Abstract
Cell surface glycans are critical mediators of cell–cell, cell–ligand, and cell–pathogen interactions. By controlling the set of glycans displayed on the surface of a cell, it is possible to gain insight into the biological functions of glycans. Moreover, control of glycan expression can be used to direct cellular behavior. While genetic approaches to manipulate glycosyltransferase gene expression are available, their utility in glycan engineering has limitations due to the combinatorial nature of glycan biosynthesis and the functional redundancy of glycosyltransferase genes. Biochemical and chemical strategies offer valuable complements to these genetic approaches, notably by enabling introduction of unnatural functionalities, such as fluorophores, into cell surface glycans. Here, we describe some of the most recent developments in glycoengineering of cell surfaces, with an emphasis on strategies that employ novel chemical reagents. We highlight key examples of how these advances in cell surface glycan engineering enable study of cell surface glycans and their function. Exciting new technologies include synthetic lipid-glycans, new chemical reporters for metabolic oligosaccharide engineering to allow tandem and in vivo labeling of glycans, improved chemical and enzymatic methods for glycoproteomics, and metabolic glycosyltransferase inhibitors. Many chemical and biochemical reagents for glycan engineering are commercially available, facilitating their adoption by the biological community.
Keywords: glycan labeling, glycoproteomics, glycosyltransferases, metabolic oligosaccharide engineering, metabolism
The set of glycans displayed on the surface of a cell determines how that cell interacts with other cells and molecules in its environment. Thus, methods to control glycan expression are valuable tools to understand and regulate the biological functions of cell surface glycoconjugates. Manipulation of glycosyltransferase gene expression is a powerful and commonly used way to control glycan expression. However, the combinatorial nature of glycan biosynthesis and the functional redundancy of glycosyltransferase genes limit the utility of genetic approaches. In addition, genetic approaches do not enable production of glycan structures containing unnatural functionalities, such as fluorophores. Fortunately, chemical strategies to glycoengineering provide a valuable complement to genetic approaches. Here we describe some of the most recent developments in glycoengineering of cell surfaces, with an emphasis on strategies that employ novel chemical reagents.
Display of synthetic glycans on cell surfaces
A conceptually straightforward way to display a glycan of choice on the surface of a cell of choice is to chemically synthesize that glycan linked to a hydrophobic anchor that facilitates presentation on the cell membrane (Figure 1A). The resulting synthetic glycolipid can be directly added to cells, or delivered via a liposome. While slightly more complicated to perform, liposomal delivery offers advantages over direct addition: glycolipids can be directed toward cell surface presentation rather than intracellular uptake and liposomes can be engineered for tissue- or cell-specific targeting. Cell surface display of synthetic glycolipids has been harnessed to investigate the impact of specific glycan structures on cell differentiation and behavior. For example, Pulsipher et al. prepared synthetic glycolipids by coupling chondroitin sulfate polymers to 2-dodecanone. Using a liposomal delivery strategy, these glycolipids were incorporated onto neuronal cell surfaces, leading to increased activation of neurotrophin-mediated signaling pathways and enhanced axonal growth (Pulsipher et al. 2014). Another synthetic proteoglycan mimic was prepared by Huang et al., who conjugated heparin sulfate glycans to a phospholipid tail (Huang et al. 2014). The resulting neoproteoglycans were used to control differentiation of embryonic stem cells: molecules with an affinity for fibroblast growth factor 2 were capable of mimicking the function of native heparin sulfate proteoglycans and promoting neural differentiation. In another example, Paszek et al. prepared polymers modified with glycan structures found in natural mucin O-glycans (Paszek et al. 2014). These structures were attached to a hydrophobic anchor and incorporated onto the surfaces of non-malignant cells. Introduction of large, bulky glycoconjugates on the cell surface induced integrin clustering. Clustering of integrins, in turn, helps circulating tumor cells survive and adhere to soft extracellular matrix, a major hurdle in the formation of metastases. Thus, these experiments suggest a mechanism by which changes in glycosylation could promote cancer metastasis. Notwithstanding such successful applications, the utility of synthetic glycolipids has been limited by their rapid removal from the cell surface by internalization. To overcome this limitation, Woods et al. investigated how the identity of the hydrophobic anchor influences internalization rate. They discovered that glycans with a cholesterylamine anchor are deposited in intracellular vesicles from where the glycans are continuously recycled to the cell surface, even throughout cell division, thereby enabling cell surface display for at least 10 days (Woods et al. 2015). The ability to prepare long-lived synthetic glycolipids promises to expand future applications of synthetic glycolipid engineering.
Fig. 1.
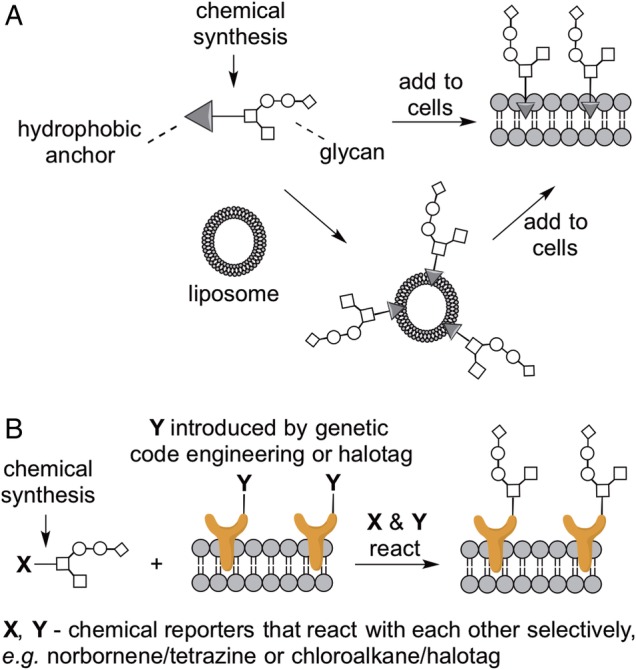
Cell surface display of defined glycans reveals glycan function and dictates cell fate. (A) Glycans linked to a hydrophobic anchor can either be directly added to cells of interest or delivered via liposomes. (B) Multiple approaches allow target cells to be engineered to display a bioorthogonal functional group. Glycans functionalized with a cognate functional group react with the target cells, yielding cell surface display of the desired glycan structures. This figure is available in black and white in print and in color at Glycobiology online.
