Abstract
Glycans are among the most intriguing carriers of biological information in living systems. The structures of glycans not only convey the cells' physiological state, but also regulate cellular communication and responses by engaging receptors on neighboring cells and in the extracellular matrix. The assembly of simple monosaccharide building blocks into linear or branched oligo- and polysaccharides gives rise to a large repertoire of diverse glycan structures. Despite their structural complexity, individual glycans rarely engage their protein partners with high affinity. Yet, glycans modulate biological processes with exquisite selectivity and specificity. To correctly evaluate glycan interactions and their biological consequences, one needs to look beyond individual glycan structures and consider the entirety of the cell-surface landscape. There, glycans are presented on protein scaffolds, or are linked directly to membrane lipids, forming a complex, hierarchically organized network with specialized functions, called the glycocalyx. Nanoscale glycomaterials, which can mimic the various components of the glycocalyx, have been instrumental in revealing how the presentation of glycans can influence their biological functions. In this review, we wish to highlight some recent developments in this area, while placing emphasis on the applications of glycomaterials providing new insights into the mechanisms through which glycans mediate cellular functions.
Keywords: glycocalyx, glycomaterials, nanomaterials, signal transduction
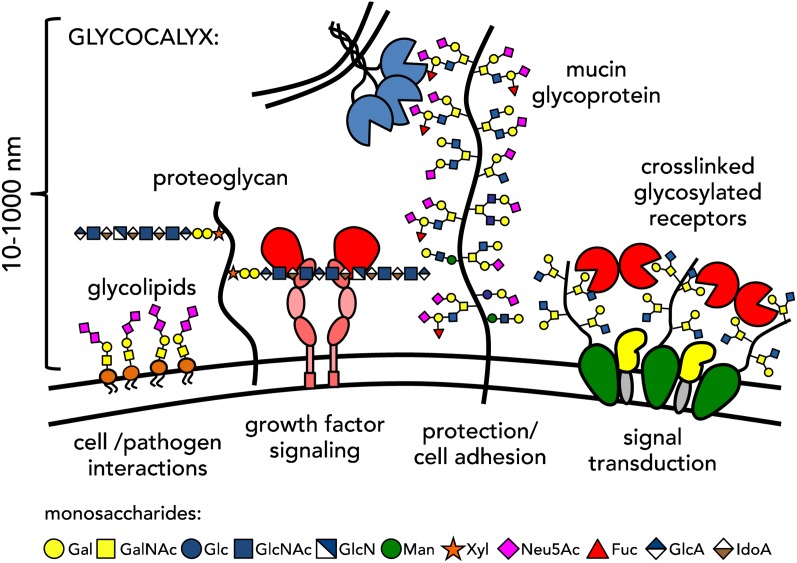
The glycocalyx is a major component of the cell-matrix interface, providing cells with a protective barrier, while also mediating cellular recognition and signaling (Varki and Lowe 2009). The glycocalyx is a collection of lipids and proteins decorated with a diversity of glycans (Figure 1) that serve as recognition elements for glycan-binding proteins (GBPs) involved in essential biological events, ranging from fertilization and embryogenesis, through tissue development and maintenance, to immunomodulation and disease development (Rademacher et al. 1988; Hart and Copeland 2010). While the structures of glycans define their molecular interactions with proteins, their presentation in multivalent ensembles in glycoconjugates is required to compensate for the relatively weak binding affinities of individual glycans toward their receptors. It is generally accepted that this characteristic of glycans, referred to as the glycoside cluster effect (Lundquist and Toone 2002) provides a mechanism for enhancing the selectivity of glycan interactions and setting thresholds for triggering signaling responses. Similarly, the diffusion of glycolipids in cellular membranes permits their organization into two-dimensional multivalent arrays exhibiting similar avidity enhancements toward GBPs (Evans and Mackenzie 1999).
Fig. 1.
The cell surface is decorated with a complex mesh of glycoconjugates, which engage their protein receptors over distances spanning tens to hundreds of nanometers and mediate cellular interactions and signaling. This figure is available in black and white in print and in color at Glycobiology online.
The multivalent presentation of glycans is mirrored in GBPs, such as lectins or antibodies, which often form oligomeric complexes with several glycan-binding sites (Sinha and Surolia 2005). The consequence of this arrangement is the ability of GBPs to engage glycans in a number of different modes, which can result in distinct biological outcomes. For instance, a lectin can bind multiple glycans on the same glycoprotein, forming a 1:1 complex or, alternatively, it could bridge glycans on two neighboring membrane-bound glycoproteins, cross-linking them together (Figure 1). While the former scenario is well suited for facilitating cell adhesion events, the latter can lead to oligomerization of membrane proteins—a process associated with the activation of multi-receptor signaling complexes. The controlling element in the formation of distinct higher-order binding interactions is not necessarily the structure of individual glycans, but rather their organization within the glycocalyx (Mager et al. 2011).
