Main conclusion
Using genome-wide association mapping, 47 SNPs within 27 significant loci were identified for four grain shape traits, and 424 candidate genes were predicted from public database.
Grain shape is a key determinant of grain yield and quality in rice (Oryza sativa L.). However, our knowledge of genes controlling rice grain shape remains limited. Genome-wide association mapping based on linkage disequilibrium (LD) has recently emerged as an effective approach for identifying genes or quantitative trait loci (QTL) underlying complex traits in plants. In this study, association mapping based on 5291 single nucleotide polymorphisms (SNPs) was conducted to identify significant loci associated with grain shape traits in a global collection of 469 diverse rice accessions. A total of 47 SNPs were located in 27 significant loci for four grain traits, and explained ~44.93–65.90 % of the phenotypic variation for each trait. In total, 424 candidate genes within a 200 kb extension region (±100 kb of each locus) of these loci were predicted. Of them, the cloned genes GS3 and qSW5 showed very strong effects on grain length and grain width in our study. Comparing with previously reported QTLs for grain shape traits, we found 11 novel loci, including 3, 3, 2 and 3 loci for grain length, grain width, grain length–width ratio and thousand grain weight, respectively. Validation of these new loci would be performed in the future studies. These results revealed that besides GS3 and qSW5, multiple novel loci and mechanisms were involved in determining rice grain shape. These findings provided valuable information for understanding of the genetic control of grain shape and molecular marker assistant selection (MAS) breeding in rice.
Electronic supplementary material
The online version of this article (doi:10.1007/s00425-016-2548-9) contains supplementary material, which is available to authorized users.
Keywords: Genome-wide association mapping, Grain shape, Rice, Single nucleotide polymorphism
Introduction
Rice (Oryza sativa L.) is one of the most important cereal crops and a staple food for more than one-half of the world’s population. Rice yield is directly determined by four major components: number of effective tillers per plant, number of grains per panicle, the ratio of filled grains, and grain weight (Sakamoto and Matsuoka 2008; Xing and Zhang 2010). Grain size, a key factor determining grain weight, is specified by its three dimensional structures: grain length (GL), grain width (GW), and grain thickness (GT) (Fan et al. 2006; Zuo and Li 2014). In breeding applications, grain size and weight are important traits for rice yield and quality.
Grain size and weight are generally recognized as quantitative traits that are controlled by multiple genes and affected by environment factors. To date, more than 400 QTLs that control grain size and weight have been detected by using various mapping populations (http://www.gramene.org/qtl). Several major QTLs controlling grain size and weight have been identified and functionally characterized, such as GS3 (Fan et al. 2006; Mao et al. 2010), GW2 (Song et al. 2007), qSW5/GW5 (Shomura et al. 2008; Weng et al. 2008), GS5 (Li et al. 2011), qGL3/qGL3.1 (Qi et al. 2012; Zhang et al. 2012), GW8 (Wang et al. 2012), TGW6 (Ishimaru et al. 2013) and GL7/GW7 (Wang et al. 2015a, b). Among these, GS3 encoding a putative transmembrane protein and qGL3/qGL3.1 encoding a protein phosphatase with Kelch-like repeat domain function as a negative regulator for grain length. For grain width, GW2 encoding a RING-type E3 ubiquitin ligase and qSW5/GW5 encoding a novel protein act as negative regulators, whereas GS5 encoding a putative serine carboxypeptidase and GW8 encoding a transcription factor with SBP domain are positive regulators. TGW6 encodes a novel protein with indole-3-acetic acid (IAA)-glucose hydrolase activity and loss of TGW6 function results in an increase in grain weight and yield. GL7/GW7 encodes a protein homologous to Arabidopsis thaliana LONGIFOLIA proteins, which regulates longitudinal cell elongation. The GL7/GW7 locus containing a 17.1-kb tandem duplication leads to upregulation of GL7/GW7 and downregulation of its nearby negative regulator, resulting in an increase in grain length and improvement of grain appearance quality. Functional characterizations of these genes have greatly enriched our knowledge of the molecular mechanisms determining grain size and weight in rice. However, additional genes controlling grain size and weight remain to be identified.
Association mapping utilized linkage disequilibrium (LD) to examine the marker-trait associations, and enabled researchers to exploit natural variation and identify novel genes for complex traits (Zhu et al. 2008). With the development of SNP assays, high throughput genotyping technologies and associated statistical methods, association mapping has been widely used in various plant species, including A. thaliana (Olsen et al. 2004; Ehrenreich et al. 2009), soybean (Jun et al. 2008), wheat (Breseghello and Sorrells 2006), maize (Palaisa et al. 2003; Wilson et al. 2004; Camus-Kulandaivelu et al. 2006), foxtail millet (Jia et al. 2013) and rice (Agrama et al. 2007; Wen et al. 2009; Huang et al. 2010; Zhao et al. 2011). Association mapping has potential advantages over conventional linkage analysis and QTL mapping, such as broader allele coverage, higher mapping resolution, shorter time-consuming and improving the cost effectiveness. Association mapping has become a useful and robust strategy complementary to classical bi-parental mapping and has the power to genetically map multiple traits simultaneously (Huang and Han 2014).
In the present study, association mapping of four traits (GL, GW, grain length–width ratio (LWR) and thousand-grain weight (TGW)) was performed with a panel of 469 accessions using a custom-designed array contained 5291 genomic SNPs. The objectives of this study were to (1) investigate the population structure and genetic diversity for grain shape in a global rice germplasm collection; (2) identify the novel SNPs and loci associated with grain shape in rice; (3) explore design of gene pyramiding breeding strategies for cultivar genetic improvement. These results will increase our understanding of the molecular mechanisms underlying grain size and weight, and may provide some new information for rice molecular design breeding.
Materials and methods
Plant materials and phenotyping
To reduce spurious genetic associations caused by population structure, a set of 469 global diverse indica accessions with rice grain traits variation were chosen to construct this genome-wide association study (GWAS) population (Supplementary Table S1). The seeds of all accessions were collected, stored and supplied by China National Center for Rice Improvement, China National Rice Research Institute (CNRRI). All the rice accessions were planted in the following four environments: the Lingshui Experiment Station of CNRRI (LS; N 18°48′, E 110°02′) in Hainan, China, in the winter of 2013–2014; the Hangzhou Experiment Station of CNRRI (HZ; N 30°32′, E 120°12′) in Zhejiang, China, in the summer of 2013–2014. For field experiments, the accessions were grown in a 6 × 6 completely randomized block design with three replications. The space was 20 cm between the plants and 25 cm between the rows. Four plants in the middle of each row were harvested individually to measure the grain traits. Twenty full-filled rice grains were randomly selected from each plant for trait measurement. GL was estimated by placing 20 grains one by one in a straight line along a ruler, and then arranged by breadth to measure grain width. The averaged GL and GW of 20 grains as the trait values of that line were used for data analysis. The LWR is equal to GL divided by its GW. TGW was calculated based on 200 grains and converted to 1000-grain weight.
DNA extraction and genotyping
Genomic DNA was extracted from leaf tissue of one plant per accession using the CTAB method (Murray and Thompson 1980). All the accessions were genotyped using the Illumina RiceSNP6k BeadChip containing 5291 SNPs, which were chosen from the Rice Haplotype Map Project Database (http://www.ncgr.ac.cn/RiceHap2) (Huang et al. 2010). Genotypes were called using the program GenomeStudio (Illumina, San Diego, Calif. USA). The quality of each SNP was confirmed manually, and low quality SNPs (call rate <80 % and minor allele frequency (MAF) <0.05) were removed from the dataset. Finally, 3951 SNPs were obtained for the association mapping (Supplementary Table S2).
