Abstract
Background: Fucose is utilized for the modification of different molecules involved in blood group determination, immunological reactions, and signal transduction pathways. We have recently reported that enhanced activity of the fucosyltransferase 3 and/or 6 promoted TGF-ß-mediated epithelial mesenchymal transition and was associated with increased metastatic potential of colorectal cancer (CRC), suggesting that fucose is required by CRC cells. With this in mind, we examined requirement of L-fucose in CRC cells and developed fucose-bound nanoparticles as vehicles for delivery of anticancer drugs specific to CRC.
Methods: In this study, we first examined the expression of fucosylated proteins in 50 cases of CRC by immunochistochemical staining with biotinylated Aleuria aurantia lectin (AAL). Then we carried out an L-fucose uptake assay using three CRC cell lines. Finally, we developed fucose-bound nanoparticles as vehicles for the delivery of an anticancer drug, SN38, and examined tumor growth inhibition in mouse xenograft model (n = 6 mice per group). All statistical tests were two-sided.
Results: We found a statistically significant relationship between vascular invasion, clinical stage, and intensity score of AAL staining (P ≤ .02). L-fucose uptake assay revealed that L-fucose incorporation, as well as fucosylated protein release, was high in cells rich in fucosylated proteins. L-fucose-bound liposomes effectively delivered Cy5.5 into CRC cells. The excess of L-fucose decreased the efficiency of Cy5.5 uptake through L-fucose-bound liposomes, suggesting an L-fucose receptor dependency. Intravenously injected, L-fucose-bound liposomes carrying SN38 were successfully delivered to CRC cells, mediating efficient tumor growth inhibition (relative tumor growth ratio: no treatment group [NT], 8.29 ± 3.09; SN38-treated group [SN38], 3.53 ± 1.47; liposome-carrying, SN38-treated group [F0], 3.1 ± 1.39; L-fucose-bound, liposome-carrying, SN38-treated group [F50], 0.94 ± 0.89; F50 vs NT, P = .003; F50 vs SN38, P = .02, F50 vs F0, P = .04), as well as prolonging survival of mouse xenograft models (log-rank test, P < .001).
Conclusions: Thus, fucose-bound liposomes carrying anticancer drugs provide a new strategy for the treatment of CRC patients.
Fucose, a deoxyhexose sugar, is utilized for modification of many different molecules in humans. For example, fucosylation plays important roles in blood group determination, immunological reactions, and signal transduction pathways (1). Fucose is synthesized via two major pathways, termed de novo and salvage (2). In the former, guanosine-diphosphate (GDP)-fucose is synthesized from GDP-mannose through two enzymatic reactions. In the latter, free fucose derived from extracellular or lysosomal sources (3) or from dietary sources (or culture medium in vitro) is transported across the plasma membrane into the cytosol. Although the precise mechanisms remain unclear, increased levels of fucose are frequently found in the serum and urine of cancer patients, suggesting increased fucosylation in cancer cells (4–6). Certain cultured cell lines have been shown to actively incorporate 14C-L-fucose from culture media and secrete fucosylated proteins (3). These observations indicated that L-fucose is highly required by various cancer cells.
Enhanced expression of fucosyltransferases (FUTs) has been reported in various cancers. FUTs are key enzymes accelerating malignant transformation through the fucosylation of different sialylated precursors. We and others have reported that enhanced activity of FUT3 is associated with increased metastatic potential of colon and pancreatic adenocarcinoma cells through the enhancement of epithelial mesenchymal transition by TGF-ß (7–9). It has been also demonstrated that other fucosyltransferases (10), such as FUT4 (11), FUT6 (12,13), FUT7 (14–16), FUT8 (17,18), mediate cancer cell migration and thereby metastasis, suggesting that fucosylation may play an important role in disease progression. Thus, these results indicate that cancer cells require L-fucose (19). However, it remains unclear whether L-fucose accumulation is indeed associated with the acceleration of the malignant potential of colorectal cancers (CRCs).
In this study, we first examined the expression of fucosylated proteins in the cancer tissues obtained from CRC patients by immunohistochemical staining using Aleuria aurantia lectin (AAL) and verified the correlation between AAL expression pattern and clinical characteristics of CRC. Furthermore, if CRC cell lines actually absorbed L-fucose and released fucosylated proteins into the media, we should try to develop cell-targeting therapy for CRC using fucose-bound liposomes carrying an anticancer drug.
Methods
Patients and Sample Collection
A total of 50 CRC patients who underwent radical resection for CRC in our hospital were recruited for this study from April 1, 2011, to March 31, 2014. All patients’ data were obtained from clinical and pathological records, including age, sex, tumor size and depth, lymph node metastasis, and distant metastasis. The postoperative pathological staging of each subject was determined according to the 7th edition of the Union for International Cancer Control tumor node metastasis (TNM) staging system for CRC (20). Tumor tissues were obtained from those patients who gave written informed consent in accordance with the guidelines (Good Clinical Practice, Ministry of Health, and Welfare of Japan). The study was approved by the ethics committees of Sapporo Medical University Hospital (registration number, 24-31: 272-1061).
Immunohistochemical Staining
Tumor tissue samples were immunostained with biotinylated AAL (J-Oil mills, Tokyo, Japan) and antihuman cleaved caspase 3 (CC3) antibodies (Cell Signaling, Beverly, MA) as primary antibodies and visualized using ABC Elite Kits (Linaris, Dossenheim, Germany). We classified pattern of staining as negative, weak positive, or strong positive according to the percentage of positive cells and staining intensity by the method of Gong et al. (21) with a minor modification. Classification of staining pattern was described in Supplementary Methods (available online).
