Abstract
Background
The aims of this study are to investigate the clinical characteristics of patients with anterior hypertensive uveitis and to compare the characteristics between patients in cytomegalovirus (CMV)-positive and CMV-negative groups in their aqueous humor samples.
Immunocompetent patients (n = 42) with a history of chronic and/or recurrent hypertensive anterior uveitis underwent ophthalmic examination and serological tests. Among the 42 patients with hypertensive anterior uveitis, aqueous humor sampling was performed in 21, and they were analyzed for viral deoxyribonucleic acids using the polymerase chain reaction (PCR).
Results
The average age of the 42 patients with hypertensive anterior uveitis was 57.6 years, and 29 (69.0 %) of the subjects were males. Of the patients, 22 (52.4 %) underwent glaucoma surgery, and the average corneal endothelial cell counts were 1908 cells/mm2. Among the 21 patients who underwent an aqueous sampling, 6 were positive for CMV-DNA, while 15 were negative. The frequency of glaucoma surgery was similar between groups (CMV positive vs. CMV negative, 66.0 vs. 66.0 %, P = 0.701). However, 66.7 % of the CMV-positive group underwent glaucoma tube shunt surgery, whereas 80 % of the CMV-negative group underwent trabeculectomy or received an ExPRESS glaucoma filtration device (Alcon, Fort Worth, TX) for glaucoma surgery (P = 0.095). The corneal endothelial cell counts were significantly lower in the CMV-positive group (CMV positive vs. CMV negative, 1245 ± 560 vs. 1981 ± 387 cells/mm2; P = 0.009).
Conclusions
CMV was found to be an etiological factor in patients with hypertensive anterior uveitis in Korea. Special caution is needed for patients with CMV-induced hypertensive anterior uveitis, considering its adverse effect on the corneal endothelium.
Electronic supplementary material
The online version of this article (doi:10.1186/s12348-016-0100-5) contains supplementary material, which is available to authorized users.
Keywords: Glaucoma, Uveitis, Cytomegalovirus, Endothelium, Cornea
Background
Herpes viruses are known to play a role in the idiopathic anterior uveitis associated with ocular hypertension [1–4]. There are three herpes viruses responsible for ocular inflammation; human cytomegalovirus (CMV), herpes simplex virus (HSV)-1, and varicella zoster virus (VZV). CMV has been recognized as a cause of morbidity and mortality, mostly in immunocompromised individuals [3, 4]. Recent reports have shown that CMV infection is an emerging cause of anterior uveitis associated with ocular hypertension in immunocompetent subjects [1, 2, 5, 6]. CMV is also an important cause of corneal endotheliitis, particularly in Asian populations [7–12]. However, the pathogenesis of CMV-induced anterior uveitis in immunocompetent patients and the systemic and ocular characteristics of the disease are not well understood.
In the present study, we investigated the clinical characteristics of patients with anterior hypertensive uveitis and compared the ocular and systemic characteristics between CMV-positive and CMV-negative patients in their aqueous humor. Finally, we investigated the factors associated with the corneal endothelial cell loss in the hypertensive anterior uveitis patients.
Methods
This was a retrospective review of patients with anterior hypertensive uveitis who were investigated at Seoul St. Mary’s Hospital from March 2009 to June 2014. This study was performed according to the tenets of the Declaration of Helsinki, and the study protocol was approved by the institutional review/ethics boards of the Seoul St. Mary’s Hospital, the Catholic University of Korea (IRB number: KC14RISI0513). All of the patients included in this study met the following criteria: (1) anterior uveitis with keratic precipitates (KPs) and (2) increased intraocular pressure (IOP). Patients with the following were excluded: (1) presence of inflammation in vitreous or retina and (2) presence of corneal endothelial changes for a known cause other than anterior uveitis.
