Abstract
Selective attention allows organisms to extract behaviorally relevant information while ignoring distracting stimuli that compete for the limited resources of their central nervous systems. Attention is highly flexible, and it can be harnessed to select information based on sensory modality, within-modality feature(s), spatial location, object identity, and/or temporal properties. In this review, we discuss the body of work devoted to understanding mechanisms of selective attention in the somatosensory system. In particular, we describe the effects of attention on tactile behavior and corresponding neural activity in somatosensory cortex. Our focus is on neural mechanisms that select tactile stimuli based on their location on the body (somatotopic-based attention) or their sensory feature (feature-based attention). We highlight parallels between selection mechanisms in touch and other sensory systems and discuss several putative neural coding schemes employed by cortical populations to signal the behavioral relevance of sensory inputs. Specifically, we contrast the advantages and disadvantages of using a gain vs. spike-spike correlation code for representing attended sensory stimuli. We favor a neural network model of tactile attention that is composed of frontal, parietal, and subcortical areas that controls somatosensory cells encoding the relevant stimulus features to enable preferential processing throughout the somatosensory hierarchy. Our review is based on data from noninvasive electrophysiological and imaging data in humans as well as single-unit recordings in nonhuman primates.
Keywords: cross-modal, noise correlations, somatosensation, spike-count correlations, spike synchrony
the natural environment generates myriads of stimuli that constantly bombard our sensory systems. To effectively function in this environment we employ a set of neural mechanisms that extract the sensory inputs most relevant to our current goals. These mechanisms, which form our selective attention system, are highly flexible and can be harnessed to select information based on the location in space (absolute or relative to the body), sensory modality (e.g., tactile), stimulus features (e.g., motion), object identity, and/or temporal characteristics (e.g., stimulus onset or temporal position in a stream of stimuli). The goal of this manuscript is to provide an ample account of the neural mechanisms mediating stimulus selection in the somatosensory system. While there have been several review articles on tactile attention (Burton and Sinclair 2000a; Eimer and Driver 2001; Johansen-Berg and Lloyd 2000; Kida and Kakigi 2008, 2015; Sambo and Forster 2011; Spence and Gallace 2007), this review provides a more recent and detailed description of the neural correlates of attention in the somatosensory system and its effects on perceptual functions. In particular, we discuss and, where appropriate, bridge many of the findings observed across species (in particular, human and nonhuman primates) and methodologies [e.g., single-unit recordings, electroencephalography (EEG), and functional magnetic resonance imaging (fMRI)]. We focus on somatotopic- and feature-based attention mechanisms (i.e., selection based on stimulus location on the body surface and stimulus properties, respectively) and the commonalities of attention mechanisms in touch with those in the auditory and visual systems. Our review is based on data from psychophysics, EEG, magnetoencephalography (MEG), and neuroimaging experiments (e.g., fMRI) in humans. EEG and MEG activity provides exquisite temporal resolution (≤1 ms level) of neural activity measured at a macroscopic level, whereas imaging methods such as fMRI offer superior spatial localization (≤1 mm3 resolution), albeit with lower temporal resolution. We also review intracranial neurophysiological studies in humans and nonhuman primates, which combine the strengths of noninvasive neurophysiological and imaging methods. We center on describing the effects of attention in primary and secondary somatosensory (SI, SII) cortexes and the neural mechanisms that mediate these effects. However, we also briefly discuss how attention modulates activity of subcortical and higher-order somatosensory neurons. We begin by providing a short description of the typical experimental paradigms used for controlling the focus of attention and behavioral state of participants in a laboratory setting.
The Cueing Paradigm: The Study of Attention in a Laboratory Setting
Experimental studies in laboratories employ cues to exogenously or endogenously control the focus of attention of participants. Exogenous attention is typically induced by presenting an abrupt stimulus (e.g., tap, sound, or light flash) that directs attention to the location of that stimulus in the absence of a volitional attention shift (Mayer et al. 2004a, 2004b; McCormick 1997; Turatto et al. 2000, 2004a, 2004b). In contrast, endogenous control of attention can be elicited with symbolic cues (e.g., visually presented arrows or sinusoidal tones) that instruct participants to direct attention to a particular sensory modality or physical characteristic of a stimulus, such as its spatial position, location on the body, or sensory feature (e.g., texture, motion direction, orientation), just to name a few examples (Forster and Eimer 2005; Fu et al. 2001; Gomez-Ramirez et al. 2014; Posner et al. 1982; Thut et al. 2006; van Velzen et al. 2002; Worden et al. 2000). Frequently, participants are cued on a trial-by-trial basis, which promotes deployments of attention that are akin to those made in natural environments (Posner et al. 1980). Attention can also be deployed on a sustained basis by instructing participants to maintain attention to the same location, feature, or sensory modality for an entire block of trials (Bradshaw et al. 1992; Eimer and Forster 2003a; Gomez-Ramirez et al. 2011; Hsiao et al. 1993; Martinez et al. 1999; Michie et al. 1987; Steinmetz et al. 2000). The validity of the symbolic cue can be manipulated such that on most trials the cue predicts the relevant discriminative property of the target (i.e., an informative cue). Cues that on most trials do not provide accurate information of the target's relevant property are termed noninformative or “neutral” cues. In this review, we focus on studying the mechanisms underlying endogenous attention in the somatosensory system.