Cell Lines
Colo205, Ls174T, and LS180 were purchased from American Type Culture Collection and authenticated in 2010 with cytogenesis. Colo205 was cultured in RPMI 1640 (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), L-glutamine, and 1% penicillin-streptomycin. Ls174T and LS180 were cultured in DMEM (Life Technologies) supplemented with 10% FBS, L-glutamine, and 1% penicillin- streptomycin. Normal human fibroblasts, human umbilical vein endothelial cells, and keratinocytes were purchased from Takara Bio Co and authenticated in 2013 by cytogenesis (Ohtsu, Japan).
Preparation of Cy5.5 and SN38 Encapsulated in Liposomes
Preparation of Cy5.5 and SN38-carrying liposomes was described in the previous report (22). The details were described in Supplementary Information (available online).
CRC Xenograft Model, Noninvasive Imaging, and Treatment Schedule
See Supplementary Methods (available online). In vivo studies were carried out in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols were approved by the Committee on the Ethics of Animal Experiments of Sapporo Medical University. All surgeries were performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering. In vivo experiments were performed using nude mice (n = 6, female age 4 to 6 weeks).
Statistical Analysis
Results are presented as the mean (± SD) for each sample. Differences between the pairs of groups were examined by unpaired and paired t test. If two groups could not be considered to be of equal variance, t test with analysis of variance was performed. χ2 and Fisher’s exact tests were used to evaluate the differences between categorical data. The correlation of fucosylated proteins and CA19-9 was tested by Pearson’s product-moment correlation coefficient. Finally, multivariable regression analysis was performed to examine the influence of each biological variable on clinical outcomes in a stepwise manner to exclude statistical confounding. We used the log-rank test for the analysis of survival data. All statistical analyses were two-sided and performed using JMP, version 12.0, for Macintosh (SAS, Cary, NC). Results were considered statistically significant when P values were less than .05.
Materials used in this study and additional experimental methods are described in the Supplementary Methods (available online).
Results
Relationship Between High Accumulation of Fucosylation and Advanced Stage in CRC
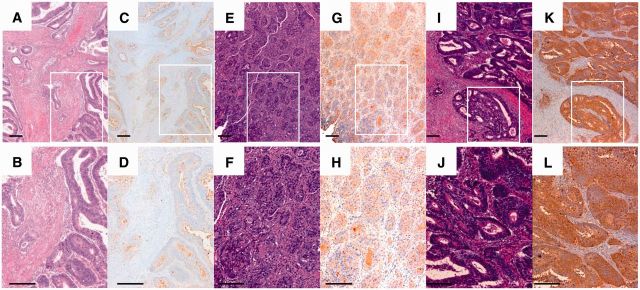
We recruited 50 patients with CRC who underwent colectomy in our hospital from April 1, 2011, to March 31, 2014 (Table 1; Supplementary Table 1, available online). Expression of AAL, with a clear intracellular localization in the epithelial cells (as evidenced by immunohistochemical brown staining) (Figure 1), was observed in 26 cases (52.0%) of the 50 CRC patients analyzed. Of these, the intensity of staining and therefore the fucosylated proteins was moderate in 21 (42.0%) patients, and strong in 5 (10.0%) patients. No statistically significant differences between fucosylated protein expression and clinicopathologic features, such as sex, age, localization, pattern of growth, differentiation, or lymph node infiltration, were found (Table 2). Nevertheless, fucosylated protein expression was more frequent in patients with advanced T factor, clinical stage, and metastatic tumors (P = .02, .02, and .002, respectively) (Supplementary Figure 1, available online). In addition, the scores of cases counted by intensity and population with moderate or strong fucosylated protein expression were higher in the cases with infiltration into the vessels (v factor–positive) than in v factor–negative cases (P = .02) (Table 2; Supplementary Figure 1, available online) although multivariable analysis did not show a statistically significant correlation. Therefore, these results suggest that fucosylated proteins might be required for disease progression in CRC and play important roles in inducing metastatic potential, as we previously reported (8). Then, we examined whether CRC cells actively absorb L-fucose into the cells.
Table 1.
Patient characteristics*
| Characteristics | No. of patients |
|---|---|
| Total | 50 |
| Male | 27 |
| Female | 23 |
| Age, y | |
| Median (range) | 72 (35–88) |
| ≤60 | 15 |
| >60 | 35 |
| Performance status | |
| 0 | 29 |
| 1 | 14 |
| 2 | 0 |
| Histology | |
| Well | 27 |
| Moderate | 22 |
| Poor | 1 |
| Tumor size, cm | |
| ≤5 | 28 |
| >5 | 22 |
| Tumor stage | |
| 0–II | 13 |
| III–IV | 37 |
| Tumor site | |
| Descending | 3 |
| Ascending | 10 |
| Transverse | 8 |
| Sigmoid | 14 |
| Rectal | 15 |
| Metastatic sites | |
| LNs | 14 |
| Distant LN | 1 |
| Liver | 9 |
| Ovary | 3 |
| Lung | 3 |
* LN = lynph node; moderate = moderately differentiated adenocarcinoma; poor = poorly differentiated adenocarcinoma; well = well-differentiated adenocarcinoma.
Figure 1.
Aleuria aurantia lectin (AAL) staining in colorectal cancer tissue. Representative microphotographs of weak (A–D), positive (E–H), and high expression (I–L) of AAL. Immunohistochemical staining for AAL was performed using a specific biotinylated AAL. A, B, E, F, I, J) Hematoxylin and eosin staining. C, D, G, H, K, L) Immunostaining for AAL. B, D, F, H, J, L) Representative areas from (A, C, E, G, I, K, and H), respectively. Scale bar = 200 μm.
Table 2.