All of the participants underwent a comprehensive ophthalmic examination, including a detailed review of medical and ocular histories, best-corrected visual acuity measurement, slit-lamp biomicroscopy, Goldmann applanation tonometry, specular microscopy using a non-contact specular microscope (Konan Noncon Robo, Konan Medical, Inc., Hyogo, Japan), dilated stereoscopic examination of the optic nerve head and fundus, stereoscopic optic disc photography and red-free retinal nerve fiber layer (RNFL) photography (Nonmyd 7; Kowa Company Ltd., Nagoya, Japan), achromatic automated perimetry using the 24–2 Swedish Interactive Threshold Algorithm Standard program (Humphrey Visual Field (VF) Analyzer; Carl Zeiss Meditec, Inc., Dublin, CA, USA), and optical coherence tomography scans (Cirrus OCT, Carl Zeiss Meditec) to measure peripapillary RNFL thickness. Peripapillary RNFL thickness was determined three times at 256 points around a set diameter (3.4 mm) circle using the fast RNFL program. Only well-focused, well-centered images without eye movement and a signal strength ≥7 were used. A global average RNFL thickness provided by the software was used for the analysis. All of the patients underwent a laboratory work-up (including complete blood count, erythrocyte sedimentation rate analysis, white cell count and differential, blood chemistry) and serologic screening for IgM and IgG anti-CMV, HSV, and VZV.
Among the 42 patients with hypertensive anterior uveitis, aqueous sampling was performed in 21. Using a 30-gauge needle, 100 μL aqueous humor was aspirated under aseptic conditions and subjected to a polymerase chain reaction (PCR) assay for CMV, HSV1, and HSV2 DNA. DNA was extracted from the aqueous humor samples using a QIAamp DNA minikit (Qiagen, Valencia, CA, USA). Quantitative CMV-DNA PCR testing was performed using an AccuPower CMV Quantitative PCR Kit (Bioneer, Daejun, Republic of Korea). For HSV PCR, the HSV 1/2 PCR Kit (Bio-Core, Seoul, Republic of Korea) was used.
Glaucoma was defined as having glaucomatous disc appearance (thinning of neuroretinal rim, peripapillary hemorrhage, or localized pallor) associated with a typical reproducible VF defect evident on standard automated perimetry. A glaucomatous VF defect was defined as a glaucoma hemifield test result outside normal limits and the presence of at least three contiguous points in the pattern deviation plot with P values <5 %, with at least one point associated with a P value <1 % on two consecutive reliable VF examinations.
Patients were classified according to the duration of active intraocular inflammation. Patients with at least 3 months of active intraocular inflammation were considered to have chronic uveitis, and other patients to have recurrent episode of acute uveitis, which was normalized between attacks [2]. Glaucoma treatment was started in a step-wise manner; anti-glaucoma medication (beta-blocker, alpha-2 agonists, topical acetazolamide, and prostaglandin), and finally, glaucoma surgery. Glaucoma surgery was chosen among conventional trabeculectomy/ExPRESS glaucoma filtration device (Alcon Laboratories, Fort Worth, TX, USA) or Ahmed glaucoma valve implant (New World Medical, Inc., Rancho Cucamonga, CA, USA) surgery, considering preoperative IOP and severity of glaucomatous optic disc damage.
Statistical analysis
For independent samples, the non-parametric Mann-Whitney U test and the χ2 test were used to compare between-group means and percentages. Multivariable analysis was performed using simple and multiple linear regressions for corneal endothelial cell counts according to the presence of CMV-DNA in the aqueous humor. First, we adjusted for age and gender (model 1). Then we adjusted for age, gender, lens status, and history of glaucoma surgery (model 2). A P value <0.05 was considered to statistically significant. All of the statistical analyses were performed using the SPSS software (ver. 14.0 for Windows; SPSS Inc.).
Results
The clinical features of our patients are summarized in Table 1. The average age of the 42 patients with hypertensive anterior uveitis was 57.6 years, and 29 (69.0 %) of the subjects were males. In total, 22 (52.4 %) patients underwent glaucoma surgery, and the mean corneal endothelial cell counts were 1908 cells/mm2.
Table 1.