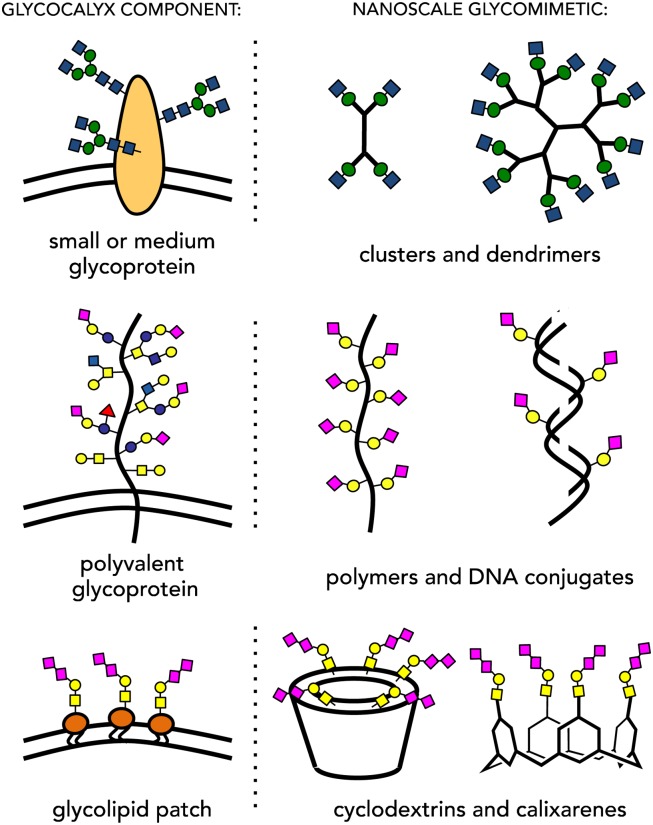
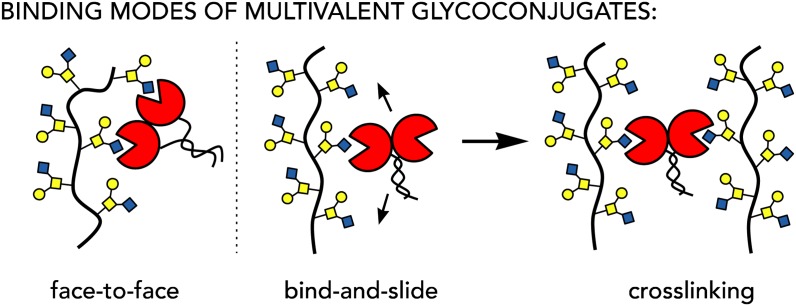
The dimensions of the glycocalyx posit that information coding by glycans is inherently a nanoscale phenomenon that requires experimental tools that operate at these length scales. In the absence of molecular biology techniques to precisely manipulate glycan presentation within the glycocalyx, chemical approaches utilizing nanoscale glycomaterials have helped fill the void. Inspired by the architectures of native glycoconjugates, nanoscale materials that recapitulate some of the key structural features of distinct classes of glycoproteins, have emerged (Figure 2). For instance, glycoclusters, glycodendrimers (Kitov and Bundle 2003), and neo-glycoproteins (Oyelaran et al. 2009) that emulate the architectures of relatively small globular glycoproteins, as well as glycopolymers (Mammen et al. 1998) that better approximate larger extended polyvalent glycoproteins, provided key early mechanistic insights into the effects of glycan valency towards binding to oligomeric lectins. In their seminal work, Whitesides and co-workers formulated models that defined the thermodynamic and kinetic parameters of multivalent interactions in glycoconjugates (Mammen et al. 1998). These models were later refined based on the results of thermodynamic analysis of binding between lectins and polyvalent mucin glycoproteins (Dam et al. 2007). There are two currently accepted models (Dam and Brewer 2008) describing lectin-glycan engagement (Figure 3): (i) the “face-to-face” model, where at least two glycans on a protein scaffold simultaneously engage multiple binding sites on a lectin, and (ii) the “bind-and-slide” model, where the lectin engages only one glycan at a time. Dissociation and rebinding to adjacent glycans on the glycoprotein increases the persistence time of the complex and, thus, its observed avidity. The second model, which can leave additional binding sites on the lectin open, also provides a mechanism for glycoprotein cross-linking.
Fig. 2.
Synthetic nanoscale glycomaterials can mimic the various components of the glycocalyx. This figure is available in black and white in print and in color at Glycobiology online.
Fig. 3.
Multivalent binding is required for glycoconjugates to engage their protein partners with high avidity. The “face-to-face” and “bind-and-slide” models have been proposed, with the latter providing a mechanism for crosslinking of membrane-bound glycoproteins. This figure is available in black and white in print and in color at Glycobiology online.
A seminal study, in which Kiessling and co-workers (Gestwicki et al. 2002) designed a set of experiments to distinguish between different higher-order binding interactions, revealed the effects of glycan valency and the underlying scaffolds on the ability of distinct soluble glycoconjugates to inhibit, cross-link, or cluster lectins. Interestingly, this study indicated that polydisperse glycopolymers and large globular glycoproteins are excellent inhibitors of lectin binding, but are less effective in facilitating protein clustering. On the other hand, glycopolymers with narrow molecular weight distributions prepared using controlled polymerization techniques, exhibited superior ability to cluster proteins. A recent study by Dam and co-workers, further isolated the effects of the underlying scaffold structure in glycoconjugates on their ability to promote the formation of higher-order complexes with lectins (Talaga et al. 2014). The authors observed that even small perturbations in the scaffold architecture of conjugates with otherwise similar glycan structures, valencies and affinities, could significantly influence the kinetics of lectin clustering. These and other studies collectively suggest that nanoscale materials with properly designed architectures may be used to intersect and analyze the mechanisms through which the various components of the glycocalyx modulate biological responses.
