Abstract
Germline BAP1 mutations predispose to several cancers, in particular malignant mesothelioma. Mesothelioma is an aggressive malignancy generally associated to professional exposure to asbestos. However, to date we found that none of the mesothelioma patients carrying germline BAP1 mutations were professionally exposed to asbestos. We hypothesized that germline BAP1 mutations might influence the asbestos-induced inflammatory response that is linked to asbestos carcinogenesis, thereby increasing the risk of developing mesothelioma after minimal exposure. Using a BAP1+/− mouse model, we found that, compared to their wild type littermates, BAP1+/− mice exposed to low-dose asbestos fibers showed significant alterations of the peritoneal inflammatory response, including significantly higher levels of pro-tumorigenic alternatively polarized M2 macrophages, and lower levels of several chemokines and cytokines. Consistent with these data, BAP1+/− mice had a significantly higher incidence of mesothelioma after exposure to very low doses of asbestos, doses that rarely induced mesothelioma in wild type mice. Our findings suggest that minimal exposure to carcinogenic fibers may significantly increase the risk of malignant mesothelioma in genetically predisposed individuals carrying germline BAP1 mutations, possibly via alterations of the inflammatory response.
Keywords: BAP1, BAP1 cancer syndrome, germline, mesothelioma, asbestos, dose, inflammation, macrophages, polarization, M1, M2
Introduction
Malignant mesothelioma (MM) is a deadly cancer usually localized to the pleural and peritoneal linings1. In the US and in the UK, ~3 200 and ~2 500 individuals are diagnosed and die with MM each year, respectively2, 3. About 60–70% of mesotheliomas have been associated to exposure to carcinogenic mineral fibers, mainly asbestos1. Nevertheless, the risk of developing MM in high-risk cohorts professionally exposed to asbestos is ~5%, suggesting that other factors contribute to MM pathogenesis1. Mineral fibers promote mesothelioma inducing a chronic inflammatory reaction: on one hand this results in the production of mutagenic oxygen and nitrogen radicals, and on the other hand it provides damaged mesothelial cells with important survival signals4. Although chronic inflammation has been associated with the pathogenesis of several cancers, competent inflammatory cells also provide immunosurveillance, the host’s protection process against nascent transformed cells expressing altered antigens5. In fact, different functional and phenotypical cell subtypes are associated to anti-tumoral or pro-tumoral immunity6. Macrophages (MΦ) can undergo different types of polarization based on the kind and levels of cytokines present in the local tissue environment. Classically activated (M1) MΦ have a pro-inflammatory anti-tumoral phenotype, while alternatively activated (M2) MΦ are involved in immunosuppression and tissue repair7. Tumor-associated macrophages (TAM) represent one of the major populations of immune cells infiltrating tumors, and usually acquire functional characteristics similar to M2 MΦ8. The ratio between M2-like and M1-like TAM has prognostic value in MM and other cancers, with the former usually associated with a worse prognosis9–11. However, the contribution of different MΦ subpopulations to the initiation of inflammation-induced cancers is still unclear. MM has a large number of TAM, suggesting that they play an important role in this malignancy12.
Recently, we identified germline mutations in the tumor suppressor gene BRCA1 associated protein-1 (BAP1) as causative of a novel hereditary cancer syndrome characterized by a very high risk of MM, uveal and cutaneous melanoma, several other malignancies, and characteristic benign melanocytic tumors we named MBAITs13–15. The penetrance of the BAP1 cancer syndrome is ~100%, and several patients carrying germline BAP1 mutations develop multiple cancers16. Notably, none of the germline BAP1 heterozygous patients who developed MM reported professional exposure to asbestos fibers13, 16, suggesting that either these MMs were not caused by asbestos, or that minimal amounts of asbestos – as in the case of some indoor exposure17 or naturally occurring outdoor environmental exposure18 – may be sufficient to cause MM in germline BAP1 mutation carriers. Here, we experimentally tested in a BAP1+/− murine model whether germline BAP1 heterozygosity would result in alterations of the asbestos-induced inflammatory response, and whether low doses of asbestos might be sufficient to cause MM.
We used constitutive BAP1+/− mice (C57BL/6 background) generated by breeding mice with loxP sites flanking BAP1 exons 4 and 5 with mice expressing a constitutive general Cre deleter19. While homozygous BAP1 deficiency in mice results in embryonic lethality19, BAP1+/− mice are viable and healthy. Compared to wild type littermates, BAP1+/− mice expressed about half the amount of BAP1 protein in relevant tissues (Suppl. Fig 1).
In our experiments, we used 10–12 weeks old mice of either sex equally distributed in the experimental groups using a computational random number generator. All the experiments were approved by the University of Hawai’i Institutional Animal Care and Use Committee (IACUC). Unless otherwise specified, results are presented as median [interquartile range].
