Memory storage in Purkinje cells is assumed to be done by changing the strength of their synapses. Here, we show that a type of synaptic plasticity in Purkinje cell synapses results in an increase in the propensity of the cell to generate action potentials, known as intrinsic excitability. Since Purkinje cells are the output of the cerebellar cortex, this type of plasticity is expected to affect the information being sent to other parts of the brain.
Keywords: synaptic throughput, dendritic integration, cerebellar function, memory
Abstract
Coding in cerebellar Purkinje cells not only depends on synaptic plasticity but also on their intrinsic membrane excitability. We performed whole cell patch-clamp recordings of Purkinje cells in sagittal cerebellar slices in mice. We found that inducing long-term depression (LTD) in the parallel fiber to Purkinje cell synapses results in an increase in the gain of the firing rate response. This increase in excitability is accompanied by an increase in the input resistance and a decrease in the amplitude of the hyperpolarization-activated cyclic nucleotide-gated (HCN) channel-mediated voltage sag. Application of a HCN channel blocker prevents the increase in input resistance and excitability without blocking the expression of synaptic LTD. We conclude that the induction of parallel fiber-Purkinje cell LTD is accompanied by an increase in excitability of Purkinje cells through downregulation of the HCN-mediated h current. We suggest that HCN downregulation is linked to the biochemical pathway that sustains synaptic LTD. Given the diversity of information carried by the parallel fiber system, we suggest that changes in intrinsic excitability enhance the coding capacity of the Purkinje cell to specific input sources.
NEW & NOTEWORTHY
Memory storage in Purkinje cells is assumed to be done by changing the strength of their synapses. Here, we show that a type of synaptic plasticity in Purkinje cell synapses results in an increase in the propensity of the cell to generate action potentials, known as intrinsic excitability. Since Purkinje cells are the output of the cerebellar cortex, this type of plasticity is expected to affect the information being sent to other parts of the brain.
synaptic plasticity is a widely assumed neuronal mechanism of learning and memory (Grasselli and Hansel 2014). However, increasing evidence suggests that intrinsic membrane excitability is also subject to modulation and that it is often accompanied by synaptic plasticity. Changes in intrinsic excitability, also known as intrinsic plasticity, have been shown to be caused by modifications of different membrane conductances that ultimately affect synaptic integration (Mahon and Charpier 2012; Ohtsuki et al. 2012; Wang et al. 2003), spiking activity (Magee and Carruth 1999; Motanis et al. 2012), and coding (Kispersky et al. 2012; Kourrich et al. 2015; Rizzo et al. 2014). Thus, to understand how neurons integrate synaptic activity, it is important to determine the expression of intrinsic excitability changes after synaptic plasticity.
The function of intrinsic excitability depends on its relationship to the polarity of the synaptic plasticity change (Mahon and Charpier 2012). This is of particular importance in cerebellar Purkinje cells, which receive >150,000 parallel fiber synapses (Shepherd 2004). Depending on the existence and relative polarity of changes in intrinsic excitability after either long-term potentiation (LTP) or long-term depression (LTD), Purkinje cells could be performing very different computational tasks (Hansel et al. 2001; Jörntell and Hansel 2006). If changes in intrinsic excitability go in the opposite direction of synaptic plasticity, a decrease for LTP and an increase for LTD, it is then considered to be a homeostatic process that allows neurons to remain in a spiking range (Aizenman et al. 2003; Brager and Johnston 2007; Schacher and Hu 2014; Turrigiano 1999). However, if intrinsic excitability follows the changes of synaptic plasticity, then this would result in an increase of firing rate for LTP and lower firing rates for LTD. Such a response could implement a mechanism of pattern separation (Armano et al. 2000; Xu et al. 2005).
In the present study, we determined whether Purkinje cells express a change in intrinsic excitability after inducing LTD in parallel fiber synapses. Using whole cell patch-clamp techniques, we quantified intrinsic excitability by measuring the firing rate in response to somatic input current before and after LTD induction. We found that LTD induction resulted in an increase in the excitability of Purkinje cells. This change followed a linear function and was accompanied by a decrease in the amplitude of voltage sag. In Purkinje cells, the sag is due to the h current that is mediated by hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. Blockade of HCN channels with ZD7288 eliminated the sag, the afterhyperpolarization (AHP), and the increase in input resistance and excitability without blocking the expression of synaptic LTD. According to a recent study, intrinsic excitability also increases in Purkinje cells after the induction of LTP, which is mediated by changes in small-conductance Ca2+-activated K+ (SK) channels (Belmeguenai et al. 2010). Thus, increases in intrinsic excitability seem to have different biochemical mechanisms depending on the type of synaptic plasticity being induced. Changes in h current could affect the synaptic integration properties of Purkinje cells and modify the processing of information in the cerebellar cortex.
MATERIALS AND METHODS
Postnatal 16- to 23-day-old wild-type (C57BL/6J) mice (https://www.jax.org/strain/000664) of either sex were decapitated after the induction of deep anesthesia with 2 ml evaporated isoflurane for at least 2 min. The cerebellum was removed, and 200-μm vermis sagittal slices were cut with a microtome (Vibratome 3000) in 2°C artificial cerebrospinal fluid (aCSF) containing the following (in mM; all from Sigma-Aldrich, St. Louis, MO): 125 NaCl, 2.5 KCl, 2 CaCl2, 1.3 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 20 d-glucose bubbled with 95% O2 and 5% CO2 (carbogen). The slices were then incubated in aCSF bubbled with carbogen at 36°C for 40 min. After removal from the incubation chamber, the slices were kept carbogenated at room temperature (Santamaria et al. 2006, 2011; Tanaka and Augustine 2008). All experiments were conducted in accordance with National Institutes of Health guidelines under a protocol approved by the University of Texas at San Antonio Institutional Animal Care and Use Committee (protocol no. MU-082).
The slices were transferred to a microscope chamber continuously perfused with carbogenated aCSF at room temperature (20–22°C). In all experiments, 10 μM gabazine (SR-95531, Tocris) was added to the aCSF to block GABAA channels. Slices were visualized with an Olympus BX51WI upright microscope equipped with a ×20/0.95W objective. Patch pipettes (2–4 MΩ) were fabricated with thin wall borosilicate glass (WPI, Saratosa, FL) using a pipette puller (PC-10, Narishige) and filled with a solution containing the following (in mM, all from Sigma-Aldrich): 130 potassium gluconate, 2 NaCl, 4 Na2ATP, 0.4 NaGTP, 4 MgCl2, 0.2 EGTA, and 30 HEPES (pH 7.3 balanced with KOH).
Motorized micromanipulators (MP225, Sutter) were used to whole cell patch clamp Purkinje cell somas under microscope guidance. Voltage- and current-clamp recordings were obtained using a Multiclamp 700B amplifier, a Digidata 1440A digitizer, and Clampex 10.3 software, all from Axon Instruments (Molecular Devices, Sunnyvale, CA). The patching procedure, monitoring of excitatory postsynaptic currents (EPSCs), and induction of LTD were performed in voltage-clamp mode, with holding potential at −60 mV. An electrode filled with aCSF was placed in the molecular layer, and EPSCs were evoked with a stimulus isolation unit (Grass Technologies, Warwick, RI) by applying current for 500 μs. The stimulation current was determined for each experiment to reliably elicit EPSCs between 50 and 200 pA for every stimulation, which resulted in a range of current of 75 to 125 μA. Intrinsic properties were monitored in current-clamp mode. A hyperpolarizing bias current was used to hold the membrane potential from −60 to −63 mV. The series resistance was compensated and kept below 30 MΩ in voltage clamp and was auto bridge balanced in current clamp. In some experiments, we blocked HCN channels with ZD7288 (Tocris). Initially, we tried intracellular delivery of this compound (up to 80 μM); however, this approach took more than 40 min to completely block the h current, making our experiments unviable. Therefore, we opted for an extracellular application of a low dose of ZD7288 (2 μM) to avoid its effects on presynaptic efficacy (Baginskas et al. 2009; Bender et al. 2007). To keep the cell quiescent during ZD7288 application, we injected a bias current, which resulted in a baseline membrane potential close to −70 mV in current clamp.