Soluble glyco-nanomaterials for probing signal transduction
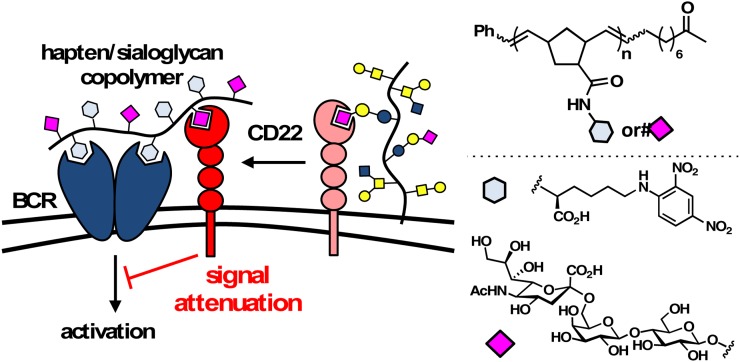
Due to their ability to promote protein clustering, precisely tailored glycopolymers have been instrumental in revealing the role of receptor organization in cellular signal transduction (Kiessling and Grim 2013; Miura et al. 2016). Early work by the Varki group using polyacrylamide glycopolymers carrying α(2,6)-sialoglycans showed that the lectin-binding activity of CD22, a receptor belonging to the Siglec (Sialic-acid binding immunoglobulin-type lectins) family, was masked in the presence of endogenous cell-surface sialoglycans (Razi and Varki 1998). Subsequent work by Kiessling and her co-workers employed similar glycopolymers to further elucidate the role of CD22 in modulating B-cell responses (Figure 4) (Courtney et al. 2009). Activation of the B-cell antigen receptors (BCRs) initiates signaling cascades that regulate B lymphocyte proliferation and differentiation. In conjunction with BCRs, CD22 co-receptors provide a mechanism to attenuate activation in response to the presentation of self-antigens. In the normal state, CD22 is engaged in binding to sialoglycans presented on the surface of B cells. This cis interaction sequesters CD22 away from the BCR complex, thus, allowing for B-cell activation. The Kiessling team showed that polymer ligands derived via the ring-opening metathesis polymerization (ROMP) technique and carrying a 2,4-dinitrophenyl (DNP) hapten, engaged BCRs and triggered activation of B cells; however, polymers presenting α(2,6)-sialoglycans in addition to DNP led to the release of CD22s from their cis-interactions and their recruitment to the BCR complex, where they attenuated B-cell signaling (Figure 4). This study pointed to the role of trans-interactions in modulating CD22 signaling and B-cell activation. This mechanism was supported by an in vivo study by Paulson and co-workers who showed that treatment of mice with polyacrylamide polymers displaying DNP and sialoglycan ligands for CD22 resulted in long-lived B-cell tolerance (Duong et al. 2010). The same group later developed liposomal nanoparticles bearing modified sialoside ligands with high affinity to CD22 to induce B-cell apoptosis in vivo (Macauley et al. 2013).
Fig. 4.
Synthetic multivalent glycopolymers were designed to elucidate the role of receptor clustering in the modulation of B-cell responses. The displayed dinitrophenyl hapten induced BCR complex formation to activate signaling, whereas the sialoglycan ligands recruited the CD22 co-receptor to suppress B-cell responses (adapted from Courtney et al. 2009). This figure is available in black and white in print and in color at Glycobiology online.
Reliance on glycan–lectin interactions appears to be widespread in controlling immunological responses, and glycomaterials have been widely applied as probes to elucidate the mechanisms of these processes (Restuccia, Fettis, et al. 2015; Restuccia, Tian, et al. 2015). One important example is the C-type lectin receptor on dendritic cells, DC-SIGN, which has garnered considerable attention for many of its functions, including antigen uptake, processing and presentation to CD4+ T cells (Unger and van Kooyk 2011). DC-SIGN is often targeted by various pathogens. For instance, the HIV-1 virus engages DC-SIGN through its highly mannosylatated envelope glycoprotein, gp120, to facilitate T-cell infection. Several groups have employed glycan nanomaterials to investigate the function of DC-SIGN. Recent examples include synthetic mannosylated glycopolymers prepared by Haddleton and colleagues that effectively inhibited HIV binding to DC-SIGN (Becer et al. 2010), or Van Kooyk's polyamidoamine (PAMAM) dendrimers carrying antigens terminated with Lewis-type glycan structures, which facilitated antigen internalization and presentation by dendritic cells and activated antigen-specific CD4+ and DC8+ T-cell proliferation (Garcia-Vallejo et al. 2013).
Glycan-mediated organization of signaling complexes provides a general mechanism for controlling biological functions from fertilization, through development, and aging. For instance, glycoconjugate co-receptors are required for the activation of growth factor-associated signaling during embryogenesis and organ development (Bishop et al. 2007). The long, highly sulfated polysaccharide appendages of cell-surface proteoglycans, called glycosaminoglycans (GAGs), assist in organizing growth factors with their receptors (Figure 1) (Venkataraman et al. 1999). The three-dimensional patterns of sulfate groups in GAGs are believed to encode protein specificity and span nanometer distances necessary to bridge and stabilize complexes between GFs and their receptor partners. Due to their size and complexity, GAGs are unwieldy synthetic targets. Early work by Seeberger and colleagues (de Paz et al. 2007) demonstrated that PAMAM dendrimers decorated with 32 well-defined short, and thus synthetically much more tractable, hexasaccharide fragments of the GAG, heparan sulfate (HS), were capable of promoting the association of the fibroblast growth factor 2 (FGF2) with its receptors, to activate a downstream MAPK-signaling pathway. The monovalent HS hexasaccharides alone showed only limited ability for activating MAPK signaling compared with the glycodendrimers, illustrating the importance of nanoscale presentation of these HS ligands. In a series of elegant studies, the Hsieh-Wilson group showed that extended linear polymers are superior scaffolds for the construction of GAG mimetics and that even disaccharides, the minimal structural motif of GAGs, when presented in a multivalent form on polymers synthesized by the ROMP technique, could reconstitute the biological activity of their parent GAGs (Rawat et al. 2008; Sheng et al. 2013). This minimalistic approach greatly facilitated access to chemical probes that provided key insights into the functions of GAGs, and their unique sulfation patterns, in axon growth and neural regeneration after injury.