Effects of Attention on Detection and Discrimination Functions of the Somatosensory System
The effects of attention are measured by comparing responses to a sensory stimulus when it is attended vs. unattended. One of the main functions of our selective attention system is to facilitate perceptual judgments by enabling processing of task-relevant inputs. Indeed, studies show that detection of tactile stimuli is faster and more accurate when attention is preallocated to the body location that is stimulated (Burton and Sinclair 2000a; Johansen-Berg and Lloyd 2000; Spence and Gallace 2007). Likewise, attention improves performance when directed toward sensory features relevant to the task, such as texture (Sinclair et al. 2000), vibration (Sinclair et al. 2000), orientation (Schweisfurth et al. 2014), and intensity (Burton and Sinclair 2000b; Burton et al. 1997).
The behavioral effects of attention comprise both costs and benefits. This was shown in a somatotopic (tactile spatial) attention study that used informative (80% valid) and neutral (50% valid) cues in separate blocks (Forster and Eimer 2005). The authors presented ∼60 Hz vibrating stimuli, which likely activated the rapidly adapting (RA) and Pacinian Corpuscles (PC) peripheral systems preferentially (Freeman and Johnson 1982; Mountcastle et al. 1972). Benefits and costs were assayed by comparing the reaction time (RT) between trials with valid vs. neutral cues and invalid vs. neutral cues, respectively. The RT data revealed behavioral benefits in the first case but behavioral costs in the second. The data also showed that RT costs were significantly larger than RT benefits (104 vs. 40 ms). Based on these findings it was suggested that somatotopic attention is mediated by facilitation of neural processing at attended body locations in combination with suppression of somatosensory stimuli at unattended locations (Forster and Eimer 2005).
The limited capacity of tactile attention.
The effects of attention on behavior are generally beneficial. This does not mean, however, that attention always decreases the RT and/or improves stimulus detection or discrimination. There are specific (and rare) instances in which deploying attention to a particular location impairs performance. One example is the “inhibition of return” (IOR) effect, which refers to the increase in RT to target stimuli that are presented in the same location as a preceding peripheral stimulus (Klein 2000; Posner and Cohen 1984; Posner et al. 1985). This effect, originally demonstrated in the visual modality, is typically observed during exogenous attention (Posner et al. 1985), and it is an integral part of computational models of attention (Itti et al. 1998; Koch and Ullman 1985). The IOR is believed to foster exploration of previously unattended stimuli by preventing the focus of attention to return to previously attended locations. The IOR is time dependent in that it is generally manifested when the target stimulus is preceded by the cue by at least 300 ms. Furthermore, studies show that the IOR is long lasting, occurring over a period of ∼1,500–2,000 ms after an active deployment of attention (Posner and Cohen 1984). Although the IOR is believed to be mediated by mechanisms of attention, some studies have suggested that the IOR may also be caused by deficits in motor pathways (Hunt and Kingstone 2003; Rafal et al. 1989). However, further investigation is needed, since recent studies have failed to find consistent and supporting evidence for this hypothesis (Chica et al. 2010; Smith et al. 2012).
One of the original reports of a tactile IOR effect was provided by Lloyd and colleagues (1999), who showed RT increases to tactile stimuli matching the body location of an nonpredictive cue (see also Cohen et al. 2005; Jones and Forster 2014, 2012; Roder et al. 2002; Tassinari and Campara 1996). Lloyd et al. (1999) showed the onset of the IOR effect around 100 ms, with the maximum effect occurring between 200 and 700 ms, which is earlier than the IOR observed in visual studies.
The IOR has also been observed in the auditory modality, indicating that it may be driven by mechanisms that are common across the senses (Spence et al. 2000a). With the use of spatially coregistered auditory, visual, and tactile stimuli on the left and right side of the body (left and right hands for tactile stimuli), it was found that RTs were significantly longer for stimuli presented on the same body side as the last target stimulus. This occurred regardless of the sensory modality of the previously presented stimulus. Interestingly, a recent study showed that the IOR in touch is abolished by engaging in a visual discrimination task (Jones and Forster 2013). In this study, participants performed a divided-attention tactile and visual task that required them to respond to a tactile (delivered to the left or right hand) or visual (a number from 2 to 9 centrally presented on a monitor) target stimulus. The tactile target stimulus was always preceded by a tactile “cue” that was presented on the same hand on 50% of the trials. The target visual stimulus was embedded within a stream of serially presented letter stimuli. On a separate set of blocks, participants engaged in the same tactile task but were asked to ignore all visual stimuli (tactile-only task). When participants performed the tactile-only task, the behavioral data revealed slower responses to tactile targets at the cued locations (i.e., the IOR effect). Furthermore, as expected, participants' RT to tactile stimuli substantially increased in the divided-attention vs. the tactile-only task. However, the data showed no difference in RTs between cued or uncued tactile targets (i.e., no IOR effect) for the divided-attention task. These findings are striking because they suggest that engaging in a visual discrimination task can withdraw significant attention resources from the somatosensory system.
Cross-modal effects in the somatosensory system are not restricted to the IOR. Psychophysical studies have reported another interesting cross-modal phenomenon, termed the Colavita visual dominance effect, which refers to the interference of visual stimuli in auditory or tactile discrimination or detection (Colavita 1974). This effect is akin to exogenous attention in that the presentation of a visual stimulus draws attention away from the tactile modality. The Colavita effect is observed in cross-modal studies of attention where participants are instructed to attend to inputs from a nonvisual channel while ignoring visual stimuli. This effect is modulated by the spatial and temporal relationship between the multisensory stimuli (Koppen and Spence 2007a, 2007b) but not by semantic or probability commonality (Koppen et al. 2008). Interestingly, recent studies show that tactile signals are also masked when visual inputs are substituted with auditory stimuli (Occelli et al. 2010). However, these effects are much weaker and depend on the spatial configuration of the multisensory inputs.