Clinical parameters of 42 patients with hypertensive anterior uveitis
| Gender (M/F) | 29:13 |
| Age (range), years | 57.6 (25–88) |
| Spherical equivalent, D | −2.6 (−11.25–0.00) |
| Glaucoma operation,% | 22 (52.4 %) |
| Corneal endothelial cell count, cells/mm2 | 1908 (625–3067) |
| Unilaterality | 39 (92.9 %) |
| KPs at baseline examination | 28 (67.8 %) |
| Anterior chamber reaction with 1+ or less | 37 (88.1 %) |
| Severe peripheral anterior synechiae | 0 (0.0 %) |
| Typical feature of P-S syndrome | 14 (33.3 %) |
Among the 21 patients who underwent aqueous sampling, six were positive for CMV-DNA, whereas 15 were negative (Table 2). The CMV-positive group was significantly younger and more myopic than the CMV-negative group (CMV positive vs. CMV negative: 47.5 ± 14.8 vs. 67.6 ± 11.8 years, P = 0.006; −3.6 ± 4.2 vs. 0.0 ± 1.6 D, P = 0.031). The frequency of glaucoma surgery was similar between the groups (CMV positive vs. CMV negative, 66.0 vs. 66.0 %, P = 0.701). However, 66.7 % of CMV-positive group had an Ahmed glaucoma valve implanted, whereas 80 % of the CMV-negative group underwent trabeculectomy or use of the ExPRESS glaucoma filtration device (P = 0.095). The corneal endothelial cell counts were significantly lower in the CMV-positive group (CMV positive vs. CMV negative: 1245 ± 560 vs. 1981 ± 387 cells/mm2; P = 0.009).
Table 2.
Comparisons of clinical and immunologic characteristics in subjects with or without CMV in aqueous humor
| Characteristics | CMV-positive subjects n = 6 | CMV-negative subjects n = 15 | P value |
|---|---|---|---|
| Demographic characteristics | |||
| Male, n (%) | 6 (100 %) | 13 (86.7 %) | 0.500 |
| Age, years | 47.5 ± 14.8 | 67.6 ± 11.8 | 0.006 |
| Ocular characteristics | |||
| Initial BCVA | 0.65 ± 0.29 | 0.58 ± 0.29 | 0.569 |
| Final BCVA | 0.47 ± 0.46 | 0.52 ± 0.30 | 0.733 |
| Spherical equivalent, D | -3.6 ± 4.2 | 0.0 ± 1.6 | 0.031 |
| Axial length, mm | 25.7 ± 1.5 | 24.4 ± 0.7 | 0.053 |
| Glaucoma, n (%) | 6 (100 %) | 13 (86.7 %) | 0.347 |
| Average RNFL thickness, μm | 66.2 ± 16.7 | 77.8 ± 19.1 | 0.132 |
| Corneal endothelial cell count, mm2 | 1245 ± 560 | 1981 ± 387 | 0.009 |
| Unilaterality, n (%) | 83.3 | 80.0 | 0.684 |
| KPs at baseline examination, n (%) | 83.3 | 73.3 | 0.550 |
| Presence of PAS, n (%) | 4 (66.7 %) | 5 (33.3 %) | 0.331 |
| Anterior chamber reaction with 1+ or less, n (%) | 100.0 | 92.9 | 0.714 |
| Lens status, phakic, n (%) | 5 (83.3 %) | 5 (33.3 %) | 0.055 |
| Baseline IOP, mmHg | 28.83 ± 8.25 | 22.73 ± 7.86 | 0.132 |
| Maximum IOP, mmHg | 37.17 ± 8.03 | 39.53 ± 13.02 | 0.622 |
| Final IOP, mmHg | 13.3 ± 3.44 | 13.5 ± 5.05 | 0.950 |
| Number of anti-glaucoma medication, n | 2.67 ± 0.81 | 2.67 ± 0.81 | 0.970 |
| Course of uveitis | 0.544 | ||
| Chronic, n (%) | 4 (66.7 %) | 13 (86.7 %) | |
| Recurrent, n (%) | 2 (33.3 %) | 2 (13.3 %) | |
| Glaucoma operation, n (%) | 4 (66.7 %) | 10 (66.7 %) | 0.701 |
| Choice of glaucoma operation | 0.095 | ||
| Ahmed valve, n (%) | 3 (75.0 %) | 2 (20.0 %) | |
| Trabeculectomy or ExPRESSⓡ, n (%) | 1 (25.0 %) | 8 (80.0 %) | |
| HSV PCR positivity, n (%) | 0 (0.0 %) | 1 (6.2 %) | 0.727 |
| CMV RQ PCR, copies/mL | 46,048.8 ± 91,334.5 | Negative | n/a |
CMV cytomegalovirus, BCVA best-corrected visual acuity, RNFL retinal nerve fiber layer, KP keratic precipitate, PAS peripheral anterior synechiae, n/a not applicable
Table 3 shows the laboratory characteristics of subjects with and without CMV-PCR(+) in the aqueous humor. In the CMV-positive subjects, the percentage of monocytes tended to be lower than in the CMV-negative subjects, with marginal significance (P = 0.055), whereas other parameters did not differ between the groups. The presence of CMV in the aqueous humor was significantly associated with corneal endothelial cell count after controlling for age and gender (P = 0.023), which was maintained after additional adjustment for lens status and history of glaucoma surgery (P < 0.001; Table 4). The clinical information of the 6 CMV+ patients was described in the Additional file 1, and two representative cases with antiviral treatment are discussed.