Precision control of glycan display in nanoscale materials
In addition to valency, the spacing of individual glycans in multivalent glycoconjugates is also a key parameter in defining interactions with GBPs. While techniques, such as ROMP, ATRP or RAFT, offer ready access to polymers with narrow chain length distributions and defined glycan valency (Miura et al. 2016), they provide limited control over the positioning of each glycan with respect to its neighbors. To circumvent this problem, Kiick and co-workers designed recombinant glycopeptides with variously spaced glutamic acid side chains amenable to chemical glycosylation with galactose residues (Polizzotti and Kiick 2006). Evaluation of these glycopeptides as inhibitors of the pentameric cholera toxin (CT), revealed, surprisingly, that the highest activity was obtained for the lowest valency glycoconjugates, in which the greater distances between galactosyl residues, presumably, best matched the organization of the five glycan-binding sites in CT.
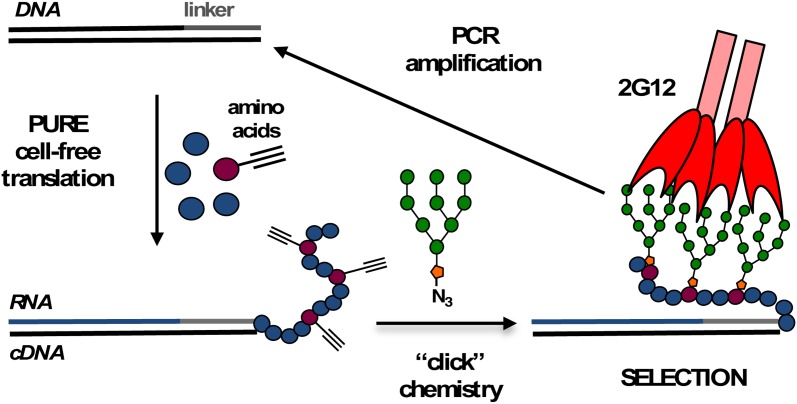
New methods are emerging that control glycan spacing in synthetic nanoscale constructs. For instance, Winssinger et al. leveraged the structural predictability of DNA assemblies to generate peptide nucleic acid-DNA hybrids displaying uniquely spaced mannose residues (Gorska et al. 2009). These materials enabled the determination of the spatial requirements for the interactions of the broadly neutralizing antibody 2G12 with the mannosylated HIV gp120. Most recently, the Krauss lab has reported direct evolution approaches for generating DNA- or peptide-supported oligomannose clusters with high avidity toward 2G12 (Figure 5) (Horiya et al. 2014; Temme et al. 2014). Interestingly, the temperature at which glycocluster selection was performed had a dramatic effect on the composition of the resulting clusters. At ambient temperature, the selection process favored higher valency (∼10) clusters with relatively low binding activity, while at 37°C, high-avidity clusters emerged carrying only 3–4 oligomannose residues, matching the number of binding sites on 2G12. The authors speculated that the elevated selection temperatures may have led to increased entropic penalties associated with statistical rebinding of high-valency clusters, thus favoring more rigid binders with properly spaced glycans. An alternative approach for generating glycomaterials that match the binding requirements of specific GBPs relies on the dynamic assembly and reorganization of glycomaterials in the presence of the target GBP (Ruff and Lehn 2008). This approach yielded new pseudopolyrotaxane (Nelson et al. 2004) or self-assembled glycopeptide nanofiber materials (Restuccia, Fettis, et al. 2015; Restuccia, Tian, et al. 2015) with enhanced abilities to sequester galectin-1 proteins and modulate T-cell agglutination and apoptosis.
Fig. 5.
Directed evolution generated multivalent ligands for the HIV antibody, 2G12. A selection among ∼1013 neo-glycopeptides, prepared through cell-free ribosomal translation and chemical glycosylation, yielded high-avidity glycopeptides with 3–4 oligomannose residues, matching the number of binding sites in the dimeric 2G12 (adapted from Horiya et al. 2014). This figure is available in black and white in print and in color at Glycobiology online.
Unlike glycoproteins, in which glycan organization is largely defined by the distribution of glycosylation sites and conformation of the central polypeptide backbone, glycolipids are monovalent glycoconjugates that are free to laterally diffuse within the plasma membrane. The dynamic assembly of glycolipids into membrane patches generates multivalent glycan patterns that can be recognized by GBPs. Elucidating how the two-dimensional (2D) nanoscale organization of glycans translates into recognition by cognate receptors remains a considerable challenge. Novel glycomaterials have been developed to pinpoint the parameters that define these interactions. Bovin and co-workers were among the first to describe glycopeptide materials which could self-assemble into nanoscale multivalent glycan displays in the presence of viruses (Bovin et al. 2004). In subsequent studies, cyclodextrins (Vico et al. 2011; Martinez et al. 2013), calixarenes (Marra et al. 2008; Sansone and Casnati 2013), macrocyclic peptide scaffolds (Amin et al. 2013; Galan et al. 2013) decorated with glycans, or glycosylated nanoparticles (Marradi et al. 2013) and lipid vesicles (Jayaraman et al. 2013) have been employed to elucidate the effects of glycan positioning in the binding of GBPs, including pathogen-associated lectins. Recently, Schaffer and co-workers generated large-scale multivalent glycan assemblies using the crystalline-cell-surface (S)-layer proteins of prokaryotic organisms (Steiner et al. 2008). The well-controlled assembly of these proteins into a 2D lattice, in conjunction with engineering of glycosylation sites, provided nanometer-scale arrays, where glycan positioning and orientation could be controlled with high precision. Lee and co-workers reported the dynamic assembly of multivalent nanofibers from simple glycosylated building blocks. The self-assembly process could be controlled by the structures of the building blocks to generate nanofibers with defined lengths (Lee et al. 2012) or to dynamically change the material's morphology from linear fibers to spherical micelles via the addition of a hydrophobic small molecule modulator (Ryu et al. 2007). Fibers decorated with mannose effectively induced the aggregation of a mutant Escherichia coli ORN 178 by engaging the pili adhesion protein, FimH. The authors showed that increased length of the nanofibers led to more extensive aggregation, which coincided with a decrease in bacterial proliferation. Interestingly, the authors observed an attenuation of E. coli mobility in the presence of longer nanofibers, presumably due to their ability to interfere with flagellar motion through cross-linking of the bacterial pili. Such materials are poised to advance our understanding of how pathogens recognize and engage cell-surface glycans and help formulate new strategies for combating pathogenic infections.