The IOR and Colavita effects highlight the commonalities between the mechanisms of attention that facilitate tactile, auditory, and visual perceptual functions. Furthermore, the Colavita effect, in particular, underscores the dominant role of the visual system over the tactile and auditory modalities. Indeed, as previous studies show, the presence of a distracting visual stimulus during a tactile discrimination task can substantially decrease performance, even when the visual stimulus shares no feature relationship with the tactile stimulus or is irrelevant to performing the task. Furthermore, these findings provide compelling evidence that, at least in primates, visual signals have priority over other sensory inputs in the attention filter that limit perceptual processing capacity. That said, under circumstances where tactile signals provide more reliable information, the sense of touch has been shown to dominate over vision (Ernst and Banks 2002; Guest and Spence 2003; Lederman et al. 1986). Indeed, it would be intriguing to assess whether similar effects hold in other species that rely more heavily on other sensory systems to sample the environment (e.g., the whisker system in rodents).
In the following sections we describe the neural correlates and mechanisms of selective attention in the tactile modality. We start by providing a brief description of the anatomical organization and information flow of neural signals in the somatosensory system.
Neural Circuitry of the Somatosensory System
A thorough understanding of the neural mechanisms mediating tactile stimulus selection requires sound knowledge of the anatomical and functional circuitry of the somatosensory system. Tactile perception begins in the periphery with the processing of individual features of somatosensory stimuli (e.g., edges, texture, vibrations, and temperature). These features are processed by specialized receptors on the glabrous (hairless) and hairy skin, which are innervated by distinct afferents that convey activity to the central nervous system. The signals carried by these afferents can be categorized into distinct functional modalities depending on the type of somatosensory feature they encode. They include: cutaneous or innocuous touch (e.g., light skin indentation), proprioception (e.g., spatial configuration of fingers enclosing an object or positions of limbs), temperature (e.g., coldness), and nociception (e.g., pain-related signals). Here, we focus on how attention modulates responses of neurons encoding cutaneous and proprioceptive inputs.
Cutaneous stimuli are predominantly encoded by mechanoreceptors. In particular, Merkel-Neurite Complex receptors, innervated by slowly adapting I (SA-I) fibers, best encode tactile spatial features such as edges, curvature, and texture (Connor et al. 1990; Connor and Johnson 1992; Goodwin et al. 1997; Johnson 1983; Yoshioka et al. 2001). Meissner's corpuscles, which are innervated by RA afferents, process motion and low-frequency vibrations (Johnson et al. 2000). PC, which are innervated by PC afferents, are specialized for high-frequency vibrations (>100 Hz) and fine texture (Harvey et al. 2013).
The sense of proprioception is initially encoded by muscle (Cordo et al. 2002; Houk and Henneman 1967; Proske and Gregory 2002; Roll et al. 1989) and skin mechanoreceptors innervated by slowly adapting II (SA-II) fibers that respond to selective patterns of skin deformation (Edin and Abbs 1991; Hallin et al. 2002; Olausson et al. 2000). As we will discuss later, the effects of attention on cutaneous processing and behavior are modulated by the spatial arrangement of the body parts (i.e., proprioception).
The classical model of somatosensory processing indicates that activity from peripheral afferents is transmitted to the brain via separate channels and then converges in “late-processing” areas (Mountcastle 2005). However, results from recent studies are at odds with this view by showing that cutaneous and proprioceptive signals are integrated at the earliest level of SI (i.e., areas 3a and 3b) and possibly even earlier (Kim et al. 2015). Furthermore, a recent study in nonhuman primates found that cutaneous signals, such as those from SA-I and RA inputs, also converge in area 3b (Pei et al. 2009). In fact, recent work using anatomical and electrophysiological methods in genetically modified animals indicates that peripheral signals are integrated even at the level of the spinal cord (Abraira and Ginty 2013). Thus, given that many submodality (e.g., RA, SA-I, and PC) and modality-specific (e.g., cutaneous and proprioceptive) peripheral afferent signals converge in subcortical and early SI, we deem that tactile selective attention operates by targeting functional, as opposed to anatomical, cell ensembles that have common neural selectivity for the relevant physical properties of sensory stimuli (e.g., spatial, feature, and temporal) (Gomez-Ramirez et al. 2014).
Although, as discussed, some modality-specific signals converge in different neural subareas before reaching cortex, the somatosensory system maintains a topographical organization that is conserved throughout SI and, to a lesser extent, SII cortex. In particular, SI cortex has a detailed topographical representation of the body, whereby neurons located more medially (i.e., toward the interhemispheric fissure) have a receptive field (RF) over the lower part of the body (e.g., toes and lower limbs), whereas neurons located more laterally (toward the lateral sulcus) have a RF over the upper body (Mountcastle 2005). In addition, SI in each hemisphere predominantly responds to inputs from the contralateral side of the body. SII cortex also has a body map. However, this representation is less well defined, and most SII cells have bilateral RFs (Fitzgerald et al. 2006a, 2004, 2006b). A major advantage of having a detailed neural representation of the body is that attention can leverage this topographical organization to select stimuli based on their somatotopic location. We discuss this mechanism in a later section of the manuscript.