Univariate and stepwise multivariable analyses of AAL expression*
| Characteristics | No. | Univariate analysis |
Multivariable analysis |
|||
|---|---|---|---|---|---|---|
| Average | SD | P | t | P | ||
| Total | ||||||
| Male | 27 | 5.3 | 2.1 | .08 | – | |
| Female | 23 | 4.2 | 2.1 | |||
| Age, y | ||||||
| ≤60 | 15 | 4.9 | 2.2 | .73 | – | |
| >60 | 35 | 4.7 | 2.2 | |||
| Location | ||||||
| Other | 21 | 4.1 | 2.1 | .06 | – | |
| R/S | 29 | 5.3 | 2.1 | |||
| Histology | ||||||
| Well | 27 | 4.6 | 2.2 | .57 | – | |
| Moderate-poor | 23 | 5.0 | 2.2 | |||
| Vascular invasion, v | ||||||
| No | 24 | 4.0 | 2.1 | .02 | 1.6 | .12 |
| Yes | 26 | 5.5 | 2.1 | |||
| Lymphatic invasion, ly | ||||||
| No | 36 | 4.8 | 2.2 | .93 | – | |
| Yes | 14 | 4.7 | 2.2 | |||
| T factor | ||||||
| I–III | 18 | 3.8 | 2.1 | .02 | 0.48 | .63 |
| IV | 32 | 5.3 | 2.1 | |||
| N factor | ||||||
| No | 33 | 4.5 | 2.2 | .22 | – | |
| Yes | 17 | 5.3 | 2.2 | |||
| M factor | ||||||
| No | 37 | 4.2 | 2.0 | .002 | 1.45 | .15 |
| Yes | 13 | 6.3 | 2.0 | |||
| Clinical stage | ||||||
| I–III | 37 | 4.2 | 2.0 | .002 | – | |
| IV | 13 | 6.3 | 2.0 | |||
* Univariate analysis was performed by t test with analysis of variance. Multivariable regression analysis was performed to examine the influence of each biological variable on clinical outcomes in a stepwise manner to exclude statistical confounding. All statistical analyses were two-sided. AAL = aleuria aurantia lectin.
Secretion and Uptake of Fucosylated Proteins in CRC Cells
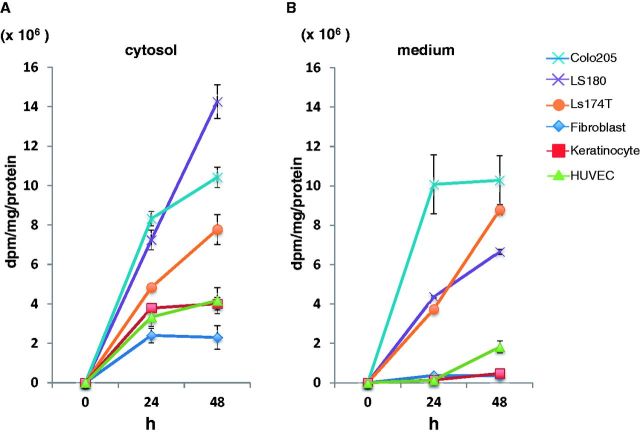
We examined the secretion and production of fucosylated proteins and glycolipids in CRC cell lines. We assessed 3H-labeled L-fucose uptake using CRC cells including normal human fibroblasts, keratinocytes, and human umbilical vein cells as controls. As shown in Figure 2, A and B, fucosylated proteins in the cells, as well as secretion of fucosylated protein in the cultured media, were elevated in Ls174T, LS180, and Colo205 cells in a time-dependent manner compared with control cells. However, uptake and secretion of fucosylated proteins was hardly detectable in normal cells. We could detect secretion of glycolipids in CRC cells but not in normal cells (Supplementary Figure 2, available online). Then, we identified fucosylated proteins by peptide mass fingerprinting (PMF). We purified the culture medium of Colo205 cells using AAL-lectin and isolated four molecules by SDS-PAGE; then, protein spots were subjected to MALDI-TOF analysis. Interestingly, two of the AAL-bound proteins, which were isolated from CRC cells, were associated to cancer cell invasion and metastasis. On the other hand, the other two molecules were identified to have kinase activity (Supplementary Figure 3, A-D, and Supplementary Table 2, available online). These data suggest that protein fucosylation may play a pivotal role in CRC cell progression. We then examined the production of CA19-9, which is sialyl Lewis A and contains fucose in a terminal glycan structure in CRC cells, and found that the cell lines (LS180, Ls174T, Colo205) secreted substantial amounts of this molecule (data not shown). Moreover, flow cytometric analysis also revealed that the amount of membrane-bound CA19-9 was high in cells that secreted CA19-9. Membrane-bound CA19-9 could not be detected by enzyme-linked immunosorbent assay in cell lines that do not secrete CA19-9 (data not shown). As shown in Supplementary Figure 4 (available online), Pearson’s product-moment correlation coefficient between the secretion of fucosylated proteins and CA19-9 production was 0.73 (95% confidence interval [CI] = 0.38 to 0.97, P = .03), indicating that CA19-9 high-producer cells may actively uptake L-fucose and synthesize fucosylated glycoproteins and glycolipids, such as CA19-9.
Figure 2.
Secretion and uptake of fucosylated proteins in colorectal cancer cells. A) Uptake of fucosylated proteins into the cytosol was increased in Ls174T, LS180, and Colo205 in a time-dependent manner. B) Secretion of fucosylated proteins in the culture medium was measured as described in Methods. Ls174T, LS180, and Colo205 secreted a substantial amount of fucosylated proteins into the medium. The experiments were performed in triplicate and repeated twice.
