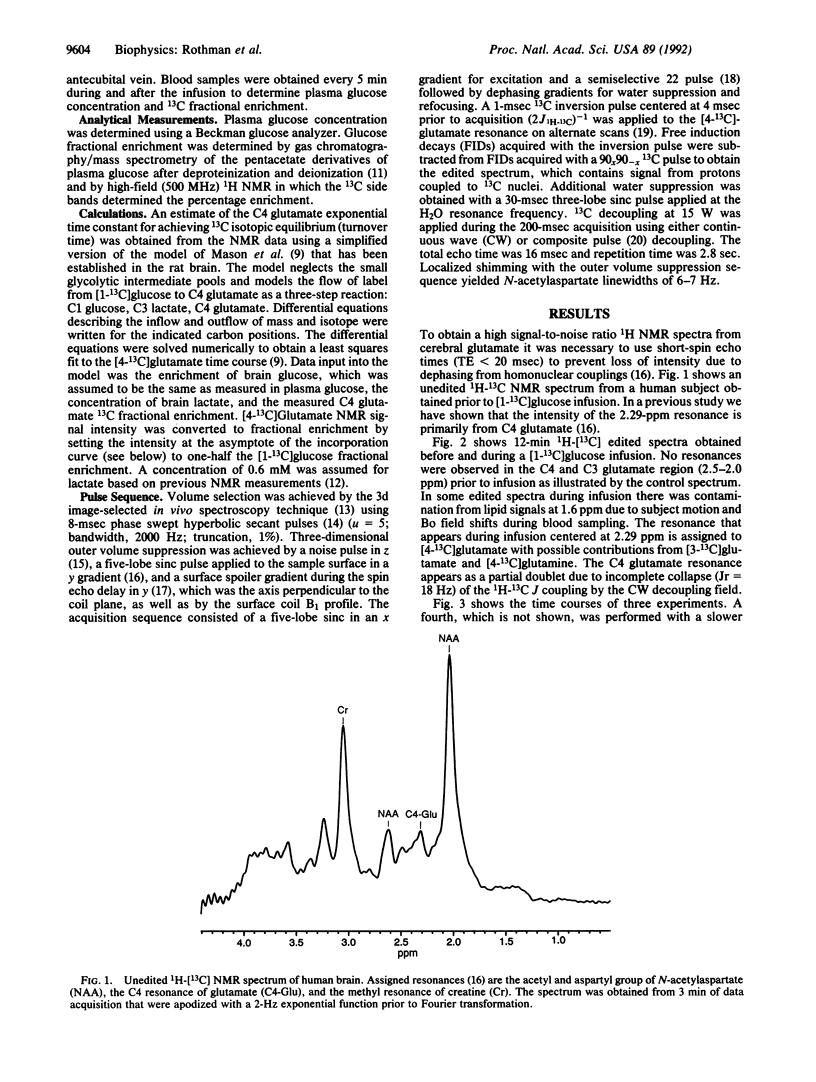
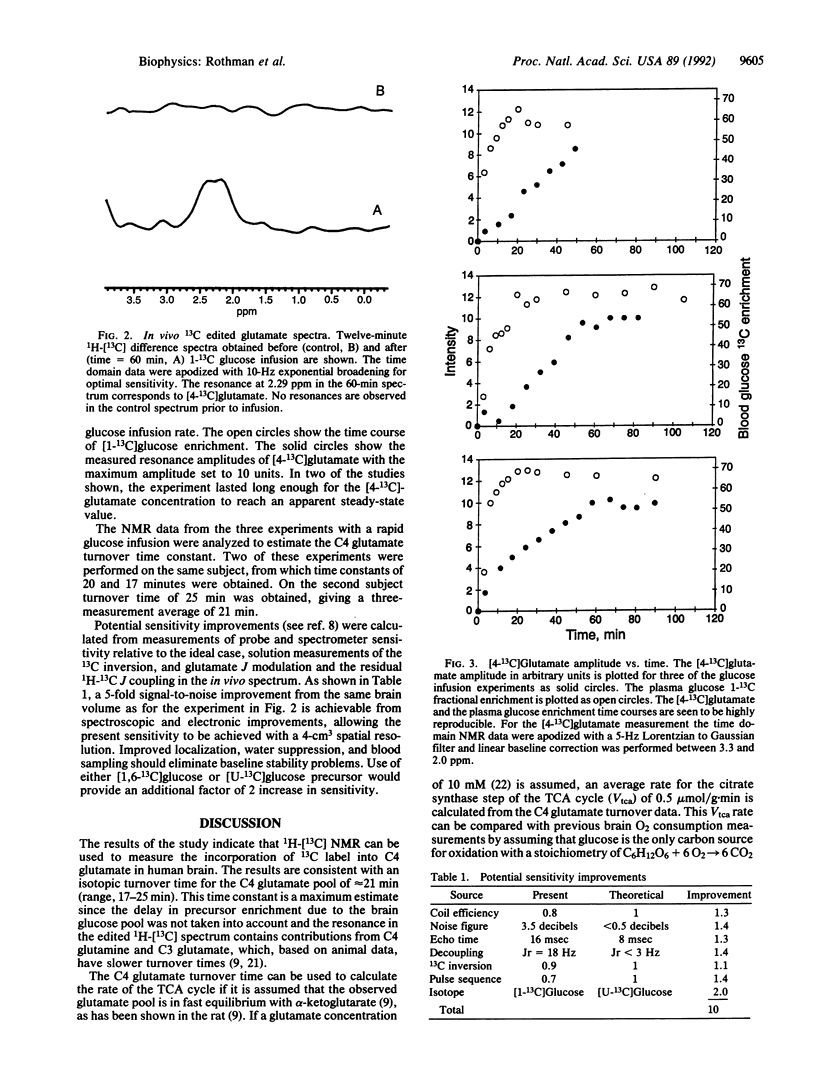
Abstract
A limitation of previous methods for studying human brain glucose metabolism, such as positron emission tomography, is that metabolic steps beyond glucose uptake cannot be studied. Nuclear magnetic resonance (NMR) has the advantage of allowing the nondestructive measurement of 13C distribution in specific carbon positions of metabolites. In this study 1H-[13C] NMR spectroscopy in conjunction with volume localization was used to measure the rate of incorporation of 13C isotope from infused enriched [1-13C]glucose to human brain [4-13C]glutamate. In three studies C4 glutamate turnover time constants of 25, 20, and 17 min were measured in a 21-cm3 volume centered in the region of the visual cortex. Based on an analysis of spectrometer sensitivity the spatial resolution of the method can be improved to < 4 cm3. In conjunction with metabolic modeling and other NMR measurements this method can provide a measure of regional rates of the brain tricarboxylic acid cycle and other metabolic pathways.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckmann N., Turkalj I., Seelig J., Keller U. 13C NMR for the assessment of human brain glucose metabolism in vivo. Biochemistry. 1991 Jul 2;30(26):6362–6366. doi: 10.1021/bi00240a002. [DOI] [PubMed] [Google Scholar]
- Behar K. L., Petroff O. A., Prichard J. W., Alger J. R., Shulman R. G. Detection of metabolites in rabbit brain by 13C NMR spectroscopy following administration of [1-13C]glucose. Magn Reson Med. 1986 Dec;3(6):911–920. doi: 10.1002/mrm.1910030611. [DOI] [PubMed] [Google Scholar]
- Chen W., Ackerman J. J. Surface coil single-pulse localization in vivo via inhomogeneous surface spoiling magnetic gradient. NMR Biomed. 1989 Apr;1(4):205–207. doi: 10.1002/nbm.1940010409. [DOI] [PubMed] [Google Scholar]
- Cooper A. J., Plum F. Biochemistry and physiology of brain ammonia. Physiol Rev. 1987 Apr;67(2):440–519. doi: 10.1152/physrev.1987.67.2.440. [DOI] [PubMed] [Google Scholar]