An alternative strategy for the installment of synthetic glycans on the cell surface is their attachment to a membrane protein by a selective chemical reaction (Figure 1B). This has been accomplished in two different ways. Kramer et al. used genetic code expansion technology to introduce a norbornene chemical handle into the extracellular portion of a membrane protein. Using a bioorthogonal conjugation reaction, this modified protein was coupled to the tetrazine handle on a synthetic mucin glycopolymer (Kramer et al. 2015). In the approach taken by the Hsieh-Wilson laboratory, a cell surface membrane protein was expressed with an appended HaloTag polypeptide sequence. The HaloTag undergoes a chemoselective reaction to form a covalent adduct with chloroalkane-functionalized molecules. Thus, the Hsieh-Wilson laboratory prepared chloroalkane-functionalized glycosaminoglycans, which were then reacted with HaloTag-displaying cells. Notably, this approach enabled cell surface display of the conjugated glycosaminoglycans for over 8 days, which induced stem cells to rapidly exit cell renewal and to differentiate into neuronal cell types (Pulsipher et al. 2015).
Metabolic oligosaccharide engineering with monosaccharide analogs
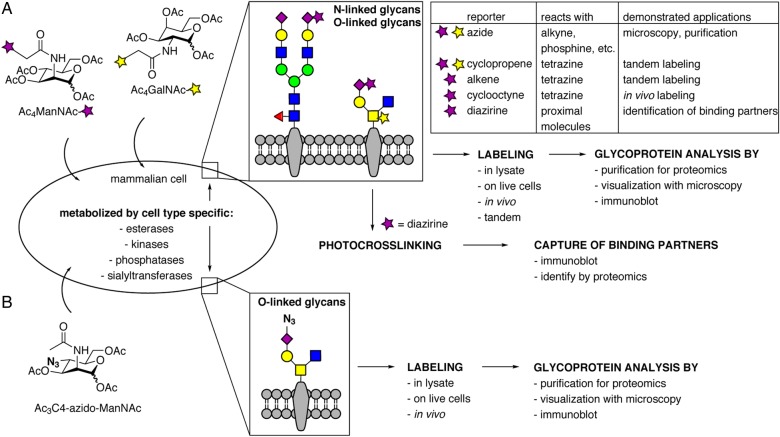
Metabolic oligosaccharide engineering (MOE) allows the introduction of chemical reporters into cellular glycans. These chemical reporters can in turn be reacted with fluorescent tags or affinity probes to enable detection, imaging, and purification of specific sets of glycoconjugates. In MOE, cells are cultured with cell-permeable analogs of natural monosaccharides. When labeling of sialic acid residues is desired, either sialic acid analogs or N-acetylmannosamine (ManNAc) analogs are used. Mammalian cells can often metabolize ManNAc analogs to the corresponding sialic acid analog, provided the unnatural modification does not interfere with enzymatic activity. N-Acetylgalactosamine (GalNAc) analogs can be used directly to label GalNAc residues; however, cellular epimerases are capable of interconverting some GalNAc and GlcNAc analogs, reducing the fidelity of labeling (Boyce et al. 2011; Zaro et al. 2011; Bateman et al. 2013). While free sugars can be used for MOE, their cell permeability is limited and millimolar concentrations are typically required. As an alternative, peracylation of the hydroxyl groups yields less polar molecules that readily cross the plasma membrane and are deprotected by intracellular esterases. Cell-permeable, peracylated sugar analogs can be used at ∼100-fold lower concentrations than free sugars, but it is important to note that the acyl groups are not innocent bystanders and can exert effects independent of their protecting group function (Almaraz et al. 2012).
The endogenous glycosylation machinery of cells constructs unnatural glycans in which natural sugars are replaced with their unnatural counterparts. While the use of MOE to introduce chemical reporters such as azides and ketones is well established (Campbell et al. 2007; Cheng et al. 2016), continuous efforts go into the introduction of new chemical reporters and improved labeling, which creates opportunities for new applications such as tandem labeling and in vivo imaging (Figure 2). At the same time, MOE also enables the introduction of other kinds of chemical functional groups that can be used to study molecular interactions and biological roles of glycans. Finally, recent studies are providing increased understanding of the roles of promiscuous enzymes that process sugar analogs into cell surface glycans.
Fig. 2.
Advances in MOE. (A) Introduction of both novel and established chemical reporters enables applications such as tandem labeling, in vivo imaging of inner organs, and identification of a glycans' binding partners. (B) A ManNAc derivative that carries an azide at the C4-position is incorporated exclusively into GalNAc-type O-linked glycans and thereby enables their study. This figure is available in black and white in print and in color at Glycobiology online.