Transfer of Fuc-Liposome-Cy5.5 Into CRC Cells
We then investigated whether fucosylated protein-producing CRC cells could be targeted by L-fucose-bound liposomes. As reported previously (22), aminated L-fucose was cross-linked via DTSSP to liposomes prepared by the modified cholate dialysis method to achieve a final concentration of 25 µg/mL (F25) or 50 µg/mL (F50). Furthermore, BS3 and Tris were then coupled to hydrophilize the liposome surface, preventing uptake by the reticuloendothelial system in the liver and spleen and by macrophages and vascular endothelial cells, and also preventing the adsorption to opsonin proteins in the plasma. Consequently, systemic retention of the liposomes is supposed to be prolonged (23). Almost all L-fucose-bound liposomes (Fuc-liposomes) were spherical in shape and, in the case of SN38- encapsulation, were approximately 150 nm in size (Supplementary Table 3, available online). The zeta potential, representing the negative electric charge of the liposome surface, was below -70 mV (Supplementary Table 3, available online): sufficiently hydrophilized for stealth function. Particle size distribution remained stable after storage at 4 °C for six months.
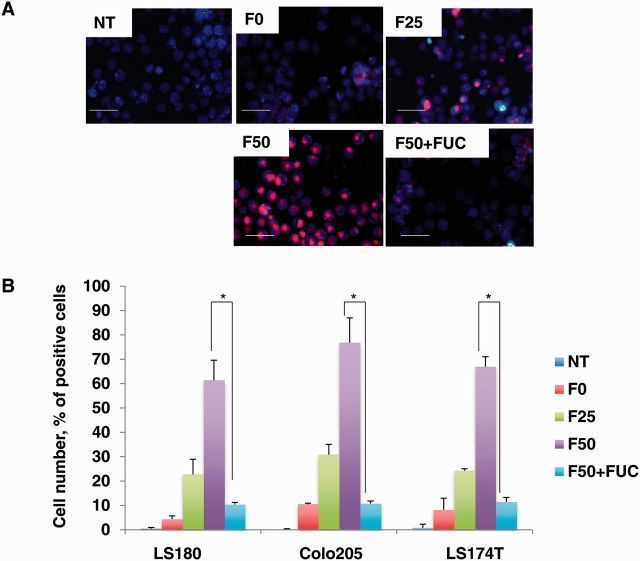
Using these Fuc-liposomes, we added Cy5.5 encapsulated L-fucose liposomes (Fuc-Liposome-Cy5.5) to fucosylated protein-producing or -nonproducing CRC cells to confirm specificity of delivery. As shown in Figure 3A, fluorescence microscopy revealed that F50-Fuc-liposomes but not F0-Fuc-liposomes effectively introduced Cy5.5 into the cytosol of Colo205 cells. Furthermore, flow cytometric analysis showed that F50-Fuc-liposomes but not F0-Fuc-liposomes effectively introduced Cy5.5 into the cytosol of LS180, Colo205, and Ls174T cells that secreted abundant fucosylated proteins within two hours (Figure 3B). Excess of L-fucose inhibited the uptake of Cy5.5 into the fucosylated protein-producing cells, suggesting an L-fucose-specific uptake. Moreover, the amount of Cy5.5 transferred into CRC cells seemed to increase proportionally with the level of fucosylated protein expression (Figure 3B). We previously found that the uptake of L-fucose is mediated by its receptors (22); in this study, the excess of L-fucose decreased the efficiency of the uptake (Figure 3B), indicating that the introduction of Cy5.5 by Fuc-liposomes is indeed L-fucose dependent.
Figure 3.
Transfer of Fuc-liposome-Cy5.5 into colorectal cancer (CRC) cell lines. A) Transfer of Fuc-liposome-Cy5.5 into CRC cells. Colo205 cells were incubated with Fuc-liposome-Cy5.5 for two hours, then washed twice with phosphate-buffered saline and visualized by fluorescence microscopy. NT = no treatment: F0, F0-liposome-Cy5.5: F25, F25-liposome-Cy5.5: F50, F50-liposome-Cy5.5: F50 + FUC, F50-liposome-Cy5.5 with excess L-fucose. Scale bar = 50 µm. B) Flow cytometric analysis of Fuc- liposome-Cy5.5-treated cells. CRC cells were treated with Fuc-liposome-Cy5.5 for two hours with or without excess of L-fucose and were analyzed by flow cytometry. Percent of Cy5.5-positive cells was shown. NT = no treatment: F0, F0-liposome-Cy5.5: F25, F25-liposome-Cy5.5: F50, F50-liposome-Cy5.5: F50 + FUC, F50-liposome-Cy5.5 with excess L-fucose. The experiments were performed in triplicate and repeated twice. Statistical analysis was performed by χ2 test (two-sided). *P < .05.