Results
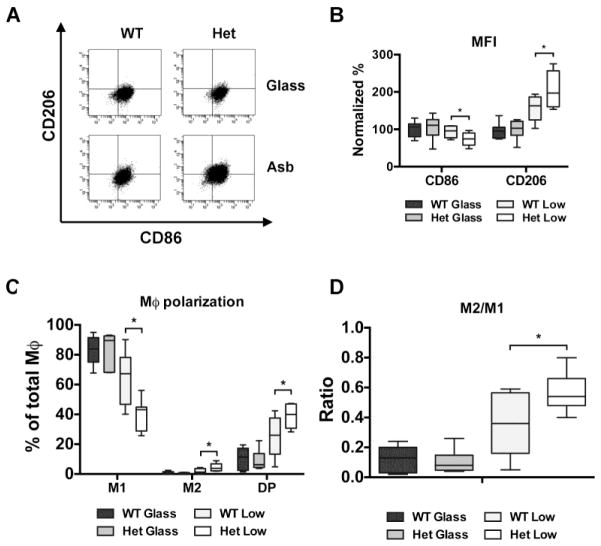
First, we exposed BAP1+/− mice and BAP1+/+ for five weeks to receive injections with glass beads or a low amount of crocidolite asbestos fibers (0.05 mg/week). After performing a peritoneal lavage, we counted the total number of peritoneal cells and determined via flow cytometry the percentage of total and subset-specific leukocytes. CD45+ leukocytes represented 95–99% of the total cells recovered in each group. In the glass control groups, macrophages and B cells represented the most abundant population, regardless of genotype (Table 1). Upon exposure to low-dose crocidolite fibers, the cellular inflammatory response was largely overlapping in mice with either genotype. We observed a significant increase in total number of leukocytes and in the relative percentage of neutrophils, and, at the same time, a significant decrease in the percentage of B cells and macrophages (Table 1). Further characterization of the cell types revealed that exposure to crocidolite fibers induced significant alterations in macrophages polarization in BAP1+/− mice (Fig. 1a). In the macrophages from BAP1+/− mice exposed to asbestos fibers, the normalized mean fluorescence intensity (MFI) for CD206 (marker of M2 macrophages) was significantly higher compared to controls (197.1% [160.6–256.8] vs 163.1% [125.4–186.7], P < 0.05), whereas the normalized MFI for CD86 (marker of M1 macrophages) was significantly lower compared to controls (74.6% [57.6–90.3] vs 95.8% [77.4–109.1], P < 0.05) (Fig. 1b). Accordingly, the percentage of M1 macrophages (CD206-CD86+ cells) was significantly lower in BAP1+/− mice (43.2% [28.9–44.9] vs 67.3% [46.7–78.2] of total macrophages, P < 0.05). On the other hand, the percentage of M2 macrophages (defined as CD206+ CD86-cells) was significantly higher in BAP1+/− mice compared to wild type littermates (3.8% [2.1–6.8] vs 1.2% [0.5–3.6%] of total macrophages, P < 0.05). Double positive (CD206+ CD86+) macrophages, which represent a transition state from M1 to M2, were also more represented in BAP1+/− mice compared to wild type littermates (40.0% [30.7–47.0] vs 26.0% [13.3–37.6] of total macrophages, P < 0.05) (Fig. 1c). Moreover, the M2/M1 ratio (overall percentage of CD206+ cells divided by overall percentage of CD86+ cells) was significantly higher in asbestos-exposed BAP1+/− mice compared to controls (0.54 [0.48–0.66] vs 0.36 [0.16–0.56], P < 0.05) (Fig. 1d).
Table 1. Major subpopulations of peritoneal leukocytes are not influenced by germline BAP1 heterozygosity.
BAP1+/− mice (n = 7 per group) and BAP1+/+ (n = 9 per group) were injected intraperitoneally every week for five weeks with 0.05 mg of inert glass beads or crocidolite asbestos fibers, for a total dose of 0.25 mg per mouse. Sample size was estimated hypothesizing a 60% difference in the levels of at least one cytokine. Full mineralogical characterization of crocidolite fibers used in these experiments was reported previously44. Next, mice were sacrificed by CO2 asphyxiation, and the abdominal cavity was washed with 5 ml of PBS. The peritoneal cells obtained were pelleted and supernatant was removed for later cytokine analysis. Cells were blindly characterized with the following antibodies: CD45 (leukocytes; anti-CD45-BV711, 563709, BD Biosciences), F4/80 (MΦ; anti-F4/80-AlexaFluor®488, MCA497A488T, AbD Serotec), Ly-6G (neutrophils; anti-Ly6G-BV421, 562737, BD Biosciences), CD3 (T cells; anti-CD3-APC, 17-0032-80, eBioscience), and B220 (B cells; anti-B220-PE, 561878, BD Biosciences). Comparisons between groups were calculated using Mann-Whitney U test for rank comparisons. Results are presented as median [interquartile range].