Tandem labeling: exploring other chemical reporters beyond azides and ketones
The advent of new bioorthogonal labeling reactions offers the possibility to introduce different chemical reporters simultaneously. However, introducing new bioorthogonal chemistry through MOE is a challenging endeavor, as a chemical reporter has to meet several requirements: (i) it needs to be small in size to be tolerated by the endogenous enzyme machinery, (ii) it needs to be inert toward all molecules that are present in cells, especially nucleophiles and enzymes, and (iii) it needs to selectively react with an orthogonal labeling partner. Harnessing considerable improvements in bioorthogonal chemistry, several examples of tandem imaging have been demonstrated, as discussed below.
Cyclopropenes
In a proof-of-principle study, Patterson et al. demonstrated that methylcyclopropenes are bioorthogonal reporters that are tolerated by cells and can be labeled with tetrazine reagents on the cell surface in an inverse electron demand Diels–Alder reaction (Patterson et al. 2012). Specifically, these researchers introduced a methylcyclopropene-labeled sialic acid into cell surface glycoconjugates and selectively labeled it using a tetrazine-biotin conjugate (Patterson et al. 2012). Most importantly, they demonstrated the feasibility of tandem labeling of cells by simultaneously introducing both methylcyclopropene- and azide-modified sialic acids followed by labeling with tetrazine and dibenzylcyclooctyne probes, respectively (Patterson et al. 2012). This labeling strategy was significantly improved with the introduction of a carbamate linkage between cyclopropene and sugar, which reduced the labeling time from 1 h to 5 min (Spate et al. 2014). Späte et al. also demonstrated tandem labeling in which two chemical reporters were used to label different monosaccharides: a cyclopropene-modified ManNAc analog was used to yield cyclopropene-modified sialic acid, which was labeled via an inverse electron Diels–Alder reaction, while GlcNAz was also incorporated and labeled using a strain-promoted 1,3-dipolar cycloaddition reaction (often informally termed “click chemistry”). Subsequently, Patterson et al. (2014) demonstrated that the carbamate linkage between the cyclopropene and the sugar leads to faster incorporation rates—incorporation can be detected after only 15 min incubation time with the cell-permeable, peracylated sugar analog. Moreover, methylcyclopropene-modified GalNAc and N-acetylglucosamine (GlcNAc) derivatives have been developed for the labeling of cell surface and intracellular glycoproteins. These sugars can be used together with analogs of other monosaccharides, considerably expanding the toolbox for tandem labeling (Patterson et al. 2014; Spate, Schart, Hafner, et al. 2014).
Initially, the use of these methylcyclopropene-modified sugars was limited due to the lack of available tetrazine-fluorophore probes, probably owing to their elaborate syntheses. Therefore, labeling has commonly been performed in a two-step procedure by reaction with a tetrazine-biotin conjugate followed by labeling with an avidin-fluorophore reagent. However, new synthetic routes to tetrazine-dye conjugates are being published, e.g. the synthesis of a tetrazine-Cy3 probe that is highly water soluble and minimizes background staining (Spate et al. 2014). Moreover, tetrazine probes are commercially available from specialized manufacturers.
Alkenes
Like cyclopropenes, terminal alkenes can be chemoselectively labeled with tetrazine reagents through an inverse electron demand Diels–Alder reaction. A mannosamine analog derivatized with a terminal alkene is metabolized to an alkene-tagged sialic acid and can be used together with azide-functionalized GalNAc (GalNAz) for the tandem labeling of cells (Niederwieser et al. 2013). One limitation of these reagents is that cells need to be incubated for long periods of time with tetrazine conjugates to achieve sufficient labeling. More recently, sugars with carbamate linkages to the alkene have been reported that offer improved reaction kinetics, reducing labeling times from 6 h to 15 min (Spate, Schart, Scholkopf, et al. 2014).
Cyclooctynes
Using an appropriate protocol, even residues as sizable as cyclooctynes can be incorporated into glycoconjugates. Agarwal et al. prepared a cyclooctyne-modified sialic acid and delivered it to zebrafish by injection. The cyclooctyne-modified sialic acid was incorporated into glycoconjugates and successfully labeled with a fluorogenic tetrazine labeling reagent to allow the in vivo imaging of glycans at inner organs with unprecedented resolution and signal-to-noise ratio (Agarwal et al. 2015).
Targeting O-linked glycans
While most sialic acid derivatives for MOE are incorporated in gangliosides, O-linked and N-linked glycans alike, Möller et al. serendipitously discovered an azide-modified sialic acid that is incorporated selectively into GalNAc-type O-linked glycans, although the mechanism of selective incorporation has not yet been defined (Moller et al. 2012). While most previously reported ManNAc derivatives were modified on the N-acyl side chain of the C2 position, these researchers asked whether chemical reporters can be incorporated at other positions of a ManNAc analog. They found that an azide modification at the C4 position is indeed tolerated during all enzymatic transformations and leads to a sialic acid analog that is azide labeled at the C7 position (Figure 2B) (Moller et al. 2012). The resulting C7-azido sialic acid was incorporated into cell surface glycans; however, the labeling was not decreased by treatment with tunicamycin or PNGase F, and indeed no labeling of individual glycoproteins that contained mostly N-linked glycans could be detected; in contrast, the C7-azido sialic acid was robustly incorporated in mucin-1, which is heavily modified with GalNAc-type O-linked glycans (Moller et al. 2012).