Effect of Fuc-Liposome-SN38 on the Growth of CRC Cell Lines
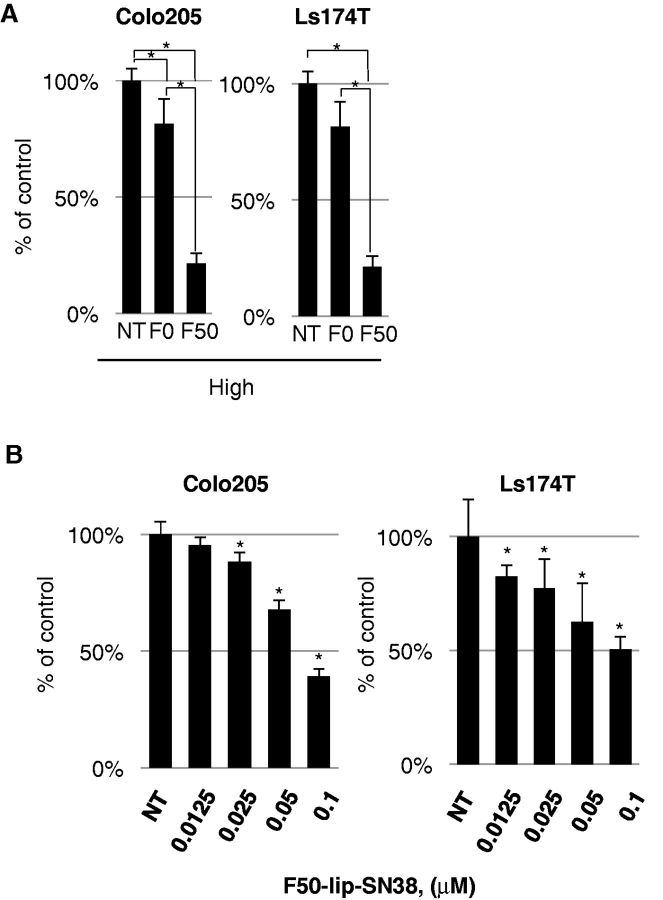
We encapsulated SN38 in Fuc-liposomes. Fuc-liposome-SN38 particles were approximately 150 nm in size, and the final concentration of SN38 was estimated at 1 mg/mL (Supplementary Table 3, available online). Nanoparticles of this size should be able to penetrate through the smallest capillary pores within the cancer vasculature by enhanced permeation and retention (EPR) effects but should not breach the blood-brain barrier (24). Cytotoxicity of Fuc-liposome-SN38 was tested using the BrdU assay (Figure 4). CRC cells were exposed to Fuc-liposome-SN38 or liposome-SN38 for two hours and then washed twice with PBS saline to test the efficacy and specificity of SN38 transfer into fucosylated protein-producing cells. Because we observed the greatest cytotoxicity using F50-liposome-SN38 (50 µg/mL Fuc-liposomes) (Colo205: F0 vs F50, 79.8±5.7 vs 26.2±5.5, P < .001; Ls174T: F0 vs F50, 87.7±4.6 vs 28.1±3.4, P < .001) (Figure 4A), we selected this condition for the following experiments. In fucosylated protein-producing cells (Colo205, Ls174T), F50-liposome-SN38 exerted a higher effect than control liposomes (F0-liposome-SN38) (Figure 4, A and B). Furthermore, these effects were inhibited by excess L-fucose, indicating a fucose-dependent cytotoxicity (Supplementary Figure 5, available online). In addition to the effects on CRC cell lines, the growth of other cancers, such as pancreatic and biliary cancer cell lines that produce fucosylated protein, were also effectively suppressed by Fuc-liposome-carrying anticancer drugs, indicating the potential applicability of this nanoparticle technology for the treatment of different types of cancer (data not shown) (22). Moreover, no cytotoxicity in normal cells, such as peripheral blood mononuclear cells, fibroblasts, human umbilical vein endothelial cells, or primary keratinocytes, was observed, probably because of their low requirement of L-fucose (22).
Figure 4.
Cytotoxic effect of Fuc-liposome-SN38 on colorectal cancer cell lines. A) Colo205, Ls174T cells were treated with Fuc-liposome-(F50) or liposome-(F0) containing SN38 (0.2 µM) for two hours, washed, incubated for 72 hours, and cell viability was measured by BrdU assay. Percent of viable cells was presented as compared with no treatment. B) Cells were treated with F50-liposome containing SN38 for two hours, then washed and incubated for 72 hours. Cell viability was measured by BrdU assay. The experiments were performed in triplicate and repeated twice. Statistical analysis was performed by t tests (two-sided); error bar = SD. *P < .05.
Augmentation of Fuc-Liposome Accumulation Into Fucosylated Protein Producing Tumors in a Xenograft Model by D-mannose Administration
We next investigated the tumor-specific delivery of Fuc- liposomes in tumor-bearing mice in vivo. It has been shown that clearance of L-fucose is delayed in D-mannose receptor–deficient mice (25), consistent with the presence of mannose/fucose receptors in the liver and Kupffer cells (26–29). Furthermore, mannose-bound liposomes administered via the tail vein accumulate in nonparenchymal cells and Kupffer cells (30). Based on these reports, we administered D-mannose before Fuc-liposomes in tumor-bearing mice to inhibit the uptake of L-fucose via the D-mannose receptor. First, we tested the effect of D-mannose on the efficiency of Fuc-mediated uptake using flow cytometry (22). Thereafter, we administered Fuc-liposomes in vivo and observed an accumulation of Cy5.5 in the tumor when D-mannose was administered before Fuc-liposome injection (Supplementary Figure 6, A and B, available online). Accumulation of Cy5.5 in the tumor was sustained up to one week after Fuc-liposome administration (Supplementary Figure 6C, available online), and no apparent side effects were observed.