Table 3.
Comparison of laboratory findings in subjects with or without CMV-PCR (+) in aqueous humor
| Characteristics | CMV-positive subjects n = 6 | CMV-negative subjects n = 15 | P value |
|---|---|---|---|
| Complete blood count | |||
| WBC count, cells/μL | 7300 ± 900 | 6,700 ± 1900 | 0.590 |
| Seg, % | 54.4 ± 8.7 | 55.9 ± 7.7 | 0.677 |
| Lymp, % | 35.0 ± 6.6 | 31.9 ± 7.1 | 0.424 |
| Mono, % | 6.5 ± 0.7 | 7.8 ± 1.6 | 0.055 |
| Eosinophil, % | 3.5 ± 1.8 | 3.8 ± 3.2 | 0.970 |
| Basophil, % | 0.6 ± 0.3 | 0.5 ± 0.2 | 0.302 |
| RBC count, 106/μL | 5.0 ± 0.5 | 4.4 ± 0.6 | 0.059 |
| Hemoglobin g/dl | 15.5 ± 1.4 | 13.9 ± 2.0 | 0.070 |
| Platelet count, 103/μL | 247.5 ± 51.0 | 220.8 ± 58.8 | 0.329 |
| ESR, mm/h | 10.5 ± 7.6 | 31.8 ± 32.7 | 0.283 |
| Blood chemistry | |||
| FBS, mg/dL | 103.7 ± 18.7 | 100.5 ± 44.1 | 0.519 |
| AST, IU/L | 23.3 ± 3.3 | 23.6 ± 10.5 | 0.367 |
| ALT, IU/L | 28.7 ± 10.5 | 27.2 ± 15.7 | 0.590 |
| BUN, mg/dL | 15.3 ± 4.7 | 17.8 ± 6.7 | 0.261 |
| Creatinine, mg/dL | 0.9 ± 0.1 | 1.0 ± 0.3 | 0.971 |
| GFR, ml/min/1.73 m2 | 91.1 ± 8.9 | 79.2 ± 19.1 | 0.178 |
| Na, mEq/L | 142.3 ± 1.2 | 141.9 ± 3.2 | 0.858 |
| K, mEq/L | 4.4 ± 0.4 | 4.3 ± 0.5 | 0.914 |
| Immunology | |||
| HSV IgM positivity, n (%) | 1 (16.7 %) | 0 (0.0 %) | 0.273 |
| HSV IgG, titer, AU/mL | 3.3 ± 1.4 | 6.5 ± 6.8 | 0.320 |
| VZV IgM positivity, n (%) | 1 (16.7 %) | 0 (0.0 %) | 0.273 |
| VZV IgG titer, AU/mL | 5.3 ± 3.1 | 5.0 ± 2.2 | 1.000 |
| CMV IgM positivity, n (%) | 0 (0.0 %) | 0 (0.0 %) | n/a |
| CMV IgG titer, AU/mL | 54.7 ± 15.3 | 55.7 ± 16.4 | 1.000 |
HSV herpes simplex virus, VZV varicella zoster virus, CMV cytomegalovirus, ESR erythrocyte sedimentation rate, FBS fasting blood sugar, AST aspartate aminotransferase, ALT alanine aminotransferase, BUN blood urea nitrogen, GFR glomerular filtration rate, n/a not applicable
Table 4.