Label-free perturbing binding events with sialic acid analogs
While the most common current application of MOE is to introduce chemical reporters, MOE was first developed to introduce sialic acid analogs with inert groups of different size at the N-acyl chain (Kayser et al. 1992). By introducing a bulky functional group on the sialic acid residue, it is possible to perturb glycan-dependent binding events. Thus, these reagents have utility in probing and modulating interactions that depend on sialic acid. Most recently, Ericson et al. performed MOE with a panel of ManNAc analogs to reveal the importance of the identity of the N-acyl group of sialic acid for the binding of murine Siglec-1 to the rodent pathogen murine leukemia virus (Erikson et al. 2015).
Covalent capture of transient binding events: ManNDAz
In addition to bioorthogonal labeling reactions to facilitate imaging and isolation of specific glycoconjugates, MOE also enables the introduction of photocross-linking monosaccharide analogs, which can be used to identify binding partners of glycoconjugates via covalent conjugation. In this application, a photocross-linking sugar is incorporated into cellular glycoconjugates and then activated by UV irradiation, leading to crosslinking between glycoconjugates and neighboring molecules, which can be identified by mass spectrometry-based proteomics methods. Four different metabolically incorporated photocross-linking sialic acid analogs have been reported (Luchansky, Goon, et al. 2004; Han et al. 2005; Tanaka and Kohler 2008; Feng et al. 2013); one of these also includes an azide to facilitate purification of the cross-linked complexes. A sialic acid analog bearing an aryl azide crosslinker has been used to identify sialylated binding partners of the sialic acid binding lectin (Siglec) CD22, both on cis and opposing cells (Han et al. 2005; Ramya et al. 2010). Alternatively, cells can be cultured with a diazirine-modified cell-permeable ManNAc analog (Ac4ManNDAz) to yield a sialic acid with the diazirine photocrosslinker (Tanaka and Kohler 2008). This photocross-linking tool allowed the investigation of the binding partners of cholera toxin in various cell types. While the known interaction of cholera toxin and the ganglioside GM1 could be demonstrated in Jurkat cells (Bond et al. 2010), use of the photocrosslinker revealed that glycoproteins, and not GM1, are the most abundant cholera toxin-binding partners in human colonic epithelial cells. Further, the use of Ac4ManNDAz enabled identification of a cholera toxin-binding glycoprotein (Wands et al. 2015).
Cell-type specificity of MOE: limitations and opportunities
A fact that is rarely emphasized in the field of MOE is that efficiency of incorporation of unnatural monosaccharides into glycoconjugates is indeed cell-type specific (Luchansky, Argade, et al. 2004; Chang et al. 2009). For example, Pham et al. evaluated metabolism of a cell-permeable diazirine-modified ManNAc analog, Ac4ManNDAz, in 15 human cell lines. Using this reagent, the efficiency of replacement of cell surface sialic acid with a diazirine-modified sialic acid analog (SiaDAz) varied widely among the cell lines, in the range of 0–65%. Further experiments suggested that the metabolic differences might be attributable to different amounts and types of esterases, kinases, phosphatases, and sialyltransferases present in the cell lines (Pham et al. 2015). Identification of the most efficient enzymes will allow the genetic engineering of cells that more effectively incorporate sugar analogs. But even in a single cell line, different sialic acid analogs may be incorporated to differing extents into α2–3- and α2–6-linked sialic acids, as demonstrated by Whitman et al. (2011).
Cell- and even tissue-specific delivery of unnatural monosaccharides is possible if they are combined with a targeting entity. Chang et al. linked Ac3ManNDAz at the C6 position to a peptide substrate that targets specifically cells that present prostate-specific antigen protease (Chang et al. 2010). Alternatively, Xie et al. demonstrated the cell-targeted delivery of unnatural monosaccharides via ligand-modified liposomes. Using folate-labeled liposomes, they delivered 9-azido sialic acid specifically to cells expressing folate receptor, which is displayed on breast cancer cells, as well as other cell types (Xie et al. 2012). The same group used cyclic RGD peptides to target liposomes loaded with 9-azido sialic acid selectively to tumors presenting the integrin αVβ3, enabling in vivo labelling as well as proteomic analysis of sialylated tumor-associated glycans (Xie et al. 2014).
Chemical and enzymatic methods to functionalize cell surface glycans
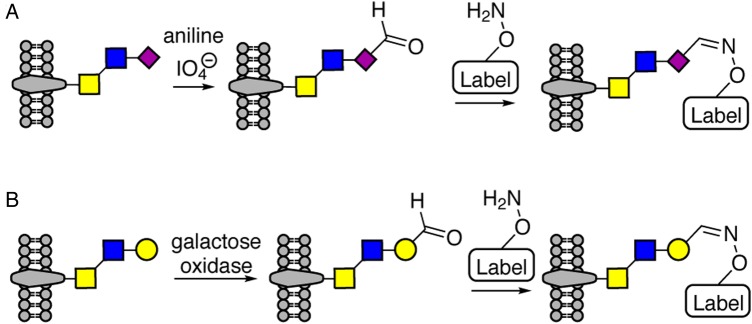
Labeling of cellular glycans using MOE is a powerful tool for proteomics studies of cells' glycosylation state. However, MOE has limited efficiency in some cell lines and is infeasible for primary human samples. Thus, chemical approaches to target subpopulations of the glycome offer a valuable alternative. In this context, the labeling of cell surface sialic acids by mild periodate oxidation to aldehydes followed by the reaction with an oxime or hydrazine tag has long been known. However, the kinetics are slow and the reaction proceeds most efficiently at pH 5–6, which is stressful to live cells. Zheng et al. considerably improved this technique by introducing aniline as catalyst, which speeds the reaction up to 10-fold, such that it works efficiently at oxime concentrations of 100 µM and at a neutral pH (Zeng et al. 2009). This approach, shown in Figure 3A, is named periodate oxidation and aniline-catalyzed oxime ligation (PAL), and allows the labeling of sialylated molecules on any cell within only 2 h. More recently, Ramya et al. developed a complementary approach to PAL that allows the tagging of a subpopulation of glycoproteins that do not carry terminal sialic acids (Ramya et al. 2013). This strategy is termed galactose oxidase and aniline-catalyzed oxime ligation (GAL) and uses galactose oxidase to create aldehydes at terminal galactose and GalNAc residues, followed by an aniline-catalyzed oxime ligation to selectively label these non-sialylated glycoconjugates (Figure 3B) (Ramya et al. 2013). Both PAL and GAL facilitate proteomics analysis of specific glycoconjugate populations, and provide complementary sets of information. In a recent example, PAL- and GAL-enabled proteomics analyses were used to identify glycoproteins desialylated by pneumoccocal neuraminidases (McCombs and Kohler 2016).