Data analysis
The mean, standard error (SE) and broad-sense heritability (H2B) were calculated using the Excel 2007. The percentage of phenotypic variation explained by population structure (R2PCA) was computed using SAS system 9.0 (SAS, Inc., Cary, NC), as well as the ANOVA. Correlation coefficients were run in R “corrgram” (https://cran.r-project.org/web/packages/corrgram/), and the best linear unbiased prediction (BLUP) was carried out in R “lme4” (https://cran.r-project.org/web/packages/lme4/) for estimating phenotypic values of each line in four environments.
Genome-wide association mapping
For association analysis of our panel, genome association and prediction integrated tool (GAPIT) with compressed mixed linear model (cMLM) and population parameters previously defined (P3D) was performed under R environment (Lipka et al. 2012; Zhang et al. 2010). And the top 10 principle components were used as a covariant. The kinship matrix estimated by these SNPs data was combined with population structure to improve statistical power of our genome-wide association mapping. As Bonferroni correction (1/3951 = 2.5E−04) was too conservation (Nakagawa 2004), a compromised threshold of P = 0.001 was used to detect the significant association signals. To obtain the loci with the lowest P value, redundant loci were filtered in a 200 kb genomic window. And the candidate gene prediction was performed from the Rice Haplotype Map Project Database (http://202.127.18.221/RiceHap2/). Finally, the allele with an increasing effect was defined as elite allele and vice versa. The elite alleles of association loci were used to evaluate the pyramiding effect.
Results
Grain shape variation among accessions
In this study, 469 indica accessions collected from 20 countries (Fig. 1a) were used as the genome wide association mapping panel. Four grain shape traits, namely GL, GW, LWR and TGW of 469 rice accessions were measured in four environments after harvested. Extensive and significant phenotypic variations were observed for the four grain traits in Hangzhou (HZ) and Lingshui (LS) during 2013–2014 (Table 1; Fig. 1b, c; Supplementary Figure S1; Supplementary Table S3). The mean of GL over the 469 accessions in each environment was 8.58, 8.51, 8.81 and 8.86, respectively. Over the four environments the minimum GL value was in 2013 HZ (6.67 cm), and the maximum value was in 2014 HZ (11.37 cm). GW had means of 2.69, 3.12, 2.81 and 2.89 in each environment, respectively. GW value ranged from 1.89 to 2.17 cm in the four environments. The mean of LWR over the 469 accessions in each environment was 3.24, 2.76, 3.19 and 3.10, respectively. Over the four environments the minimum LWR value was in 2013 LS (1.94), and the maximum value was in 2013 HZ (4.98). TGW had means of 23.94, 28.44, 25.04 and 26.88 g in each environment, respectively. TGW value ranged from 14.35 to 50.75 g in the four environments. The distribution of the four grain traits in all four environments showed continuous variation, and the phenotypic segregation approximately fit a normal distribution (Fig. 1d–g). This indicated that grain shape traits were governed by multiple loci in this association panel. The broad-sense heritability (H2B %), averaged across four environments, of GL, GW, LWR and TGW, was 79.1, 84.4, 89.6 and 83.7 %, respectively. The ANOVA indicated that effects of genotype (G), environment (E) and their interaction were high significantly (P < 0.001) (Table 1).
Fig. 1.
Material distribution and grain shape traits diversity. a The worldwide distribution of 469 indica accessions. The black dots represent the country-specific distribution. b Different grain length in the germplasm. Bar 1 cm. c Different grain width in the germplasm. Bar 1 cm. d Histogram of grain length in multiple environments. e Histogram of grain width in multiple environments. f Histogram of grain length–width ratio in multiple environments. g Histogram of thousand-grain weight in multiple environments. LS Lingshui, HZ Hangzhou
Table 1.
Phenotypic variation for grain traits across four environments in 469 indica accessions
| Trait | Environment | Mean ± SE | Range | R 2PCA (%)a | H 2B (%)b | G × Ec |
|---|---|---|---|---|---|---|
| GL (cm) | 2013, HZ | 8.58 ± 0.03 | 6.67–10.92 | 29.10 | 79.1 | *** |
| 2013, LS | 8.51 ± 0.03 | 6.83–10.67 | 27.24 | |||
| 2014, HZ | 8.81 ± 0.03 | 6.92–11.37 | 31.65 | |||
| 2014, LS | 8.86 ± 0.03 | 7.11–11.20 | 32.22 | |||
| GW (cm) | 2013, HZ | 2.69 ± 0.01 | 1.89–3.79 | 37.50 | 84.4 | *** |
| 2013, LS | 3.12 ± 0.01 | 2.17–3.93 | 31.10 | |||
| 2014, HZ | 2.81 ± 0.01 | 1.92–3.90 | 39.37 | |||
| 2014, LS | 2.89 ± 0.01 | 2.02–3.90 | 32.53 | |||
| LWR | 2013, HZ | 3.24 ± 0.02 | 2.17–4.98 | 41.23 | 89.6 | *** |
| 2013, LS | 2.76 ± 0.02 | 1.94–4.11 | 38.52 | |||
| 2014, HZ | 3.19 ± 0.02 | 2.15–4.74 | 43.73 | |||
| 2014, LS | 3.10 ± 0.02 | 1.97–4.75 | 38.26 | |||
| TGW (g) | 2013, HZ | 23.94 ± 0.16 | 14.50–38.85 | 19.16 | 83.7 | *** |
| 2013, LS | 28.44 ± 0.18 | 16.70–50.75 | 21.82 | |||
| 2014, HZ | 25.04 ± 0.15 | 14.35–41.75 | 18.69 | |||
| 2014, LS | 26.88 ± 0.19 | 16.45–47.80 | 23.96 |
GL grain length, GW grain width, LWR grain length–width ratio, TGW thousand-grain weight, LS Lingshui, HZ Hangzhou, SE standard error
*** Significant at P = 0.001
aPhenotypic variation explained by population structure
bHeritability in the broad sense
cG × E, interaction of genotype and environment
The results of Pearson correlation analysis showed that the phenotypic correlations between traits were similar in the four testing environments (Fig. 2). GW showed significantly negative correlation with GL and LWR, and highly positive correlation with TGW in all environments. TGW exhibited significantly or very significantly negative correlation with LWR, and highly positive correlation with GW. The correlation coefficients ranged from -0.88 between LWR and TGW, to 0.71 between LWR and GL (Fig. 2). These results demonstrated that rice grain traits were highly related to each other, and this provided valuable knowledge for rice grain shape modifications.
Fig. 2.