Association between the presence CMV DNA in aqueous and corneal endothelial cell count in patients with anterior hypertensive uveitis
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Unadjusted | Adjusted for age and gender | Adjusted for age, gender, lens status, and history of glaucoma surgery | |
| B (CI) | B (CI) | B (CI) | |
| CMV positivity | 672.4 | 734.4 | 1034.2 |
| (77.0–1267.9) | (116.4–1352.4) | (631.3–1437.1) | |
| P value | 0.029 | 0.023 | <0.001 |
B beta coefficient, CI confidence interval
Case reports
Patient 1
A 68-year-old man had hypertensive anterior uveitis of his left eye with a recurrence rate of one to two times a month since 2010. On slit-lamp examination at Seoul St. Mary’s Hospital in October 2012, fine KPs in the lower part of the cornea and mild inflammatory reaction in the anterior chamber were seen. The corneal endothelial cell count of the left eye was 2141 cells/mm2, and the IOP was 22 mmHg with two topical antiglaucoma medications. He showed glaucomatous optic disc changes, with a mean deviation of −9.50 dB. Despite the use of topical steroids and three antiglaucoma medications for 5 months, his IOP was not controlled, and his corneal endothelial cell count in the left eye decreased to 1245 cells/mm2. A CMV-quantitative PCR analysis of aqueous humor samples for the left eye revealed 229,000 copies/mL CMV-DNA, but no HSV-DNA. Thus, an oral course of valacyclovir (90 mg/day) was started. The ExPRESS glaucoma filtration device (P-200) was implanted under a scleral flap. Oral valacyclovir (900 mg/day) was continued for 10 days, and anterior chamber tapping revealed 66,600 copies/mL CMV-DNA. Oral valacyclovir treatment (450 mg/day) was continued for another 14 days, and repeated anterior chamber tapping revealed 630 copies/mL CMV-DNA. After glaucoma surgery, the anterior chamber had a rare to +1 grade inflammation, and IOP was well controlled in a range of 6–9 mmHg. However, 5 months later, his corneal endothelial cell count was found to have decreased further, to 981 cells/mm2 (Fig. 1).
Fig. 1.
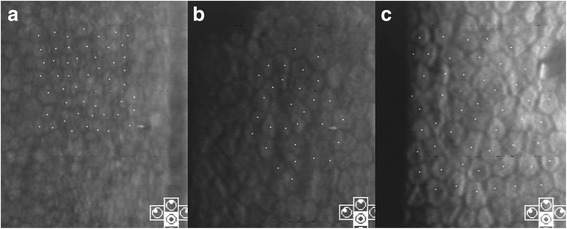
Case 1: serial follow-up of specular microscopy. At the initial visit, the corneal endothelial count in the left eye was 2141 cells/mm2 (a). After topical steroid and three antiglaucoma medications for 5 months, the corneal endothelial cell count decreased to 1245 cells/mm2 (b). A CMV-quantitative PCR analysis of aqueous humor samples revealed 229,000 copies/mL CMV-DNA. After valacyclovir treatment for 1 month, repeated anterior chamber tapping revealed 630 copies/mL CMV-DNA. However, the corneal endothelial cell count had decreased further, to 981 cells/mm2 (c)
Patient 2
A 64-year-old man had hypertensive anterior uveitis of his left eye with a recurrence rate of one to two times a month since 1991. He had been diagnosed with Posner-Schlossman syndrome, but chronic inflammation in the anterior chamber with uncontrolled IOP elevation was seen, and he was referred to Seoul St. Mary’s Hospital for glaucoma surgery. At the initial visit, mutton-fat keratic precipitates were noted in the central cornea with minimal anterior chamber reaction (Fig. 2). IOP was 21 mmHg with three antiglaucoma medications, and high pigmentation was noted in the anterior chamber angle. Severe glaucomatous optic disc was seen, with a mean deviation of −18.20 dB. The corneal endothelial cell count of the left eye was 1310 cells /mm2. A CMV-quantitative PCR analysis of an aqueous humor sample for the left eye revealed 44,609 copies/mL CMV-DNA, but no HSV-DNA. Oral valacyclovir treatment (900 mg/day) was started, and an Ahmed glaucoma valve was implanted. After 14 days of oral valacyclovir treatment, the aqueous humor showed 6980 copies/mL CMV-DNA. Oral valacyclovir was continued for 14 days, and further anterior chamber tapping showed 450 copies/mL CMV-DNA. After 1 month, the corneal endothelial cell count of the left eye was 1310 cells/mm2.
Fig. 2.