| Cells | WT Glass | WT Asb | Het Glass | Het Asb | P value | |||
|---|---|---|---|---|---|---|---|---|
| WT (G vs A) | Het (G vs A) | Glass (WT vs Het) | Asb (WT vs Het) | |||||
| Total leukocytes (× 106) | 2.7 [1.3–3.6] | 6.1 [3.5–14.2] | 2.7 [1.3–4.9] | 8.5 [4.9–12.7] | < 0.01 | < 0.05 | ns | ns |
| Neut (%) | 1.8 [1.6–2.4] | 13.0 [11.3–16.4] | 1.1 [0.8–2.2] | 10.4 [9.9–16.6] | < 0.0001 | < 0.001 | ns | ns |
| B cells (%) | 20.4 [17.5–26.3] | 12.7 [9.9–14.2] | 19.4 [17.8–21.3] | 10.3 [8.6–12.6] | < 0.01 | < 0.01 | ns | ns |
| T cells (%) | 7.0 [5.1–10.4] | 5.0 [3.8–6.4] | 6.4 [4.1–10.8] | 7.7 [4.3–8.4] | ns | ns | ns | ns |
| MF (%) | 33.4 [27.0–38.5] | 21.3 [18.6–27.5] | 24.2 [20.1–45.2] | 19.2 [14.6–22.8] | < 0.01 | < 0.05 | ns | ns |
Figure 1. MΦ polarization is altered in BAP1+/− mice exposed to low doses of asbestos fibers.
Macrophages and macrophage subtypes were identified using a separate tube of peritoneal cells stained for general MΦ markers CD11b (anti-CD11b-Bv711, 563168, BD Biosciences) and F4/80, CD206 (M2 marker; anti-CD206-APC, 141707, BioLegend), and CD86 (M1 marker; anti-CD86-PE, 561963, BD Biosciences). (a) Representative flow cytometry dot plot of peritoneal MΦ in BAP1+/− mice and wild type littermates after short-term treatment with glass beads or crocidolite asbestos. (b) Mean fluorescence intensities of CD86 and CD206. (c) Percentage of MΦ subpopulations: M1 (CD86+ CD206-), M2 (CD86-CD206+), Double positive (DP) (CD86+ CD206+). (d) M2/M1 ratio (overall percentage of CD206+ cells divided by overall percentage of CD86+ cells). Comparisons between heterozygous and wild-type groups were calculated using Mann-Whitney U test for rank comparisons. * (P < 0.05). The experiment was replicated two times.
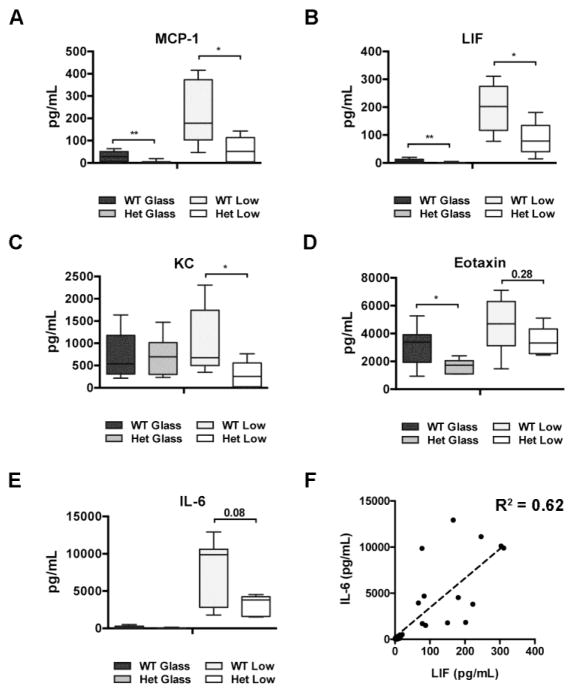
Next, we compared the profiles of cytokines and chemokines present in peritoneal lavages of these same mice. Compared to wild type littermates, the levels of monocyte chemoattractant protein-1 (MCP-1) were significantly lower in BAP1+/− mice exposed to glass (2.5 pg/mL [2.3–5.2] vs 33.6 pg/mL [6.5–51.7], P < 0.01) and in BAP1+/− mice exposed to asbestos (52.4 pg/mL [4.7–113.4] vs 178.5 pg/mL [102.9–373.2], P < 0.05) (Fig. 2a). Analogously, compared to wild type littermates, the levels of leukemia inhibitory factor (LIF) were significantly lower in the BAP1+/− mice exposed to glass (0.9 pg/mL [0.9–1.0] vs 6.9 pg/mL [1.1–13.5], P < 0.01), and in the BAP1+/− mice exposed to asbestos (78.2 pg/mL [41.0–134.4] vs 201.9 pg/mL [116.9–274.8], P < 0.05) (Fig. 2b). Moreover, lavages from BAP1+/− mice exposed to asbestos contained significantly lower amounts of keratinocyte-derived chemokine (KC) compared to wild type littermates (253.4 pg/mL [19.5–557.1] vs 675.3 pg/mL [469.8–1741.5], P < 0.05) (Fig. 2c). We also observed that eotaxin levels were significantly lower in BAP1+/− mice compared to wild type littermates in the glass exposed control group (1.73 ng/mL [1.11–2.06] vs 3.27 ng/mL [1.94–3.92], P < 0.05); the same trend, although non-significant, was retained following asbestos exposure (3.33 ng/mL [2.56–4.33] vs 4.70 ng/mL [3.13–6.30], P = 0.28) (Fig. 2d). Levels of IL-6 also differed between genotypes upon asbestos exposure, though this difference did not reach nominal significance (P = 0.08) (Fig. 2e). Both IL-6 and LIF belong to the IL-6 family of cytokines, and in our samples their levels significantly correlated (R2 = 0.62, P < 0.0001) (Fig. 2f). Finally, levels of G-CSF, IL-5, IP-10, and VEGF significantly increased after asbestos exposure, independently of the genotype (Suppl. Fig 2a-d). Levels of several other cytokines were below the lower limit of detection of our assay. Together, these results indicated that germline BAP1 heterozygosity significantly influenced the peritoneal inflammatory response upon asbestos exposure.
