Abstract
Purpose:
Accurate tracking of anatomical changes and computation of actually delivered dose to the patient are critical for successful adaptive radiation therapy (ART). Additionally, efficient data management and fast processing are practically important for the adoption in clinic as ART involves a large amount of image and treatment data. The purpose of this study was to develop an accurate and efficient Software platform for CUmulative Dose Assessment (scuda) that can be seamlessly integrated into the clinical workflow.
Methods:
scuda consists of deformable image registration (DIR), segmentation, dose computation modules, and a graphical user interface. It is connected to our image PACS and radiotherapy informatics databases from which it automatically queries/retrieves patient images, radiotherapy plan, beam data, and daily treatment information, thus providing an efficient and unified workflow. For accurate registration of the planning CT and daily CBCTs, the authors iteratively correct CBCT intensities by matching local intensity histograms during the DIR process. Contours of the target tumor and critical structures are then propagated from the planning CT to daily CBCTs using the computed deformations. The actual delivered daily dose is computed using the registered CT and patient setup information by a superposition/convolution algorithm, and accumulated using the computed deformation fields. Both DIR and dose computation modules are accelerated by a graphics processing unit.
Results:
The cumulative dose computation process has been validated on 30 head and neck (HN) cancer cases, showing 3.5 ± 5.0 Gy (mean±STD) absolute mean dose differences between the planned and the actually delivered doses in the parotid glands. On average, DIR, dose computation, and segmentation take 20 s/fraction and 17 min for a 35-fraction treatment including additional computation for dose accumulation.
Conclusions:
The authors developed a unified software platform that provides accurate and efficient monitoring of anatomical changes and computation of actually delivered dose to the patient, thus realizing an efficient cumulative dose computation workflow. Evaluation on HN cases demonstrated the utility of our platform for monitoring the treatment quality and detecting significant dosimetric variations that are keys to successful ART.
Keywords: software platform, adaptive radiotherapy, deformable registration, segmentation, dose computation, graphics processing unit
1. INTRODUCTION
Intensity-modulated radiation therapy (IMRT) is widely used to treat cancer patients due to its ability to generate steep dose gradients to conform the radiotherapy dose to complex target volumes while minimizing the dose to surrounding normal tissues.1–4 Due to the sharp dose fall-off between the target tumor and surrounding normal tissues, accurate monitoring of the anatomical changes of both the target and organs at risk (OARs) is essential for successful IMRT treatment. Although the initial plan is optimized on the planning CT, the location, shape, and size of the tumor and OARs change during the course of therapy, often significantly, due to daily positioning error and response to the therapy during 6–7 weeks of treatment.5
Adaptive radiation therapy (ART) is a process to improve radiation treatment by monitoring treatment variations and incorporating them to reoptimize the treatment planning during the course of treatment.6,7 It has been decades since the concept of ART was first proposed. Although not a new concept, technical and practical challenges have limited its routine clinical application. To successfully execute ART, accurate tracking of the actual delivered dose to the target and surrounding OARs is critical. Since daily CBCT is typically taken at the time of treatment to monitor the anatomical changes of the patient and correct the setup error, daily CBCT can be leveraged to assess the delivered dose to the patient. However, CBCT’s poor image quality makes it difficult to segment the target tumor and critical structures of interest. In addition, dose computation on CBCT is not reliable because the reconstructed CBCT image intensity is not calibrated as CT number in general, even though several approaches to directly map CBCT intensity to Hounsfield unit exist.8–10
Deformable image registration (DIR) of the planning CT to daily CBCT has been widely used to map CBCT intensity to electron density for dose computation as well as to automatically segment the structures of interest in CBCT.11,12 In this approach, uncertainties in DIR directly influence the successive segmentation, dose computation, and dosimetric parameter estimation. Most DIR methods use intensity-based approaches based on well-known similarity metrics such as mutual information (MI) and cross correlation (CC). These conventional techniques often fail to achieve sufficient accuracy in CT–CBCT registration due to the poor quality of CBCT images and significant reconstruction artifacts therein. Recent studies demonstrated that the CT–CBCT registration could be significantly improved by actively correcting the CBCT intensity during the registration process.13–16
Another practical challenge in integrating the ART process within the existing clinical workflow is the complexity of various databases and associated data management and computational burden. Patient’s image data, treatment plan, and treatment information such as patient setup, couch shift, and beam data are often stored in separate databases, and transferring such data between different platforms requires tedious manual steps that are also susceptible to errors. Furthermore, ART requires a large amount of computations as the planning CT has to be registered to every daily CBCT on which daily delivered dose has to be computed and then accumulated (note that there are 30–40 fractions for a typical head and neck intensity-modulated radiotherapy). Therefore, a unified software platform that communicates with clinical databases, automatically collects necessary data, and processes and visualizes each processing step as well as the final results has long been desired for a seamless integration of the ART workflow within the existing radiotherapy workflow.