Fig. 3.
Selective chemical labeling of cell surface glycans. Aldehydes are formed on sialic acid residues by mild periodate treatment (PAL; A) or on galactose residues by galactose oxidase treatment (GAL; B). Subsequent labeling of oxidized glycans with biotin or flurorescent reporters is performed by aniline-catalyzed oxime ligation. This figure is available in black and white in print and in color at Glycobiology online.
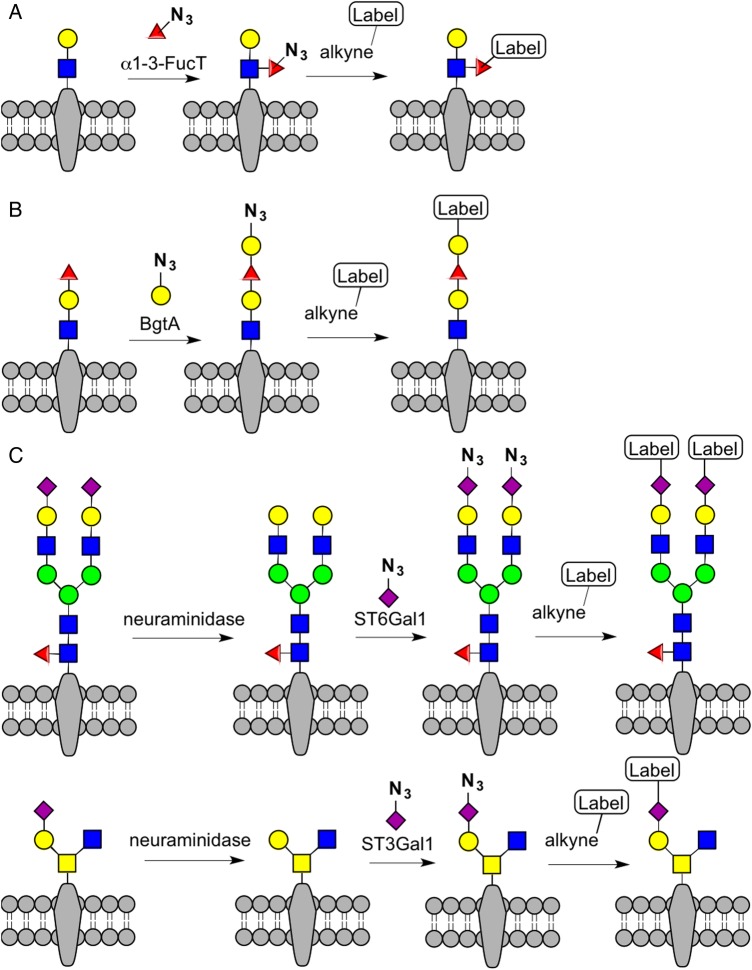
Glycosyltransferases can also be used for cell surface glycan engineering, a concept demonstrated by Zheng et al. These researchers used an α1–3-fucosyltransferase to selectively label terminal LacNAc residues on the cell surface with C5-modified fucose analogs that could be fluorescently marked in a second step. This two-step labeling procedure enabled tracking of LacNAc levels on mammalian cells and also on zebrafish embryos during the tail bud stage (Figure 4A) (Zheng et al. 2011). Furthermore, Hsieh-Wilson and co-workers used a bacterial homolog of the human blood group A antigen glycosyltransferase (BgtA) to selectively add a functionalized Gal or GalNAc analog to α1–2-fucosylated glycans on the surface of cells (Chaubard et al. 2012), thus enabling both proteomics and imaging applications (Figure 4B). Similarly, Mbua et al. have used ST6Gal1, a sialyltransferase from rat, for selective labeling of N-linked glycans with an azide-functionalized sialic acid derivative in an approach they term selective exo-enzymatic labeling (SEEL) (Figure 4C) (Mbua et al. 2013). Recently, the efficiency of SEEL was increased by treating cells with neuraminidase prior to SEEL; also, the scope of SEEL was expanded by labeling GalNAc-type O-linked glycans with ST3Gal1 (Yu et al. 2016). In the same work, Yu et al. demonstrated that SEEL-enabled proteomics could detect more sialylated proteins than MOE-enabled proteomics, possibly due to limitations in unnatural sugar metabolism (Yu et al. 2016). Glycosyltransferase-enabled cell surface glycoengineering can be used to control the behavior of cells in vivo. For example, Sackstein and co-workers used an α1–3-fucosyltransferase to assemble the glycoform of the hematopoietic cell E-selectin/L-selectin ligand (HCELL) on native CD44 receptors on mesenchymal stem cells, thereby inducing their trafficking to and infiltration of the bone marrow (Sackstein et al. 2008). More recently, the same group used an α1–3-fucosyltransferase to present HCELL ligands on neural stem cells, which improved the efficacy of stem cell treatment in mice by increased neurotropism, reduced inflammation, and better axonal integrity (Merzaban et al. 2015).