Glycocalyx engineering with nanoscale glycomaterials
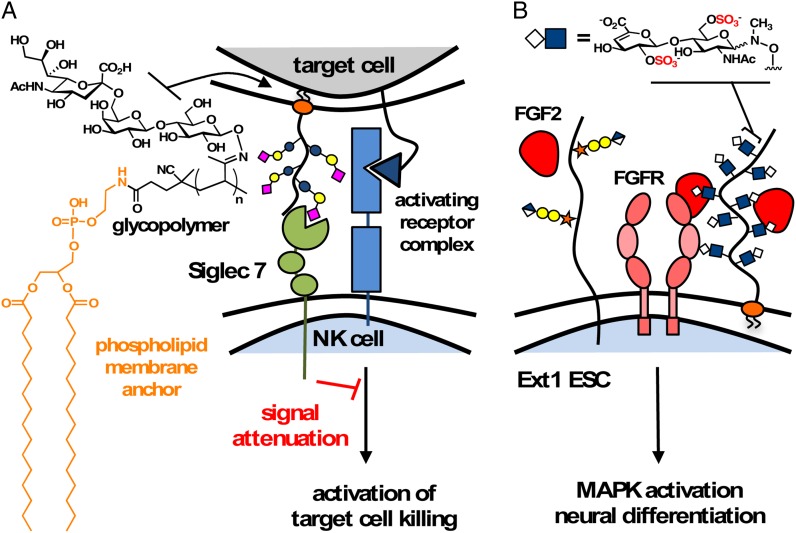
It is becoming increasingly evident that new methods are needed to evaluate glycan interactions in the context of the entire cellular glycocalyx. Bertozzi and co-workers reported a simple approach for glycocalyx remodeling by passive insertion of lipid-functionalized mimetics of mucins, a family of large, heavily glycosylated glycoproteins populating epithelial cells (Figure 6) (Rabuka et al. 2008; Mauris et al. 2013). In one study, the authors introduced high-valency sialoglycans to the surfaces of Jurkat cells to mimic the hypersialylation state of many adenocarcinoma cells. The higher levels of sialic acids on the remodeled cells decreased their susceptibility to the cytotoxic effects of natural killer (NK) cells (Figure 6A) (Hudak et al. 2013). Mechanistic studies identified that the glycopolymers targeted a protein receptor on NK cells, Siglec 7, which when engaged in a trans interaction, attenuated the NK-cell response. The ability to access mucin mimetics with well-defined glycosylation patterns and dimensions enabled the investigation of the nanoscale properties of the glycocalyx, as well as the biophysical pertubations that bulky glycoproteins, such as mucins, may exert on cell-matrix interactions. A recent study by Paszek, Weaver, Bertozzi and their coworkers uncovered a new functional role for mucins in cell-matrix interactions (Paszek et al. 2014). These groups showed that the mechanical forces exerted by the mucin glycocalyx drives integrin clustering into focal adhesion complexes required for cell proliferation and survival. Mucin mimetic glycopolymers of increasing lengths were used to remodel mammary epithelial cells, and evaluated for their ability to induce integrin clustering. While short polymers (∼3 nm) showed no discernible effects, longer polymers (∼80 nm) induced clustering and allowed for cells to adhere and proliferate on soft hydrogel matrices. Mucin production is upregulated in many types of cancers and this study provided a possible functional role for these glycoproteins in enabling tumor metastasis and survival in soft tissues. While passive insertion of lipid-terminated polymers offers a facile method for the remodeling of the glycan component on the outer leaflet of the plasma membrane, most mucins also contain cytosolic domains involved in intracellular signaling. The Bertozzi group has now developed bio-orthogonal chemical strategies to generate chimeras of membrane proteins carrying well-defined mucin mimetic domains, thus increasing the precision with which glycocalyx engineering can be accomplished (Kramer et al. 2015).
Fig. 6.
Nanoscale glycomaterials armed with lipid anchors can be introduced directly into the cellular glycocalyx via insertion into the extracellular leaflet of the plasma membrane. (A) Surface engineering with polymers carrying sialoglycans to mimic the hypersialylation state of cancer cells revealed the role of sialylation in evading surveillance by NK cells. Trans-interactions between cancer cell sialoglycans and the NK cell-surface receptor, Siglec 7, attenuated NK cell cytotoxicity. (B) Glycopolymers with affinity for the FGF2 restored FGF-mediated signaling in Ext1−/− ESCs lacking functional proteoglycan co-receptors and enabled neural differentiation. This figure is available in black and white in print and in color at Glycobiology online.