There have been several efforts to develop such a unified platform. Early efforts include cerr,17 plunc,18 and mmctp.19 cerr has basic functions such as importing dicom RT data and displaying dose–volume histograms (DVHs) for radiotherapy, but it has limited computational functionalities. plunc (or PLanUNC) and mmctp are planning systems equipped with a dose engine, but were not designed for cumulative dose computation (CDC). More recently, software platforms applicable to CDC and ART such as dirart (Ref. 12) and SlicerRT (Ref. 20) were introduced. Especially, SlicerRT offers various modules for creating accumulated dose based on DIR, comparing dose volumes, and displaying DVH. However, using them for clinical cases directly has limitations because they do not provide a streamlined workflow and require a lot of user interventions. Additionally, both dirart and SlicerRT do not have a dose computation engine, and therefore compute the accumulated dose by simply warping the planning dose through DIR rather than computing the actual dose. The commercial software system, RayStation (Raysearch Laboratories, Sweden), can support CDC and ART workflow. It provides integrated functions of RT planning as well as dose computation and accumulation using daily CBCT images. Table I summarizes available functionalities in different platforms.
TABLE I.
Comparison of functionalities for existing (A)RT systems (O: available, —: not available).
| cerr | plunc | mmctp | dirart | RayStation | SlicerRT | scuda | |
|---|---|---|---|---|---|---|---|
| Platform/language (distribution arrangement) | Matlab, c++ (open source) | c++, Perl (binary distributable) | REALBasic (binary distributable) | Matlab (Matlab toolbox) | Commercial SW (licensing) | 3DSlicer/Python, c++ (open source) | c++, cuda (licensing) |
| CT–CBCT DIR | — | — | — | O | O | O | O |
| Dose computation | — | O | O | — | O | — | O |
| Dose accumulation | — | — | — | O | O | O | O |
| Automatic segmentation | — | — | — | O | O | — | O |
| Data retrieval | — | — | — | — | O | O | O |
Despite the existence of these tools, it is still challenging to implement a CDC or ART workflow within current clinical workflows as none of them except for RayStation are equipped with all requisite functions. Moreover, their computational efficiency is insufficient for large data processing required for CDC or ART as most of them run on CPU without parallelization. Currently, RayStation provides the most functionalities, but as a commercial system, it does not lend itself to necessary modifications to connect multiple clinical platforms and accommodate various data formats and clinical workflows. Furthermore, these tools use a conventional DIR method which is not optimized for CT–CBCT registration. Our goal is to design and develop a software system that addresses the aforementioned issues and satisfies the following aspects:
-
•
Integrated environment: Importing/exporting images, RT structures, RT dose, treatment information including patient setup, couch shift, and beam information. Integrated functionalities of DIR, segmentation, and dose computation/accumulation. Efficient graphical user interface (GUI) to visualize 2D/3D images/dose and to monitor and assess each processing step and the results.
-
•
Accuracy and computational efficiency: Accurate CT–CBCT DIR with iterative CBCT intensity correction. GPU acceleration for DIR, dose computation, and segmentation. Efficient workflow with minimal user interaction.
-
•
Seamless interface with clinical systems: Automatic data query/retrieval from image PACS and clinical informatics database.
