Abstract
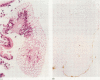
A non-radioactive DNA in situ hybridisation (DISH) protocol was developed. It requires the use of biotinylated Campylobacter pylori DNA as the probe to detect C pylori DNA in routinely embedded stomach biopsy specimens. In sequential tissue samples from a 58 year old woman with recurrent chronic active gastritis the C pylori probe hybridised with bacteria whenever they were histologically visible. When no bacteria were visible histologically, hybridisation was negative with one exception. In a single biopsy specimen without visible C pylori, hybridisation occurred with the surface of the antrum epithelium, while control hybridisation with a heterologous probe remained negative. From a parallel biopsy specimen taken at the same time the C pylori culture was positive. It is concluded that DISH, although more laborious than routine staining techniques, may be more sensitive in that it detects bacteria very easily, even when sections are not counterstained or when they are present in low numbers, and that bacteria which do hybridise are unequivocally identified as C pylori and not Campylobacter-like organisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaney R. P., Lammertsma A. A., Jones T., McKenzie C. G., Halnan K. E. Positron emission tomography for in-vivo measurement of regional blood flow, oxygen utilisation, and blood volume in patients with breast carcinoma. Lancet. 1984 Jan 21;1(8369):131–134. doi: 10.1016/s0140-6736(84)90063-1. [DOI] [PubMed] [Google Scholar]
- Jones D. M., Lessells A. M., Eldridge J. Campylobacter like organisms on the gastric mucosa: culture, histological, and serological studies. J Clin Pathol. 1984 Sep;37(9):1002–1006. doi: 10.1136/jcp.37.9.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
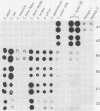
- Langenberg W., Rauws E. A., Widjojokusumo A., Tytgat G. N., Zanen H. C. Identification of Campylobacter pyloridis isolates by restriction endonuclease DNA analysis. J Clin Microbiol. 1986 Sep;24(3):414–417. doi: 10.1128/jcm.24.3.414-417.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffeld R. J., Loffeld B. C., Arends J. W., Flendrig J. A., van Spreeuwel J. P. Retrospective study of Campylobacter-like organisms in patients undergoing partial gastrectomy. J Clin Pathol. 1988 Dec;41(12):1313–1315. doi: 10.1136/jcp.41.12.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B. J. Campylobacter pyloridis and gastritis. J Infect Dis. 1986 Apr;153(4):650–657. doi: 10.1093/infdis/153.4.650. [DOI] [PubMed] [Google Scholar]
- Musgrove C., Bolton F. J., Krypczyk A. M., Temperley J. M., Cairns S. A., Owen W. G., Hutchinson D. N. Campylobacter pylori: clinical, histological, and serological studies. J Clin Pathol. 1988 Dec;41(12):1316–1321. doi: 10.1136/jcp.41.12.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaniuk P. J., Zoltowska B., Trust T. J., Lane D. J., Olsen G. J., Pace N. R., Stahl D. A. Campylobacter pylori, the spiral bacterium associated with human gastritis, is not a true Campylobacter sp. J Bacteriol. 1987 May;169(5):2137–2141. doi: 10.1128/jb.169.5.2137-2141.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983 Jun 4;1(8336):1273–1275. [PubMed] [Google Scholar]
- van den Berg F. M., Jiwa M., Rook R., Geelen J. L. Analysis and isolation of cytomegalovirus DNA by field inversion gel electrophoresis. J Gen Virol. 1988 Mar;69(Pt 3):699–704. doi: 10.1099/0022-1317-69-3-699. [DOI] [PubMed] [Google Scholar]