Data were sampled at 20 kHz and filtered below 3 kHz in voltage-clamp mode and 10 kHz in current clamp mode. Current and voltage traces were analyzed with custom-built Matlab code (Natick, MA). All data are reported as means ± SE unless otherwise noted. All data were tested to be described by a normal distribution using the Lilliefors test (“lillietest” function in Matlab); thus, most statistical tests were done using a t-test and paired t-test as reported in the text. In the cases that the data failed the Lilliefors test, we performed nonparametric tests as indicated. Linear fits to the data were done using Matlab functions and are reported as ±95% confidence intervals. The code and data are available upon request.
RESULTS
In the present study, we investigated whether the induction of LTD in the parallel fiber to Purkinje cell synapses resulted in changes in Purkinje cell intrinsic excitability, characterized by the firing rate response to current injection. We whole cell patch clamped Purkinje cells and used an extracellular electrode to evoke parallel fiber synaptic activity. We induced LTD in voltage-clamp mode by stimulating parallel fibers concomitantly with a 50-ms-long depolarization of the Purkinje cell to 0 mV at a rate of 1 Hz for 180 s. We measured EPSCs 25 min before and 45 min after LTD induction (n = 12 different cells from 11 animals; Fig. 1A, top). The amplitude of electrically evoked EPSCs was reduced by 36.25 ± 4.03% (Fig. 1A, bottom). We quantified the excitability in current clamp mode by measuring the firing rate of Purkinje cells in response to step current injections of different amplitude (0–250 pA, 600 ms) 15 min before and 40 min after LTD induction (Fig. 1B). The firing rate response for each cell was well fitted by a linear function. The average of the calculated slopes before LTD was 0.13 ± 0.01 Hz/pA (95% confidence intervals) and 0.19 ± 0.01 Hz/pA after LTD (Fig. 1C, top). A paired t-test for the average fitted slope for each neuron before and after LTD induction showed a significant difference in the firing rate gain (P = 0.04). Another form of analyzing the same data is to calculate the difference in firing rate for each input current before and after LTD. This analysis also resulted in a linear relationship with a slope change equal to the gain increase of intrinsic excitability of ΔG = 0.05 ± 0.01 Hz/pA (Fig. 1C, bottom). The same analysis in control experiments (in which we measured firing rate responses 55 min apart without any LTD stimulation) showed no increase in gain (control, n = 9 cells from 8 animals, ΔG = 0.00 ± 0.01 Hz/pA; Fig. 1C, bottom). As opposed to what has been shown in other brain areas (Campanac et al. 2008), the magnitude of LTD was not correlated with the change in excitability. The correlation coefficient between the LTD amplitude and the change in firing rate was r = −0.46 (P = 0.13, not significant). We also tested whether somatic depolarization or parallel fiber stimulation by themselves could cause changes in Purkinje cell excitability. When only the Purkinje cell soma was depolarized (n = 10 cells from 9 animals; Fig. 1C, bottom, Dep), there was no increase in excitability (0.12 ± 0.03 Hz/pA before somatic stimulation and 0.14 ± 0.03 Hz/pA after stimulation, P = 0.62 by paired t-test, not significant). We obtained the same lack of change in excitability after exclusively delivering parallel fiber stimulation (0.15 ± 0.02 before parallel fiber stimulation and 0.16 ± 0.02 after stimulation, n = 7 from 6 animals, P = 0.94 by paired t-test, not significant; Fig. 1C, bottom, PF).
Fig. 1.
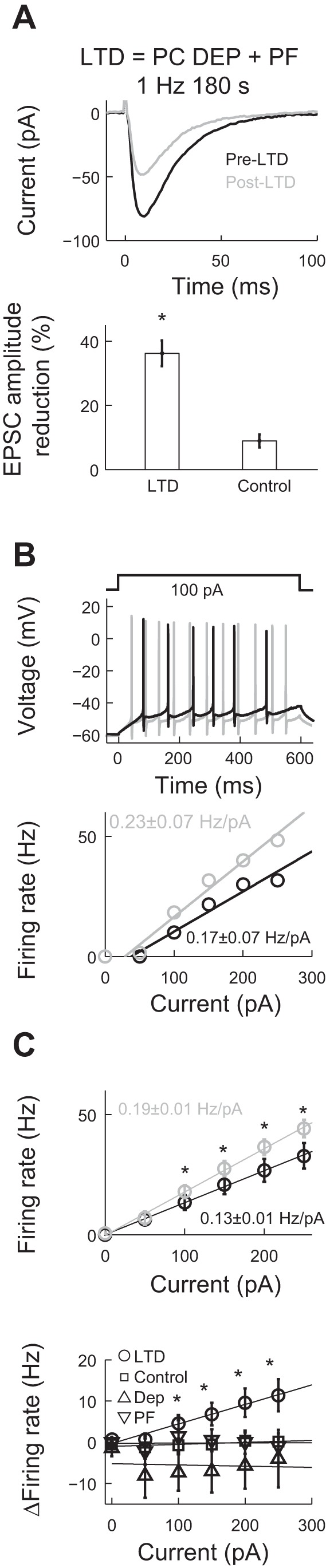
Induction of long-term depression (LTD) in the parallel fiber to Purkinje cell (PC) synapse results in an increase of intrinsic excitability. A, top: representative excitatory postsynaptic currents (EPSCs) measured before (pre) and after (post) LTD induction. Bottom, summary graph showing the EPSC amplitude reduction in LTD (n = 12) and control (n = 4) groups. B, top: representative action potential (AP) traces to the same input current before (pre) and after (post) LTD induction. Bottom, firing rate versus injected current curves for the same cell (fits ± 95% confidence intervals). C, top: average firing rate response before (pre) and after (post) LTD induction (n = 12). Bottom, change in firing rate for all experimental groups. Values are means ± SE; control: n = 9; only somatic depolarization (DEP): n = 10; only parallel fiber stimulation (PF): n = 7. Average of fits to the LTD group showed a slope of ΔG = 0.05 ± 0.01 Hz/pA. *P < 0.05.
To avoid spontaneous firing in the Purkinje cells and to keep the baseline voltage at the same value before and after LTD induction, we injected a bias current to hold the membrane potential between −63 and −60 mV. The average bias current before LTD induction was −96.25 ± 16.70 pA, which changed to −69.58 ± 17.78 pA after LTD induction (average change of 26.67 ± 13.09 pA, P = 0.07 by paired t-test, not significant). The same measurement in the control group showed a change in bias current of 12.78 ± 18.18 pA (P = 0.5 by paired t-test, not significant). We repeated the analysis of firing rate taking into account the bias current. For this purpose, we analyzed the firing versus the total current (bias current + input step). Total current values ranged from −100 to 250 pA. We fitted a line to each of the firing rate versus total current curves before and after LTD induction. We then evaluated all the fits at equally spaced values of the total current. A linear regression analysis of these data showed that ΔG of the control group was 0.00 ± 0.01 Hz/pA, whereas that of the LTD group was 0.05 ± 0.01 Hz/pA, with the slopes being statistically different (P = 1 × 10−3 by t-test). This analysis was in good agreement with the analysis we performed using only the input current steps. Taken together, our results show that the concomitant parallel fiber stimulation and somatic depolarization resulted in both synaptic LTD and an increase of Purkinje cell excitability.