Fig. 4.
Selective enzymatic labeling of cell surface glycans. (A) Terminal LacNAc residues are selectively tagged using an α1–3-fucosyl transferase. (B) Glycans containing α1–2 fucose can be selectively labeled with Gal or GalNAc analogs using a bacterial glycosyltransferase, BgtA. (C) Non-sialylated N-linked glycans and O-linked glycans can be selectively labeled with a sialic acid analog using a sialyltransferase. Prior neuraminidase treatment makes additional glycans accessible for labeling. This figure is available in black and white in print and in color at Glycobiology online.
Remodeling cell surface glycosylation by metabolic inhibition
In some cases, it is desirable to control the total level of specific sugars or glycan structures on the surface of cells. This can be difficult to achieve genetically, in part due to the functional redundancy of glycosyltransferase family members. As an alternative, Marathe et al. offer a cell-permeable fluorinated GalNAc derivative—peracylated 4F-GalNAc—that metabolically interrupts biosynthesis of GalNAc-type O-linked glycosylation and sialyl Lewis-X, thereby reducing leukocyte binding to selectins. Intravenous delivery of 4F-GalNAc decreased leukocyte migration to the peritoneum in a murine model of peritonits (Marathe et al. 2010). In a related approach, Rillahan et al. developed small molecule reagents that cause metabolic inhibition of sialylation and fucosylation in cells and in vivo. These cell-permeable fluorinated sugars—peracylated 2-fluorofucose (2F-Fuc) and peracylated 3-fluoro sialic acid (3Fax-Neu5Ac)—are metabolized intracellularly into substrate-based inhibitors. Via metabolic feedback loops, the resulting inhibitors induce global shutdown of sialyl- or fucosyltransferase activity (Rillahan et al. 2012). In initial proof-of-principle experiments, the utility of 2F-Fuc and 3Fax-Neu5Ac was demonstrated by reducing the expression of sialyl Lewis X on myeloid cells, which led to loss of selectin binding and impaired leukocyte rolling (Rillahan et al. 2012). Both inhibitors have found multiple applications in cells and in vivo, including the following examples. Use of 2F-Fuc in a sickle cell mouse model reduces obstruction of blood vessels by sickled red blood cells, a pathology that depends on P- and E-selectin adhesion to sialyl Lewis X (Belcher et al. 2015). In a mouse tumor model, 2F-Fuc resulted in significantly decreased tumor growth and even protection from tumor engraftment when used in combination with other therapies (Okeley et al. 2013). Finally, global inhibition of sialylation in mice through the use of 3Fax-Neu5Ac leads to kidney failure and liver dysfunction, confirming the essential role of sialic acids in vivo (Macauley et al. 2014).
We conclude that, due to the development of valuable methodologies in the recent years, today the glycobiologist can choose from a range of methods to engineer a cells' surface glycans according to need. In cell culture and in vivo, metabolic inhibitors of glycosyltransferases allow the phenotypes of glycan depletion to be studied. The introduction of new chemical reporters to MOE enables tandem and in vivo labeling. For glycoproteomics experiments, the toolbox of chemical and enzymatic glycan labeling methodologies is continuously increasing and enables study of specific glycan subsets. Enzymatic remodeling and synthetic glycans enable cell surface presentation of glycans of choice. Taken together, these new biochemical methods will continue to propel glycoscience forward, by both revealing the biological functions of naturally occurring glycans and offering new glycan-based methods to regulate biological processes.
Funding
The authors acknowledge funding from in the NIH (R01GM090271) and the Welch Foundation (I-1686).
Conflict of interest statement
None declared.
Abbreviation
GalNAc, N-acetylgalactosamine; HCELL, hematopoietic cell E-selectin/L-selectin ligand; MOE, metabolic oligosaccharide engineering; SEEL, selective exo-enzymatic labeling.
Acknowledgements
Due to the brief format, this review is not comprehensive; we apologize to those authors whose work has not been cited here.