2. METHODS
scuda (Software platform for CUmulative Dose Assessment) is a stand-alone software system optimized for tracking anatomical changes and accumulating dose to support CDC for ART. It consists of three core computation components: (1) deformable image registration, (2) dose computation and accumulation, and (3) automatic segmentation. The overall platform is implemented in c++, and the computationally intensive modules of DIR and dose computation are parallelized and accelerated in the GPU for efficient computation. Each component is independent and the entire pipeline is modular so that we can easily test, modify, and change each component with different algorithms.
As shown in Fig. 1, the CDC workflow requires two additional steps (indicated as shaded boxes) between successive treatment fractions.21 To automatically perform these activities, data locations, formats, and input/output (I/O) protocols in the current clinical systems and databases have to be identified and properly set up in the processing platform. These setups are institution-dependent as different institutions use different planning systems, PACS, and clinical informatics systems. Table II summarizes the required data, their formats, storage locations, and query/retrieval protocols used in Radiation Oncology at Johns Hopkins Hospital (JHH). All required data including images, radiotherapy plan, beam, and patient treatment information are automatically extracted from clinical databases through dicom and sql query/retrieval. Although we set up our system to be compatible with the clinical setups at JHH, it can be easily modified to be compatible with other clinical workflow as scuda is modular and only I/O module needs to be updated.
FIG. 1.
CDC workflow. Our system supports two additional steps (indicated as shaded boxes) to the general radiotherapy workflow.
TABLE II.
Required data and information (Radiation Oncology at Johns Hopkins Hospital).
| Step | Activity | Data | Type | Server | Protocol |
|---|---|---|---|---|---|
| Planning | Delineation of OAR/target definition | Image (CT/MRI) | dicom | TeraMedicaa | dicom |
| Contours | dicom (RT structure) | TeraMedica | dicom | ||
| Treatment planning | Plan | dicom (RT plan) | TeraMedica | dicom | |
| Streamed data | Mosaiqb | sql | |||
| Computed dose | dicom (RT dose) | TeraMedica | dicom | ||
| Treatment | Patient setup | Image (CBCT) | dicom | TeraMedica | dicom |
| XVI | XVIc | XVI | |||
| Isocenter | xyz coordinate | Mosaiq | sql | ||
| Adapt position | Setup info | Couch shift: xyz coordinate | Mosaiq | sql | |
| Couch rotation: Euler angle |
TeraMedica: PACS (Fujifilm Medical Systems, Inc.).
Mosaiq: information management system/server (Elekta).
XVI: CBCT imaging system (Elekta).
2.A. System architecture
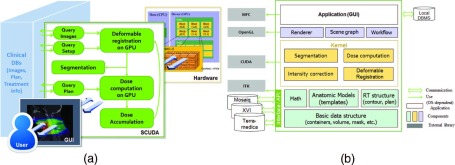
Our CDC workflow is described in Fig. 2(a). The CDC process begins with necessary data import (planning CT, contours, plan, and treatment information) from clinical databases followed by DIR of the planning CT to daily CBCTs. Contours of the tumor and OARs are propagated by applying the computed deformation vector fields. Daily dose delivered to the patient is computed on the deformed CT and accumulated to the current target fraction. These steps are repeated for every fraction through the last available treatment fraction. The accumulated dose for a case is updated at every fraction and passed to the next fraction computation. Because we query and retrieve the plan information for each fraction, correct plan is automatically extracted and applied even if the treatment plan is changed due to replanning.
FIG. 2.
(a) CDC workflow, and (b) scuda system architecture.
As shown in Fig. 2(b), each core component is modular for easy and independent test and update. Basic level layer consists of basic data structures and mathematical classes such as matrices, vectors, and bounding boxes based on which advanced algorithms are built in a modular way. As external libraries, Insight Toolkit (ITK)22 for dicom import/export and NVIDIA CUDA SDK (Ref. 23) for GPU computing, and Microsoft foundation classes (MFC) for GUI are used. Note that the internal data structures are independent of these external libraries.