Our own lab has utilized glycocalyx engineering to control cell-surface glycan interactions required for neural differentiation in embryonic stem cells (ESCs) (Figure 6B) (Huang et al. 2014). By introducing lipidated polymer mimetics of HS GAGs with affinity for the growth factor FGF2 to the surfaces of mutant Ext1−/− ECSs deficient in exostosin 1, a key glycosyltransferase enzyme responsible for the assembly of HS GAGs, we were able to reactivate FGF2-mediated MAPK signaling and induce neural differentiation. GAG polysaccharides derived from natural sources can be applied in a similar fashion as demonstrated by Hsieh-Wilson and her co-workers, who reported a liposomal delivery of lipidated chondroitin sulfate GAGs to the surfaces of cortical neurons, where they stimulated neutrophin signaling and promoted neural outgrowths (Pulsipher et al. 2014). This lab has also demonstrated that pre-functionalized HS GAGs can be covalently grafted to ESCs presenting recombinant HaloTag proteins to increase cell-membrane residence time of the exogenous GAGs and accelerate the ESC differentiation into the neuroectoderm (Pulsipher et al. 2015). These early studies demonstrated the potential of this cell-surface glycan engineering approach in assessing the impact of individual glycocalyx components on cellular interactions and functions.
The limited scope of this review certainly fails to capture the breadth and creativity of the field of nanoscale glycomaterials. For instance, outside the focus of this article are elegant studies utilizing glyco-nanoparticles as bioimaging agents or as vaccine delivery vehicles (Marradi et al. 2013). However, we hope that we have not failed in conveying the significant impact that nanoscale glycomaterials have already made in illuminating the many biological roles of the glycocalyx. Despite significant progress, outstanding challenges in this field still remain. At the molecular level, the effects of glycan valency and presentation on receptor binding and signal transduction are beginning to emerge; however, the next frontier is to translate this information within the complexity of in vivo systems. We anticipate that future advances in the precision synthesis of nanomaterials, driven by technical improvements in protein engineering and chemoenzymatic or chemical synthesis of carbohydrates, will continue to expand the repertoire of experimental tools to allow systems level evaluation of the role of glycan presentation on cellular signaling. In particular, capturing the hierarchical organization of the glycocalyx in analytical platforms will enable cataloguing of the collective contributions of heterogeneous glycan microenvironments. As well, engineering strategies to edit the glycocalyx composition with nanometer precision in all three dimensions will be needed to elucidate the biological effects of both the architecture of glycoconjugate scaffolds, as well as the dynamics of their organization within membranes. Coordinated research efforts combining advances in glycomaterials design and bioconjugation chemistries together with the power of genetic and molecular biology tools are likely to identify new mechanisms of glycan function, providing a more complete picture of the Glycome.
Funding
This work was supported by startup funds from the University of California-San Diego and the National Institutes of Health Pathway to Independence Award (National Institute of Biomedical Imaging and Bioengineering 5 R00 EB013446-05).
Conflict of interest statement
None declared.
Abbreviations
BCRs, B-cell antigen receptors; CT, cholera toxin; DNP, 2,4-dinitrophenyl; ESCs, embryonic stem cells; FGF2, fibroblast growth factor 2; GAGs, glycosaminoglycans; GBPs, glycan-binding proteins; HS, heparan sulfate; NK, natural killer; PAMAM, polyamidoamine; ROMP, ring-opening metathesis polymerization.
References
- Amin MN, McLellan JS, Huang W, Orwenyo J, Burton DR, Koff WC, Kwong PD, Wang L-X. 2013. Synthetic glycopeptides reveal the glycan specificity of HIV-neutralizing antibodies. Nat Chem Biol. 9(8):521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becer CR, Gibson MI, Geng J, Ilyas R, Wallis R, Mitchell DA, Haddleton DM. 2010. High-affinity glycopolymer binding to human DC-SIGN and disruption of DC-SIGN interactions with HIV envelope glycoprotein. J Am Chem Soc. 132(43):15130–15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop JR, Schuksz M, Esko JD. 2007. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 446(7139):1030–1037. [DOI] [PubMed] [Google Scholar]
- Bovin N, Tuzikov AB, Chinarev AA, Gambaryan AS. 2004. Multimeric glycotherapeutics: New paradigm. Glycoconj J. 21(8):471–478. [DOI] [PubMed] [Google Scholar]
- Courtney AH, Puffer EB, Pontrello JK, Yang ZQ, Kiessling LL. 2009. Sialylated multivalent antigens engage CD22 in trans and inhibit B cell activation. Proc Natl Acad Sci USA. 106(8):2500–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam TK, Brewer CF. 2008. Effects of clustered epitopes in multivalent ligand-receptor interactions. Biochemistry. 47(33):8470–8476. [DOI] [PubMed] [Google Scholar]