Somatosensory activity beyond SII is believed to be processed in two distinct, but interrelated, functional pathways. One pathway, which is related to object grasping and manipulation, includes areas in the posterior parietal cortex (e.g., areas 5 and 7), and it is tightly connected with motor and premotor neural areas. The other pathway, which is involved in tactile object recognition and aesthetics, includes more anterior structures such as the insula and cingulate. These two functional pathways are thought to operate in parallel and in concert during natural object grasping, manipulation, and recognition (Hsiao and Gomez-Ramirez 2011).
Neurophysiological Correlates of Attention in the Somatosensory System
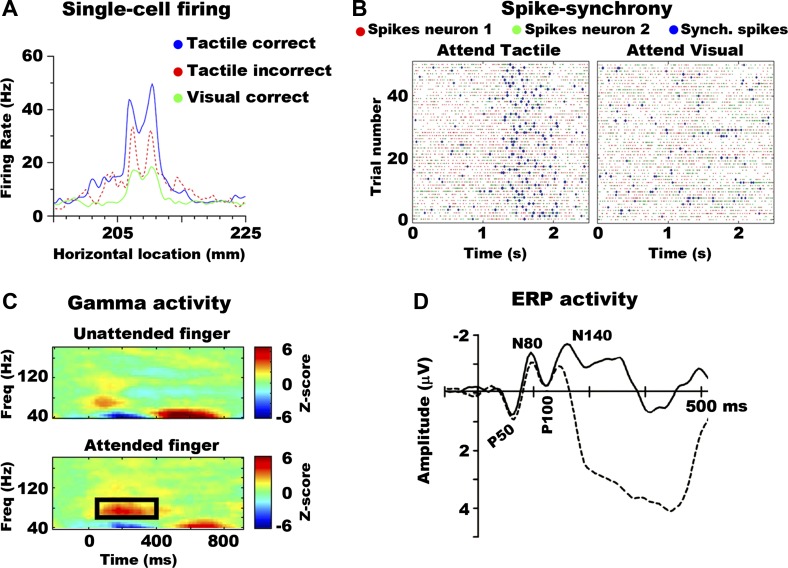
The effects of tactile attention on behavior are thought to be mediated by the enhancement of activity in somatosensory cortical areas with RFs over attended stimuli (Forster and Eimer 2005; Gomez-Ramirez et al. 2014; Hsiao et al. 1993; Michie 1984; Michie et al. 1987; Steinmetz et al. 2000; van Velzen et al. 2002). In a seminal study, Hsiao and his colleagues found that attention modulated activity of neurons in SI and SII cortex when attention was directed to touch vs. vision (Hsiao et al. 1993). In this study, animals switched between performing a tactile letter and visual luminance discrimination task. During attend-visual blocks, animals were presented with the same tactile letter stimuli as in the tactile blocks but were trained to ignore these letters and focus attention on visual stimuli. The data revealed greater firing rate in SI and SII neurons when performing the tactile vs. visual task. Furthermore, it was found that the firing rates of a subset of cells were additionally enhanced during correct vs. incorrect trials. These findings are shown in Fig. 1A.
Fig. 1.
Attention effects on neural activity. A: instantaneous mean firing rate of secondary somatosensory (SII) neurons responding to a tactile stimulus when it was attended and the behavioral response was correct (blue) or incorrect (red) and when the stimulus was unattended (green; in this case attention was to a visual stimulus, and behavioral response to the latter was correct). Firing rate is plotted as a function of time, which is represented here for technical reasons as the location of the drum used to present the tactile stimuli. Because the drum moved with constant velocity, there is a one-to-one mapping between drum location and time, with 1 s corresponding to a distance of 15 mm. Adapted with permission from Hsiao et al. 1993. B: raster plots of two neurons in SII cortex when attention was directed to the tactile (left) and visual (right) modality. Green and red dots indicate individual spikes for each neuron, whereas blue dots are coincident spikes between both neurons within 2.5 ms of each other. There are substantially more coincident spikes between SII neurons when attention is directed to touch (left) vs. vision (right). Adapted with permission from Steinmetz et al. (2000). C: time-frequency plots over somatosensory areas for unattended (top) and attended (bottom) stimuli. The black frame in the bottom highlights the difference in the γ-band between the two conditions. Adapted with permission from Bauer et al. (2006). D: event-related potential (ERP) activity to attended (broken line) vs. unattended (solid line) stimuli. The voltage polarity of the evoked potential is indicated by the first letter (“N” for negative, “P” for positive), whereas the timing of the peak activity of the ERP component is indicated by the numbers. Thus, the N80 refers to a negative deflection occurring 80 ms after the onset of the tactile stimulus. Adapted with permission from Forster and Eimer (2004).
In addition to modulating firing rates, attention can also modulate the correlated spiking activity between somatosensory cells. This was originally shown by Steinmetz and colleagues who trained animals to perform a tactile and visual discrimination task and recorded from multiple cells while animals directed attention to tactile or visual stimuli (Steinmetz et al. 2000). It was found that tactile attention increased the synchronized spiking activity (spikes within approximately ±5 ms; see Fig. 1B) between neural pairs in SII cortex. Enhanced spike synchrony was also observed in a more recent attention study in animals trained to discriminate tactile stimuli based on their orientation and/or frequency (Gomez-Ramirez et al. 2014). It was also found that the spike synchrony between feature-selective cells was tightly correlated with animals' performance, whereby greater correlated spiking was associated with higher performance.