References
- Agarwal P, Beahm BJ, Shieh P, Bertozzi CR. 2015. Systemic fluorescence imaging of zebrafish glycans with bioorthogonal chemistry. Angew Chem. 54:11504–11510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almaraz RT, Aich U, Khanna HS, Tan E, Bhattacharya R, Shah S, Yarema KJ. 2012. Metabolic oligosaccharide engineering with N-acyl functionalized ManNAc analogs: cytotoxicity, metabolic flux, and glycan-display considerations. Biotechnol Bioeng. 109:992–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman LA, Zaro BW, Chuh KN, Pratt MR. 2013. N-Propargyloxycarbamate monosaccharides as metabolic chemical reporters of carbohydrate salvage pathways and protein glycosylation. Chem Commun. 49:4328–4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher JD, Chen C, Nguyen J, Abdulla F, Nguyen P, Nguyen M, Okeley NM, Benjamin DR, Senter PD, Vercellotti GM. 2015. The fucosylation inhibitor, 2-fluorofucose, inhibits vaso-occlusion, leukocyte-endothelium interactions and NF-kB activation in transgenic sickle mice. PLoS ONE. 10:e0117772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond MR, Whitman CM, Kohler JJ. 2010. Metabolically incorporated photocrosslinking sialic acid covalently captures a ganglioside-protein complex. Mol Biosyst. 6:1796–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M, Carrico IS, Ganguli AS, Yu SH, Hangauer MJ, Hubbard SC, Kohler JJ, Bertozzi CR. 2011. Metabolic cross-talk allows labeling of O-linked beta-N-acetylglucosamine-modified proteins via the N-acetylgalactosamine salvage pathway. Proc Natl Acad Sci USA. 108:3141–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CT, Sampathkumar SG, Yarema KJ. 2007. Metabolic oligosaccharide engineering: Perspectives, applications, and future directions. Mol Biosyst. 3:187–194. [DOI] [PubMed] [Google Scholar]
- Chang PV, Chen X, Smyrniotis C, Xenakis A, Hu T, Bertozzi CR, Wu P. 2009. Metabolic labeling of sialic acids in living animals with alkynyl sugars. Angew Chem. 48:4030–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PV, Dube DH, Sletten EM, Bertozzi CR. 2010. A strategy for the selective imaging of glycans using caged metabolic precursors. J Am Chem Soc. 132:9516–9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaubard JL, Krishnamurthy C, Yi W, Smith DF, Hsieh-Wilson LC. 2012. Chemoenzymatic probes for detecting and imaging fucose-alpha(1–2)-galactose glycan biomarkers. J Am Chem Soc. 134:4489–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B, Xie R, Dong L, Chen X. 2016. Metabolic remodeling of cell-surface sialic acids: Principles, applications, and recent advances. Chembiochem. 17:11–27. [DOI] [PubMed] [Google Scholar]
- Erikson E, Wratil PR, Frank M, Ambiel I, Pahnke K, Pino M, Azadi P, Izquierdo-Useros N, Martinez-Picado J, Meier C et al. 2015. Mouse Siglec-1 mediates trans-infection of surface-bound murine leukemia virus in a sialic acid N-acyl side chain-dependent manner. J Biol Chem. 290:27345–27359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Hong S, Rong J, You Q, Dai P, Huang R, Tan Y, Hong W, Xie C, Zhao J et al. 2013. Bifunctional unnatural sialic acids for dual metabolic labeling of cell-surface sialylated glycans. J Am Chem Soc. 135:9244–9247. [DOI] [PubMed] [Google Scholar]
- Han S, Collins BE, Bengtson P, Paulson JC. 2005. Homomultimeric complexes of CD22 in B cells revealed by protein-glycan cross-linking. Nat Chem Biol. 1:93–97. [DOI] [PubMed] [Google Scholar]
- Huang ML, Smith RA, Trieger GW, Godula K. 2014. Glycocalyx remodeling with proteoglycan mimetics promotes neural specification in embryonic stem cells. J Am Chem Soc. 136:10565–10568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser H, Zeitler R, Kannicht C, Grunow D, Nuck R, Reutter W. 1992. Biosynthesis of a nonphysiological sialic acid in different rat organs, using N-propanoyl-D-hexosamines as precursors. J Biol Chem. 267:16934–16938. [PubMed] [Google Scholar]
- Kramer JR, Onoa B, Bustamante C, Bertozzi CR. 2015. Chemically tunable mucin chimeras assembled on living cells. Proc Natl Acad Sci U S A. 112:12574–12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchansky SJ, Argade S, Hayes BK, Bertozzi CR. 2004. Metabolic functionalization of recombinant glycoproteins. Biochemistry. 43:12358–12366. [DOI] [PubMed] [Google Scholar]
- Luchansky SJ, Goon S, Bertozzi CR. 2004. Expanding the diversity of unnatural cell-surface sialic acids. Chembiochem. 5:371–374. [DOI] [PubMed] [Google Scholar]
- Macauley MS, Arlian BM, Rillahan CD, Pang PC, Bortell N, Marcondes MC, Haslam SM, Dell A, Paulson JC. 2014. Systemic blockade of sialylation in mice with a global inhibitor of sialyltransferases. J Biol Chem. 289:35149–35158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marathe DD, Buffone A Jr, Chandrasekaran EV, Xue J, Locke RD, Nasirikenari M, Lau JT, Matta KL, Neelamegham S. 2010. Fluorinated per-acetylated GalNAc metabolically alters glycan structures on leukocyte PSGL-1 and reduces cell binding to selectins. Blood. 115:1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbua NE, Li X, Flanagan-Steet HR, Meng L, Aoki K, Moremen KW, Wolfert MA, Steet R, Boons GJ. 2013. Selective exo-enzymatic labeling of N-glycans on the surface of living cells by recombinant ST6Gal I. Angew Chem. 52:13012–13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCombs JE, Kohler JJ. 2016. Pneumococcal neuraminidase substrates identified through comparative proteomics enabled by chemoselective labeling. Bioconj Chem. doi:10.1021/acs.bioconjchem.6b00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzaban JS, Imitola J, Starossom SC, Zhu B, Wang Y, Lee J, Ali AJ, Olah M, Abuelela AF, Khoury SJ et al. 2015. Cell surface glycan engineering of neural stem cells augments neurotropism and improves recovery in a murine model of multiple sclerosis. Glycobiology. 25:1392–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller H, Bohrsch V, Bentrop J, Bender J, Hinderlich S, Hackenberger CP. 2012. Glycan-specific metabolic oligosaccharide engineering of C7-substituted sialic acids. Angew Chem. 51:5986–5990. [DOI] [PubMed] [Google Scholar]