- Dam TK, Gerken TA, Cavada BS, Nascimento KS, Moura TR, Brewer CF. 2007. Binding studies of alpha-GalNAc-specific lectins to the alpha-GalNAc (Tn-antigen) form of porcine submaxillary mucin and its smaller fragments. J Biol Chem. 282(38):28256–28263. [DOI] [PubMed] [Google Scholar]
- de Paz JL, Noti C, Bohm F, Werner S, Seeberger PH. 2007. Potentiation of fibroblast growth factor activity by synthetic heparin oligosaccharide glycodendrimers. Chem Biol. 14(8):879–887. [DOI] [PubMed] [Google Scholar]
- Duong BH, Tian H, Ota T, Completo G, Han S, Vela JL, Ota M, Kubitz M, Bovin N, Paulson JC et al. 2010. Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo. J Exp Med. 207(1):173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SV, Mackenzie CR. 1999. Characterization of protein-glycolipid recognition at the membrane bilayer. J Mol Recognit. 12(3):155–168. [DOI] [PubMed] [Google Scholar]
- Galan MC, Dumy P, Renaudet O. 2013. Multivalent glyco(cyclo)peptides. Chem Soc Rev. 42(11):4599–4612. [DOI] [PubMed] [Google Scholar]
- Garcia-Vallejo JJ, Ambrosini M, Overbeek A, van Riel WE, Bloem K, Unger WWJ, Chiodo F, Bolscher JG, Nazmi K, Kalay H et al. 2013. Multivalent glycopeptide dendrimers for the targeted delivery of antigens to dendritic cells. Mol Immunol. 53(4):387–397. [DOI] [PubMed] [Google Scholar]
- Gestwicki JE, Cairo CW, Strong LE, Oetien KA, Kiessling LL. 2002. Influencing receptor-ligand binding mechanisms with multivalent ligand architecture. J Am Chem Soc. 124(50):14922–14933. [DOI] [PubMed] [Google Scholar]
- Gorska K, Huang K-T, Chaloin C, Winssinger N. 2009. DNA-templated homo- and heterodimerization of peptide nucleic acid encoded oligosaccharides that mimick the carbohydrate epitope of HIV. Angew Chem Int Ed. 48(41):7695–7700. [DOI] [PubMed] [Google Scholar]
- Hart GW, Copeland RJ. 2010. Glycomics hits the big time. Cell. 143(5):672–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiya S, Bailey JK, Temme SJ, Guillen-Schlippe YV, Krauss IJ. 2014. Directed evolution of multivalent glycolpeptides tightly recognized by HIV antibody 2G12. J Am Chem Soc. 136(14):5407–5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ML, Smith RAA, Trieger GW, Godula K. 2014. Glycocalyx remodeling with proteoglycan mimetics promotes neural specification in embryonic stem cells. J Am Chem Soc. 136(30):10565–10568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudak JE, Canham SM, Bertozzi CR. 2013. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoinvasion. Nat Chem Biol. 10(1):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman N, Maiti K, Naresh K. 2013. Multivalent glycoliposomes and micelles to study carbohydrate-protein and carbohydrate-carbohydrate interactions. Chem Soc Rev. 42(11):4640–4656. [DOI] [PubMed] [Google Scholar]
- Kiessling LL, Grim JC. 2013. Glycopolymer probes of signal transduction. Chem Soc Rev. 42(10):4476–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitov PI, Bundle DR. 2003. On the nature of the multivalency effect: A thermodynamic model. J Am Chem Soc. 125(52):16271–16284. [DOI] [PubMed] [Google Scholar]
- Kramer JR, Onoa B, Bustamante C, Bertozzi CR. 2015. Chemically tunable mucin chimeras assembled on living cells. Proc Natl Acad Sci USA. 112(41):12574–12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D-W, Kim T, Park I-S, Huang Z, Lee M. 2012. Multivalent nanofibers of a controlled length: Regulation of bacterial cell agglutination. J Am Chem Soc. 134:14722–14725. [DOI] [PubMed] [Google Scholar]
- Lundquist JJ, Toone EJ. 2002. The cluster glycoside effect. Chem Rev. 102(2):555–578. [DOI] [PubMed] [Google Scholar]
- Mager MD, LaPointe V, Stevens MM. 2011. Exploring and exploiting chemistry at the cell surface. Nat Chem. 3(8):582–589. [DOI] [PubMed] [Google Scholar]
- Macauley MS, Pfrengle F, Rademacher C, Nycholat CM, Gale AJ, von Drygalski A, Paulson JC. 2013. Antigenic liposomes displaying CD22 ligands induce antigen-specific B cell apoptosis. J Clin Invest. 123:3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammen M, Choi S-K, Whitesides GM. 1998. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed. 37(20):2754–2794. [DOI] [PubMed] [Google Scholar]
- Marra A, Moni L, Pazzi D, Corallini A, Bridi D, Dondoni A. 2008. Synthesis of sialoclusters appended to calix[4]arene platforms via multiple azide-alkyne cycloaddition. New inhibitors of hemaglutinnation and cytopathic effect mediated by BK and influenza A viruses. Org Biomol Chem. 6(8):1396–1409. [DOI] [PubMed] [Google Scholar]
- Marradi M, Chiodo F, García I, Penadés S. 2013. Glyconanoparticles as multifunctional and multimodal carbohydrate systems. Chem Soc Rev. 42(11):4728–4745. [DOI] [PubMed] [Google Scholar]
- Martinez A, Mellet CO, Fernandez JMG. 2013. Cyclodextrin-based multivalent glycodisplays: Covalent and supramolecular conjugates to assess carbohydrate-protein interactions. Chem Soc Rev. 42(11):4746–4773. [DOI] [PubMed] [Google Scholar]
- Mauris J, M.antelli F, Woodward AM, Cao Z, Bertozzi CR, Panjwani N, Godula K, Argueso P. 2013. Modulation of ocular surface glycocalyx barrier function by a galectin-3 N-terminal deletion mutant and membrane-anchored synthetic glycopolymers. PLoS ONE. 8(8):e72304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y, Hoshino Y, Seto H. 2016. Glycopolymer nanobiotechnology. Chem Rev. 116(4):1673–1692. [DOI] [PubMed] [Google Scholar]