Figure 2. Several cytokines and chemokines are differentially expressed in lavage from BAP1+/− mice.
The supernatants recovered from the peritoneal lavages were concentrated 45–60 times using Amicon Ultra Centrifuge Filters with a 3,000 Dalton cutoff. Levels of 32 cytokines and chemokines were detected in concentrated lavages using human cytokine multiplex kits (EMD Millipore Corporation, Billerica, MA). Levels of MCP-1 (a), LIF (b), KC (c), eotaxin (d) and IL-6 (e) in lavages from BAP1 wild type and heterozygous mice after short-term exposure to glass beads or crocidolite fibers. Comparisons between heterozygous and wild type groups were calculated using Mann-Whitney U test for rank comparisons. * (P < 0.05), ** (P < 0.01) (f) Correlation of IL-6 and LIF levels (both belonging to the IL-6 family of cytokines) calculated using linear regression. The experiment was replicated two times.
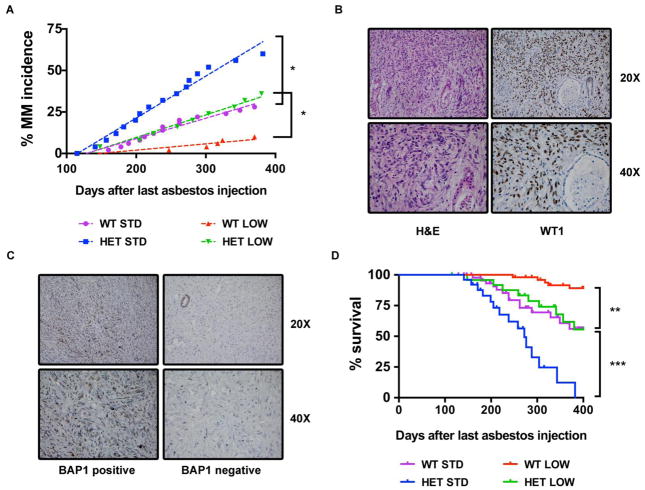
Therefore, we sought to experimentally study the relationship between asbestos dosage and MM carcinogenesis in the context of BAP1 heterozygosis. Based on previous publications on murine models20, 21, and on our own experience (Carbone, unpublished observations), doses of asbestos ranging from 3 to 5 mg induce MM in ~20–40% of exposed animals, while 0.5 mg of asbestos induce MM in 0–10% of exposed animals. BAP1+/+ mice and BAP1+/− mice received ten weekly injections of 0.5 mg of crocidolite asbestos fibers (total of 5 mg, referred to as “standard-dose” as it is the dose most commonly used to induced MM in rodents), 0.05 mg of crocidolite fibers (total of 0.5 mg, referred to as “low-dose”), or 0.5 mg of inert glass beads (total of 5 mg, negative control). During the 13 months of follow up after the last injection, we did not observe MM or any other spontaneous tumor in the glass control groups. In mice exposed to asbestos fibers, MM was the only malignancy observed. In the low-dose group, crocidolite fibers caused pathologically confirmed MM in 9/25 (36.0%) BAP1+/− mice compared to 5/50 (10.0%) BAP1+/+ mice (P = 0.010). Similarly, in the standard-dose group, MM was diagnosed in 15/25 (60.0%) BAP1+/− mice compared to 14/50 (28.0%) BAP1+/+ mice (P = 0.011) (Fig. 3a). Immunohistochemical staining of the tumors revealed expression of the mesothelial marker WT1 (Fig. 3b), supporting the histologic diagnosis of MM. In sporadic human MM, somatic BAP1 inactivation is one of the most frequent events, and it has been reported in about 40–60% of the cases13, 22–27. Consistent with these human data, BAP1 nuclear staining was absent in all MM analyzed arising from BAP1+/− mice and in 66.7% from BAP1+/+ mice (Fig. 3c). With regard to histology, all the MMs we observed in human germline BAP1 mutation carriers were epithelioid13. In sporadic human MMs, several groups have reported that mutations of BAP1 occur primarily in epithelioid MM24, 25, although this is not unequivocal28. All the MMs we observed in our BAP1+/− and BAP1+/+ mice displayed, totally or partially, sarcomatoid features. This is likely due to interspecies differences, since sarcomatoid features, contrary to what happens in human MMs, were also prevalent in MMs arising from other independent murine models of asbestos-induced MM29, 30. BAP1+/− mice had also a significantly shorter survival, i.e. life-span, compared to BAP1+/+ mice, both in the low-dose (P < 0.01) and the standard-dose group (P < 0.001) (Fig. 3d).
Figure 3. BAP1+/− mice develop more MMs and have shorter survival compared to wild type littermates.