Tactile attention can also influence synchronized neural activity measured at macroscopic levels [e.g., EEG, MEG, electrocorticography (ECoG), and local field potentials]. This was originally shown in a delayed-to-match sample task of somatotopic attention (Bauer et al. 2006). Specifically, Bauer and colleagues showed increases in γ-band (30–80 Hz) activity over contralateral somatosensory cortexes when attention was directed to the left vs. right hand that commenced around 100 ms after stimulus onset (Fig. 1C). Similar findings were observed in a cross-modal attention task using ECoG recordings in humans (Ray et al. 2008). Specifically, it was shown that attention toward touch compared with audition increased high γ-band activity (80–150 Hz) over the postcentral gyrus. Conversely, increased high γ-activity was observed over auditory cortexes when attention was deployed to audition. These findings indicate that enhancement of synchronized γ-band oscillations represents a neural correlate of signaling behavioral relevance across all sensory modalities.
Anatomical and temporal distribution of attention effects in the somatosensory system.
Attention effects on somatosensory cells can occur early in the neural processing stream. Neural responses of somatosensory neurons can be modulated by behavioral states at the levels of the thalamus (Bushnell and Duncan 1987; Morrow and Casey 2000, 1992; Tremblay et al. 1993) and even the medullary dorsal horn (Dubner et al. 1981; Hayes et al. 1981; Hoffman et al. 1981). Hayes and colleagues (1981) reported that neurons in the dorsal horn increased their responses when animals performed a thermal (innocuous or noxious stimuli) vs. visual discrimination task. Furthermore, they found that neural responses were enhanced already during the initial volley of afferent activity, indicating that attention can modulate the neural activity of dorsal horn neurons in anticipation of peripheral stimulation.
We just described that attention can modulate neural activity of somatosensory neurons in the early phases of stimulus processing. However, similar to the visual system (Desimone and Duncan 1995; Luck et al. 1997; Martinez et al. 1999; Reynolds et al. 1999), the magnitude and prevalence of these effects increase throughout the sensory hierarchy. For instance, Hsiao and colleagues (1993) showed that attention modulated the responses of ∼50% cells in SI and 80% of neurons SII. A similar percentage difference was observed in a separate study that trained animals to perform a tactile texture and visual discrimination task (Meftah el et al. 2002). Approximately 25% of cells in SI and 62% of SII neurons were modulated by attention. This pattern of attention effects has also been observed in human imaging studies, which show greater attention modulations in hemodynamic responses of SII and insula compared with SI (Burton et al. 1999; Chen et al. 2010, 2008; Hamalainen et al. 2002). However, in the somatosensory system, it is unknown whether attention effects continue to scale across associative areas (e.g., beyond SII cortex), a finding that has been observed in the visual system (Luck et al. 1997).
To date, there are only a handful of studies that have investigated the effects of tactile attention on neural activity beyond SII cortex. In particular, a study in nonhuman primates showed that most neurons (∼60%) in area 7b are enhanced by attention (Burton et al. 1997). The sample size in this study was small (n = 22 cells), but these findings are supported by several human imaging studies that show increased responses in higher-order neural areas encoding the attended stimuli (Burton et al. 1999; Goltz et al. 2013). Studies in humans have also shown that attention increases the functional connectivity between high- and low-order somatosensory areas. In particular, a combined EEG and fMRI study in humans found enhanced coupling between the intraparietal sulcus and somatosensory cortexes during sustained attention to tactile stimuli. These findings indicate that attention synchronizes activity between high- and low-order areas in parietal cortex as a means to enhance the relevant sensory signals (Goltz et al. 2015).
An interesting phenomenon observed in single-unit studies is the heterogeneity of attention effects across somatosensory areas. Hsiao and colleagues (Hsiao et al. 1993) reported that, of those SI neurons that showed attention effects (∼50% of the entire SI population), all had greater activity when attention was deployed to touch vs. vision. In contrast, attention effects on SII neurons were found to be variable, with only 73% of attention-modulated cells exhibiting greater responses when attention was directed to touch vs. vision, whereas 27% of the remaining set of cells modulated by attention showed enhanced responses when attention was deployed to vision vs. touch (Hsiao et al. 1993). Such diversity of attention effects in SII has also been observed in other tactile attention studies (Burton et al. 1997; Gomez-Ramirez et al. 2014). The cause of this heterogeneity is unknown, but one plausible hypothesis is that attention imposes different selection filters across neural areas. That is, it is possible that attention effects on SI cells are driven by spatial selection mechanisms that operate by solely enhancing cells' responses when the RF is aligned with the spatial focus of attention, regardless of their selectivity for the attended stimulus feature. In contrast, because of the diversity of feature encoding of SII neurons (Gomez-Ramirez et al. 2014), attention might impose feature selection filters in SII (or a composite of features and spatial selection filters) such that a cell's response is further modulated according to its affinity for the attended stimulus features. Thus, neurons that are selective for attended features are enhanced, whereas neurons that encode task-irrelevant features are suppressed.
The temporal incidence of tactile attention effects has been extensively investigated in human studies using event-related potential (ERP) and MEG methods. These techniques afford exquisite temporal resolution of neural activity albeit at a macroscopic spatial scale. Studies show that attention can modulate the amplitude of early and midlatency tactile ERP components (e.g., P55, N80, P100, N140 where “N” indicates negative, “P” indicates positive, and the numbers indicate the timing of the peak activity of the ERP component in ms) as well as later-occurring components (see, e.g., Fig. 1D) (Eimer and Forster 2003a; Eimer et al. 2004b; Forster and Eimer 2004, 2005; Garcia-Larrea et al. 1995; Kennett et al. 2001; Kida et al. 2011, 2004; Michie 1984; Michie et al. 1987). With the use of inverse source modeling, EEG and MEG studies report that the N80 component arises from contralateral neural ensembles in SI cortex, whereas the P100 response emerges bilaterally in SII (Allison et al. 1992, 1989; Inui et al. 2004; Mauguiere et al. 1997; Mima et al. 1998). These data confirm single-unit findings in nonhuman primates that tactile attention modulates responses during early phases of stimulus processing.