There are two major transformations that need to be considered when computing the actually delivered dose based on daily CBCTs. First, there is a transformation between the planning CT and daily CBCT as these two systems use different frames of reference. This transformation allows us to match the isocenter marked in the plan to that of the linac. Additionally, we have to apply the couch shift performed to properly set up and align the patient. These transformations are stored in Mosaiq and automatically copied to scuda by sql query/retrieval. To handle local transformations efficiently, we adopt a scene graph structure that is widely used for rendering. Volumes or data objects in the same coordinate systems are grouped as children of the same transformation group, which stores the local coordinate system. The computed rigid transformation is saved as a transformation matrix, and the deformation vector fields are also saved to compute the contour propagation. All the data required for any computation or rendering (i.e., visualization) are managed by the graph, and it is implemented as the Singleton pattern.24
2.B. Deformable image registration of CT and CBCT
Deformable image registration of planning CT and daily CBCT may be considered well-posed as it is intrasubject and between the same imaging modalities. Most existing approaches use intensity-based methods based on well-known similarity metrics such as MI and CC. However, these conventional intensity-based registration methods often fail due to the poor image quality and spatially varying image contrast in CBCT.
CBCT reconstruction is typically computed by a filtered backprojection from a series of projections obtained by a single rotation of a diverging x-ray source. Therefore, the mid-plane (plane of source-detector rotation) can be exactly reconstructed, but off-midplanes are only approximately reconstructed. It is known that different off-midplanes undergo different amount of missing information, showing different artifacts.25 To address this issue and improve the registration accuracy, we use our in-house DIR algorithms that iteratively match CBCT and CT image intensities by anisotropic patch-based local histogram matching during the DIR process.16,26 In this method, intensity correction step is independent of registration method, and therefore can be combined with any DIR method. Three widely used DIR algorithms, hierarchical B-Spline,27 double force demons,28 and optical flow,29 are currently available in scuda. Their performances have been extensively evaluated in Ref. 26, showing superior performance over conventional and state-of-the-art methods.
2.C. Automatic segmentation
Segmentation of the tumor and OARs can be computed by propagating contours from the planning CT to daily CBCTs using the deformation fields computed by DIR. Since each contour in dicom RT structure set is defined as a set of points on 2D slices of the planning CT, we create a triangular surface mesh for each structure and apply deformation to the vertices of the surface mesh to generate the deformed structure. The propagated contours can be obtained by clipping the deformed surface mesh at each slice plane of the target CBCT as shown in Fig. 3.
FIG. 3.
Segmentation by contour propagation. (a) Daily CBCT. (b) Planning CT with physician’s contour (right nodal gross tumor volume). (c) 3D surface extraction from the original contours. (d) Registered CT. (e) Deformation field. (f) Deformed surface. (g) 3D mesh – 2D (axial) plane clipping. (h) Propagated contour on the CBCT.
2.D. Dose computation and accumulation
Accurate dose computation based on the patient’s anatomy and treatment setup at the time of treatment is critical for the CDC and ART processes. However, most existing platforms do not provide online dose computation functionality, and therefore estimate daily and accumulated doses by deforming the planning dose to daily CBCTs.11,19 The deformed and accumulated dose cannot be an accurate representation of the actual delivered dose especially when there are significant anatomical changes. In contrast, scuda computes the daily delivered dose on the deformed planning CT that is registered to the daily CBCT and corrected for the couch shift rather than deforming the planning dose. We use a superposition/convolution dose computation algorithm that has been previously developed in our group.30
Dose accumulation is computed by summing daily computed dose volumes. The accumulated dose of the ith fraction is the summation of the ith daily dose and the (i − 1)th accumulated dose deformed through DIR of the (i − 1)th CBCT to ith CBCT. The computed accumulated dose can be assessed by overlaying it on the CBCT and the associated propagated contours or through DVH as shown in Fig. 5(b). DVHs computed with the original plan and the accumulated dose can be plotted together for comparison.
FIG. 5.
Comparison of DVHs associated with planned and accumulated doses. The prefixes “R” and “L” represent right and left, and the suffixes yellow “P” (dashed line) and “C” (solid line) represent the planning and computed accumulated doses, respectively. “Parotid” and “submand.” represent parotid glands and submandibular glands. In these cases, parotid glands and submandibular glands received higher dose than the initial plan.