The increase in Purkinje cell excitability after LTD induction could be mediated by the modulation of several conductances (Brager and Johnston 2007; Frick et al. 2004; Ohtsuki et al. 2012; Xu and Kang 2005). We analyzed spiking responses to test whether conductances involved in the first spike latency after the stimulus onset contributed to the increase in excitability. We calculated the time to first action potential after the onset of the stimulation for current steps ≥100 pA, which reliably generated spikes in all traces and neurons tested. This analysis showed that in the LTD group, there was a significant decrease of −21.12 ± 4.01 ms (P = 0.03 by paired t-test) in the time to first spike only at an input current of 100 pA but not for the rest of the input currents (Fig. 2A). Thus, there was no general shift in the time to first action potential, which could have indicated modulation in the activity of A-type K+ current (Molineux et al. 2005; Shibata et al. 2000). To determine if the increase in excitability was due to a reduction of the spiking voltage threshold, we performed a phase plane analysis of the evoked action potentials. The threshold was defined as the voltage at which the first derivative of the membrane potential was larger than 10 mV/ms (Fig. 2B) (Bean 2007; Muñoz and Fuentealba 2012; Ritter et al. 2012). We determined the thresholds for each one of the four input current steps in the control and LTD groups. In the control group, the first measurement of the voltage action potential threshold ranged from an average of −46.05 ± 0.36 mV with a 100-pA input to −43.93 ± 0.28 mV at 250 pA (P = 1.9 × 10−5, significant). We tested whether there was a change in the voltage threshold in the control experiments that could be attributed to the duration of the experiment. Thus, we repeated the voltage threshold measurement after 55 min and quantified the difference in voltage threshold (ΔVth). This analysis showed a slight change in voltage threshold depending on the current input. For a current input of 100 pA, ΔVth = −1.80 ± 0.57 mV (P = 0.01 by paired t-test, significant) and for a current input of 250 pA, ΔVth = −0.832 ± 0.57 mV (P = 0.18 by paired t-test, not significant; Fig. 2C, top). We repeated this same analysis in the LTD group. For a current input of 100 pA, we found that ΔVth = −1.77 ± 0.46 mV (P = 0.01 by paired t-test, significant) and for a current input of 250 pA, ΔVth = −0.94 ± 0.67 mV (P = 0.68 by paired t-test, not significant; Fig. 2C, bottom). As expected, a comparison of the values of ΔVth between the control and LTD groups for each one of the input currents showed no significance (all P > 0.71 by t-test). Thus, the increase of excitability was not due to a change of the spiking threshold of the Purkinje cell after LTD induction.
Fig. 2.
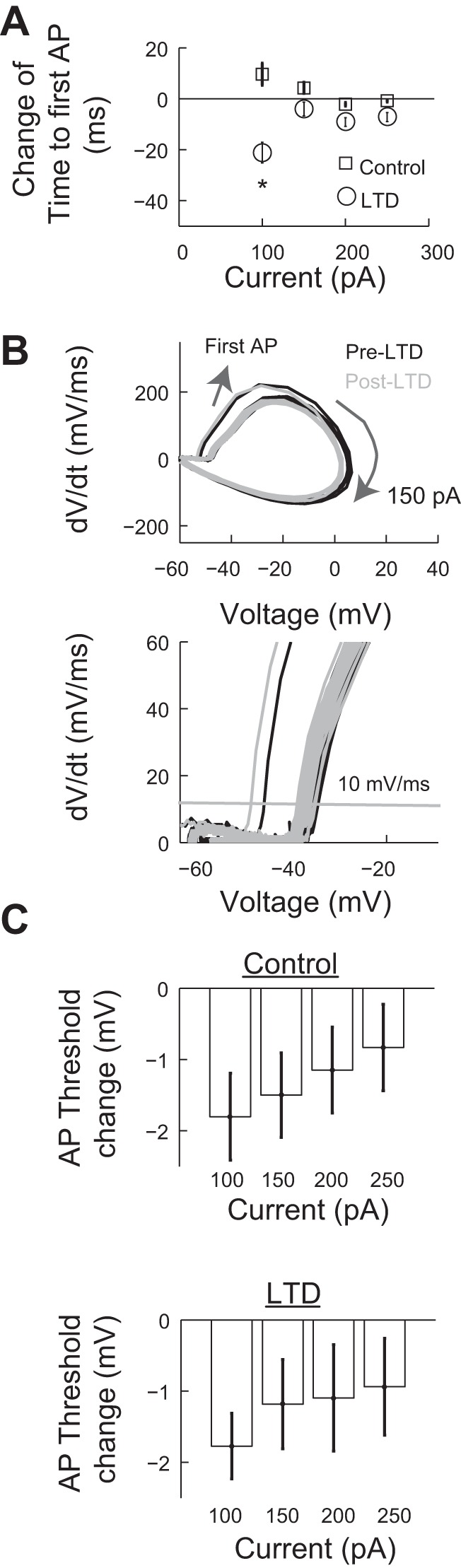
PC AP properties do not change after LTD induction. A: change in time to first spike in the control (n = 9) and LTD groups (n = 8 cells). *P < 0.05. B, top: representative AP phase plane plot generated with the same input current before (pre) and after (post) LTD induction. Bottom, detail from the top phase plane showing the threshold line. C, top: change in AP thresholds in the control group for each input current amplitude (n = 9). Bottom, same measurement for the LTD group (n = 8). Values are means ± SE. dV/dt, change in voltage over time.
To characterize changes in excitability not directly related to the spiking threshold, we applied a series of hyperpolarizing current steps before and after LTD induction. The hyperpolarization of the Purkinje cell revealed the presence of a voltage sag (Fernandez et al. 2007; Williams et al. 2002). By the end of an 800-ms stimulus, the voltage reached a stable value (Fig. 3A, top). We determined that the plots of the steady-state voltage versus input current between −300 and 0 pA were well fitted by a line with an average r2 value of 0.96 ± 0.01 for the LTD and control groups (Fig. 3A, bottom). We considered the value of the calculated slope from the fits a measure of the input resistance of the cell. The average slope was 63.84 ± 3.73 MΩ before and 71.91 ± 3.72 MΩ after LTD induction (n = 10; Fig. 3B). We also calculated the difference in input resistance in all the other experimental groups. This analysis showed that only the LTD group had a significant increase of 13.35 ± 4.78% (P < 0.03 by paired t-test; Fig. 3C). Thus, LTD induction is followed by an increase in the Purkinje cell input resistance.
Fig. 3.