Correlation of four grain shape traits in four environments. The upper panel contains the correlation coefficients, and the lower panel contains the distributions of the four traits. The diagonal represents the density line of the traits. *, ** and *** represent significant at 0.05, 0.01 and 0.001, respectively. GL, GW, LWR and TGW represent grain length, grain width, grain length–width ratio and thousand grain weight, respectively. HZ and LS represent Hangzhou and Lingshui, respectively
Genetic structure and linkage disequilibrium estimation
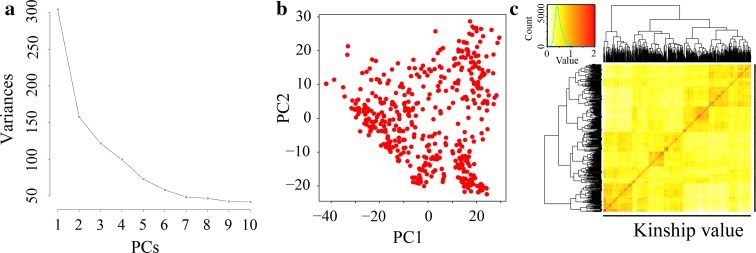
STRUCTURE software was used to assess the subpopulation genetic structure of the 469 indica rice accessions based on the distribution of the 3,951 SNPs across 12 rice chromosomes. Our previous study showed that the indica panel could be classified into four subpopulations (Lu et al. 2015). In this GWAS mapping, the population effect showed by a principal component analysis (PCA) component and relatedness estimated automatically from the genotypic data were evaluated using GAPIT as well as GWAS (Lipka et al. 2012; Zhang et al. 2010). The scree plot generated through GAPIT indicated the first two PCs as informative, and then decreased gradually (Fig. 3a, b). Moreover, until the 10th PC component, the variance value almost unchanged, thus the top 10 PCs were used as a covariate to adjust the GWAS results (Fig. 3a). In addition, the heatmap of kinship value showed that most of the value concentrated at ~0.5 level indicating a weak relatedness in the complex GWAS panel (Fig. 3c). This evaluation is consistent with our previous study suggesting that the GAPIT is credible for population structure and relative kinship estimations (Lu et al. 2015). In the present study, the linkage disequilibrium (LD) decay distance was ~109.37 kb at which the LD parameter (r2) dropped to half of its maximum value. In addition, the LD decay distance differed among chromosomes and ranged from 96.15 kb on chromosome 5 to 421.39 kb on chromosome 7.
Fig. 3.
Population structure of current association panel which consisted mostly of the indica accessions. a Scree plot from GAPIT showing the selection of PCs for association study. b Variation of the top two principal components. c Kinship values
Genome-wide association analysis
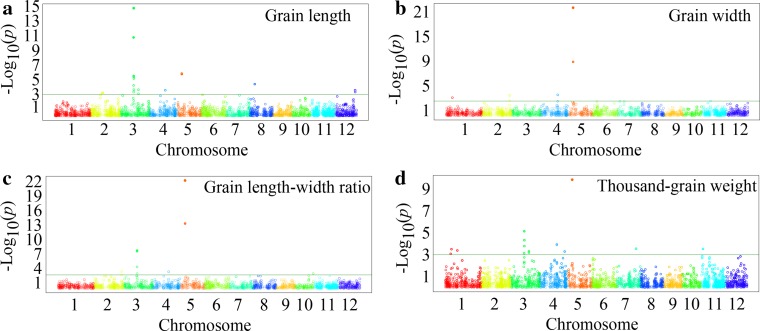
In this study, the association analysis was performed using BLUP method predicted for each accession to reduce the environment effects and simplify the calculations. The stringent criterion of −log10 (P) ≥3.0 under four environments was used for determining the association significance of the four grain traits. Using the GWAS approach, we successfully identified both known genes and previously reported QTLs from rice as well as novel candidate loci in the rice genome. The results of our genome-wide association scans were summarized in Fig. 4 and Table 2 where we showed the SNP trait associations discovered in the association panel. A total of 27 significant loci were identified for the four grain traits. For the 27 significant loci, 424 candidate genes were obtained within a 200-kb genomic region (±100 kb from the peak SNP) from the Rice Haplotype Map Project Database (http://202.127.18.221/RiceHap2/) (Supplementary Table S4). In addition, the distributions of the 3951 SNPs were analyzed and 47 SNPs were located in the 27 significant loci for the four grain traits (Supplementary Table S2, Table S4). Of the 27 loci, GS3 and qSW5 showed very strong effects on grain length and width. Simultaneously, in order to investigate environment-specific and common loci under multiple environments, we performed association mapping using TASSEL version 4.0 (Bradbury et al. 2007) under each environment (Supplementary Table S5).
Fig. 4.
Manhattan plots of genome wide association mapping for four rice grain traits. a Grain length, b grain width, c grain length–width ratio, d thousand grain weight. Green lines represent the threshold at −log10 (P) ≥3
Table 2.
Summary of SNPs significantly associated with grain traits using the BLUP method
| Trait | Marker | Chr. | Position | Allelea | MAF | P value | R 2 (%)b | Known QTL | Marker | References |
|---|---|---|---|---|---|---|---|---|---|---|
| GL | seq-rs918 | 2 | 13760905 | G/A | 0.17 | 6.68E−04 | 50.23 | |||
| seq-rs919 | 2 | 14722011 | T/G | 0.17 | 6.68E−04 | |||||
| seq-rs1614 | 3 | 16939138 | T/C | 0.25 | 1.87E−15 | GS3 | Fan et al. (2006) | |||
| seq-rs1697c | 3 | 22579680 | G/T | 0.21 | 2.63E−04 | qGL3a | RM15456 | Li et al. (2010) | ||
| seq-rs2123 | 4 | 19733128 | C/T | 0.05 | 2.89E−04 | qGL4b | RM5586 | Kato et al. (2011) | ||
| gwseq-rs14d | 5 | 5377176 | T/C | 0.21 | 1.91E−06 | qSW5 | Shomura et al. (2008) | |||
| seq-rs3750 | 8 | 5964991 | C/T | 0.06 | 4.35E−05 | |||||
| seq-rs5920 | 12 | 25594275 | G/A | 0.36 | 2.91E−04 | qGL12 | RM2854 | Li et al. (2010) | ||
| GW | seq-rs196 | 1 | 7562923 | A/G | 0.29 | 2.24E−04 | 58.57 | |||
| seq-rs1303 | 2 | 33850349 | T/C | 0.25 | 7.62E−05 | |||||
| seq-rs2139 | 4 | 21062959 | A/G | 0.42 | 6.02E−05 | |||||
| seq-rs2427d | 5 | 5359498 | G/A | 0.32 | 1.12E−22 | qSW5 | Shomura et al. (2008) | |||
| LWR | seq-rs1303 | 2 | 33850349 | T/C | 0.25 | 2.75E−04 | 65.90 | |||
| seq-rs1614 | 3 | 16939138 | T/C | 0.25 | 9.46E−09 | GS3 | Fan et al. (2006) | |||
| seq-rs2123 | 4 | 19733128 | C/T | 0.05 | 3.03E−04 | qLWR4 | RM6997 | Ying et al. (2012) | ||
| seq-rs2427 | 5 | 5359498 | G/A | 0.32 | 1.48E−23 | qSW5 | Shomura et al. (2008) | |||
| seq-rs4717 | 10 | 22631657 | T/C | 0.08 | 7.37E−04 | |||||
| TGW | seq-rs184 | 1 | 6667379 | A/G | 0.45 | 9.17E−04 | 44.93 | Gw1-1 | RM10398 | Yu et al. (2008) |
| seq-rs196 | 1 | 7562923 | A/G | 0.29 | 3.70E−04 | QGwt1c, qBRW1a | RM259 | Xu et al. (2002), Wang et al. (2003) | ||
| seq-rs288 | 1 | 14827946 | C/T | 0.30 | 4.76E−04 | |||||