Most ERP studies in humans also indicate that feature-based attention effects (e.g., effects related to deploying attention to oriented or textural features) are observed after spatial selection of somatosensory stimuli. However, the evidence is not unequivocal. An example where spatial and feature attention effects occurred at the same time was published previously (Forster and Eimer 2004). In this study, human participants were instructed to perform frequency and intensity discrimination tasks with stimuli delivered to the right or left hand. Participants were told to attend to specific combinations of stimulus location (left or right hand) and nonspatial feature (frequency or intensity) on different blocks. The data showed that the onset of spatial attention effects occurred around 140 ms (i.e., in the period related to the N140 component). Furthermore, it was shown that ERP attention effects on nonspatial features modulated activity around the same latency range and that these feature attention effects did not vary as a function of the spatial focus of attention. This pattern of effects led the authors to conclude that feature and spatial selection in the somatosensory system are neural processes that occur in parallel and are independent from each other (Forster and Eimer 2004). However, as noted earlier, several studies found that somatotopic (spatial) attention modulates neural activity at ∼100 ms posttactile stimulus (Eimer and Forster 2003a, 2003b), and even earlier (∼55 and 80 ms) (Eimer and Forster 2003a; Michie et al. 1987) (see also Fig. 1D). The range of differences in the onsets of spatial attention effects between the Forster and Eimer (2004) study and the aforementioned studies is quite substantial (∼40 and 85 ms). The reasons for these dramatically different time courses are unclear and not addressed in the literature. Thus, further studies are needed to bridge the discrepancies between these datasets. However, as it stands right now, the aggregated evidence supports the thesis that somatotopic (or spatial) selection is a neural process that precedes the effects of feature attention by at least ∼40 ms.
Neural Mechanisms Mediating Selection of Tactile Sensory Stimuli
The attentional system is highly flexible and involves separable mechanisms of both enhancement and suppression. As noted earlier, γ-band oscillations are associated with enhancement of neural activity encoding attended stimuli (see Fig. 1C) (Bauer et al. 2006; Fries et al. 2001; Karns and Knight 2009; Ray et al. 2008; van Ede et al. 2014). In contrast, α-band (8–14 Hz) oscillations are linked to suppression of activity encoding stimuli outside the focus of attention, a phenomenon that is commonly referred to as the α-suppression effect (Foxe and Snyder 2011). However, unlike γ-band oscillations, which have been shown to arise from local recurrent excitatory/inhibitory effects (Cardin et al. 2009; Siegle et al. 2014; Whittington and Traub 2003; Whittington et al. 1995), the mechanisms driving α-oscillations are not fully determined (but see Contreras et al. 1992, 1996, 1997a, 1997b; Destexhe et al. 1998; Sherman 2001, 2005; Sherman and Guillery 2002 for putative models). In the next section, we describe in more detail the role of this α-oscillatory effect in the somatosensory system. In what follows we provide a description of the mechanisms mediating spatial selection of somatosensory stimuli. Particularly, we focus on describing the neural mechanisms that establish and maintain the spatial focus of attention and how proprioceptive inputs can influence neural representations of cutaneous sensory signals.
Somatotopic (spatial) selection in the somatosensory system.
In vision, attention has been proposed to function like a “spotlight” by enhancing all relevant information within its focus and changing its size (Eriksen and Hoffman 1972; Eriksen and St. James 1986). Furthermore, fMRI data show that tasks requiring attention to multiple spatial locations, at the same time, lead to multiple peaks in the hemodynamic response of areas selective for those spatial locations. This finding led to the suggestion that the spotlight of attention can be simultaneously split across different spatial locations to maximize the likelihood that only relevant stimuli receive preferential processing (McMains and Somers 2004; Morawetz et al. 2007). Indeed, a similar phenomenon has been described in the tactile modality (Eimer and Forster 2003b).
In vision, there is also evidence of a limited degree of autonomy of the cerebral hemispheres in the control of selective attention. The classical multiobject tracking results by Pylyshyn and Storm (1988) showed that humans can track about four independent objects. Later it was shown that this number is actually two plus two, with two objects being tracked in each visual hemifield (Alvarez and Cavanagh 2005; Cavanagh and Alvarez 2005).
In touch, discrimination of spatial patterns diminishes when distracters and targets are presented to the same hand as compared when they are presented to different hands (Craig 1985). For stimuli presented on the same hand, this interference effect increases as the distance between targets and distracters decreases. Specifically, discrimination of stimuli presented to the little finger (digit 5) diminished when distracter stimuli were presented on the ring finger vs. the index finger (digit 4 vs. digit 2). However, this interference effect decreased when distracters were presented on the opposite hand, and it was not further modulated by the spatial distance between the hands (Evans et al. 1992). These psychophysical data indicate that distracters presented on the same hand may not be fully suppressed. This effect was confirmed by ERP studies that also showed that there is no clear modulation of attention when deployed to either whole fingers or individual phalanxes (Eimer and Forster 2003b). Whereas ERP attention modulations generally decayed with distance from the focus of attention, the decay was gradual, and no discrete jumps were observed at the boundaries of phalanxes or fingers. Whether there are any other categorical distinctions in the spread of tactile attention merits further examination.