Briefly, BAP1+/+ mice (n = 50 per group) and BAP1+/− mice (n = 25 per group) were injected intraperitoneally every week for ten weeks with 0.05 mg (low dose) or 0.5 mg (standard dose) of UICC crocidolite. 0.5 mg of glass beads were injected at the same schedule as control. Sample size was estimated to detect a difference in MM incidence between the low-exposed groups ≥ 25%. Mice were monitored daily for clinical evidence of abdominal swelling, and euthanized in presence of respiratory distress, gait instability, unresponsiveness to pain stimuli, or when tumor burden was obvious. Upon detection of illness, mice were sacrificed by CO2 asphyxiation, and all the major organs were evaluated histologically. (a) MM incidence in BAP1+/− mice and wild type littermates after long-term exposure to glass beads or asbestos fibers (standard and low dose) was compared using Fisher’s exact test. * (P < 0.05) (b) Formalin-fixed/paraffin-embedded samples were cut into 5 μm sections and stained with Hematoxylin and Eosin (H&E) according to standard procedure. The pathological diagnosis of mesothelioma was based on H&E staining and supported by WT1 nuclear staining in tumor cells. H&E and immunostainings were blindly interpreted by M.C and A.P., both US board specialized pathologists with expertise in human and animal mesotheliomas14, 45, 46 (c) Tumors were also stained with a rabbit polyclonal anti-BAP1 antibody to evaluate presence and localization of BAP1. (d) Survival curves of BAP1+/− mice and wild type littermates after long-term exposure to asbestos fibers (standard and low dose) were compared using log-rank (Mantel-Cox) test. . ** (P < 0.01), *** (P < 0.001). The experiment was performed one time.
Discussion
Taken together, our results showed that germline BAP1 heterozygosity is associated with a significantly altered peritoneal inflammatory response upon exposure to asbestos fibers and to an increased risk of MM following exposure to minimal amounts of asbestos that rarely cause MM in wild type animals. BAP1 is a nuclear deubiquitinating enzyme and an important epigenetic regulator via deubiquitination of histone H2A31. Originally discovered in 199832, it has several cell-intrinsic tumor suppressive functions, such as regulation of gene transcription33, cell cycle and replication34–36, and DNA damage response37, 38. BAP1 knockdown in MM cell lines has been paradoxically associated to a decreased proliferation, with an accumulation of cells in the S phase of the cell cycle22, suggesting that BAP1 loss might promote tumorigenesis inducing a delayed, but more permissive, G1/S checkpoint22. Heterozygous germline mutations of other important tumor suppressor genes, such as BRCA1, CDKN2A, and RB1, increase risk of cancer specifically to one or very few anatomical sites39. One of the few tumor suppressor genes whose germline heterozygosity, similar to BAP1, is associated to increased risk of cancer to several sites is TP53, which encodes p5339. Besides its well-known intrinsic functions, recently a novel non-cell-autonomous tumor suppressor effect of p53 has been described, via regulation of macrophage polarization and cytokine release40. Our results suggest that germline BAP1 heterozygosity, similarly to TP53, influences in vivo macrophage polarization and cytokine release. Indeed, BAP1+/− mice exposed to asbestos had significantly more M2-like pro-tumoral macrophages. Also, the chemokines MCP-1 and KC, and two cytokines of the IL-6 family (IL-6 itself and LIF) are soluble mediators significantly reduced in BAP1+/− mice exposed to asbestos. MCP-1 and IL-6 have been reported to increase following asbestos exposure and have been linked to asbestos pathogenesis41, 42. Our results support these findings and also suggest that this inflammatory response might be associated with increased immunosurveillance, since lower levels of these and other inflammatory mediators in BAP1+/− mice are associated with higher M2/M1 macrophage ratio and higher MM incidence following asbestos exposure. Interestingly, BAP1 has been recently showed to regulate the myeloid stem cell compartment via complex alterations of the transcriptional profile, possibly via its interaction with transcriptional co-regulators such as Host Cell Factor-1 (HCF-1) and Additional Sex Combs Like-1 (ASXL1)19.
Altogether, our results suggest a novel, complex model of asbestos-induced MM pathogenesis, in which the chronic inflammatory response can have preferentially anti-tumoral or pro-tumoral roles, depending on the cellular and soluble mediators involved. To explain the observed intra- and inter-familial variability of cancer types in germline BAP1 mutated carriers, we hypothesized that MM might be more prevalent in individuals/families exposed to low levels of asbestos15, levels that are not, or only marginally, carcinogenic for the population at large. Our results support our hypothesis, as we found that 36% of BAP1+/− mice exposed to low doses of asbestos developed MM, compared to 10% of wild type mice. Moreover, we found that MM is significantly more frequent in BAP1+/− mice exposed to standard doses of asbestos. This finding corroborates the recent results of Xu et al. that were obtained in an independent murine model29. Both studies found a shorter lifespan of asbestos exposed BAP1 heterozygous mice compared to wild type littermates, suggesting that BAP1+/− mice might develop MM at an earlier age compared to wild type littermates. Similarly, individuals carrying germline BAP1 mutations are diagnosed with MM at a much younger age compared to sporadic MM cases (mean age 55 years vs 72 years, respectively)16. Accordingly, although MMs in carriers of germline BAP1 mutations are less aggressive and are associated with survivals from diagnosis of 5–10 years16, compared to an average of 1 year in sporadic MM patients, the former die at an earlier age compared to the latter. Survival from diagnosis could not be evaluated in our model, as per IACUC requirements, mice were euthanized at the first clinical evidences of disease.