| seq-rs1614 | 3 | 16939138 | T/C | 0.25 | 9.00E−06 | GS3 | Fan et al. (2006) | |||
| seq-rs1698c | 3 | 22582110 | C/A | 0.21 | 5.42E−04 | qTGW3-4, qGW3.8 | RM16 | Marathi et al. (2012), Zhang et al. (2013) | ||
| seq-rs2139 | 4 | 21062959 | A/G | 0.42 | 1.41E−04 | |||||
| seq-rs2255 | 4 | 30599877 | A/G | 0.18 | 5.67E−04 | qTGW4-1 | RM3276 | Marathi et al. (2012) | ||
| seq-rs2427 | 5 | 5359498 | G/A | 0.32 | 2.03E−10 | qSW5 | Shomura et al. (2008) | |||
| seq-rs3526 | 7 | 23662096 | C/A | 0.07 | 3.19E−04 | |||||
| seq-rs4745 | 11 | 3031650 | T/C | 0.34 | 3.40E−04 | GWt11 | RM332 | Yoshida et al. (2002) |
GL grain length, GW grain width, LWR grain length–width ratio, TGW thousand-grain weight
aMajor allele/minor allele
bPhenotypic variation explained by all of the significant loci
cThe two SNPs can be considered as the same locus (~2 kb)
dThe two SNPs can be considered as the same locus (~17 kb)
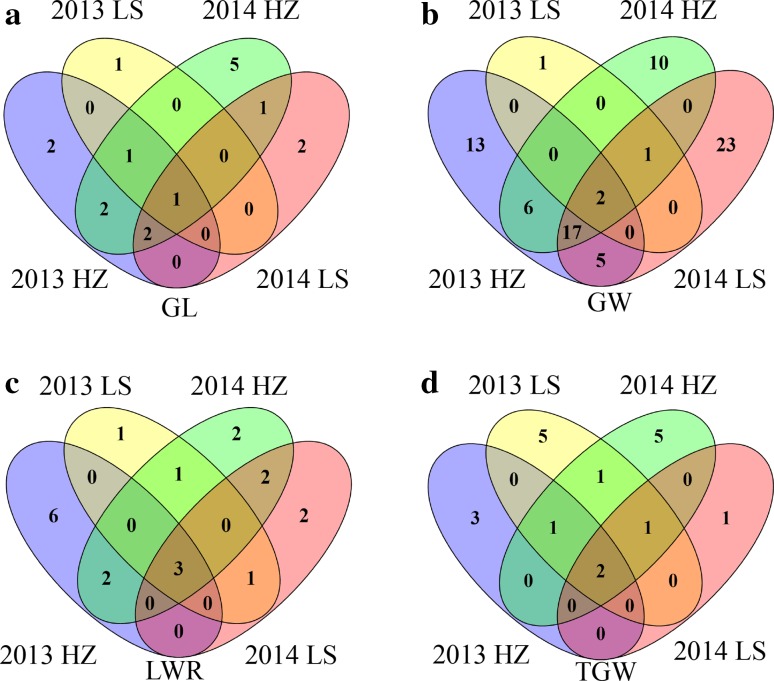
For the GL trait, 8 major loci, explaining ~50.23 % of the phenotypic variation, were identified on chromosomes 2, 3, 4, 5, 8 and 12 (Table 2). Among them, the GS3 locus (seq-rs1614) was confirmed and explained ~8.50–14.73 % of the total phenotypic variation under four environments (Fig. 5a; Supplementary Table S6), and this result was consistent with previous reports (Huang et al. 2010; Zhao et al. 2011). Furthermore, the loci, seq-rs1610, seq-rs2061 and seq-rs4660, on chromosomes 3, 4 and 10 were identified across three environments (Fig. 5a; Supplementary Table S6). Additionally, the loci, seq-rs1604 and seq-rs2427, on chromosomes 3 and 5 were detected in both 2013 and 2014 HZ environments (Fig. 5a; Supplementary Table S6). The locus (seq-rs2718) on chromosome 6 was identified in both 2014 HZ and LS environments (Fig. 5a; Supplementary Table S6).
Fig. 5.
Venn diagrams showing unique and shared SNPs for grain shape traits among four environments. a GL grain length. b GW grain width. c LWR grain length–width ratio. d TGW thousand grain weight. LS and HZ represent Lingshui and Hangzhou, respectively
For the GW trait, 4 major loci, explaining ~58.57 % of the phenotypic variation, were identified on chromosomes 1, 2, 4 and 5 (Table 2). Among them, the previously cloned qSW5 locus showed very strong effect on GW and had high R2 values (~13.6–27.79 %) in the four environments (Supplementary Table S5), and this result was consistent with previous reports (Huang et al. 2010; Zhao et al. 2011). The locus (seq-rs2434) as well as the qSW5 (seq-rs2427), on chromosome 5 was detected across four environments (Fig. 5b; Supplementary Table S6). In addition, eleven and eighteen significant loci were identified across two and three environments, respectively (Fig. 5b; Supplementary Table S6). Moreover, the loci, seq-rs196, seq-rs1303 and seq-rs2139, on chromosomes 1, 2 and 4 were also detected for TGW, LWR and TGW, respectively (Table 2).
In our study, marker-trait association analyses revealed that 5 loci associated with LWR, locating on chromosomes 2, 3, 4, 5 and 10 (Table 2; Fig. 4c). These loci could explain up to 65.90 % of the total phenotypic variation (Table 2). Among them, the known genes GS3 and qSW5 were showed clear signal for LWR in all the four environments (Fig. 5c; Supplementary Table S6). The locus (seq-rs1610) on chromosome 3 was also detected across four environments (Supplementary Table S6). Additionally, the loci (seq-rs944 and seq-rs2123) on chromosomes 2 and 4 were detected in both 2013 and 2014 HZ, and the loci (seq-rs3533 and seq-rs3537) on chromosome 7 were identified in both 2013 and 2014 LS (Fig. 5c; Supplementary Table S6).
For the TGW trait, 10 major loci, explaining ~44.93 % of phenotypic variation, were detected on chromosomes 1, 3, 4, 5, 7 and 11, and six loci were located in the adjacent or overlapping regions previously reported to be associated with TGW (Table 2). Moreover, the loci (seq-rs288 and seq-rs2139) on chromosomes 1 and 4 were identified across three environments (Fig. 5d; Supplementary Table S6). Furthermore, we also identified the known genes (GS3 and qSW5) for TGW in all four environments (Fig. 5d; Supplementary Table S6). Additionally, the locus (seq-rs5793) on chromosome 12 was detected in both 2013 LS and 2014 HZ (Fig. 5d; Supplementary Table S6).
Gene linkage or pleiotropy
Gene linkage and pleiotropy are common phenomena in plant genetics. A matrix summarizing the linkage or pleiotropy of the loci associated with four grain traits were shown in Fig. 6. In our study, 12 known or new candidate SNPs showed clear signal linkage or pleiotropy that was associated with multiple grain traits in the four environments. Among them, the loci seq-rs1614 and seq-rs2427 underlying grain size (GS3 and qSW5) were shown to be significantly associated with GL, LWR, TGW and all four grain traits, respectively (Fig. 6). Previous studies showed that GS3 had pleiotropic effects on grain length and weight (Fan et al. 2006; Mao et al. 2010), and qSW5 was associated with grain width and weight (Shomura et al. 2008; Weng et al. 2008). These associations were also supported by Pearson correlation analysis based on traits measured in all four environments (Fig. 2). In addition, we detected the locus (seq-rs2123) on chromosome 4 that were significantly associated with both GL and LWR, and we found a locus (seq-rs196) on chromosome 2 that was significantly associated with GW and TGW. Moreover, the average correlation coefficients of GL-LWR (r = 0.71, P < 0.001) and GW-TGW (r = 0.57, P < 0.001) were highly significant in all four environments. Additionally, some new candidate loci possessing gene linkage or pleiotropy were observed as well (Fig. 6). Exploring and utilizing the linkage or pleiotropy of the loci underlying grain traits would be beneficial for rice grain yield improvement.