Effect of Fuc-Liposomes Carrying SN38 on the Tumor Growth and Survival in Tumor-Bearing Mice
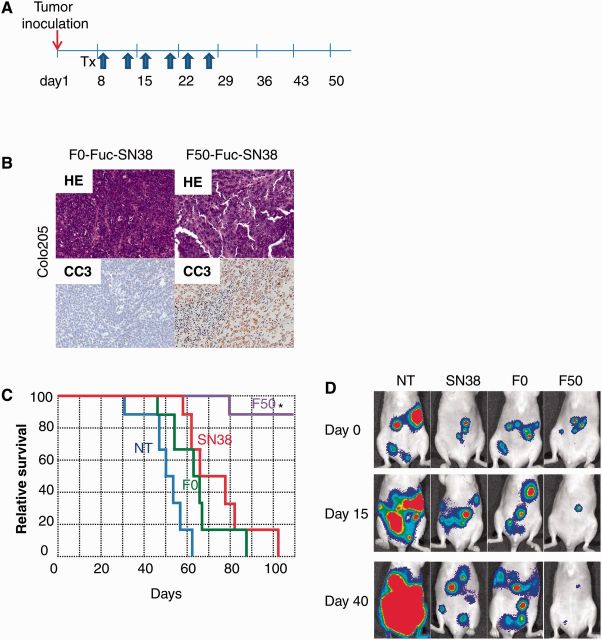
In order to test the effects of Fuc-liposome-SN38 on tumor growth in vivo, we developed a CRC xenograft model in mice. These mice were treated with Fuc-liposome-SN38 twice a week for three weeks (Figure 5A). Tumor growth was statistically inhibited by treatment with F50-liposome-SN38 compared with no treatment, F0-liposome-SN38, or SN38 alone in Colo205 tumor-bearing mice (relative tumor growth ratio: no treatment group [NT], 8.29 ± 3.09; SN38 treated group [SN38], 3.53 ± 1.47; liposome-carrying, SN38-treated group [F0], 3.1 ± 1.39; L-fucose-bound, liposome-carrying, SN38-treated group [F50], 0.94 ± 0.89; F50 vs NT, P = .003; F50 vs SN38, P = .02, F50 vs F0, P = .04), suggesting that F50-Fuc-liposome could specifically and efficiently deliver SN38 (Supplementary Figure 7, available online). In the hematoxylin and eosin staining of tumor tissues, many viable cells were observed in untreated mice. The number of tumor cells decreased in SN38-treated and F0-liposome-SN38-treated mice compared with untreated mice. However, in mice treated with F50-liposome-SN38, tumor cells almost completely disappeared. CC3 staining revealed the presence of a greater number of apoptotic cells in tumors treated with F50-liposome-SN38 than in controls (Figure 5B), possibly because of the marked accumulation of SN38 in the tumor tissue (Supplementary Figure 8, available online). We also tested the effects of Fuc-liposome-SN38 on survival in a peritoneal dissemination model in vivo using Colo205-Luc cells (Figure 5, C and D). As shown in Figure 5C, survival of mice treated with Fuc-liposome-SN38 was statistically significantly prolonged compared with untreated mice or with mice treated with SN38 alone or F0-liposome-SN38 (median survival days: NT, 50.3±4.8; SN38, 65±8.6; F0, 60.4±5.1; F50, not reached, log-rank test P < .001). Conversely, the tumor size in mice treated with Fuc-liposome-SN38 was decreased, as shown by in vivo imaging (total flux on day 40 [photon/sec]: NT, 8.1±1.5 x 106; SN38, 3.9±1.7 x 106; F0, 2.7±2.2 x 106; F50, 0.3±0.5 x 106; NT vs SN38, P = .01; NT vs F0, P = .02, NT vs F50, P = .001; SN38 vs F50, P < .001; F0 vs F50, P = .02) (Figure 5D). No adverse effects, including body weight changes, attributable to the administration of either D-mannose or F50-liposome-SN38 were observed during this study (data not shown).
Figure 5.
Fuc-liposome-SN38 suppressed tumor growth and prolonged the survival in a peritoneal dissemination model. A) Treatment schedule for the CRC xenograft mouse model. SN38 (1 mg/kg), F0-liposome-SN38 (1 mg/kg), or F50-liposome-SN38 solution (1 mg/kg) was administered via tail vein injection twice a week for three weeks. B) Tumor tissue was prepared on day 22 after the beginning of the treatment. Hematoxylin and eosin staining and CC3 staining in Colo205 tumors are shown. C) Survival rate of the mice treated with SN38 alone, F0-liposome-SN38, and F50-liposome-SN38 in the peritoneal dissemination model. Statistical analysis was performed by log-rank test. *P < .01 compared with NT, SN38, and F0. D) Representative image of Colo205 tumor-bearing mice. On days 15 and 40 after the start of the treatment, luciferase activity was monitored using the IVIS imaging system.
Discussion
Accumulated evidence suggests that L-fucose accelerates malignant potential of cancer cells, although the biological roles of L-fucose remain unclear. In physiological conditions, L-fucose concentration is low in the serum, but its concentration has been reported to increase in cancer patients (4,6). Elevation of L-fucose in the serum and body fluids may be attributed to the release of preformed glycoproteins from the tissue as a result of cell destruction, or it may be caused by local synthesis and secretion of glycoproteins by tumor cells. However, several investigators support the hypothesis that an increase in the serum levels reflects tissue proliferation rather than tissue destruction (31). Thus, these previous reports supported our findings that cancer cells directly uptake L-fucose and secrete fucosylated proteins.
The mechanism of L-fucose cellular uptake is still controversial, with both the diffusion and the active transport system proposed as the major pathways for the uptake of L-fucose into the cells (3), although the structure of the L-fucose transporter was identified in E. coli (32). In a previous report, we conducted a receptor-binding assay and demonstrated L-fucose-specific, high-affinity receptors on pancreatic cancer cells (22). The Fuc-liposomes penetrate into fucosylated protein-producing cells immediately and are inhibited by the excess of L-fucose, indicating the existence of a transporter or internalization system mediated by specific receptors on the cells, rather than merely a nonspecific diffusion system. However, further investigation is required to resolve this issue.
We identified several fucosylated proteins secreted in culture media by CRC cells: agrin, perlecan, M6 kinase, quiescin Q6, and lectin-binding galactoside (Supplementary Figure 3, A-D, and Supplementary Table 2, available online). It has been recently reported that agrin and perlecan enhance tumor progression by activating cell migration and invasion in oral cancer cells (33). Further investigation is required to determine the role of the other molecules, which may also participate in tumor progression.
SN38 is an active form derived from camptothecin-11 (CPT-11, irinotecan). CPT-11 is a component of first-line combination therapy with 5-fluorouracil and leucovorin for the treatment of patients with metastatic CRC (34,35). Although CPT-11 is one of the most powerful anticancer drugs for metastatic CRC, it has several limitations; therefore, chemical modifications may further improve the therapeutic index of the compound. To exert activity, CPT-11 needs to be converted into SN38; this conversion depends on carboxylesterase activity (36). The active form of SN38 still has limitations because of poor water solubility in any pharmaceutically acceptable excipient. In order to overcome these issues, chemical modification of drug carriers with certain synthetic polymers has been frequently employed in an attempt to decrease the limitations of this drug (37).