Mechanistically, Xu et al. suggest that the increased MM incidence in BAP1 heterozygous mice was partially related to BAP1-dependent transcriptional regulation of the tumor suppressor retinoblastoma protein29. Our findings expand what was previously reported by implicating novel tumor suppressor effects of BAP1 mediated via the microenvironment.
Moreover, we discovered that BAP1+/− mice exposed to low doses of asbestos developed MMs at a similar rate as BAP1+/+ mice exposed to 10 times higher doses. Therefore, although it is not possible to directly compare the low-dose exposure in mice to indoor and/or outdoor environmental exposure in humans, our findings support our hypothesis that germline BAP1 heterozygosity increases susceptibility to the carcinogenic effects of low doses of asbestos.
Based on our results, we suggest that prevention programs of MM in individuals carrying germline BAP1 mutations should focus on reducing exposure to even minimal sources of carcinogenic fibers, levels that are within the acceptable “safe” limits for the population at large (0.1 fibers/cc of air as an eight-hour time-weighted average, as per US Occupational Safety & Health Administration standards43). Finally, while our model focuses on asbestos as a trigger, this novel non-cell-autonomous tumor suppressive function of BAP1 may not be restricted to the peritoneal compartment or to the asbestos stimulation, and may contribute to the large numbers and diverse types of tumors that arises in carriers of the BAP1 cancer syndrome.
Supplementary Material
BAP1 protein levels in peritoneal macrophages and mesothelial cells from heterozygous and wild type mice. Antibodies used are: rabbit anti-BAP1 (D7W7O; Cell Signaling Technology) and mouse anti-β-actin (C4; Santa Cruz Biotechnology). The experiment was replicated three times.
Material and methods are the same as Fig. 2. (a), G-CSF (b), VEGF (c), IL-5 (d) IP-10 in lavages from BAP1 wild type and heterozygous mice after short-term exposure to glass beads or crocidolite fibers. Comparisons between heterozygous and wild type groups were calculated using Mann-Whitney U test for rank comparisons. No statistically significant differences were observed. The experiment was replicated two times.
Acknowledgments
This work was supported by National Institute of Health [grant numbers R01CA106567, P01CA114047, P30CA071789 to MC and R01CA160715-0A to HY]; the DoD CDMRP PRMRP Career Development Award to HY, and the V Foundation to MC and HY, the P30 CA071789 (UHCC Pathology Shared Resource); the Mesothelioma Applied Research Foundation to HY, the United-4 A Cure, the Hawai’i Community Foundation to HY, and the University of Hawai’i Foundation, which received donations to support mesothelioma research from Honeywell International Inc., to MC.
Abbreviations
- BAP1
BRCA1 associated protein-1
- G-CSF
granulocyte-colony stimulating factor
- IL-5
interleukin 5
- IL-6
interleukin 6
- IP-10
interferon gamma-induced protein 10, CXCL10
- LIF
leukemia inhibitory factor
- MCP-1
monocyte chemoattractant protein-1, CCL2
- MFI
mean fluorescence intensity
- MM
malignant mesothelioma
- MΦ
macrophages
- KC
keratinocyte-derived chemokine, CXCL1
- TAM
tumor-associated macrophages
- VEGF
vascular endothelial growth factor
Footnotes
Competing interests
M. Carbone has pending patent applications on BAP1 and provides consultation for mesothelioma expertise and diagnosis. The remaining authors declare no competing financial interests.