A related question is whether the focus of attention can be split in two noncontiguous regions separated by an unattended region. This question was investigated in a human ERP study that compared ERP responses with tactile stimuli when attention was directed to two adjacent fingers vs. two nonneighboring stimuli (e.g., attention to digit 3 and digit 4 vs. digit 2 and digit 5) (Eimer and Forster 2003b). It was hypothesized that, if two separate attention foci are maintained, one would expect a difference in ERP signals in these two cases, whereas a contiguous focus of attention should result in similar ERP signals. The experimental result showed that the former scenario was correct. Specifically, the P100 component in response to stimuli delivered to attended fingers was modulated by attention, but attention did not modulate the responses to stimuli presented to the unattended finger located between attended fingers (Eimer and Forster 2003b). Although ERPs afford a very crude spatial representation of intracranial neural activity, it is notable that the authors were still able to find a differential response between these two conditions. Studies that resolve neural activity at the single-cell level are needed to further understand the mechanisms underlying this spatial selection effect.
As noted earlier, the brain may use neural oscillatory mechanisms in the α-band to suppress sensory information that is irrelevant to the current goals of the task (the α-suppression effect). Selective attention can control the focus and magnitude of these α-oscillations according to the spatial location and perceptual load of the distracting stimulus (Foxe and Snyder 2011). This oscillatory suppression mechanism was initially reported in the visual modality (Banerjee et al. 2011; Foxe et al. 1998; Fu et al. 2001; Worden et al. 2000), but it is also observed in somatosensation (Haegens et al. 2011a, 2012, 2011b; Jones et al. 2010; van Ede et al. 2011, 2014) and audition (Banerjee et al. 2011; Fu et al. 2001; Gomez-Ramirez et al. 2011). In touch, this effect was originally reported in a MEG study of somatotopic attention (Jones et al. 2010). Participants were cued to direct attention to their left hand or foot and report whether a vibratory stimulus was presented to that location. The data showed increased α-band amplitude over areas responsive to stimulation of the hand in anticipation of a tactile stimulus delivered to the left foot, that is, α-amplitude increased over the representation of the unattended body location. The data further revealed that α-power over unattended areas was positively correlated with detection of tactile stimuli at the single-trial level. In separate studies, it was shown that the α-suppression effect increased systematically as a function of cue validity, with higher cue validity leading to greater α-power over unattended areas (Haegens et al. 2011a). Similar to the findings by Jones et al. (2010), Haegens and colleagues found a relationship between α-power and performance (Haegens et al. 2011a, 2011b, 2012). Taken together, these data indicate that the α-suppression effect is a supramodal neural mechanism controlled by attention to suppress distracting stimuli regardless of the sensory identity of the stimulus.
Much is known about how somatotopic (spatial) attention modulates behavior and neural processing of tactile stimuli (Burton et al. 1997, 1999; Burton and Sinclair 2000a, 2000b; Eimer and Forster 2003a; Forster and Eimer 2004; Gherri and Forster 2014; Gomez-Ramirez et al. 2014; Hsiao et al. 1993; Meftah el et al. 2002; Michie 1984; Michie et al. 1987; Sinclair et al. 1991; Steinmetz et al. 2000; van Velzen et al. 2002). However, the neural mechanisms that establish and maintain the control of attention in somatosensory cortexes are less well understood. Several human studies have characterized a series of ERP components that are associated with establishing and maintaining attention in space and body locations (Eimer et al. 2003b, 2004a; Eimer and Van Velzen 2002; Harter et al. 1989; Hopf and Mangun 2000; Kelly et al. 2009; Praamstra et al. 2005; Praamstra and Kourtis 2010; Seiss et al. 2009; Simpson et al. 2006; van Velzen and Eimer 2003). These studies have identified several early and late lateralized ERP components that emerge in anticipation of the target stimulus (i.e., during the cue-to-target interval). These components are observed in auditory, visual, and tactile attention tasks in normal healthy individuals (Eimer and Van Velzen 2002; Eimer et al. 2002) and in blind individuals performing spatial attention tasks in the tactile modality (Van Velzen et al. 2006). These data indicate that these anticipatory ERP components may reflect supramodal neural correlates of attention control.
The early directing attention negativity (EDAN) is one of the earliest ERP components (∼250 ms postcue) that show cue-related attention effects (here, we refer to attention modulations of neural activity in response to the cue stimulus itself. These effects should not be confused with the attention effects mentioned earlier that reflect modulations in neural responses to attended and unattended target stimuli, which may occur as early as 55 ms after stimulus onset). The EDAN consists of a negative deflection over posterior electrodes that is contralateral to the direction of the cue stimulus and is believed to reflect decoding of the cue and initiation of an attention shift. However, some studies indicate that the EDAN may reflect processing of the physical attributes of a sensory cue stimulus (van Velzen and Eimer 2003). The EDAN is followed by the anterior directing attention negativity (ADAN), which is an enhanced negativity over the frontal scalp that is contralateral to the direction of attention. The ADAN is believed to index the control of spatial attention by neural populations in higher-order frontal cortexes. It emerges around 300 ms after the onset of the cue stimulus and lasts several hundred milliseconds. Furthermore, the ADAN is modulated by distracter stimuli (Seiss et al. 2009) and occurs even when visual cue stimuli are replaced by auditory spatial cues (Van Velzen et al. 2006). The ADAN has also been shown to reverse polarity when the upper limbs are crossed, indicating that it operates in a proprioceptive-centered reference frame because it follows the spatial location of the attended hand (Eardley and van Velzen 2011; Eimer et al. 2003a). However, in a separate study, it was found that this polarity reversal emerged during the later phase of the ADAN component (700–900 ms postcue), suggesting that neural activity during the latter part of ADAN (the so-called late ADAN or late somatosensory negativity) might represent a different mechanism of attention orienting compared with the early phase of the ERP component (Gherri and Forster 2012a). In particular, it was suggested that the early ADAN (300–500 ms) operates according to an external frame of reference that does not reverse polarity with crossed hands, whereas the late ADAN (or late somatosensory negativity) reflects selective activation of somatosensory neurons in anticipation of an upcoming tactile stimulus (Gherri and Forster 2012a).