Fig. 6.
Summary of significant trait-marker associations for four grain traits across genomic regions. GL, GW, LWR and TGW represent grain length, grain width, grain length–width ratio and thousand grain weight, respectively
Pyramiding elite alleles for breeding
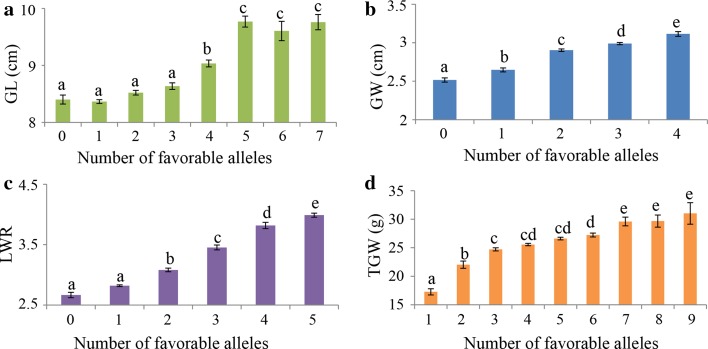
The objective of gene pyramiding in molecular breeding is to combine a series of target alleles in a specific line or variety (Servin et al. 2004). We examined the efficacy of pyramiding elite alleles into an individual plant for four grain traits (Supplementary Table S7). Besides GS3 (seq-rs1614) and qSW5 (seq-rs2427), such as the eilte allele of the locus seq-rs1698 showed potential breeding value for GL and TGW (Supplementary Table S7). This result was also confirmed by classical QTL mapping in different bi-parental mapping populations (Li et al. 2010; Marathi et al. 2012; Zhang et al. 2013). Without considering the interaction effects among these loci, mean values for phenotypic traits except GL significantly increased linearly with pyramiding more elite alleles in rice cultivars (Fig. 7). For the GL trait, when pyramiding 0–3 elite alleles, the mean phenotypic values had no significant changes, and the mean phenotypic values increased significantly with pyramiding 4 elite alleles, and the mean phenotypic values were significantly higher when pyramiding 5–7 elite alleles in rice varieties (Fig. 7). These results indicated that enhancing the frequency of elite alleles has a significant phenotypic effect on rice grain traits improvements.
Fig. 7.
Pyramid effect analysis for different numbers of elite alleles. X-axis represents the number of elite alleles carried by the accessions and Y-axis represents trait mean value GL, GW, LWR and TGW represent grain length, grain width, grain length–width ratio and thousand grain weight, respectively. All bars represent standard error
Discussion
Rice is one of the most staple foods in the world, feeding more than half of the global population especially in Asian countries. It has been estimated that a 40 % increase in rice production by 2030 will be needed to meet the predicted demand of the growing world population (Khush 2005). Rice grain shape is one of the most important factors determining rice yield (Huang et al. 2013). Thus, identification of major QTLs for grain shape and grain weight is an important objective of rice genetic research and breeding programs. In this study, genome-wide association mapping was performed to identify the loci for four grain shape traits in 469 rice accessions. The sample size of our study is larger or similar to the number of accessions used in genome wide association study in rice (Huang et al. 2010; Zhao et al. 2011). The 469 diverse accessions were from 20 countries, which represented all major rice growing regions of the world (Fig. 1a). Four grain shape traits including GL, GW, LWR and TGW were measured in four environments. We successfully identified 27 loci for the four grain traits, comprising 17 distinct regions distributed on all 12 chromosomes except chromosomes 6 and 9.
In rice, more than 400 QTLs that control grain shape traits have been detected by using various mapping population (Huang et al. 2013). To date, 20 genes associated with grain size and weight have been isolated by map-based cloning strategies (Huang et al. 2013; Zuo and Li 2014; Duan et al. 2013; Hu et al. 2015; Liu et al. 2015a, b; Wang et al. 2015a, b). We compared the positions of the significant loci identified in this study with the positions of the reported genes or QTLs for the four grain traits. Of the 27 identified loci, the positions of 16 loci were close to those reported in previous studies, including 5, 1, 3 and 7 loci for GL, GW, LWR and TGW, respectively (Table 2).
As is known, GS3 and qSW5 are major QTLs for GL and GW (Fan et al. 2006; Shomura et al. 2008), which showed very strong effect for the same phenotypes in our study. This result was consistent with the GWAS results for GL and GW by Huang et al. (2010) and Zhao et al. (2011). Kato et al. (2011) mapped a GL QTL (qGL4b) to a 3.0 Mb region on chromosome 4 between marker RM5586 and RM3524. Interestingly, we detected a locus (seq-rs2123) for both GL and LWR in this chromosome region (Table 2), within which overlapped with a QTL for LWR reported by Ying et al. (2012). In addition, we detected 2 loci (seq-rs1697 and seq-rs5920) for GL on chromosome 3 and 12, respectively (Table 2), which have also been located in similar positions in different mapping population (Li et al. 2010). Moreover, Yu et al. (2008) mapped a TGW QTL (Gw1-1) to a 392.9 kb region on chromosome 1 between marker RM10376 and RM10398. We also detected a locus (seq-rs184) for TGW in the overlapped region. In the present study, 2 significant loci (seq-rs1698 and seq-rs2255) for TGW were located in the same genomic regions as those previously reported by Marathi et al. (2012). Additionally, three out of eight associated loci for TGW were mapped in adjacent intervals on the same chromosome as those reported in previous studies (Table 2). These results demonstrated that our 6K SNP array could capture the major common variants responsible for grain shape traits. However, no significant loci for grain traits were detected on chromosomes 6 and 9. One possible explanation was that the QTL effects were too small to be detected in our association panel, and another reason might be due to the lack of SNP coverage in the potential QTL regions. On the other hand, we identified 11 novel loci, including 3, 3, 2 and 3 loci for GL, GW, LWR and TGW, respectively (Table 2). Validation of these new loci would be performed in the future studies.
QTL× environment interaction is an important component for quantitative traits (Xing et al. 2002). In the present study, we observed some significant loci were only detected in one environment. For example, the associated loci, seq-rs3156, seq-rs4859, seq-rs768 and seq-rs2255 for GL, GW, LWR and TGW, respectively, were only detected in 2013 HZ. Result of ANOVA also showed that the G × E interactions were highly significant for all rice grain traits. Therefore, the QTL× environment interaction should not be ignored if molecular marker assistant selection (MAS) is applied for the manipulation of rice grain shape traits. However, the stable expression of a QTL across a broad range of agrometeorological conditions is a critical factor when breeding for wide adaptation (Maccaferri et al. 2008). In this study, the major loci (GS3 and qSW5) were detected across all four environments. These results were also proved by those obtained NILs carrying various alleles in the same background of ZhenShan97 (Lu et al. 2013) and supported by other GWAS experiments (Huang et al. 2010; Zhao et al. 2011). These results suggested that the major loci were first fixed in cultivated variety free of environmental influences due to strong human selection. Additionally, 3, 18 and 2 significant loci for GL, GW and TGW, respectively, were detected across three environments (Fig. 5; Supplementary Table S6). Moreover, 3, 11, 6 and 1 significant loci for GL, GW, LWR and TGW, respectively, were detected across two environments (Fig. 5; Supplementary Table S6). These stable significant loci affecting grain traits across different environments may be helpful in MAS of rice grain shape breeding.