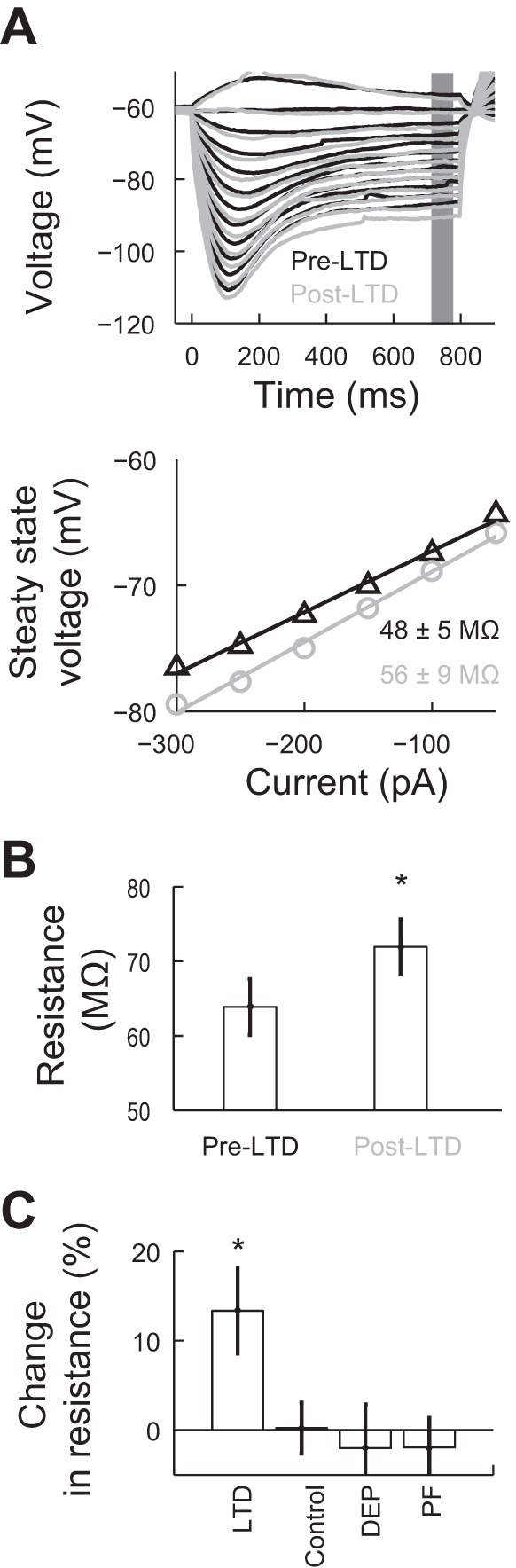
PC input resistance increases after LTD induction. A, top: representative traces of the response of a PC to multiple current steps before (pre) and after (post) LTD induction. The gray area corresponds to the section of the voltage used to generate the voltage versus input current plot. Bottom, linear fit to these measurements before (pre) and after (post) LTD induction (slope ± 95% confidence interval). B: summary graph of changes in input resistance in the LTD group (n = 10). C: change in input resistance in all experimental groups. Values are means ± SE; LTD: n = 10; control: n = 10; DEP: n = 9, and PF: n = 12. *P < 0.05.
The increase in input resistance indicates a downregulation of conductances after LTD induction. In our experiment, we observed a prominent voltage sag that was generated by the activation of HCN channels upon membrane hyperpolarization (Biel et al. 2009). Purkinje cells show high expressions of HCN1 (Nolan et al. 2003; Santoro et al. 2000) and, at smaller amounts, of HCN2 (Notomi and Shigemoto 2004; Santoro et al. 2000). We compared the amplitude of the sag before and after LTD induction. To perform this comparison, it was necessary to take into account the changes in input resistance, which could result in a different voltage response and HCN activation to the same input current (Brager and Johnston 2007). Therefore, we matched the maximum voltage deflection (peak) before and after LTD induction (Fig. 4A). We then calculated the sag ratio [SR = 100 (sag/peak)] for each trace and computed the percentage change between the SR before (SRpre) and after (SRpost) LTD induction {ΔSR = 100[(SRpost − SRpre)/SRpre]}. The average ΔSR was −10.93 ± 1.18% in the LTD group (n = 11 cells, 99 trace pairs for LTD, P = 0.02 by Wilcoxon signed-rank test, significant; Fig. 4B) and 2.74 ± 0.78% in the control group (17 trace pairs, P = 0.74 by Wilcoxon signed-rank test, not significant). The decrease in ΔSR in the LTD group was significantly different than in the control group (P = 1 × 10−5 by Wilcoxon rank-sum test). Taken together, these analyses show that the voltage sag decreased after LTD induction. This indicates a decrease of h current, and this loss of conductance could result in an increase of the input resistance of the Purkinje cell.
Fig. 4.
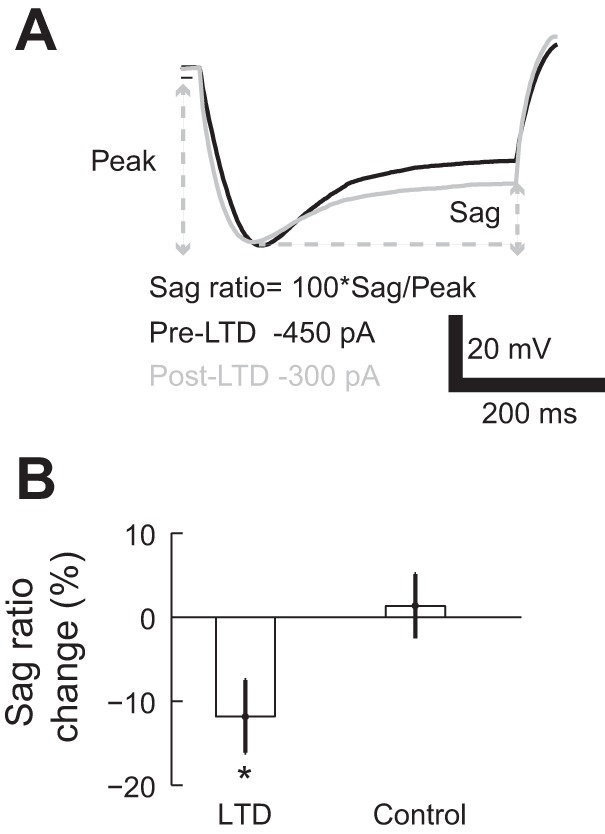
PC voltage sag decreases after LTD induction. A: representative traces showing the comparison of sag potential before (pre) and after (post) LTD induction. The comparisons were made based on traces with similar peak amplitude. B: percentage change in sag ratio in the LTD and control groups (*P < 0.05). Values are means ± SE.
The AHP evoked following a spike train is usually called the slow AHP (sAHP) (Schmolesky et al. 2002). Changes in the properties of sAHP have been implicated in intrinsic plasticity (Daoudal and Debanne 2003; Xu et al. 2005). In Purkinje cells, sAHP has been attributed to Ca2+-activated K+ currents, including SK currents (Hosy et al. 2011; Schmolesky et al. 2002). However, HCN-mediated h current has also been found to contribute to sAHP in other neuronal types (Bonin et al. 2013; Gulledge et al. 2013; Oswald et al. 2009; Steuber et al. 2007). We quantified the effect of LTD induction on sAHP by averaging four voltage responses of the cell to 250-pA, 500-ms-long stimuli that generated spiking (Fig. 5A, top). We calculated the percent change in amplitude, time to the maximum value, and area below resting potential over a window of 1,500 ms in the LTD and control groups (Fig. 5A, bottom). In the control group, none of these measurements had a significant change. In the LTD group, the only value that had a significant change was the time to the maximum value (−8.79 ± 2.79%, from 1069 ± 30 ms before LTD to 972 ± 29 ms after LTD, n = 8 cells, P = 0.02 by t-test). The amplitude of sAHP did not increase even when the average firing rate increased by 22 ± 13% after LTD induction. We performed the same analysis in which the firing rate was matched before and after LTD in the same cell (the maximum absolute difference in firing rate was 10%, n = 14 pairs, 8 cells). This analysis showed that while sAHP amplitude did not change (P = 0.09 by paired t-test, not significant), the time to peak decreased by −8.62 ± 2.00% from 1,228 ± 26 ms before LTD to 1,132 ± 22 ms after LTD induction (P = 0.01 by paired t-test, significant). Therefore, our results show that after LTD induction, there is a decrease in the amplitude of the voltage sag and time to peak of the AHP.
Fig. 5.