- Nelson A, Belitsky JM, Vidal S, Joiner CS, Baum LG, Stoddart JF. 2004. A self-assmebled multivalent pseudopolyrotaxane for binding galectin-1. J Am Chem Soc. 126(38):11914–11922. [DOI] [PubMed] [Google Scholar]
- Oyelaran O, Li Q, Farnsworth D, Gildersleeve JC. 2009. Micorarrays with varying carbohydrate density reveal distinct subpopulations of serum antibodies. J Proteome Res. 8(7):3529–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, DuFort CC, Rossier O, Bainer R, Mouw JK, Godula K, Hudak JE, Lakins JN, Wijekoon AC, Cassereau L et al. 2014. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature. 511(7509):319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polizzotti BD, Kiick KL. 2006. Effects of polymer structure on the inhibition of the cholera toxin by linear poplypeptide-based glycopolymers. Biomacromolecules. 7(2):483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsipher A, Griffin ME, Stone SE, Brown JM, Hsieh-Wilson LC. 2014. Directing neuronal signaling through cell-surface glycan engineering. J Am Chem Soc. 136(10):6794–6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsipher A, Griffin ME, Stone SE, Hsieh-Wilson LC. 2015. Long-lived engineering of glycans to direct stem cell fate. Angew Chem Int Ed Engl. 54(5):1466–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabuka D, Forstner MB, Groves JT, Berttozi CR. 2008. Noncovalent cell surface engineering: Incorporation of bioactive synthetic glycopolymers into cellular membranes. J Am Chem Soc. 130(18):5947–5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher TW, Parekh RB, Dwek RA. 1988. Glycobiology. Annu Rev Biochem. 57:785–838. [DOI] [PubMed] [Google Scholar]
- Rawat M, Gama CI, Matson JB, Hsieh-Wilson LC. 2008. Neuroactive chondroitin sulfate glycomimetics. J Am Chem Soc. 130(10):2959–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razi N, Varki A. 1998. Masking and unmasking of the sialic acid-binding lectin activity of CD22 (Siglec-2) on B lymphocytes. Proc Natl Acad Sci USA. 95:7469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restuccia A, Fettis MM, Hudalla GA. 2015. Glycomaterials for immunomodulation, immunotherapy, and infection prophylaxis. doi:10.1039/c5tb01780g. [DOI] [PubMed] [Google Scholar]
- Restuccia A, Tian YF, Collier JH, Huddala GA. 2015. Self-assembled glycopeptide nanofibers as modulators of galectin-1 bioactivity. Cell Mol Bioeng. 8(3):471–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff Y, Lehn J-M. 2008. Glycodynamers: Fluorescent dynamic analogues of polysaccharides. Angew Chem Int Ed Engl. 47(19):3556–3559. [DOI] [PubMed] [Google Scholar]
- Ryu J-H, Lee E, Lim Y, Lee M. 2007. Carbohydrate-coated supramolecular structures: Tranformation of nanofibers into spherical micelles triggered by guest encapsulation. J Am Chem Soc. 129:4808–4814. [DOI] [PubMed] [Google Scholar]
- Sansone F, Casnati A. 2013. Multivalent glycocalixarenes for recognition of biological macromolecules: Glycocalyx mimics capable of multitasking. Chem Soc Rev. 42(11):4623–4639. [DOI] [PubMed] [Google Scholar]
- Sheng GJ, Oh YI, Chang SK, Hsieh-Wilson LC. 2013. Tunable heparan sulfate mimetics for modulating chemokine activity. J Am Chem Soc. 135(30):10898–10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Surolia A. 2005. Oligomerization endows enormous stability to soybean agglutinin: A comparison of the stability of the monomer and tetramer of soybean agglutinin. Biophys J. 88(6):4243–4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner K, Hanreich A, Kainz B, Hitchen PG, Dell A, Messner P, Schaffer C. 2008. Recombinant glycans on an S-layer self-assembly protein: A new dimension for nanopatterned biomaterials. Small. 4(10):1728–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaga ML, Fan N, Fueri AL, Brown RK, Chabre YM, Bandyopadhyay P, Roy R, Dam TK. 2014. Significant other half of a glycoconjugate: Contributions of scaffolds to lectin-glycoconjugate interactions. Biochemistry. 53(27):4445–4454. [DOI] [PubMed] [Google Scholar]
- Temme JS, MacPherson IS, DeCourcey JF, Krauss IJ. 2014. High temperature SELMA: Evolution of DNA-supported oligomannose clusters which are tightly recognized by HIV bnAb 2G12. J Am Chem Soc. 136(5):1726–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger WWJ, van Kooyk Y. 2011. Dressed for success C-type lectin receptors for the delivery of glycol-vaccines to dendritic cells. Curr Opin Immunol. 23(1):131–137. [DOI] [PubMed] [Google Scholar]
- Varki A, Lowe JB. 2009. Biological roles of glycans. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. 2nd ed, Chapter 6. Cold Spring Harbor: (NY: ): Cold Spring Harbor Laboratory Press. [PubMed] [Google Scholar]
- Venkataraman G, Raman R, Sasisekharan V, Sasisekharan R. 1999. Molecular characteristics of fibroblastgrowth factor-fibroblast growth factor receptor-heparin-like glycosaminoglycan complex. Proc Natl Acad Sci USA. 96(7):3658–3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vico RV, Voskuhl J, Ravoo BJ. 2011. Multivalent interaction of cyclodextrin vesicles, carbohydrate guests, and lectins: A kinetic investigation. Langmuir. 27(4):1391–1397. [DOI] [PubMed] [Google Scholar]