The colocation of QTLs for multiple traits may be due to either pleiotropy or tightly linked genes controlling related traits (Brown 2002; Chen and Lubberstedt 2010). Our results showed that the associated loci GS3 and qSW5 exhibited strong pleiotropic effects on grain size and grain weight. Besides, 5 overlapped regions (seq-rs2123, seq-rs184, seq-rs196, seq-rs1698 and seq-rs2255) were found to be associated with grain traits in this study (Table 2). Among them, the locus (seq-rs2123) was detected for both GL and LWR, which was closely to the position identified by Kato et al. (2011) for GL and Ying et al. (2012) for LWR. In addition, the seq-rs1697 locus was detected for GL and TGW on chromosome 3. Its position was coincident with previously identified QTLs (Li et al. 2010; Marathi et al. 2012). Moreover, we identified a new locus (seq-rs2139) on chromosome 4 for GW and TGW across three environments. The Pearson correlation analysis also proved that the traits with co-localized loci were correlated with each other in all four environments (Fig. 2). Accumulation of favorable alleles in linkage blocks is very important and useful for breeders to implement grain shape improvement programs. Characterization and validation studies involving joint linkage and association mapping, combining with fine mapping to identify the novel genes and alleles underlying our association hits, would help us more clearly understand the relationship between these candidate genes and the phenotypes observed in our study, and provide breeders with the effective genetic tools to break unfavorable linkages and exploit these valuable alleles.
In the present study, we identified 27 loci associated with four grain traits via GWAS and convincingly demonstrated that the rice grain shape is complex trait controlled by many genes of major or minor effect. We also refined candidate chromosomal regions for the known QTLs, including the cloned genes GS3 and qSW5. Moreover, new candidate loci were obtained, and genetic variations combined with phenotypic variances were observed. Results of the present study demonstrated that genome-wide association mapping in rice could complement and enhance the information from linkage-based QTL studies toward the implementation of MAS in breeding programs. Considering the effect and distribution of novel loci associated with grain shape in our study, further validation is needed to unearth the relationship between these candidate loci and the phenotypes and facilitate exploring the molecular mechanisms governing grain shape suitable for rice breeding programs.
Author contribution statement
YF and XHW designed the experiments. YF, QL, RRZ and MCZ performed the phenotyping. QX, YF, RRZ and YLY carried out the genetic studies. XPY, HYY and YPW managed the materials. XHW designed the overall project. YF analyzed the data and drafted the manuscript. SW and YLY helped to revise the manuscript. All of the authors read and approved the final manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure S1: Box plot of four grain shape traits in four environments. (a) GL, grain length. (b) GW, grain width. (c) LWR, grain length–width ratio. (d) TGW, thousand grain weight. LS and HZ represent Lingshui and Hangzhou, respectively (TIFF 471 kb)
Supplementary Table S1: The origin of 469 indica accessions (XLSX 25 kb)
Supplementary Table S2: Summary of 3951 SNPs information (XLSX 147 kb)
Supplementary Table S3: Phenotypic data for four grain traits in Hangzhou and Lingshui during 2013–2014 (XLSX 107 kb)
Supplementary Table S4: Summary of 424 candidate genes for four grain traits (XLSX 38 kb)
Supplementary Table S5: Summary of SNPs significant associated with four grain traits in Hangzhou and Lingshui during 2013–2014 (XLSX 24 kb)
Supplementary Table S6: Summary of common loci associated with grain shape traits under multiple environments (XLSX 16 kb)
Supplementary Table S7: Summary of elite allele and phenotypic effect (XLSX 16 kb)
Acknowledgments
We sincerely thank all the participants. This work was supported by Zhejiang Provincial Natural Science Foundation of China (LY15C130005), the National Natural Science Foundation of China (31200916), the Agricultural Sciences and Technologies Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS) and the Ministry of Science and Technology of China (2013CBA01404 and 2013BAD01B02-14).
Abbreviations
- GL
Grain length
- GW
Grain width
- GT
Grain thickness
- TGW
Thousand grain weight
- LWR
Grain length–width ratio
- QTL
Quantitative trait loci
- LD
Linkage disequilibrium
- SNP
Single nucleotide polymorphism
- GWAS
Genome-wide association study
- CTAB
Hexadecyltrimethy ammonium bromide
- MAF
Minor allele frequency
- SE
Standard error
- ANOVA
Analysis of variance
- BLUP
Best linear unbiased prediction
- cMLM
Compressed mixed linear model
- PCA
Principle component analysis
- G × E
Interaction of genotype and environment
- GAPIT
Genome association and prediction integrated tool
- MAS
Molecular assistant selection
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Yue Feng and Qing Lu contributed equally to this work.
References
- Agrama HA, Eizenga GC, Yan W. Association mapping of yield and its components in rice cultivars. Mol Breed. 2007;19:341–356. doi: 10.1007/s11032-006-9066-6. [DOI] [Google Scholar]
- Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics. 2007;23:2633–2635. doi: 10.1093/bioinformatics/btm308. [DOI] [PubMed] [Google Scholar]
- Breseghello F, Sorrells ME. Association mapping of kernel size and milling quality in wheat (Triticum aestivum L.) cultivars. Genetics. 2006;172:1165–1177. doi: 10.1534/genetics.105.044586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JKM. Yield penalties of disease resistance in crops. Curr Opin Plant Biol. 2002;5:339–344. doi: 10.1016/S1369-5266(02)00270-4. [DOI] [PubMed] [Google Scholar]
- Camus-Kulandaivelu L, Veyrieras JB, Madur D, Combes V, Fourmann M, Barraud S, Dubreuil P, Gouesnard B, Manicacci D, Charcosset A. Maize adaptation to temperate climate: relationship between population structure and polymorphism in the Dwarf8 gene. Genetics. 2006;172:2449–2463. doi: 10.1534/genetics.105.048603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lubberstedt T. Molecular basis of trait correlations. Trends Plant Sci. 2010;15:454–461. doi: 10.1016/j.tplants.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Duan P, Rao Y, Zeng D, Yang Y, Xu R, Zhang B, Dong G, Qian Q, Li Y. SMALL GRAIN 1, which encodes a mitogen-activated protein kinase kinase 4, influences grain size in rice. Plant J. 2013;77:547–557. doi: 10.1111/tpj.12405. [DOI] [PubMed] [Google Scholar]