References
- 1.Carbone M, Ly BH, Dodson RF, Pagano I, Morris PT, Dogan UA, et al. Malignant mesothelioma: facts, myths, and hypotheses. Journal of cellular physiology. 2012;227:44–58. doi: 10.1002/jcp.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henley SJ, Larson TC, Wu M, Antao VC, Lewis M, Pinheiro GA, et al. Mesothelioma incidence in 50 states and the District of Columbia, United States, 2003–2008. International journal of occupational and environmental health. 2013;19:1–10. doi: 10.1179/2049396712Y.0000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Networks UC. Mesothelioma (C) European age standardised incidence rates, 2008–2010 [Google Scholar]
- 4.Carbone M, Yang H. Molecular pathways: targeting mechanisms of asbestos and erionite carcinogenesis in mesothelioma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:598–604. doi: 10.1158/1078-0432.CCR-11-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. The Journal of pathology. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 8.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pantano F, Berti P, Guida FM, Perrone G, Vincenzi B, Amato MM, et al. The role of macrophages polarization in predicting prognosis of radically resected gastric cancer patients. Journal of cellular and molecular medicine. 2013;17:1415–1421. doi: 10.1111/jcmm.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, et al. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. Journal of ovarian research. 2014;7:19. doi: 10.1186/1757-2215-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornelissen R, Lievense LA, Maat AP, Hendriks RW, Hoogsteden HC, Bogers AJ, et al. Ratio of intratumoral macrophage phenotypes is a prognostic factor in epithelioid malignant pleural mesothelioma. PloS one. 2014;9:e106742. doi: 10.1371/journal.pone.0106742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burt BM, Rodig SJ, Tilleman TR, Elbardissi AW, Bueno R, Sugarbaker DJ. Circulating and tumor-infiltrating myeloid cells predict survival in human pleural mesothelioma. Cancer. 2011;117:5234–5244. doi: 10.1002/cncr.26143. [DOI] [PubMed] [Google Scholar]
- 13.Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nature genetics. 2011;43:1022–1025. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbone M, Ferris LK, Baumann F, Napolitano A, Lum CA, Flores EG, et al. BAP1 cancer syndrome: malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. Journal of translational medicine. 2012;10:179. doi: 10.1186/1479-5876-10-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carbone M, Yang H, Pass HI, Krausz T, Testa JR, Gaudino G. BAP1 and cancer. Nature reviews Cancer. 2013;13:153–159. doi: 10.1038/nrc3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumann F, Flores E, Napolitano A, Kanodia S, Taioli E, Pass H, et al. Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis. 2015;36:76–81. doi: 10.1093/carcin/bgu227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sebastien P, Bignon J, Martin M. Indoor airborne asbestos pollution: from the ceiling and the floor. Science. 1982;216:1410–1412. doi: 10.1126/science.6283630. [DOI] [PubMed] [Google Scholar]
- 18.Baumann F, Buck BJ, Metcalf RV, McLaurin BT, Merkler D, Carbone M. The presence of asbestos in the natural environment is likely related to mesothelioma in young individuals and women from Southern Nevada. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2015 doi: 10.1097/JTO.0000000000000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dey A, Seshasayee D, Noubade R, French DM, Liu J, Chaurushiya MS, et al. Loss of the tumor suppressor BAP1 causes myeloid transformation. Science. 2012;337:1541–1546. doi: 10.1126/science.1221711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marsella JM, Liu BL, Vaslet CA, Kane AB. Susceptibility of p53-deficient mice to induction of mesothelioma by crocidolite asbestos fibers. Environmental health perspectives. 1997;105(Suppl 5):1069–1072. doi: 10.1289/ehp.97105s51069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis MR, Manning LS, Whitaker D, Garlepp MJ, Robinson BW. Establishment of a murine model of malignant mesothelioma. International journal of cancer Journal international du cancer. 1992;52:881–886. doi: 10.1002/ijc.2910520609. [DOI] [PubMed] [Google Scholar]
- 22.Bott M, Brevet M, Taylor BS, Shimizu S, Ito T, Wang L, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21. 1 losses in malignant pleural mesothelioma. Nature genetics. 2011;43:668–672. doi: 10.1038/ng.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasu M, Emi M, Pastorino S, Tanji M, Powers A, Luk H, et al. High Incidence of Somatic BAP1 Alterations in Sporadic Malignant Mesothelioma. Journal of Thoracic Oncology. 2015 doi: 10.1097/JTO.0000000000000471. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshikawa Y, Sato A, Tsujimura T, Emi M, Morinaga T, Fukuoka K, et al. Frequent inactivation of the BAP1 gene in epithelioid-type malignant mesothelioma. Cancer science. 2012;103:868–874. doi: 10.1111/j.1349-7006.2012.02223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Reynies A, Jaurand MC, Renier A, Couchy G, Hysi I, Elarouci N, et al. Molecular classification of malignant pleural mesothelioma: identification of a poor prognosis subgroup linked to the epithelial-to-mesenchymal transition. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:1323–1334. doi: 10.1158/1078-0432.CCR-13-2429. [DOI] [PubMed] [Google Scholar]
- 26.Lo Iacono M, Monica V, Righi L, Grosso F, Libener R, Vatrano S, et al. Targeted next-generation sequencing of cancer genes in advanced stage malignant pleural mesothelioma: a retrospective study. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2015;10:492–499. doi: 10.1097/JTO.0000000000000436. [DOI] [PubMed] [Google Scholar]