3. RESULTS
Figure 4 shows the GUI of scuda. A user can select a specific patient from the patient list, and visualize images, dose, and contours of the selected patient. A user can select one of three different DIR algorithms depending on the target site. In our evaluation on HN cases, we used demons algorithm due to its known robust and reliable performance on HN regions.14,15 To compute the daily and accumulated doses and segment CBCTs, the user only needs to choose treatment fractions to be processed and click the “Compute” button [see Fig. 4(b)]. scuda automatically connects to PACS and clinical databases, and using the patient’s ID, transfers the planning CT, RT plan, contours, daily CBCTs, and patient treatment information such as treatment time and couch shift. Once all necessary data are transferred, the platform automatically performs DIR, segmentation of CBCT, and daily and accumulated dose computation. The user can monitor each step from the progress window as well as visually from the slice viewer.
FIG. 4.
Graphical user interface of scuda. (a) Patient list/data management and visualization. (b) CDC processing and assessment. The progress window on the right shows the progress of each step.
Each computation module has been extensively tested in our previous studies. Our DIR has been tested on HN cancer data sets and its performance was compared with conventional as well as state-of-the-art DIR algorithms,16,31 showing superior registration accuracy and computational efficiency. Our method produced overall normalized mutual information of 0.59, normalized cross correlation of 0.96, and structural similarity index of 0.93, outperforming existing methods by up to 3.6%, 2.4%, and 2.8%, respectively. Our DIR-based automatic segmentation has been evaluated on six HN cancer cases with ground truth manual segmentations independently drawn by multiple raters. Our DIR-based autosegmentation showed good agreement with the ground truth, while conventional methods failed to track anatomical changes especially for later fractions where there were larger anatomical changes.26 Our approach significantly reduced the volume estimation error by 38% over the conventional mutual information based method. For the details of the evaluation results, we refer readers to our previous publications.16,26,31 These evaluations are based on HN cases, and the results may vary for different regions not only because the organ motions/deformations are different but also due to different image artifacts, e.g., severe truncation artifacts on thoracic or abdominal regions. Therefore, no single DIR algorithm performs the best for all sites, and a specific DIR for each target site needs to be chosen and tuned to achieve the best performance. scuda allows the user to adjust DIR parameters by changing the DIR configuration so that the DIR algorithms can be optimized for the image data to be processed and target anatomical site.
One of the main goals of CDC is to assess the treatment quality by properly monitoring uncertainties caused by setup errors and the patient’s anatomical changes. To demonstrate the utility of scuda, we compared the actual delivered dose to the tumor and OARs with the initial planned dose on 30 HN cancer patients. In general, DVH for target tumor regions does not significantly change for HN cases because the target tumor does not significantly move its anatomical position but change its volume in response to radiation, and PTV covers it well. However, nearby normal tissues may significantly move due to the tumor volume shrinkage and be exposed to high dose radiation than the original plan, resulting in significant DVH changes. Figure 5 shows an example of DVH plots for two cases showing significant deviation from the initial plan in the actual delivered doses.
To quantitatively compare the accumulated dose to the initial planning dose, we computed dosimetric parameters for one of the critical structures, parotid glands. CBCT images were acquired using an Elekta Synergy on-board imager (Elekta, Inc., Maryland Heights, MO, USA) and CT images were acquired using a Philips Brilliance Big Bore CT scanner (Philips Medical Systems, Cleveland, OH, USA). CT image has the size of 512 × 512 × (129–161) voxels with a voxel size of 1.236 × 1.236 × 3 mm3. CBCTs were exported as dicom images using the approximately the same voxel grid as CT [image size of 270 × 270 ×(88–264) voxels with a pixel size of 1 × 1 × (1–3) mm3] from Elekta XVI imaging software. Our platform ran on a desktop computer with Intel Core i7 3.4 GHz CPU, 16 GB RAM, and NVIDIA GeForce GTX 980.