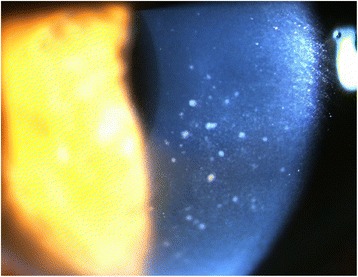
Case 2: slit lamp photograph showing diffuse mutton-fat keratic precipitates within the central area of the cornea. There was mild inflammation in the anterior chamber
Discussions
We reported the clinical characteristics of hypertensive anterior uveitis (Table 1), which occurred unilaterally (93 %), predominantly in middle-aged (57.6 ± 17.0 years) males (69.0 %; Table 1). Despite seemingly mild intraocular inflammation of the anterior chamber in the majority of patients (88.1 %) and the absence of peripheral anterior synechiae (0 %), glaucoma surgeries were required in over half of the patients (52.4 %). The male dominance in hypertensive anterior uveitis is consistent with previous results in non-Korean populations [4, 7]. Among the patients who underwent aqueous sampling, six (28.6 %) patients were positive for CMV-DNA. This prevalence is similar to the previous studies, reporting 22.8 % of anterior uveitis associated with raised IOP [1, 4].
Notably, the corneal endothelial cell count in the CMV-positive subjects was significantly lower than in the CMV-negative subjects (P = 0.009; Table 2). This association between the presence of CMV-DNA and the corneal endothelial cell count was more evident after adjusting for age, gender, lens status, and history of glaucoma surgery (P < 0.001; Table 3). Previous studies demonstrated that CMV is an important pathogen for chronic corneal endotheliitis, particularly in Asian populations [7–9, 13]. This study confirmed that CMV is a major cause of corneal endothelial cell loss in patients with hypertensive anterior uveitis.
Evidence of valacyclovir’s efficacy was seen in our study, using serial follow-up of CMV-DNA copy number and corneal endothelial cell counts in two case patients. After 1 month of therapy, CMV-DNA copy numbers decreased dramatically in both patients. Considering that patient 1 showed very high CMV-DNA copy numbers (44,609 copies/mL), it seems that one episode of acutely increased viral load is sufficient to cause extensive corneal endothelial cell loss. Consistent with our study, Kandori et al. [11] also reported a significant correlation between CMV viral load and corneal endothelial cell loss in CMV-associated uveitis.
We found that the proportion of monocytes tended to be lower in CMV-positive subjects, with marginal significance (P = 0.053; Table 2). Monocytes are the primary cell type of CMV persistence within the peripheral blood mononuclear cells [14]. CMV infection of monocytes induces trans-endothelial migration and monocyte to macrophage differentiation, which is productive for CMV replication [15]. Although the reason is unclear, CMV-induced differentiation of monocytes into macrophages may be associated with the lower proportion of monocytes in CMV-positive subjects in this study.
We found that the CMV-positive subjects were significantly younger (P = 0.006, Table 2). Generally, CMV seropositivity increases with age and serves as an immune risk phenotype, which is associated with survival in an older population [16, 17]. The current understanding of the mechanisms by which CMV reactivates in relatively young immunocompetent subjects is very limited. CMV is unlikely to cause clinically significant symptoms as long as it is maintained in balance by the host immune system [18]. However, recent literature suggests that CMV is associated with the pathogenesis of cardiovascular diseases, such as atherosclerosis, autoimmune disease, and even certain cancers [19, 20]. It is known that CMV can be reactivated when circulating monocytes with latent CMV are recruited to sites of inflammation [20]. For this reactivation, inflammatory cytokines, such as tumor necrosis factor and interferon, are known to play a role [21, 22]. In this regard, it is suggested that chronic inflammation in the anterior chamber may trigger CMV reactivation, which in turn, aggravates the inflammatory condition. In addition, CMV anterior uveitis is recalcitrant to topical steroid treatment [3, 4]. Recent reports show evidence that local immunosuppression by topical steroid use increases the risk of CMV infection in immunocompetent patients [23–26]. In addition, there is possibility that the frequent use of prostaglandin eyedrops have play an important role for worsening, including the uncontrolled glaucoma, considering previous reports regarding the complications from glaucoma eyedrops in uveitis [27–29].
Conclusions
In conclusion, we found that CMV was a significant etiological factor in hypertensive anterior uveitis patients in Korea. In CMV-associated uveitis, extensive corneal endothelial cell damage may occur, even with effective anti-viral medication. CMV-associated hypertensive anterior uveitis patients were younger compared to CMV-negative uveitis patients. Special caution is needed for patients with CMV-positive hypertensive anterior uveitis, given its adverse effects on the corneal endothelium.