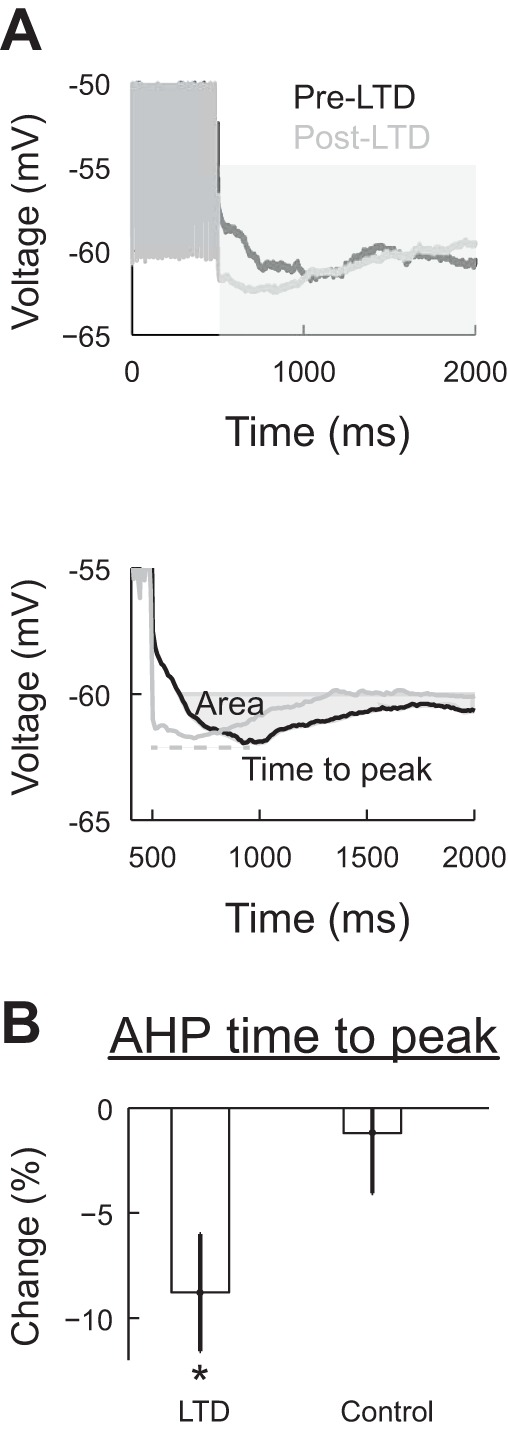
The time to peak of the afterhyperpolarization (AHP) decreases after LTD induction. A, top: representative traces of the evoked AHP after a 600-ms, 250-pA stimulation before (pre) and after (post) LTD induction. The gray area shows the section used for the AHP measurements. Bottom, average of four traces before and after LTD induction. The area was measured below the prestimulus potential (−60 mV), and the time to peak was calculated relative to the end of the stimulus. B: comparison of the change in time to peak in the LTD and control groups. Values are means ± SE; n = 7 cells in both groups. *P < 0.05.
Our results suggest that HCN channels might be involved in the increase of excitability of Purkinje cells after LTD induction. To test this hypothesis, we performed LTD experiments in the presence of an extracellularly applied HCN blocker (ZD7822) at a concentration of 2 μM (Harnett et al. 2015). Under these conditions, our LTD protocol resulted in an EPSC amplitude decrease of 50.23 ± 0.03% (n = 7 different cells, P = 2.5 × 10−4 by paired t-test, significant; Fig. 6A) (Guli et al. 2012). Application of ZD7822 compound in control group, where no LTD stimulation was applied, resulted in no significant decrease of the EPSC (control, n = 5, P = 0.30 by paired t-test, significant). The spiking response versus injected current plot showed statistically identical linear relationships with the slope being 0.20 ± 0.01 Hz/pA before and after LTD with no statistical difference in amplitude (Fig. 6B, top). Control experiments did not show a significant change in excitability (Fig. 6B, bottom). There was no change in the input resistance (181.31 ± 7.72 MΩ before LTD and 207.24 ± 10.47 MΩ after LTD, n = 4 cells; Fig. 6C, top), although the input resistance was three times more than the input resistance measured without the compound (cf. Fig. 3B), suggesting high levels of HCN channel expression in Purkinje cells (Fig. 6C, bottom). Application of 10 μM ZD7288 blocked sAHP (n = 12 cells, P = 2 × 10−4 by Wilcoxon rank-sum test, significant; Fig. 6D). We conclude that LTD induction decreases HCN-mediated h current, which results in an increase of the input resistance and excitability of the Purkinje cell.
Fig. 6.
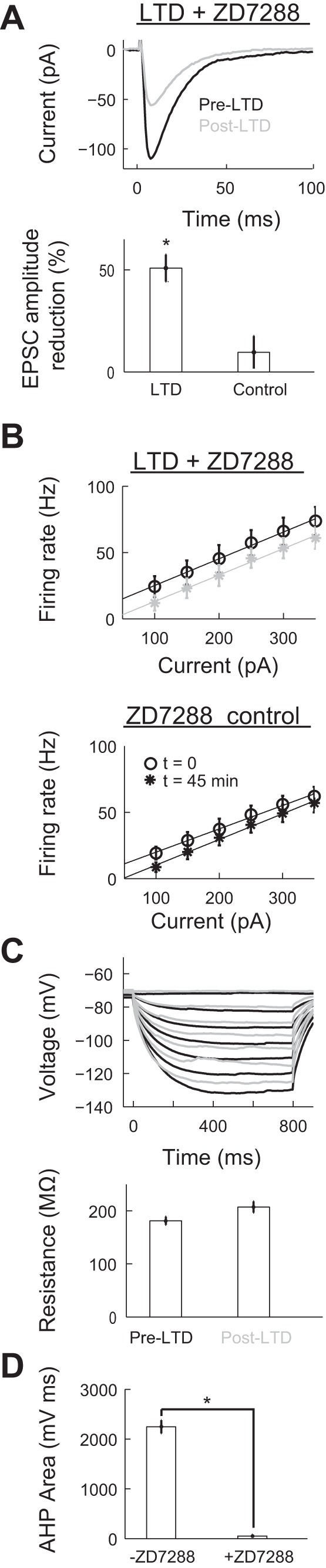
Blockade of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels prevents the increase in excitability after LTD induction without blocking LTD expression. A, top: representative traces of EPSCs before (pre) and after (post) LTD induction in the presence of an extracellularly added HCN channel blocker (ZD7288; 2 μM). Bottom, summary graph of the EPSC amplitude reduction in LTD (n = 7) and ZD7288 control (n = 5) groups. *P < 0.05. B: average firing rate versus injected current responses showed identical slopes in the LTD (top) and control (bottom) groups, both in the presence of ZD7288. C, top: representative trace of voltage in response to multiple input current steps before and after LTD in the presence of ZD7288. Bottom, average input resistance before and after the induction of LTD (n = 4). D: AHP area below the holding potential in the absence (−ZD7288, n = 12) and presence (+ZD7288, 10 μM, n = 11) of ZD7288 compound.
DISCUSSION
We have shown that the induction of LTD in parallel fiber to Purkinje cell synapses results in an increase of intrinsic excitability. This intrinsic plasticity is accompanied by a reduction of the voltage sag and an increase of input resistance. We also showed that concomitant somatic depolarization and parallel fiber stimulation were required for the observed increase in excitability. Blockade of HCN channels with ZD7288 compound eliminated the voltage sag and AHP and blocked the increase in excitability after LTD induction. Our data suggest that LTD induction results in a downregulation of HCN-mediated h current. While changes in intrinsic excitability could be homeostatic, they could also be part of nonsynaptic long-term plasticity mechanisms that could affect synaptic integration and function. This is of particular importance in the cerebellar cortex since Purkinje cells are the sole output and express changes in excitability in pathological conditions (Roselli and Caroni 2015; Shakkottai et al. 2009; Tsai et al. 2012).
Activity-dependent changes in intrinsic excitability are prevalent across the brain and could contribute to long-term information storage (Aizenman and Linden 2000; Armano et al. 2000; Brager and Johnston 2007; Contractor et al. 2015; Fan et al. 2005; Kourrich et al. 2015; McKay et al. 2013; Ohtsuki et al. 2012; Xu et al. 2005). In particular, the modulation of HCN has been implicated in cellular mechanisms of memory storage (Nolan et al. 2003; Rinaldi et al. 2013; Schreurs et al. 1998; Schreurs et al. 1997). The effects of this intrinsic plasticity on Purkinje cell output depend on its relative direction with respect to synaptic plasticity. While our data show that LTD induction resulted in an increase in intrinsic excitability, recent work has shown that LTP is also accompanied by an increase in excitability (Belmeguenai et al. 2010). In the case of LTP, changes in intrinsic excitability are mediated by a downregulation of SK channels, while in our case, it is HCN channels that are downregulated after LTD induction. Thus, depending on the type of synaptic stimulation, increases in intrinsic excitability can be mediated by modulation of different conductances through multiple biochemical pathways.