We compared the mean dose and D90 values to the left and right parotid glands between the planning and accumulated doses. The absolute differences of the mean dose and D90 are 3.5 ± 5.0 Gy and 5.5 ± 7.5 Gy (mean ± standard deviation), respectively. The absolute mean dose differences for the left and right submandibular glands are 3.4 ± 4.2 Gy and 3.6 ± 4.1 Gy, respectively. Such differences imply that there could be normal tissue toxicities caused by excessive radiation. We tested the classification power from dosimetric parameters to severe xerostomia (severe when grade ≥2 vs less severe when grade <2), which is one of the most common complications of HN radiotherapy. D90, D50, D10, and mean, standard deviation, minimum and maximum dose values are extracted from the parotid and submandibular glands. With these parameters, the actual delivered dose was found to be a better predictor of severe xerostomia than the original planned done. The actual delivered dose was better by 11%, 5%, and 30% in terms of higher overall accuracy, sensitivity, and specificity, respectively.
Our platform is computationally efficient, thanks to significant parallelization provided by the GPU. Each CT–CBCT registration, daily dose computation, and dose accumulation by CBCT–CBCT registration take approximately 7–10 s, 8–10 s, and 2–3 s, respectively (approximately 20 s combined). Computation of an entire HN IMRT case with 35 fractions therefore takes approximately 17 min; 12 min for DIR, segmentation, and daily dose computation (20 s/fraction × 35 fractions) plus additional ∼5 min for dose accumulation as the previous CBCT has to be registered to current CBCT (note that CBCT–CBCT registration is much faster than CT–CBCT registration). In practice, each fraction can be processed and stored as soon as each daily treatment is completed, so only the new fraction needs to be processed each day, which requires only 20 s.
4. CONCLUSION
We have developed an integrated software platform to support CDC and ART. The DIR in our platform iteratively corrects CBCT intensities during the registration, thus significantly improving the CT–CBCT registration over existing methods. Accurate CT–CBCT registration leads to more accurate segmentation of the tumor and OARs based on daily CBCT. Additionally, the core components (DIR, segmentation, and dose computation) are parallelized with GPU, enabling near real-time processing of each treatment fraction data. Convenient GUI and seamless connection to existing databases allow for efficient management of raw/processed data as well as monitoring individual processing steps and final results. With the improved accuracy and computational workflow efficiency, our platform can remove the roadblocks to successful implementation for CDC and ART processes in the clinic. Our platform provides key functions to assess the quality of radiotherapy. Dosimetric and anatomical variations occurring throughout the course of radiotherapy will help physicians determine the need for replanning and also predict the clinical outcome. Finally, our platform can be further extended if combined with automatic planning,32,33 in which case, the entire time-consuming adaptive replanning task can be performed with only a few mouse-clicks, thus significantly reducing tedious human steps.
ACKNOWLEDGMENTS
This work was supported in part by NIH/NCI under Grant No. R42CA137886, in part by National Science Foundation under Grant No. EEC9731748, and in part by Johns Hopkins University internal funds.
CONFLICT OF INTEREST DISCLOSURE
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Intensity Modulated Radiation Therapy Collaborative Working Group, “Intensity-modulated radiotherapy: Current status and issues of interest,” Int. J. Radiat. Oncol., Biol., Phys. (4), 880–914 (2001). 10.1016/s0360-3016(01)01749-7 [DOI] [PubMed] [Google Scholar]