- Niederwieser A, Spate AK, Nguyen LD, Jungst C, Reutter W, Wittmann V. 2013. Two-color glycan labeling of live cells by a combination of Diels-Alder and click chemistry. Angew Chem. 52:4265–4268. [DOI] [PubMed] [Google Scholar]
- Okeley NM, Alley SC, Anderson ME, Boursalian TE, Burke PJ, Emmerton KM, Jeffrey SC, Klussman K, Law CL, Sussman D et al. 2013. Development of orally active inhibitors of protein and cellular fucosylation. Proc Natl Acad Sci USA. 110:5404–5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, DuFort CC, Rossier O, Bainer R, Mouw JK, Godula K, Hudak JE, Lakins JN, Wijekoon AC, Cassereau L et al. 2014. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature. 511:319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson DM, Jones KA, Prescher JA. 2014. Improved cyclopropene reporters for probing protein glycosylation. Mol Biosyst. 10:1693–1697. [DOI] [PubMed] [Google Scholar]
- Patterson DM, Nazarova LA, Xie B, Kamber DN, Prescher JA. 2012. Functionalized cyclopropenes as bioorthogonal chemical reporters. J Am Chem Soc. 134:18638–18643. [DOI] [PubMed] [Google Scholar]
- Pham ND, Fermaintt CS, Rodriguez AC, McCombs JE, Nischan N, Kohler JJ. 2015. Cellular metabolism of unnatural sialic acid precursors. Glycoconj J. 32:515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsipher A, Griffin ME, Stone SE, Brown JM, Hsieh-Wilson LC. 2014. Directing neuronal signaling through cell-surface glycan engineering. J Am Chem Soc. 136:6794–6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsipher A, Griffin ME, Stone SE, Hsieh-Wilson LC. 2015. Long-lived engineering of glycans to direct stem cell fate. Angew Chem. 54:1466–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramya TN, Weerapana E, Cravatt BF, Paulson JC. 2013. Glycoproteomics enabled by tagging sialic acid- or galactose-terminated glycans. Glycobiology. 23:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramya TN, Weerapana E, Liao L, Zeng Y, Tateno H, Liao L, Yates JR III, Cravatt BF, Paulson JC. 2010. In situ trans ligands of CD22 identified by glycan-protein photocross-linking-enabled proteomics. Mol Cell Proteomics. 9:1339–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillahan CD, Antonopoulos A, Lefort CT, Sonon R, Azadi P, Ley K, Dell A, Haslam SM, Paulson JC. 2012. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat Chem Biol. 8:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, Wohlgemuth R. 2008. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 14:181–187. [DOI] [PubMed] [Google Scholar]
- Spate AK, Busskamp H, Niederwieser A, Schart VF, Marx A, Wittmann V. 2014. Rapid labeling of metabolically engineered cell-surface glycoconjugates with a carbamate-linked cyclopropene reporter. Bioconjug Chem. 25:147–154. [DOI] [PubMed] [Google Scholar]
- Spate AK, Schart VF, Hafner J, Niederwieser A, Mayer TU, Wittmann V. 2014. Expanding the scope of cyclopropene reporters for the detection of metabolically engineered glycoproteins by Diels-Alder reactions. Beilstein J Org Chem. 10:2235–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spate AK, Schart VF, Schollkopf S, Niederwieser A, Wittmann V. 2014. Terminal alkenes as versatile chemical reporter groups for metabolic oligosaccharide engineering. Chemistry. 20:16502–16508. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kohler JJ. 2008. Photoactivatable crosslinking sugars for capturing glycoprotein interactions. J Am Chem Soc. 130:3278–3279. [DOI] [PubMed] [Google Scholar]
- Wands AM, Fujita A, McCombs JE, Cervin J, Dedic B, Rodriguez AC, Nischan N, Bond MR, Mettlen M, Trudgian DC et al. 2015. Fucosylation and protein glycosylation create functional receptors for cholera toxin. eLife. 4:e09545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman CM, Yang F, Kohler JJ. 2011. Modified GM3 gangliosides produced by metabolic oligosaccharide engineering. Bioorg Med Chem Lett. 21:5006–5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods EC, Yee NA, Shen J, Bertozzi CR. 2015. Glycocalyx engineering with a recycling glycopolymer that increases cell survival in vivo. Angew Chem. 54:15782–15788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie R, Dong L, Huang R, Hong S, Lei R, Chen X. 2014. Targeted imaging and proteomic analysis of tumor-associated glycans in living animals. Angew Chem. 53:14082–14086. [DOI] [PubMed] [Google Scholar]
- Xie R, Hong S, Feng L, Rong J, Chen X. 2012. Cell-selective metabolic glycan labeling based on ligand-targeted liposomes. J Am Chem Soc. 134:9914–9917. [DOI] [PubMed] [Google Scholar]
- Yu SH, Zhao P, Sun T, Gao Z, Moremen KW, Boons GJ, Wells L, Steet R. 2016. Selective exo-enzymatic labeling detects increased cell surface sialoglycoprotein expression upon megakaryocytic differentiation. J Biol Chem. doi:10.1074/jbc.M115.700369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaro BW, Yang YY, Hang HC, Pratt MR. 2011. Chemical reporters for fluorescent detection and identification of O-GlcNAc-modified proteins reveal glycosylation of the ubiquitin ligase NEDD4-1. Proc Natl Acad Sci USA. 108:8146–8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Ramya TN, Dirksen A, Dawson PE, Paulson JC. 2009. High-efficiency labeling of sialylated glycoproteins on living cells. Nat Methods. 6:207–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng T, Jiang H, Gros M, del Amo DS, Sundaram S, Lauvau G, Marlow F, Liu Y, Stanley P, Wu P. 2011. Tracking N-acetyllactosamine on cell-surface glycans in vivo. Angew Chem. 50:4113–4118. [DOI] [PMC free article] [PubMed] [Google Scholar]