- Dzubow L. M., Garfinkel D. A simulation study of brain compartments. II. Atom-by-atom simulation of the metabolism of specifically labeled glucose and acetate. Brain Res. 1970 Oct 28;23(3):407–417. doi: 10.1016/0006-8993(70)90066-1. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick S. M., Hetherington H. P., Behar K. L., Shulman R. G. The flux from glucose to glutamate in the rat brain in vivo as determined by 1H-observed, 13C-edited NMR spectroscopy. J Cereb Blood Flow Metab. 1990 Mar;10(2):170–179. doi: 10.1038/jcbfm.1990.32. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E., Mintun M. A., Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988 Jul 22;241(4864):462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Gruetter R., Novotny E. J., Boulware S. D., Rothman D. L., Mason G. F., Shulman G. I., Shulman R. G., Tamborlane W. V. Direct measurement of brain glucose concentrations in humans by 13C NMR spectroscopy. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1109–1112. doi: 10.1073/pnas.89.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanstock C. C., Rothman D. L., Prichard J. W., Jue T., Shulman R. G. Spatially localized 1H NMR spectra of metabolites in the human brain. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1821–1825. doi: 10.1073/pnas.85.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis R., Ross B. D., Farrow N. A., Ackerman Z. Metabolic disorders of the brain in chronic hepatic encephalopathy detected with H-1 MR spectroscopy. Radiology. 1992 Jan;182(1):19–27. doi: 10.1148/radiology.182.1.1345760. [DOI] [PubMed] [Google Scholar]
- Mason G. F., Rothman D. L., Behar K. L., Shulman R. G. NMR determination of the TCA cycle rate and alpha-ketoglutarate/glutamate exchange rate in rat brain. J Cereb Blood Flow Metab. 1992 May;12(3):434–447. doi: 10.1038/jcbfm.1992.61. [DOI] [PubMed] [Google Scholar]
- Matthews P. M., Andermann F., Arnold D. L. A proton magnetic resonance spectroscopy study of focal epilepsy in humans. Neurology. 1990 Jun;40(6):985–989. doi: 10.1212/wnl.40.6.985. [DOI] [PubMed] [Google Scholar]