- 2.Chui C.-S. and Spirou S. V., “Inverse planning algorithm for external beam radiation therapy,” Med. Dosim. (2), 189–197 (2001). 10.1016/S0958-3947(01)00069-3 [DOI] [PubMed] [Google Scholar]
- 3.Leibel S. A., Fuks Z., Zelefsky M. J., Hunt M., Burman C. M., Mageras G. S., Chui C.-S., Jackson A., Amols H. I., and Ling C. C., “Technological advances in external-beam radiation therapy for the treatment of localized prostate cancer,” Semin. Oncol. (5), 596–615 (2003). 10.1016/S0093-7754(03)00354-3 [DOI] [PubMed] [Google Scholar]
- 4.Lee N., Puri D. R., Blanco A. I., and Clifford Chao K. S., “Intensity-modulated radiation therapy in head and neck cancers: An update,” Head Neck (4), 387–400 (2007). 10.1002/hed.20332 [DOI] [PubMed] [Google Scholar]
- 5.Schwarts D. L. and Dong L., “Adaptive radiation therapy for head and neck cancer—Can an old goal evolve into a new standard?,” J. Oncol. , 1–13. 10.1155/2011/690595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan D., Vicini F., Wong J., and Martinez A., “Adaptive radiation therapy,” Phys. Med. Biol. , 123–132 (1997). 10.1088/0031-9155/42/1/008 [DOI] [PubMed] [Google Scholar]
- 7.Schwartz D. L., “Current progress in adaptive radiation therapy for head and neck cancer,” Curr. Oncol. Rep. , 139–147 (2012). 10.1007/s11912-012-0221-4 [DOI] [PubMed] [Google Scholar]
- 8.Yoo S. and Yin F., “Dosimetric feasibility of cone-beam CT-based treatment planning compared to CT-based treatment planning,” Int. J. Radiat. Oncol., Biol., Phys. (5), 1553–1561 (2006). 10.1016/j.ijrobp.2006.08.031 [DOI] [PubMed] [Google Scholar]
- 9.Hatton J., McCurdy B., and Greer P. B., “Cone beam computerized tomography: The effect of calibration of the Hounsfield unit number to electron density on dose calculation accuracy for adaptive radiation therapy,” Phys. Med. Biol. (15), N329–N346 (2009). 10.1088/0031-9155/54/15/N01 [DOI] [PubMed] [Google Scholar]
- 10.Fotina I., Hopfgartner J., Stock M., Steininger T., Lutgendorf-Causcig C., and Georg D., “Feasibility of CBCT-based dose calculation: Comparative analysis of HU adjustment techniques,” Radiother. Oncol. , 249–256 (2012). 10.1016/j.radonc.2012.06.007 [DOI] [PubMed] [Google Scholar]
- 11.Chao M., Xie Y., and Xing L., “Auto-propagation of contours for adaptive prostate radiation therapy,” Phys. Med. Biol. , 4533–4542 (2008). 10.1088/0031-9155/53/17/005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang D., Brame S., Naqa I. E., Adiya A., Wu Y., Goddu S. M., Mutic S., Deasy J. O., and Low D. A., “Technical note: DIRART—A software suite for deformable image registration and adaptive radiotherapy research,” Med. Phys. (1), 67–77 (2011). 10.1118/1.3521468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou J., Guerreo M., Chen W., and D’Souza W. D., “Deformable planning CT to cone-beam CT image registration in head-and-neck cancer,” Med. Phys. , 2088–2094 (2011). 10.1118/1.3554647 [DOI] [PubMed] [Google Scholar]
- 14.Nithiananthan S., Schafer S., Uneri A., Mirota D. J., Stayman J. W., Zbijewski W., Brock K. K., Daly M. J., Chan H., Irish J. C., and Siewerdsen J. H., “Demons deformable registration of CT and cone-beam CT using an iterative intensity matching approach,” Med. Phys. (4), 1785–1798 (2011). 10.1118/1.3555037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhen X., Gu X., Yan H., Zhou L., Jia X., and Jiang S. B., “CT to cone-beam CT deformable registration with simultaneous intensity correction,” Phys. Med. Biol. , 6807–6826 (2012). 10.1088/0031-9155/57/21/6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park S., Plishker W., Shekhar R., Quon H., Wong J., and Lee J., “Deformable registration of CT and cone-beam CT by local CBCT intensity correction,” Proc. SPIE , 941333 (2015). 10.1117/12.2082485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deasy J. O., Blanco A. I., and Clark V. H., “CERR: A computational environment for radiotherapy research,” Med. Phys. (5), 979–985 (2003). 10.1118/1.1568978 [DOI] [PubMed] [Google Scholar]
- 18.PLANUNC, http://planunc.radonc.unc.edu/.
