1):169–177. [DOI] [PubMed] [Google Scholar]
- 9.Allison RD, Tong X, Moorman AC, et al. Increased incidence of cancer and cancer-related mortality among persons with chronic hepatitis C infection, 2006-2010. J Hepatol. 2015;63(4):822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee MH, Yang HI, Lu SN, et al. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206(4):469–477. [DOI] [PubMed] [Google Scholar]
- 11.Torres HA, Adachi JA, Roach LR, et al. Hepatitis C clinic operated by infectious disease specialists at a comprehensive cancer center: help is on the way. Clin Infect Dis. 2012;54(5):740–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. International classification of diseases for oncology, third edition, topographical codes. http://codes.iarc.fr/topography. Accessed December 3, 2015.
- 13.Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99(10):777–789. [DOI] [PubMed] [Google Scholar]
- 14.Agudo A, Bonet C, Travier N, et al. Impact of cigarette smoking on cancer risk in the European prospective investigation into cancer and nutrition study. J Clin Oncol. 2012;30(36):4550–4557. [DOI] [PubMed] [Google Scholar]
- 15.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932–954. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki K, Elkind MS, Boden-Albala B, et al. Moderate alcohol consumption is associated with better endothelial function: a cross sectional study. BMC Cardiovasc Disord. 2009;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang CC, Qiu JT, Kashima ML, Kurman RJ, Wu TC. Generation of type-specific probes for the detection of single-copy human papillomavirus by a novel in situ hybridization method. Mod Pathol. 1998;11(10):971–977. [PubMed] [Google Scholar]
- 19.Ambinder RF, Mann RB. Epstein-Barr-encoded RNA in situ hybridization: diagnostic applications. Hum Pathol. 1994;25(6):602–605. [DOI] [PubMed] [Google Scholar]
- 20.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J Royal Stat Soc Series B (Method). 1995;57(1):289–300. [Google Scholar]
- 21.Omland LH, Farkas DK, Jepsen P, Obel N, Pedersen L. Hepatitis C virus infection and risk of cancer: a population-based cohort study. Clin Epidemiol. 2010;2:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iqbal T, Mahale P, Turturro F, Kyvernitakis A, Torres HA. Prevalence and association of hepatitis C virus infection with different types of lymphoma. Int J Cancer. 2016;138(4):1035–1037. [DOI] [PubMed] [Google Scholar]
- 23.Kyvernitakis A, Mahale P, Popat U, et al. Hepatitis C virus infection in patients undergoing hematopoietic cell transplantation in the era of direct-acting antiviral agents. Biol Blood Marrow Transplant. 2016; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. [DOI] [PubMed] [Google Scholar]
- 25.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chien YC, Chen JY, Liu MY, et al. Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med. 2001;345(26):1877–1882. [DOI] [PubMed] [Google Scholar]
- 27.Nobles J, Wold C, Fazekas-May M, Gilbert J, Friedlander PL. Prevalence and epidemiology of hepatitis C virus in patients with squamous cell carcinoma of the head and neck. Laryngoscope. 2004;114(12):2119–2122. [DOI] [PubMed] [Google Scholar]
- 28.Su FH, Chang SN, Chen PC, et al. Positive association between hepatitis C infection and oral cavity cancer: a nationwide population-based cohort study in Taiwan. PLoS One. 2012;7(10):e48109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrozzo M. Oral diseases associated with hepatitis C virus infection. Part 1. sialadenitis and salivary glands lymphoma. Oral Dis. 2008;14(2):123–130. [DOI] [PubMed] [Google Scholar]
- 30.Porter SR, Lodi G, Chandler K, Kumar N. Development of squamous cell carcinoma in hepatitis C virus-associated lichen planus. Oral Oncol. 1997;33(1):58–59. [DOI] [PubMed] [Google Scholar]
- 31.Carrozzo M, Carbone M, Gandolfo S, et al. An atypical verrucous carcinoma of the tongue arising in a patient with oral lichen planus associated with hepatitis C virus infection. Oral Oncol. 1997;33(3):220–225. [DOI] [PubMed] [Google Scholar]
- 32.Stanley M. Immune responses to human papillomavirus. Vaccine 2006;24(Suppl 1):S16–S22. [DOI] [PubMed] [Google Scholar]
- 33.McGivern DR, Lemon SM. Virus-specific mechanisms of carcinogenesis in hepatitis C virus associated liver cancer. Oncogene. 2011;30(17):1969–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White MK, Pagano JS, Khalili K. Viruses and human cancers: a long road of discovery of molecular paradigms. Clin Microbiol Rev. 2014;27(3):463–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sturgis EM, Wei Q, Spitz MR. Descriptive epidemiology and risk factors for head and neck cancer. Semin Oncol. 2004;31(6):726–733. [DOI] [PubMed] [Google Scholar]
- 36.Reepalu A, Blome MA, Bjork J, Widell A, Bjorkman P. The risk of cancer among persons with a history of injecting drug use in Sweden - a cohort study based on participants in a needle exchange program. Acta Oncol. 2012;51(1):51–56. [DOI] [PubMed] [Google Scholar]
- 37.Baumann JL, Cohen S, Evjen AN, et al. Human papillomavirus in early laryngeal carcinoma. Laryngoscope. 2009;119(8):1531–1537. [DOI] [PubMed] [Google Scholar]
- 38.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467–475. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Gao L, Li H, et al. Human papillomavirus infection and laryngeal cancer risk: a systematic review and meta-analysis. J Infect Dis. 2013;207(3):479–488. [DOI] [PubMed] [Google Scholar]
- 40.Berkson J. Limitations of the application of fourfold table analysis to hospital data. Biometrics. 1946;2(3):47–53. [PubMed] [Google Scholar]
- 41.Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–714. [DOI] [PubMed] [Google Scholar]
- 42.Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terrault NA, Dodge JL, Murphy EL, et al. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology. 2013;57(3):881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47(Rr-19):1–39. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