- Ehrenreich IM, Hanzawa Y, Chou L, Roe JL, Kover PX, Purugganan MD. Candidate gene association mapping of Arabidopsis flowering time. Genetics. 2009;183:325–335. doi: 10.1534/genetics.109.105189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Xing Y, Mao H, Lu T, Han B, Xu C, Li X, Zhang Q. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor Appl Genet. 2006;112:1164–1171. doi: 10.1007/s00122-006-0218-1. [DOI] [PubMed] [Google Scholar]
- Hu J, Wang Y, Fang Y, Zeng L, Xu J, Yu H, Shi Z, Pan J, Zhang D, Kang S, Zhu L, Dong G, Guo L, Zeng D, Zhang G, Xie L, Xiong G, Li J, Qian Q. A rare allele of GS2 enhances grain size and grain yield in rice. Mol Plant. 2015;8:1455–1465. doi: 10.1016/j.molp.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Huang X, Han B. Natural variations and genome-wide association studies in crop plants. Ann Rev Plant Biol. 2014;65:531–551. doi: 10.1146/annurev-arplant-050213-035715. [DOI] [PubMed] [Google Scholar]
- Huang X, Wei X, Sang T, Zhao Q, Feng Q, Zhao Y, Li C, Zhu C, Lu T, Zhang Z, Li M, Fan D, Guo Y, Wang A, Wang L, Deng L, Li W, Lu Y, Weng Q, Liu K, Huang T, Zhou T, Jing Y, Li W, Lin Z, Buckler ES, Qian Q, Zhang Q, Li J, Han B. Genome-wide association studies of 14 agronomic traits in rice landraces. Nat Genet. 2010;42:961–967. doi: 10.1038/ng.695. [DOI] [PubMed] [Google Scholar]
- Huang R, Jiang L, Zheng J, Wang T, Wang H, Huang Y, Hong Z. Genetic bases of rice grain shape: so many genes, so little known. Trends Plant Sci. 2013;18:218–226. doi: 10.1016/j.tplants.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Ishimaru K, Hirotsu N, Madoka Y, Murakami N, Hara N, Onodera H, Kashiwagi T, Ujiie K, Shimizu B, Onishi A, Miyagawa H, Katoh E. Loss of function of the IAA-glucose hydrolase gene TGW6 enhances rice grain weight and increases yield. Nat Genet. 2013;45:707–711. doi: 10.1038/ng.2612. [DOI] [PubMed] [Google Scholar]
- Jia G, Huang X, Zhi H, Zhao Y, Zhao Q, Li W, Chai Y, Yang L, Liu K, Lu H, Zhu C, Lu Y, Zhou C, Fan D, Weng Q, Guo Y, Huang T, Zhang L, Lu T, Feng Q, Hao H, Liu H, Lu P, Zhang N, Li Y, Guo E, Wang S, Wang S, Liu J, Zhang W, Chen G, Zhang B, Li W, Wang Y, Li H, Zhao B, Li J, Diao X, Han B. A haplotype map of genomic variations and genome-wide association studies of agronomic traits in foxtail millet (Setaria italica) Nat Genet. 2013;45:957–961. doi: 10.1038/ng.2673. [DOI] [PubMed] [Google Scholar]
- Jun TH, Van K, Kim MY, Lee SH, Walker DR. Association analysis using SSR markers to find QTL for seed protein content in soybean. Euphytica. 2008;162:179–191. doi: 10.1007/s10681-007-9491-6. [DOI] [Google Scholar]
- Kato T, Segami S, Toriyama M, Kono I, Ando T, Yano M, Kitano H, Miura K, Iwasaki Y. Detection of QTLs for grain length from large grain rice (Oryza sativa L.) Breed Sci. 2011;61:269–274. doi: 10.1270/jsbbs.61.269. [DOI] [Google Scholar]
- Khush GS. What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol Biol. 2005;59:1–6. doi: 10.1007/s11103-005-2159-5. [DOI] [PubMed] [Google Scholar]
- Li M, Xu L, Ren J, Cao G, Yu L, He H, Han L, Koh H. Identification of quantitative trait loci for grain traits in japonica rice. Agric Sci China. 2010;9:929–936. doi: 10.1016/S1671-2927(09)60173-5. [DOI] [Google Scholar]
- Li Y, Fan C, Xing Y, Jiang Y, Luo L, Sun L, Shao D, Xu C, Li X, Xiao J, He Y, Zhang Q. Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat Genet. 2011;43:1266–1269. doi: 10.1038/ng.977. [DOI] [PubMed] [Google Scholar]
- Lipka AE, Tian F, Wang Q, Peiffer J, Li M, Bradbury PJ, Gore MA, Buckler ES, Zhang Z. GAPIT: genome association and prediction integrated tool. Bioinformatics. 2012;28:2397–2399. doi: 10.1093/bioinformatics/bts444. [DOI] [PubMed] [Google Scholar]
- Liu L, Tong H, Xiao Y, Che R, Xu F, Hu B, Liang C, Chu J, Li J, Chu C. Activation of Big Grain1 significantly improves grain size by regulating auxin transport in rice. Proc Natl Acad Sci. 2015;112:11102–11107. doi: 10.1073/pnas.1512748112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Hua L, Dong S, Chen H, Zhu X, Jiang J, Zhang F, Li Y, Fang X, Chen F. OsMAPK6, a mitogen-activated protein kinase, influences rice grain size and biomass production. Plant J. 2015;84:672–681. doi: 10.1111/tpj.13025. [DOI] [PubMed] [Google Scholar]
- Lu L, Shao D, Qiu X, Sun L, Yan W, Zhou X, Yang L, He Y, Yu S, Xing Y. Natural variation and artificial selection in four genes determine grain shape in rice. New Phytol. 2013;200:1269–1280. doi: 10.1111/nph.12430. [DOI] [PubMed] [Google Scholar]
- Lu Q, Zhang M, Niu X, Wang S, Xu Q, Feng Y, Wang C, Deng H, Yuan X, Yu H, Wang Y, Wei X. Genetic variation and association mapping for 12 agronomic traits in indica rice. BMC Genom. 2015;16:1067. doi: 10.1186/s12864-015-2245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri M, Sanguineti MC, Corneti S, Ortega JLA, Salem MB, Bort J, DeAmbrogio E, Moral LFG, Demontis A, El-Ahmed A, Maalouf F, Machlab H, Martos V, Moragues M, Motawaj J, Nachit M, Nserallah N, Quabbou H, Royo C, Slama A, Tuberosa R. Quantitative trait loci for grain yield and adaptation of durum wheat (Triticum durum Desf.) across a wide range of water availability. Genetics. 2008;178:489–511. doi: 10.1534/genetics.107.077297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H, Sun S, Yao J, Wang C, Yu S, Xu C, Li X, Zhang Q. Linking differential domain functions of the GS3 protein to natural variation of grain size in rice. Proc Natl Acad Sci. 2010;107:19579–19584. doi: 10.1073/pnas.1014419107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marathi B, Guleria S, Mohapatra T, Parsad R, Mariappan N, Kurungara VK, Atwal SS, Prabhu KV, Singh NK, Singh AK. QTL analysis of novel genomic regions associated with yield and yield related traits in new plant type based recombinant inbred lines of rice (Oryza sativa L.) BMC Plant Biol. 2012;12:137. doi: 10.1186/1471-2229-12-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucl Acid Res. 1980;8:4321–4326. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav Eol. 2004;15:1044–1045. doi: 10.1093/beheco/arh107. [DOI] [Google Scholar]