To date, several drug delivery systems for CPT-11 have been developed, and some of them are now under clinical evaluation. However, recent evidence has shown that polyethylene glycol, which was previously considered to be biologically inert, can induce certain adverse effects through activation of the complement system (38). Other approaches using polymer-based or organic nanoparticles (Abraxan) are used in the clinic, but these are limited by the lack of controlled drug release at specific sites because of longevity in the bloodstream, leading to adverse effects (39–41). Although these adverse effects will also need to be screened for Fuc-liposomes carrying other anticancer drugs, such as doxorubicin, we suspect that the EPR effect and toxicity to bystander cells will be minimal, given the limited requirement of normal cells for L-fucose and its limited delivery via normal blood vessels.
Our study is not without limitations. We have examined only 50 patients with CRC using immunohistochemical analysis and performed an in vitro study; the findings presented in this article must be verified using a larger number of CRC patients. However, the present report confirms that targeted delivery of a cytotoxic drug as an L-fucose nanoconjugate can efficiently inhibit in vivo growth of CRC cells. Furthermore, the L-fucose nanoparticles that we developed can be exploited as delivery vehicles for other anticancer drugs. This strategy could be extended as a generalized approach for the treatment of a wide variety of other fucosylated protein-producing carcinomas, such as biliary tract (70%∼80% positive) and gastric carcinomas (20%∼50% positive) (42), in addition to CRC (∼80% positive) (43,44). In conclusion, Fuc-liposome-containing anticancer drugs represent a promising new strategy for an active anticancer targeted therapy.
Note
No potential of conflicts of interest were disclosed by the authors.
Supplementary Material
References
- 1.Becker DJ, Lowe JB. Fucose: biosynthesis and biological function in mammals. Glycobiology. 2003;13(7):41R–53R. [DOI] [PubMed] [Google Scholar]
- 2.Tonetti M, Sturla L, Bisso A, et al. The metabolism of 6-deoxyhexoses in bacterial and animal cells. Biochimie. 1998;80(11):923–931. [DOI] [PubMed] [Google Scholar]
- 3.Wiese TJ, Dunlap JA, Yorek MA. L-fucose is accumulated via a specific transport system in eukaryotic cells. J Biol Chem. 1994;269(36):22705–22711. [PubMed] [Google Scholar]
- 4.Sawke NG, Sawke GK. Serum fucose level in malignant diseases. Indian J Cancer. 2010;47(4):452–457. [DOI] [PubMed] [Google Scholar]
- 5.Sakai T, Yamamoto K, Hino F, et al. [Clinical significance of L-fucose assay as a tumor marker]. Nihon Rinsho. 1990;48(Suppl):1025–1027. [PubMed] [Google Scholar]
- 6.Deyasi SK, Aikat BK, Sengupta U. Serum fucose in the diagnosis of malignancy, and its relative merits. Indian J Pathol Bacteriol. 1975;18(1):16–20. [PubMed] [Google Scholar]
- 7.Aubert M, Panicot-Dubois L, Crotte C, et al. Peritoneal colonization by human pancreatic cancer cells is inhibited by antisense FUT3 sequence. Int J Cancer. 2000;88(4):558–565. [DOI] [PubMed] [Google Scholar]
- 8.Hirakawa M, Takimoto R, Tamura F, et al. Fucosylated TGF-beta receptors transduces a signal for epithelial-mesenchymal transition in colorectal cancer cells. Br J Cancer. 2014;110(1):156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weston BW, Hiller KM, Mayben JP, et al. Expression of human alpha(1,3)fucosyltransferase antisense sequences inhibits selectin-mediated adhesion and liver metastasis of colon carcinoma cells. Cancer Res. 1999;59(9):2127–2135. [PubMed] [Google Scholar]
- 10.Miyoshi E, Moriwaki K, Nakagawa T. Biological function of fucosylation in cancer biology. J Biochem. 2008;143(6):725–729. [DOI] [PubMed] [Google Scholar]
- 11.Yan X, Lin Y, Liu S, et al. Fucosyltransferase IV (FUT4) as an effective biomarker for the diagnosis of breast cancer. Biomed Pharmacother. 2015;70:299–304. [DOI] [PubMed] [Google Scholar]
- 12.Muinelo-Romay L, Vazquez-Martin C, Villar-Portela S, et al. Expression and enzyme activity of alpha(1,6)fucosyltransferase in human colorectal cancer. Int J Cancer. 2008;123(3):641–646. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Guillebon AD, Hsu JW, et al. Human fucosyltransferase 6 enables prostate cancer metastasis to bone. Br J Cancer. 2013;109(12):3014–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu F, Qi HL, Chen HL. Regulation of differentiation- and proliferation-inducers on Lewis antigens, alpha-fucosyltransferase and metastatic potential in hepatocarcinoma cells. Br J Cancer. 2001;84(11):1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu F, Qi HL, Zhang Y, et al. Transfection of the c-erbB2/neu gene upregulates the expression of sialyl Lewis X, alpha1,3-fucosyltransferase VII, and metastatic potential in a human hepatocarcinoma cell line. Eur J Biochem. 2001;268(12):3501–3512. [DOI] [PubMed] [Google Scholar]
- 16.Koike T, Kimura N, Miyazaki K, et al. Hypoxia induces adhesion molecules on cancer cells: A missing link between Warburg effect and induction of selectin-ligand carbohydrates. Proc Natl Acad Sci U S A. 2004;101(21):8132–8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto K, Yokote H, Arao T, et al. N-Glycan fucosylation of epidermal growth factor receptor modulates receptor activity and sensitivity to epidermal growth factor receptor tyrosine kinase inhibitor. Cancer Sci. 2008;99(8):1611–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen CY, Jan YH, Juan YH, et al. Fucosyltransferase 8 as a functional regulator of nonsmall cell lung cancer. Proc Natl Acad Sci U S A. 2013;110(2):630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv Cancer Res. 1989;52:257–331. [DOI] [PubMed] [Google Scholar]
- 20.Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of Malignant Tumors, 7th edition. In; 2009.