- Novotny E. J., Jr, Ogino T., Rothman D. L., Petroff O. A., Prichard J. W., Shulman R. G. Direct carbon versus proton heteronuclear editing of 2-13C ethanol in rabbit brain in vivo: a sensitivity comparison. Magn Reson Med. 1990 Dec;16(3):431–443. doi: 10.1002/mrm.1910160310. [DOI] [PubMed] [Google Scholar]
- Ordidge R. J. Random noise selective excitation pulses. Magn Reson Med. 1987 Jul;5(1):93–98. doi: 10.1002/mrm.1910050113. [DOI] [PubMed] [Google Scholar]
- Petroff O. A., Spencer D. D., Alger J. R., Prichard J. W. High-field proton magnetic resonance spectroscopy of human cerebrum obtained during surgery for epilepsy. Neurology. 1989 Sep;39(9):1197–1202. doi: 10.1212/wnl.39.9.1197. [DOI] [PubMed] [Google Scholar]
- Phelps M. E., Huang S. C., Hoffman E. J., Selin C., Sokoloff L., Kuhl D. E. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol. 1979 Nov;6(5):371–388. doi: 10.1002/ana.410060502. [DOI] [PubMed] [Google Scholar]
- Raichle M. E., Larson K. B., Phelps M. E., Grubb R. L., Jr, welch M. J., Ter-Pogossian M. M. In vivo measurement of brain glucose transport and metabolism employing glucose- -11C. Am J Physiol. 1975 Jun;228(6):1936–1948. doi: 10.1152/ajplegacy.1975.228.6.1936. [DOI] [PubMed] [Google Scholar]
- Rothman D. L., Behar K. L., Hetherington H. P., den Hollander J. A., Bendall M. R., Petroff O. A., Shulman R. G. 1H-Observe/13C-decouple spectroscopic measurements of lactate and glutamate in the rat brain in vivo. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1633–1637. doi: 10.1073/pnas.82.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman D. L., Hanstock C. C., Petroff O. A., Novotny E. J., Prichard J. W., Shulman R. G. Localized 1H NMR spectra of glutamate in the human brain. Magn Reson Med. 1992 May;25(1):94–106. doi: 10.1002/mrm.1910250110. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Rothman D. L., Jue T., Stein P., DeFronzo R. A., Shulman R. G. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990 Jan 25;322(4):223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- Silver M. S., Joseph R. I., Chen C. N., Sank V. J., Hoult D. I. Selective population inversion in NMR. Nature. 1984 Aug 23;310(5979):681–683. doi: 10.1038/310681a0. [DOI] [PubMed] [Google Scholar]