- 27.Guo G, Chmielecki J, Goparaju C, Heguy A, Dolgalev I, Carbone M, et al. Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer research. 2015;75:264–269. doi: 10.1158/0008-5472.CAN-14-1008. [DOI] [PubMed] [Google Scholar]
- 28.Zauderer MG, Bott M, McMillan R, Sima CS, Rusch V, Krug LM, et al. Clinical characteristics of patients with malignant pleural mesothelioma harboring somatic BAP1 mutations. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013;8:1430–1433. doi: 10.1097/JTO.0b013e31829e7ef9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J, Kadariya Y, Cheung M, Pei J, Talarchek J, Sementino E, et al. Germline mutation of Bap1 accelerates development of asbestos-induced malignant mesothelioma. Cancer research. 2014;74:4388–4397. doi: 10.1158/0008-5472.CAN-14-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altomare DA, Menges CW, Xu J, Pei J, Zhang L, Tadevosyan A, et al. Losses of both products of the Cdkn2a/Arf locus contribute to asbestos-induced mesothelioma development and cooperate to accelerate tumorigenesis. PloS one. 2011;6:e18828. doi: 10.1371/journal.pone.0018828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheuermann JC, de Ayala Alonso AG, Oktaba K, Ly-Hartig N, McGinty RK, Fraterman S, et al. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature. 2010;465:243–247. doi: 10.1038/nature08966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen DE, Proctor M, Marquis ST, Gardner HP, Ha SI, Chodosh LA, et al. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene. 1998;16:1097–1112. doi: 10.1038/sj.onc.1201861. [DOI] [PubMed] [Google Scholar]
- 33.Yu H, Mashtalir N, Daou S, Hammond-Martel I, Ross J, Sui G, et al. The ubiquitin carboxyl hydrolase BAP1 forms a ternary complex with YY1 and HCF-1 and is a critical regulator of gene expression. Molecular and cellular biology. 2010;30:5071–5085. doi: 10.1128/MCB.00396-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Misaghi S, Ottosen S, Izrael-Tomasevic A, Arnott D, Lamkanfi M, Lee J, et al. Association of C-terminal ubiquitin hydrolase BRCA1-associated protein 1 with cell cycle regulator host cell factor 1. Molecular and cellular biology. 2009;29:2181–2192. doi: 10.1128/MCB.01517-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HS, Lee SA, Hur SK, Seo JW, Kwon J. Stabilization and targeting of INO80 to replication forks by BAP1 during normal DNA synthesis. Nature communications. 2014;5:5128. doi: 10.1038/ncomms6128. [DOI] [PubMed] [Google Scholar]
- 36.Zarrizi R, Menard JA, Belting M, Massoumi R. Deubiquitination of gamma-Tubulin by BAP1 Prevents Chromosome Instability in Breast Cancer Cells. Cancer research. 2014;74:6499–6508. doi: 10.1158/0008-5472.CAN-14-0221. [DOI] [PubMed] [Google Scholar]
- 37.Yu H, Pak H, Hammond-Martel I, Ghram M, Rodrigue A, Daou S, et al. Tumor suppressor and deubiquitinase BAP1 promotes DNA double-strand break repair. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:285–290. doi: 10.1073/pnas.1309085110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ismail IH, Davidson R, Gagne JP, Xu ZZ, Poirier GG, Hendzel MJ. Germline mutations in BAP1 impair its function in DNA double-strand break repair. Cancer research. 2014;74:4282–4294. doi: 10.1158/0008-5472.CAN-13-3109. [DOI] [PubMed] [Google Scholar]
- 39.Cybulski C, Nazarali S, Narod SA. Multiple primary cancers as a guide to heritability. International journal of cancer Journal international du cancer. 2014;135:1756–1763. doi: 10.1002/ijc.28988. [DOI] [PubMed] [Google Scholar]
- 40.Lujambio A, Akkari L, Simon J, Grace D, Tschaharganeh DF, Bolden JE, et al. Non-cell-autonomous tumor suppression by p53. Cell. 2013;153:449–460. doi: 10.1016/j.cell.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka S, Choe N, Iwagaki A, Hemenway DR, Kagan E. Asbestos exposure induces MCP-1 secretion by pleural mesothelial cells. Experimental lung research. 2000;26:241–255. doi: 10.1080/019021400404528. [DOI] [PubMed] [Google Scholar]
- 42.Simeonova PP, Toriumi W, Kommineni C, Erkan M, Munson AE, Rom WN, et al. Molecular regulation of IL-6 activation by asbestos in lung epithelial cells: role of reactive oxygen species. J Immunol. 1997;159:3921–3928. [PubMed] [Google Scholar]
- 43.Occupational Safety & Health Administration. OSHA FactSheet: Asbestos. 2014 http://www.osha.gov/Publications/OSHA3507.pdf.
- 44.Qi F, Okimoto G, Jube S, Napolitano A, Pass HI, Laczko R, et al. Continuous exposure to chrysotile asbestos can cause transformation of human mesothelial cells via HMGB1 and TNF-alpha signaling. The American journal of pathology. 2013;183:1654–1666. doi: 10.1016/j.ajpath.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kroczynska B, Cutrone R, Bocchetta M, Yang H, Elmishad AG, Vacek P, et al. Crocidolite asbestos and SV40 are cocarcinogens in human mesothelial cells and in causing mesothelioma in hamsters. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14128–14133. doi: 10.1073/pnas.0604544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Comertpay S, Pastorino S, Tanji M, Mezzapelle R, Strianese O, Napolitano A, et al. Evaluation of clonal origin of malignant mesothelioma. Journal of translational medicine. 2014;12:301. doi: 10.1186/s12967-014-0301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BAP1 protein levels in peritoneal macrophages and mesothelial cells from heterozygous and wild type mice. Antibodies used are: rabbit anti-BAP1 (D7W7O; Cell Signaling Technology) and mouse anti-β-actin (C4; Santa Cruz Biotechnology). The experiment was replicated three times.
Material and methods are the same as Fig. 2. (a), G-CSF (b), VEGF (c), IL-5 (d) IP-10 in lavages from BAP1 wild type and heterozygous mice after short-term exposure to glass beads or crocidolite fibers. Comparisons between heterozygous and wild type groups were calculated using Mann-Whitney U test for rank comparisons. No statistically significant differences were observed. The experiment was replicated two times.