- 19.Alexander A., DeBlois F., Stroian G., Al-Yahya K., Heath E., and Seintjens J., “MMCTP: A radiotherapy research environment for Monte Carlo and patient-specific treatment planning,” Phys. Med. Biol. , N297–N308 (2007). 10.1088/0031-9155/52/13/N03 [DOI] [PubMed] [Google Scholar]
- 20.Pinter C., Lasso A., Wang A., Jaffray D., and Fichtinger G., “SlicerRT: Radiation therapy research toolkit for 3D slicer,” Med. Phys. (10), 6332–6338 (2012). 10.1118/1.4754659 [DOI] [PubMed] [Google Scholar]
- 21.Mullaney T., Pettersson H., Nyholm T., and Stolterman E., “Thinking beyond the cure: A case for human-centered design in cancer case,” Int. J. Des. (3), 27–39 (2012). [Google Scholar]
- 22.Ibanez L., Schroeder W., Ng L., and Cates J., The ITK Software Guide (Kitware Inc., New York, NY, 2003). [Google Scholar]
- 23.CUDA, https://developer.nvidia.com/cuda-zone.
- 24.Johnson R., Vlissides J., Helm R., and Gamma E., Design Patterns: Elements of Reusable Object-Oriented Software (Pearson Education, NJ, 1994). [Google Scholar]
- 25.Ramamurthi K., “Cone-beam tomography using C-arm x-ray projections: Complete trajectories and integration of prior CT information,” Ph.D. dissertation, Johns Hopkins University, 2006. [Google Scholar]
- 26.Park S., Plishker W., Robinson A., Zaki G., Shekhar R., McNutt T., Quon H., Wong J., and Lee J., “A hardware-accelerated software platform for adaptive radiation therapy,” in World Congress on Medical Physics and Biomedical Engineering, June 7-12, 2015, Toronto, Canada,IFMBE Proceedings (Springer International Publishing, Switzerland, 2015), Vol. 51, pp. 509–512. 10.1007/978-3-319-19387-8_125 [DOI] [Google Scholar]
- 27.Xie Z. and Farin G. E., “Image registration using hierarchical B-Spline,” IEEE Trans. Visualization Comput. Graphics (1), 85–94 (2004). 10.1109/tvcg.2004.1260760 [DOI] [PubMed] [Google Scholar]
- 28.Wang H., Dong L., O’Dinel J., Mohan R., Garden A. S., Ang K. K., Kuban D. A., Bonnen M., Chang J. Y., and Cheung R., “Validation of accelerated ‘demons’ algorithm for deformable image registration in radiation therapy,” Phys. Med. Biol. , 2887–2905 (2005). 10.1088/0031-9155/50/12/011 [DOI] [PubMed] [Google Scholar]
- 29.Noe K., de Senneville B., Elstrom U., Tanderup K., and Sorensen T., “Acceleration and validation of optical flow based deformable registration for image-guided radiotherapy,” Acta Oncol. , 1286–1293 (2008). 10.1080/02841860802258760 [DOI] [PubMed] [Google Scholar]
- 30.Jacques R., Wong J., Taylor R., and McNutt T., “Real-time dose-computation: GPU-accelerated source modeling and superposition/convolution,” Med. Phys. , 294–305 (2011). 10.1118/1.3483785 [DOI] [PubMed] [Google Scholar]
- 31.Park S., Robinson A., Quon H., Kiess A. P., Shen C., Wong J., Plishker W., Shekhar R., and Lee J., “Accurate tracking of tumor volume change during radiotherapy by CT-CBCT registration with intensity correction,” Proc. SPIE , 97860P (2016). 10.1117/12.2217047 [DOI] [Google Scholar]
- 32.Hong T. S., Craft D. L., Carlsson F., and Bortfeld T., “Multicriteria optimization in intensity-modulated radiation therapy treatment planning for locally advanced cancer of the pancreatic head,” Int. J. Radiat. Oncol., Biol., Phys. , 1208–1214 (2008). 10.1016/j.ijrobp.2008.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertsimas D., Cacchiani V., Craft D., and Nohadani O., “A hybrid approach to beam angle optimization in intensity-modulated radiation therapy,” Comput. Oper. Res. (9), 2187–2197 (2013). 10.1016/j.cor.2012.06.009 [DOI] [Google Scholar]