Acknowledgements
Not applicable.
Funding
The authors wish to acknowledge of the financial support of the National Research Foundation of Korea Grant funded by the Korean government (MSIP) (No. NRF- 2016R1C1B1011287).
Authors’ contributions
JAC and CKP contributed to the conception and design. JAC, KSK, and YHJ contributed to the acquisition of data and analysis and interpretation of data. JAC contributed in drafting the manuscript. HYLP contributed in revising the manuscript. CKP gave the final approval of the version to be published. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval
The study protocol was approved by the institutional review/ethics boards of Seoul St. Mary’s Hospital, the Catholic University of Korea (IRB number: KC14RISI0513).
Additional file
Demographic data and clinical manifestations of patients with the presence of cytomegalovirus in the aqueous. (DOCX 15 kb)
Contributor Information
Jin A Choi, Email: jinah616@daum.net.
Kyu Seop Kim, Email: immib@catholic.ac.kr.
Younhea Jung, Email: write2une@catholic.ac.kr.
Hae Young Lopilly Park, Email: lopilly@catholic.ac.kr.
Chan Kee Park, Email: ckpark@catholic.ac.kr.
References
- 1.Chee SP, Bacsal K, Jap A, Se-Thoe SY, Cheng CL, Tan BH. Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. Am J Ophthalmol. 2008;145:834–840. doi: 10.1016/j.ajo.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 2.van Boxtel LA, van der Lelij A, van der Meer J, Los LI. Cytomegalovirus as a cause of anterior uveitis in immunocompetent patients. Ophthalmology. 2007;114:1358–1362. doi: 10.1016/j.ophtha.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 3.Jap A, Chee SP. Emerging forms of viral uveitis in the developing world. Int Ophthalmol Clin. 2010;50:155–171. doi: 10.1097/IIO.0b013e3181d26f2c. [DOI] [PubMed] [Google Scholar]
- 4.Jap A, Chee SP. Viral anterior uveitis. Curr Opin Ophthalmol. 2011;22:483–488. doi: 10.1097/ICU.0b013e32834be021. [DOI] [PubMed] [Google Scholar]
- 5.Park SW, Yu HG. Association of cytomegalovirus with idiopathic chronic anterior uveitis with ocular hypertension in Korean patients. Ocul Immunol Inflamm. 2013;21:192–196. doi: 10.3109/09273948.2012.754908. [DOI] [PubMed] [Google Scholar]
- 6.Knox DL. Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. Am J Ophthalmol. 2008;146:625. doi: 10.1016/j.ajo.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Koizumi N, Inatomi T, Suzuki T, et al. Clinical features and management of cytomegalovirus corneal endotheliitis: analysis of 106 cases from the Japan corneal endotheliitis study. Br J Ophthalmol. 2015;99:54–58. doi: 10.1136/bjophthalmol-2013-304625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi A, Yokogawa H, Higashide T, Nitta K, Sugiyama K. Clinical significance of owl eye morphologic features by in vivo laser confocal microscopy in patients with cytomegalovirus corneal endotheliitis. Am J Ophthalmol. 2012;153:445–453. doi: 10.1016/j.ajo.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 9.Chee SP, Bacsal K, Jap A, Se-Thoe SY, Cheng CL, Tan BH. Corneal endotheliitis associated with evidence of cytomegalovirus infection. Ophthalmology. 2007;114:798–803. doi: 10.1016/j.ophtha.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 10.Sungur GK, Hazirolan D, Yalvac IS, Ozer PA, Aslan BS, Duman S. Incidence and prognosis of ocular hypertension secondary to viral uveitis. Int Ophthalmol. 2010;30:191–194. doi: 10.1007/s10792-009-9305-z. [DOI] [PubMed] [Google Scholar]
- 11.Kandori M, Miyazaki D, Yakura K, Komatsu N, Touge C, Ishikura R, Inoue Y (2013) Relationship between the number of cytomegalovirus in anterior chamber and severity of anterior segment inflammation. Jpn J Ophthalmol 57(6):497–502 [DOI] [PubMed]