- Olsen KM, Halldorsdottir SS, Stinchcombe JR, Weinig C, Schmitt J, Purugganan MD. Linkage disequilibrium mapping of Arabidopsis CRY2 flowering time alleles. Genetics. 2004;167:1361–1369. doi: 10.1534/genetics.103.024950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaisa KA, Morgante M, Williams M, Rafalski A. Contrasting effects of selection on sequence diversity and linkage disequilibrium at two phytoene synthase loci. Plant Cell. 2003;15:1795–1806. doi: 10.1105/tpc.012526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi P, Lin YS, Song XJ, Shen JB, Huang W, Shan JX, Zhu MZ, Jiang L, Gao JP, Lin HX. The novel quantitative trait locus GL3.1 controls rice grain size and yield by regulating Cyclin-T1;3. Cell Res. 2012;22:1666–1680. doi: 10.1038/cr.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Matsuoka M. Identifying and exploiting grain yield genes in rice. Curr Opin Plant Biol. 2008;11:209–214. doi: 10.1016/j.pbi.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Servin B, Martin OC, Mezard M, Hospital F. Toward a theory of marker-assisted gene pyramiding. Genetics. 2004;168:513–523. doi: 10.1534/genetics.103.023358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomura A, Izawa T, Ebana K, Ebitani T, Kangegae H, Konishi S, Yano M. Deletion in a gene associated with grain size increased yields during rice domestication. Nat Genet. 2008;40:1023–1028. doi: 10.1038/ng.169. [DOI] [PubMed] [Google Scholar]
- Song XJ, Huang W, Shi M, Zhu MZ, Lin HX. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat Genet. 2007;39:623–630. doi: 10.1038/ng2014. [DOI] [PubMed] [Google Scholar]
- Wang B, Lan T, Wu W. Identification of QTLs underlying grain traits in rice using SSLP linkage map. Fujian J Agric Sci. 2003;18:11–15. [Google Scholar]
- Wang S, Wu K, Yuan Q, Liu X, Liu Z, Lin X, Zeng R, Zhu H, Dong G, Qian Q, Zhang G, Fu X. Control of grain size, shape and quality by OsSPL16 in rice. Nat Genet. 2012;44:950–954. doi: 10.1038/ng.2327. [DOI] [PubMed] [Google Scholar]
- Wang S, Li S, Liu Q, Wu K, Zhang J, Wang S, Wang Y, Chen X, Zhang Y, Gao C, Wang F, Huang H, Fu X. The OsSPL16-GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nat Genet. 2015;47:949–954. doi: 10.1038/ng.3352. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xiong G, Hu J, Jiang L, Yu H, Xu J, Fang Y, Zeng L, Xu E, Xu J, Ye W, Meng X, Liu R, Chen H, Jing Y, Wang Y, Zhu X, Li J, Qian Q. Copy number variation at the GL7 locus contributes to grain size diversity in rice. Nat Genet. 2015;47:944–948. doi: 10.1038/ng.3346. [DOI] [PubMed] [Google Scholar]
- Wen W, Mei H, Feng F, Yu S, Huang Z, Wu J, Chen L, Xu X, Luo L. Population structure and association mapping on chromosome 7 using a diverse panel of Chinese germplasm of rice (Oryza sativa L.) Theor Appl Genet. 2009;119:459–470. doi: 10.1007/s00122-009-1052-z. [DOI] [PubMed] [Google Scholar]
- Weng J, Gu S, Wan X, Gao H, Guo T, Su N, Lei C, Zhang X, Cheng Z, Guo X, Wang J, Jiang L, Zhai H, Wan J. Isolation and initial characterization of GW5, a major QTL associated with rice grain width and weight. Cell Res. 2008;18:1199–1209. doi: 10.1038/cr.2008.307. [DOI] [PubMed] [Google Scholar]
- Wilson LM, Whitt SR, Ibanez AM, Rocheford TR, Goodman MM, Buckler ES. Dissection of maize kernel composition and starch production by candidate gene association. Plant Cell. 2004;16:2719–2733. doi: 10.1105/tpc.104.025700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Zhang Q. Genetic and molecular bases of rice yield. Annu Rev Plant Biol. 2010;61:421–442. doi: 10.1146/annurev-arplant-042809-112209. [DOI] [PubMed] [Google Scholar]
- Xing Y, Tan Y, Hua J, Sun X, Xu C, Zhang Q. Characterization of the main effects, epistatic effects and their environmental interactions of QTLs on the genetic basis of yield traits in rice. Theor Appl Genet. 2002;105:248–257. doi: 10.1007/s00122-002-0952-y. [DOI] [PubMed] [Google Scholar]
- Xu J, Xue Q, Luo L, Li Z. Genetic dissection of grain weight and its related traits in rice (Oryza sativa L.) Chin J Rice Sci. 2002;16:6–10. [Google Scholar]
- Ying JZ, Gao JP, Shan JX, Zhu MZ, Shi M, Lin HX. Dissecting the genetic basis of extremely large grain shape in rice cultivar ‘JZ1560’. J Genet Genom. 2012;39:325–333. doi: 10.1016/j.jgg.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Ikegami M, Kuze J, Sawada K, Hashimoto Z, Ishii T, Nakamura C, Kamijima O. QTL analysis for plant and grain characters of sake-brewing rice using a doubled haploid population. Breed Sci. 2002;52:309–317. doi: 10.1270/jsbbs.52.309. [DOI] [Google Scholar]
- Yu SW, Yang CD, Fan YY, Zhuang JY, Li XM. Genetic dissection of a thousand-grain weight quantitative trait locus on rice chromosome 1. Chin Sci Bull. 2008;53:2326–2332. [Google Scholar]
- Zhang Z, Ersoz E, Lai CQ, Todhunter RJ, Tiwari HK, Gore MA, Bradbury PJ, Yu J, Arnett DK, Ordovas JM, Buckler ES. Mixed linear model approach adapted for genome-wide association studies. Nat Genet. 2010;42:355–360. doi: 10.1038/ng.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang J, Huang J, Lan H, Wang C, Yin C, Wu Y, Tang H, Qian Q, Li J, Zhang H. Rare allele of OsPPKL1 associated with grain length causes extra-large grain and a significant yield increase in rice. Proc Natl Acad Sci. 2012;109:21534–21539. doi: 10.1073/pnas.1219776110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wang H, Qian Y, Xia J, Li Z, Shi Y, Zhu L, Ali J, Gao Y, Li Z. Simultaneous improvement and genetic dissection of grain yield and its related traits in a backbone parent of hybrid rice (Oryza sativa L.) using selective introgression. Mol Breed. 2013;31:181–194. doi: 10.1007/s11032-012-9782-z. [DOI] [Google Scholar]
- Zhao K, Tung CW, Eizenga GC, Wright MH, Ali ML, Price AH, Norton GJ, Islam MR, Reynolds A, Mezey J, McClung AM, Bustamante CD, McCouch SR. Genome-wide association mapping reveals a rich genetic architecture of complex traits in Oryza sativa. Nat Commun. 2011;2:467. doi: 10.1038/ncomms1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Gore M, Buckler ES, Yu J. Status and prospects of association mapping in plants. Plant Genome. 2008;1:5–20. doi: 10.3835/plantgenome2008.02.0089. [DOI] [Google Scholar]
- Zuo J, Li J. Molecular genetic dissection of quantitative trait loci regulating rice grain size. Ann Rev Genet. 2014;48:99–118. doi: 10.1146/annurev-genet-120213-092138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: Box plot of four grain shape traits in four environments. (a) GL, grain length. (b) GW, grain width. (c) LWR, grain length–width ratio. (d) TGW, thousand grain weight. LS and HZ represent Lingshui and Hangzhou, respectively (TIFF 471 kb)
Supplementary Table S1: The origin of 469 indica accessions (XLSX 25 kb)
Supplementary Table S2: Summary of 3951 SNPs information (XLSX 147 kb)
Supplementary Table S3: Phenotypic data for four grain traits in Hangzhou and Lingshui during 2013–2014 (XLSX 107 kb)
Supplementary Table S4: Summary of 424 candidate genes for four grain traits (XLSX 38 kb)
Supplementary Table S5: Summary of SNPs significant associated with four grain traits in Hangzhou and Lingshui during 2013–2014 (XLSX 24 kb)
Supplementary Table S6: Summary of common loci associated with grain shape traits under multiple environments (XLSX 16 kb)
Supplementary Table S7: Summary of elite allele and phenotypic effect (XLSX 16 kb)