- 21.Gong W, Wang L, Yao JC, et al. Expression of activated signal transducer and activator of transcription 3 predicts expression of vascular endothelial growth factor in and angiogenic phenotype of human gastric cancer. Clin Cancer Res. 2005;11(4):1386–1393. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida M, Takimoto R, Murase K, et al. Targeting anticancer drug delivery to pancreatic cancer cells using a fucose-bound nanoparticle approach. PLoS One. 2012;7(7):e39545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirai M, Minematsu H, Kondo N, et al. Accumulation of liposome with Sialyl Lewis X to inflammation and tumor region: application to in vivo bio-imaging. Biochem Biophys Res Commun. 2007;353(3):553–558. [DOI] [PubMed] [Google Scholar]
- 24.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7(11):653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SJ, Evers S, Roeder D, et al. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science. 2002;295(5561):1898–1901. [DOI] [PubMed] [Google Scholar]
- 26.Haltiwanger RS, Hill RL. The ligand binding specificity and tissue localization of a rat alveolar macrophage lectin. J Biol Chem. 1986;261(33):15696–15702. [PubMed] [Google Scholar]
- 27.Haltiwanger RS, Hill RL. The isolation of a rat alveolar macrophage lectin. J Biol Chem. 1986;261(16):7440–7444. [PubMed] [Google Scholar]
- 28.Haltiwanger RS, Lehrman MA, Eckhardt AE, et al. The distribution and localization of the fucose-binding lectin in rat tissues and the identification of a high affinity form of the mannose/N-acetylglucosamine-binding lectin in rat liver. J Biol Chem. 1986;261(16):7433–7439. [PubMed] [Google Scholar]
- 29.Lehrman MA, Haltiwanger RS, Hill RL. The binding of fucose-containing glycoproteins by hepatic lectins. The binding specificity of the rat liver fucose lectin. J Biol Chem. 1986;261(16):7426–7432. [PubMed] [Google Scholar]
- 30.Kawakami S, Wong J, Sato A, et al. Biodistribution characteristics of mannosylated, fucosylated, and galactosylated liposomes in mice. Biochim Biophys Acta. 2000;1524(2-3):258–265. [DOI] [PubMed] [Google Scholar]
- 31.Shetlar MR, Foster JV, et al. The serum polysaccharide level in malignancy and in other pathological conditions. Cancer Res. 1949;9(9):515–519. [PubMed] [Google Scholar]
- 32.Dang S, Sun L, Huang Y, et al. Structure of a fucose transporter in an outward-open conformation. Nature. 2010;467(7316):734–738. [DOI] [PubMed] [Google Scholar]
- 33.Kawahara R, Granato DC, Carnielli CM, et al. Agrin and perlecan mediate tumorigenic processes in oral squamous cell carcinoma. PLoS One. 2014;9(12):e115004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343(13):905–914. [DOI] [PubMed] [Google Scholar]
- 35.Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):1041–1047. [DOI] [PubMed] [Google Scholar]
- 36.Guichard SM, Danks MK. Topoisomerase enzymes as drug targets. Curr Opin Oncol. 1999;11(6):482–489. [DOI] [PubMed] [Google Scholar]
- 37.Sapra P, Zhao H, Mehlig M, et al. Novel delivery of SN38 markedly inhibits tumor growth in xenografts, including a camptothecin-11-refractory model. Clin Cancer Res. 2008;14(6):1888–1896. [DOI] [PubMed] [Google Scholar]
- 38.Moein Moghimi S, Hamad I, Bunger R, et al. Activation of the human complement system by cholesterol-rich and PEGylated liposomes-modulation of cholesterol-rich liposome-mediated complement activation by elevated serum LDL and HDL levels. J Liposome Res. 2006; 16(3):167–174. [DOI] [PubMed] [Google Scholar]
- 39.Damascelli B, Cantu G, Mattavelli F, et al. Intraarterial chemotherapy with polyoxyethylated castor oil free paclitaxel, incorporated in albumin nanoparticles (ABI-007): Phase I study of patients with squamous cell carcinoma of the head and neck and anal canal: preliminary evidence of clinical activity. Cancer. 2001;92(10):2592–2602. [DOI] [PubMed] [Google Scholar]
- 40.Batist G, Gelmon KA, Chi KN, et al. Safety, pharmacokinetics, and efficacy of CPX-1 liposome injection in patients with advanced solid tumors. Clin Cancer Res. 2009;15(2):692–700. [DOI] [PubMed] [Google Scholar]
- 41.Matsumura Y. Preclinical and clinical studies of NK012, an SN-38-incorporating polymeric micelles, which is designed based on EPR effect. Adv Drug Deliv Rev. 2011;63(3):184–192. [DOI] [PubMed] [Google Scholar]
- 42.Sturgeon CM, Duffy MJ, Hofmann BR, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for use of tumor markers in liver, bladder, cervical, and gastric cancers. Clin Chem. 2010;56(6):e1–e48. [DOI] [PubMed] [Google Scholar]
- 43.Koprowski H, Herlyn M, Steplewski Z, et al. Specific antigen in serum of patients with colon carcinoma. Science. 1981;212(4490):53–55. [DOI] [PubMed] [Google Scholar]
- 44.Magnani JL, Brockhaus M, Smith DF, et al. A monosialoganglioside is a monoclonal antibody-defined antigen of colon carcinoma. Science. 1981;212(4490):55–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.