- 12.Lewkowicz D, Willermain F, Relvas LJ, et al. Clinical outcome of hypertensive uveitis. J Ophthalmol. 2015;2015:974870. doi: 10.1155/2015/974870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chee SP, Jap A. Immune ring formation associated with cytomegalovirus endotheliitis. Am J Ophthalmol. 2011;152:449–453. doi: 10.1016/j.ajo.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991;72:2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- 15.Smith MS, Bentz GL, Alexander JS, Yurochko AD. Human cytomegalovirus induces monocyte differentiation and migration as a strategy for dissemination and persistence. J Virol. 2004;78:4444–4453. doi: 10.1128/JVI.78.9.4444-4453.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wikby A, Johansson B, Olsson J, Löfgren S, Nilsson BO, Ferguson F. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp Gerontol. 2002;37:445–453. doi: 10.1016/S0531-5565(01)00212-1. [DOI] [PubMed] [Google Scholar]
- 17.Solana R, Tarazona R, Aiello AE, et al. CMV and immunosenescence: from basics to clinics. Immun Ageing. 2012;9:23. doi: 10.1186/1742-4933-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeves MB, MacAry PA, Lehner PJ, Sissons JG, Sinclair JH. Latency, chromatin remodeling, and reactivation of human cytomegalovirus in the dendritic cells of healthy carriers. Proc Natl Acad Sci U S A. 2005;102:4140–4145. doi: 10.1073/pnas.0408994102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halenius A, Hengel H. Human cytomegalovirus and autoimmune disease. Biomed Res Int. 2014;2014:472978. doi: 10.1155/2014/472978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Söderberg-Nauclér C. HCMV microinfections in inflammatory diseases and cancer. J Clin Virol. 2008;41:218–223. doi: 10.1016/j.jcv.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Prösch S, Heine AK, Volk HD, Krüger DH. CCAAT/enhancer-binding proteins alpha and beta negatively influence the capacity of tumor necrosis factor alpha to up-regulate the human cytomegalovirus IE1/2 enhancer/promoter by nuclear factor kappaB during monocyte differentiation. J Biol Chem. 2001;276:40712–40720. doi: 10.1074/jbc.M009815200. [DOI] [PubMed] [Google Scholar]
- 22.Prösch S, Staak K, Stein J, et al. Stimulation of the human cytomegalovirus IE enhancer/promoter in HL-60 cells by TNFalpha is mediated via induction of NF-kappaB. Virology. 1995;208:197–206. doi: 10.1006/viro.1995.1143. [DOI] [PubMed] [Google Scholar]
- 23.Sims JL, Chee SP. Cytomegalovirus endotheliitis following fluocinolone acetonide (Retisert) implant. Eye (Lond) 2010;24:197–198. doi: 10.1038/eye.2009.54. [DOI] [PubMed] [Google Scholar]
- 24.Tugal-Tutkun I, Araz B, Cagatay A. CMV retinitis after intravitreal triamcinolone acetonide injection in a patient with Behçet’s uveitis. Int Ophthalmol. 2010;30:591–593. doi: 10.1007/s10792-009-9332-9. [DOI] [PubMed] [Google Scholar]
- 25.Ufret-Vincenty RL, Singh RP, Lowder CY, Kaiser PK. Cytomegalovirus retinitis after fluocinolone acetonide (Retisert) implant. Am J Ophthalmol. 2007;143:334–335. doi: 10.1016/j.ajo.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Park UC, Kim SJ, Yu HG. Cytomegalovirus endotheliitis after fluocinolone acetonide (Retisert) implant in a patient with Behçet uveitis. Ocul Immunol Inflamm. 2011;19:282–283. doi: 10.3109/09273948.2011.580075. [DOI] [PubMed] [Google Scholar]
- 27.Goyal R, Ram AR. Brimonidine tartarate 0.2 % (Alphagan) associated granulomatous anterior uveitis. Eye (Lond) 2000;14:908–910. doi: 10.1038/eye.2000.250. [DOI] [PubMed] [Google Scholar]
- 28.Cates CA, Jeffrey MN. Granulomatous anterior uveitis associated with 0.2 % topical brimonidine. Eye (Lond) 2003;17:670–671. doi: 10.1038/sj.eye.6700392. [DOI] [PubMed] [Google Scholar]
- 29.Warwar RE, Bullock JD, Ballal D. Cystoid macular edema and anterior uveitis associated with latanoprost use. Experience and incidence in a retrospective review of 94 patients. Ophthalmology. 1998;105:263–268. doi: 10.1016/S0161-6420(98)92977-3. [DOI] [PubMed] [Google Scholar]