The effect of h current on the Purkinje cell firing rate has also been observed in studies using HCN1 knockout (HCN1−/−) mice. In particular, when using bidirectional current-ramp stimulation designed with a hyperpolarizing and depolarizing phases, a study showed that in the depolarizing phase, the slope of the firing rate versus input current was larger in HCN1−/− Purkinje cells than in the control [cf. Fig. 7E (Nolan et al. 2003)], which is consistent with our results. The effect of the voltage sag reduction on the firing gain increase could be due to voltage-dependent activation properties of the h current. This current has a bidirectional rectification effect on both hyperpolarization and depolarization (Biel et al. 2009). Membrane hyperpolarization activates HCN channels, causing an inward depolarizing current that drives the membrane potential back to the initial state. In contrast, membrane depolarization deactivates HCN and the total h current decreases, resulting in a membrane potential closer to the original value. As the membrane is further depolarized, less h current flows into the cell. Thus, the firing rate gain decreases due to the effect of the h current. Consistent with this explanation, our results showed an increase in firing rate gain due to the reduction of h current.
Despite the depolarizing nature of h current, HCN channels usually exert a shunting effect in dendrites, causing a reduction of amplitude and duration of excitatory and inhibitory postsynaptic potentials (Atherton et al. 2010; Kase and Imoto 2012). As a consequence, the downregulation of h current can increase excitatory postsynaptic potential temporal summation and enhance excitatory postsynaptic potential-spike coupling (Magee 1998; Shah et al. 2004). Considering the spontaneously active state of Purkinje cells and the activation of HCN at more hyperpolarized membrane potentials, the reduction of h current may have a larger impact on inhibitory input integration (Atherton et al. 2010; Kase and Imoto 2012).
The combination of our electrophysiological and pharmacological manipulations allows us to conclude that h current downregulation causes intrinsic plasticity after LTD induction. However, to determine the current magnitude and kinetic changes, it would require voltage-clamp studies. Another aspect to take into account in interpreting our experiments is that we performed them at room temperature. While there are temperature-dependent effects in the activation of HCN conductance, these changes have been reported to be nonsignificant at the membrane potential range we used as the holding potential (−63 to −60 mV) (Gambardella et al. 2012; Yanagida et al. 2000). However, more hyperpolarized values, such as −80 mV, HCN current shows a strong temperature dependence (Magee 1998). Other variables that have to be considered to compare with our results are animal age and the use of negative bias current.
After LTD induction there was no reduction in sAHP amplitude, which could be due to compensation between the increase in input resistance and the reduction of h current. However, we found that the timing to maximum voltage of sAHP was reduced after LTD. The mechanism of decrease in sAHP timing is not well understood, but it could be due to a stronger activation of repolarizing channels. We are currently studying this with a computational model.
In our results, there was an apparent lack of SK contribution to sAHP. This could be due to the expression patterns of SK channels in mice, which have been reported to cluster around parallel fiber synapses (Ballesteros-Merino et al. 2014). The failure of action potential backpropagation in Purkinje cells (Vetter et al. 2001) would then result in few SK channels being activated by a train of somatically evoked action potentials.
Several studies have shown that ZD7288 also inhibits low-threshold T-type Ca2+ channels (IT) (Felix et al. 2003; Sanchez-Alonso et al. 2008). However, complete blockade of this channel requires considerably higher concentrations of ZD7288 than the concentrations used in our study (IC50 of ∼40 μM in hippocampal pyramidal cell). It has been shown that at 10 μM, the reduction of IT by ZD7288 is ∼0.8% (Sanchez-Alonso et al. 2008); thus, at the 2 μM concentration we used to occlude intrinsic plasticity, we expect the IT reduction by ZD7288 to be undetectable. Furthermore, considering the inactivation properties of IT, its contribution to AHP after 600 ms of spike train activity should be small. Therefore, in our case, ZD7288-sensitive AHP was mostly mediated by HCN channels.
Intrinsic excitability changes can be spatially distributed. As mentioned in methods, intracellular delivery of ZD7288 compound took >40 min to block the voltage sag, suggesting that the compound was slow to diffuse and that HCN channels could be distributed throughout the Purkinje cell dendrite, consistent with a previous report (Angelo et al. 2007). Since we did not know if the changes responsible for the decrease in SR were local or global, we bath applied the HCN blocker. There are three basic possibilities for the distribution of HCN downregulation. First, the h current could decrease local to the site of dendritic stimulation. This would affect the conditioned synapses and those synapses distal from the site of stimulation. A second possibility is that the change is across multiple branches or the entire dendrite. A third possibility is that the decrease in HCN could take place at the soma, where it would affect the throughput of all synaptic activity. It has been suggested that an increase in excitability after LTP is a mechanism to reduce the effect of the potentiated synapses on the output of the Purkinje cell, which has been referred to as decrease in the signal-to-noise ratio (Belmeguenai et al. 2010). Following this interpretation, the increase in excitability after LTD enhances the contrast between depressed and unconditioned synapses and thus increases the signal-to-noise ratio of the depressed synapses. Intrinsic excitability in the Purkinje cell could be a mechanism that preferentially enhances the information encoded in depressed synapses.
The biochemical pathway linking LTD induction and decrease of the h current is not known. Since the increase in excitability is during the same time window of LTD expression, we expect that the effects of LTD on HCNs are through biochemical reactions rather than on protein expression regulation. In Purkinje cells, LTD has been described as a positive feedback loop between PKC and MAPK (Tanaka and Augustine 2008; Tanaka et al. 2007). This biochemical reaction results activated PKC phosphorylating α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, which are later internalized. It has recently been shown that HCN can be phosphorylated by PKC, reducing HCN surface expression (Biel et al. 2009; Williams et al. 2015). Our results suggest that the increase in intracellular Ca2+ due to somatic depolarization or parallel fiber stimulation alone is not sufficient to trigger intrinsic plasticity. Therefore, we propose that the combined activation of PKC due to somatic and synaptic stimulation is required to downregulate the function of HCN channels. This model predicts that the time course of the increase in excitability follows the expression of LTD, consistent with our results. Furthermore, this model also suggests that the changes in excitability are expected to happen close to the site of LTD induction.
Most theories of cerebellar function assume that the planar dendrite of a Purkinje cell acts as a matrix where synaptic plasticity stores patterns of parallel fibers that are, mostly, linearly integrated and translated by the soma into trains of action potentials (Albus 1971; Brunel et al. 2004; Clopath and Brunel 2013; Clopath et al. 2012; Marr 1969). Individual Purkinje cells receive inputs from multiple somatosensory or proprioceptive areas (Santamaria et al. 2007; Shambes et al. 1978; Shumway et al. 2005). Thus, bundles of axons synapsing close to each other on a Purkinje cell could carry information from the same somatosensory patch. Therefore, increases of intrinsic excitability after LTD induction could be a form of priming a Purkinje cell to enhance its firing rate to further input from the same somatosensory patch or change the threshold for synaptic plasticity (Narayanan and Johnston 2010; Tsay et al. 2007). As a consequence, plasticity of intrinsic excitability can be a mechanism to steer the coding capacity of the Purkinje cell to specific input sources.
GRANTS
This work was supported by National Science Foundation Grants NSF 1208029 and NSF 1137897 and by National Center on Minority Health and Health Disparities Grant G12-MD-007591 (Computational Biology Core).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Z.Y. and F.S. conception and design of research; Z.Y. performed experiments; Z.Y. and F.S. analyzed data; Z.Y. and F.S. interpreted results of experiments; Z.Y. and F.S. drafted manuscript; Z.Y. and F.S. edited and revised manuscript; Z.Y. and F.S. approved final version of manuscript; F.S. prepared figures.
ACKNOWLEDGMENTS
The authors thank Dr. Keiko Tanaka-Yamamoto for comments on a presubmission version of the manuscript.
REFERENCES
- Aizenman CD, Akerman CJ, Jensen KR, Cline HT. Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron 39: 831–842, 2003. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Linden DJ. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat Neurosci 3: 109–111, 2000. [DOI] [PubMed] [Google Scholar]
- Albus JS. A theory of cerebellar function. Math Biosci 10: 25–61, 1971. [Google Scholar]
- Angelo K, London M, Christensen SR, Häusser M. Local and global effects of Ih distribution in dendrites of mammalian neurons. J Neurosci 27: 8643–8653, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armano S, Rossi P, Taglietti V, D'angelo E. Long-term potentiation of intrinsic excitability at the mossy fiber-granule cell synapse of rat cerebellum. J Neurosci 20: 5208–5216, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton JF, Kitano K, Baufreton J, Fan K, Wokosin D, Tkatch T, Shigemoto R, Surmeier DJ, Bevan MD. Selective participation of somatodendritic HCN channels in inhibitory but not excitatory synaptic integration in neurons of the subthalamic nucleus. J Neurosci 30: 16025–16040, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baginskas A, Palani D, Chiu K, Raastad M. The H-current secures action potential transmission at high frequencies in rat cerebellar parallel fibers. Eur J Neurosci 29: 87–96, 2009. [DOI] [PubMed] [Google Scholar]
- Ballesteros-Merino C, Martínez-Hernández J, Aguado C, Watanabe M, Adelman JP, Luján R. Localization of SK2 channels relative to excitatory synaptic sites in the mouse developing Purkinje cells. Front Neuroanat 8: 154, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci 8: 451–465, 2007. [DOI] [PubMed] [Google Scholar]
- Belmeguenai A, Hosy E, Bengtsson F, Pedroarena CM, Piochon C, Teuling E, He Q, Ohtsuki G, De Jeu MT, Elgersma Y, De Zeeuw CI, Jorntell H, Hansel C. Intrinsic plasticity complements long-term potentiation in parallel fiber input gain control in cerebellar Purkinje cells. J Neurosci 30: 13630–13643, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender RA, Kirschstein T, Kretz O, Brewster AL, Richichi C, Rüschenschmidt C, Shigemoto R, Beck H, Frotscher M, Baram TZ. Localization of HCN1 channels to presynaptic compartments: novel plasticity that may contribute to hippocampal maturation. J Neurosci 27: 4697–4706, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev 89: 847–885, 2009. [DOI] [PubMed] [Google Scholar]
- Bonin RP, Zurek AA, Yu J, Bayliss DA, Orser BA. Hyperpolarization-activated current (In) is reduced in hippocampal neurons from Gabra5−/− mice. PLos One 8: e58679, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager DH, Johnston D. Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in Ih in hippocampal CA1 pyramidal neurons. J Neurosci 27: 13926–13937, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunel N, Hakim V, Isope P, Nadal JP, Barbour B. Optimal information storage and the distribution of synaptic weights: perceptron versus Purkinje cell. Neuron 43: 745–757, 2004. [DOI] [PubMed] [Google Scholar]
- Campanac E, Daoudal G, Ankri N, Debanne D. Downregulation of dendritic Ih in CA1 pyramidal neurons after LTP. J Neurosci 28: 8635–8643, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clopath C, Brunel N. Optimal properties of analog perceptrons with excitatory weights. PLos Comput Biol 9: e1002919, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clopath C, Nadal JP, Brunel N. Storage of correlated patterns in standard and bistable Purkinje cell models. PLos Comput Biol 8: e1002448, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor A, Klyachko VA, Portera-Cailliau C. Altered neuronal and circuit excitability in fragile X syndrome. Neuron 87: 699–715, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoudal G, Debanne D. Long-term plasticity of intrinsic excitability: learning rules and mechanisms. Learn Mem 10: 456–465, 2003. [DOI] [PubMed] [Google Scholar]
- Fan Y, Fricker D, Brager DH, Chen X, Lu HC, Chitwood RA, Johnston D. Activity-dependent decrease of excitability in rat hippocampal neurons through increases in Ih. Nat Neurosci 8: 1542–1551, 2005. [DOI] [PubMed] [Google Scholar]
- Felix R, Sandoval A, Sanchez D, Gomora JC, De la Vega-Beltran JL, Trevino CL, Darszon A. ZD7288 inhibits low-threshold Ca2+ channel activity and regulates sperm function. Biochem Biophys Res Commun 311: 187–192, 2003. [DOI] [PubMed] [Google Scholar]
- Fernandez FR, Engbers JD, Turner RW. Firing dynamics of cerebellar purkinje cells. J Neurophysiol 98: 278–294, 2007. [DOI] [PubMed] [Google Scholar]
- Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat Neurosci 7: 126–135, 2004. [DOI] [PubMed] [Google Scholar]
- Gambardella C, Pignatelli A, Belluzzi O. The h-current in the substantia nigra pars compacta neurons: a re-examination. PLos One 7: e52329, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G, Hansel C. Cerebellar long-term potentiation: cellular mechanisms and role in learning. Int Rev Neurobiol 117: 39–51, 2014. [DOI] [PubMed] [Google Scholar]
- Guli X, Tokay T, Rohde M, Bender RA, Köhling R, Kirschstein T. ZD7288 enhances long-term depression at early postnatal medial perforant path-granule cell synapses. Neural Plasticity 2012: 237913, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Dasari S, Onoue K, Stephens EK, Hasse JM, Avesar D. A sodium-pump-mediated afterhyperpolarization in pyramidal neurons. J Neurosci 33: 13025–13041, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel C, Linden DJ, D'Angelo E. Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat Neurosci 4: 467–475, 2001. [DOI] [PubMed] [Google Scholar]
- Harnett MT, Magee JC, Williams SR. Distribution and function of HCN channels in the apical dendritic tuft of neocortical pyramidal neurons. J Neurosci 35: 1024–1037, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosy E, Piochon C, Teuling E, Rinaldo L, Hansel C. SK2 channel expression and function in cerebellar Purkinje cells. J Physiol 589: 3433–3440, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörntell H, Hansel C. Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron 52: 227–238, 2006. [DOI] [PubMed] [Google Scholar]
- Kase D, Imoto K. The Role of HCN channels on membrane excitability in the nervous system. J Signal Transduct 2012: 619747, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispersky TJ, Caplan JS, Marder E. Increase in sodium conductance decreases firing rate and gain in model neurons. J Neurosci 32: 10995–11004, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Calu DJ, Bonci A. Intrinsic plasticity: an emerging player in addiction. Nat Rev Neurosci 16: 173–184, 2015. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci 18: 7613–7624, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Carruth M. Dendritic voltage-gated ion channels regulate the action potential firing mode of hippocampal CA1 pyramidal neurons. J Neurophysiol 82: 1895–1901, 1999. [DOI] [PubMed] [Google Scholar]
- Mahon S, Charpier S. Bidirectional plasticity of intrinsic excitability controls sensory inputs efficiency in layer 5 barrel cortex neurons in vivo. J Neurosci 32: 11377–11389, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol 202: 437–470, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BM, Oh MM, Disterhoft JF. Learning increases intrinsic excitability of hippocampal interneurons. J Neurosci 33: 5499–5506, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molineux ML, Fernandez FR, Mehaffey WH, Turner RW. A-type and T-type currents interact to produce a novel spike latency-voltage relationship in cerebellar stellate cells. J Neurosci 25: 10863–10873, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motanis H, Maroun M, Barkai E. Learning-induced bidirectional plasticity of intrinsic neuronal excitability reflects the valence of the outcome. Cerebral Cortex 24: 1075–1087, 2014. [DOI] [PubMed] [Google Scholar]
- Muñoz F, Fuentealba P. Dynamics of action potential initiation in the GABAergic thalamic reticular nucleus in vivo. PLos One 7: e30154, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R, Johnston D. The h current is a candidate mechanism for regulating the sliding modification threshold in a BCM-like synaptic learning rule. J Neurophysiol 104: 1020–1033, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Lee KH, Gibbs E, Dudman JT, Santoro B, Yin D, Thompson RF, Siegelbaum SA, Kandel ER. The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell 115: 551–564, 2003. [DOI] [PubMed] [Google Scholar]
- Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol 471: 241–276, 2004. [DOI] [PubMed] [Google Scholar]
- Ohtsuki G, Piochon C, Adelman JP, Hansel C. SK2 channel modulation contributes to compartment-specific dendritic plasticity in cerebellar Purkinje cells. Neuron 75: 108–120, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald MJ, Oorschot DE, Schulz JM, Lipski J, Reynolds JN. IH current generates the afterhyperpolarisation following activation of subthreshold cortical synaptic inputs to striatal cholinergic interneurons. J Physiol 587: 5879–5897, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi A, Defterali C, Mialot A, Garden DL, Beraneck M, Nolan MF. HCN1 channels in cerebellar Purkinje cells promote late stages of learning and constrain synaptic inhibition. J Physiol 591: 5691–5709, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter DM, Ho C, O'Leary ME, Covarrubias M. Modulation of Kv3.4 channel N-type inactivation by protein kinase C shapes the action potential in dorsal root ganglion neurons. J Physiol 590: 145–161, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo V, Richman J, Puthanveettil SV. Dissecting mechanisms of brain aging by studying the intrinsic excitability of neurons. Front Aging Neurosci 6: 337, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli F, Caroni P. From intrinsic firing properties to selective neuronal vulnerability in neurodegenerative diseases. Neuron 85: 901–910, 2015. [DOI] [PubMed] [Google Scholar]
- Sanchez-Alonso JL, Halliwell JV, Colino A. ZD 7288 inhibits T-type calcium current in rat hippocampal pyramidal cells. Neurosci Lett 439: 275–280, 2008. [DOI] [PubMed] [Google Scholar]
- Santamaria F, Tripp PG, Bower JM. Feedforward inhibition controls the spread of granule cell-induced Purkinje cell activity in the cerebellar cortex. J Neurophysiol 97: 248–263, 2007. [DOI] [PubMed] [Google Scholar]
- Santamaria F, Wils S, De Schutter E, Augustine GJ. Anomalous diffusion in Purkinje cell dendrites caused by spines. Neuron 52: 635–648, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria F, Wils S, De Schutter E, Augustine GJ. The diffusional properties of dendrites depend on the density of dendritic spines. Eur J Neurosci 34: 561–568, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Chen S, Lüthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci 20: 5264–5275, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacher S, Hu JY. The less things change, the more they are different: contributions of long-term synaptic plasticity and homeostasis to memory. Learn Mem 21: 128–134, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmolesky MT, Weber JT, De Zeeuw CI, Hansel C. The making of a complex spike: ionic composition and plasticity. Ann NY Acad Sci 978: 359–390, 2002. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Gusev PA, Tomsic D, Alkon DL, Shi T. Intracellular correlates of acquisition and long-term memory of classical conditioning in Purkinje cell dendrites in slices of rabbit cerebellar lobule HVI. J Neurosci 18: 5498–5507, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Tomsic D, Gusev PA, Alkon DL. Dendritic excitability microzones and occluded long-term depression after classical conditioning of the rabbit's nictitating membrane response. J Neurophysiol 77: 86–92, 1997. [DOI] [PubMed] [Google Scholar]
- Shah MM, Anderson AE, Leung V, Lin X, Johnston D. Seizure-induced plasticity of h channels in entorhinal cortical layer III pyramidal neurons. Neuron 44: 495–508, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakkottai VG, Xiao M, Xu L, Wong M, Nerbonne JM, Ornitz DM, Yamada KA. FGF14 regulates the intrinsic excitability of cerebellar Purkinje neurons. Neurobiol Dis 33: 81–88, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shambes GM, Gibson JM, Welker W. Fractured somatotopy in granule cell tactile areas of rat cerebellar hemispheres revealed by micromapping. Brain Behav Evol 15: 94–140, 1978. [DOI] [PubMed] [Google Scholar]
- Shepherd GM. The Synaptic Organization of the Brain. Oxford: Oxford Univ. Press, 2004, p. xiv. [Google Scholar]
- Shibata R, Nakahira K, Shibasaki K, Wakazono Y, Imoto K, Ikenaka K. A-type K+ current mediated by the Kv4 channel regulates the generation of action potential in developing cerebellar granule cells. J Neurosci 20: 4145–4155, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumway CA, Morissette J, Bower JM. Mechanisms underlying reorganization of fractured tactile cerebellar maps after deafferentation in developing and adult rats. J Neurophysiol 94: 2630–2643, 2005. [DOI] [PubMed] [Google Scholar]
- Steuber V, Mittmann W, Hoebeek FE, Silver RA, De Zeeuw CI, Häusser M, De Schutter E. Cerebellar LTD and pattern recognition by Purkinje cells. Neuron 54: 121–136, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Augustine GJ. A positive feedback signal transduction loop determines timing of cerebellar long-term depression. Neuron 59: 608–620, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Khiroug L, Santamaria F, Doi T, Ogasawara H, Ellis-Davies GC, Kawato M, Augustine GJ. Ca2+ requirements for cerebellar long-term synaptic depression: role for a postsynaptic leaky integrator. Neuron 54: 787–800, 2007. [DOI] [PubMed] [Google Scholar]
- Tsai PT, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, Sahin M. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature 488: 647–651, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay D, Dudman JT, Siegelbaum SA. HCN1 channels constrain synaptically evoked Ca2+ spikes in distal dendrites of CA1 pyramidal neurons. Neuron 56: 1076–1089, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci 22: 221–227, 1999. [DOI] [PubMed] [Google Scholar]
- Vetter P, Roth A, Häusser M. Propagation of action potentials in dendrites depends on dendritic morphology. J Neurophysiol 85: 926–937, 2001. [DOI] [PubMed] [Google Scholar]
- Wang Z, Xu NL, Wu CP, Duan S, Poo MM. Bidirectional changes in spatial dendritic integration accompanying long-term synaptic modifications. Neuron 37: 463–472, 2003. [DOI] [PubMed] [Google Scholar]
- Williams AD, Jung S, Poolos NP. Protein kinase C bidirectionally modulates Ih and hyperpolarization-activated cyclic nucleotide-gated (HCN) channel surface expression in hippocampal pyramidal neurons. J Physiol 593: 2779–2792, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Christensen SR, Stuart GJ, Häusser M. Membrane potential bistability is controlled by the hyperpolarization-activated current IH in rat cerebellar Purkinje neurons in vitro. J Physiol 539: 469–483, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kang J. The mechanisms and functions of activity-dependent long-term potentiation of intrinsic excitability. Rev Neurosci 16: 311–323, 2005. [DOI] [PubMed] [Google Scholar]
- Xu J, Kang N, Jiang L, Nedergaard M, Kang J. Activity-dependent long-term potentiation of intrinsic excitability in hippocampal CA1 pyramidal neurons. J Neurosci 25: 1750–1760, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida H, Inoue R, Tanaka M, Ito Y. Temperature-sensitive gating of cation current in guinea pig ileal muscle activated by hyperpolarization. Am J Physiol Cell Physiol 278: C40–C48, 2000. [DOI] [PubMed] [Google Scholar]