The ADAN is followed by the late-directing attention positivity (LDAP), which is a contralateral positivity over the posterior scalp that is maintained until the presentation of the target stimulus. The LDAP is thought to reflect the activation of visual (or multisensory) areas in anticipation of stimuli at the attended locations. The LDAP is modulated by the distance between hands, with increased amplitude as the hands are placed farther apart (Eimer et al. 2004a). In contrast to the ADAN component, the LDAP does not reverse polarity when limbs are crossed, indicating that the LDAP operates in an external frame of reference (e.g., visual-spatial coordinate frame) (Eimer et al. 2003a, 2004a; Gherri and Forster 2012b).
Although it is predominantly believed that spatial attention is controlled by a frontoparietal network (Corbetta and Shulman 2002; Doesburg et al. 2008; Hopfinger et al. 2000; Kida and Kakigi 2013; Petersen and Posner 2012; Reynolds and Chelazzi 2004), recent work in nonhuman primates provides compelling evidence that spatial selection is also mediated by subcortical circuitry in the superior colliculus (SC). With the use of combined correlative (electrophysiological) and causal (pharmacological) methods, it was found that inactivation of the intermediate and deep layers of the SC via muscimol (a GABA agonist) led to profound impairments in selecting the visual stimulus informative of the behavioral response. What is more striking is that, while inactivation of the SC led to severe deficits in behavioral performance, neurons in neocortex that encode the stimulus features of the task (e.g., mediotemporal cortex for motion) still exhibited robust attention effects in their firing rates (Lovejoy et al. 2009; Zenon and Krauzlis 2012). This pattern of effects led to the hypothesis that spatial attention effects of SC on behavior are mediated by neural mechanisms that are independent of neocortical response modulations (Krauzlis et al. 2013, 2014). From a multisensory perspective, this model of spatial selection is attractive because the intermediate and deep layers of SC are composed of neurons with audio, tactile, and/or visual RF that are spatially aligned. Certainly, this spatially organized system is advantageous for facilitating spatial stimulus selection regardless of sensory identity of stimuli.
A fundamental question in the somatosensory field is whether tactile stimuli are selected in a somatotopic frame of reference (body location and/or proprioceptive coordinates), an external spatial frame (visual-spatial coordinates or extrapersonal space), or a combination of both. This question was initially addressed in a study that cued participants to two body locations in sequence and then asked participants to judge whether a puff of air was presented in the location indicated by the second cue (Lakatos and Shepard 1997). The first and second cues were presented at similar locations in 70% of trials, which increased the likelihood that attention was maintained on the first location. It was found that RTs to stimuli presented across hemifields (i.e., left to right body locations, or vice versa) were significantly slower than RTs to stimuli within the same hemifield. Moreover, and perhaps more importantly, RTs increased linearly with the Euclidean distance between body locations. These findings support the hypothesis that attention operates in a visual-spatial coordinate reference frame. Further supporting this view, studies show that tactile attention effects are modulated by the position of the eyes. Specifically, it was found that attention effects were reduced in eccentric compared with central fixation conditions (Gherri and Forster 2014).
Several ERP and behavioral studies indicate that proprioception (e.g., body posture) also plays a fundamental role in tactile spatial selection (Eimer et al. 2001; Gherri and Forster 2012b; Kennett et al. 2001; Roder et al. 2008; Schicke and Roder 2006; Yamamoto and Kitazawa 2001). In particular, crossing the upper limbs (i.e., placing the left and right hands on the right and left sides of the body, respectively) can lead to increased RTs and decreased discrimination (Spence et al. 2000b; Yamamoto and Kitazawa 2001). This tactile remapping effect also occurs for crossing of lower body parts (e.g., feet) (Schicke and Roder 2006). Furthermore, reduced performance due to crossed limbs is accompanied by a reduction (or even absence) of early attention effects on target stimuli measured with ERPs (Eimer et al. 2001). Moreover, recent behavioral studies indicate that this tactile remapping takes place around 180–360 ms after stimulus presentation (Azanon et al. 2010; Azanon and Soto-Faraco 2008), indicating that this somatotopic reconfiguration occurs in higher-order somatosensory cortical areas or is mediated by cortico-cortico feedback to early somatosensory areas. In support of the latter hypothesis, a recent neurophysiological study in nonhuman primates revealed a subpopulation in SI whose responses to tactile stimuli were modulated by proprioception after the initial response to cutaneous stimuli (after 100 ms) (Kim et al. 2015). Taken together, these data indicate that stimulus selection in the tactile modality is not solely based on a somatotopic reference frame but rather on the interplay between proprioceptive, visual-spatial, and anatomically defined coordinates.
Feature selection in the somatosensory system.
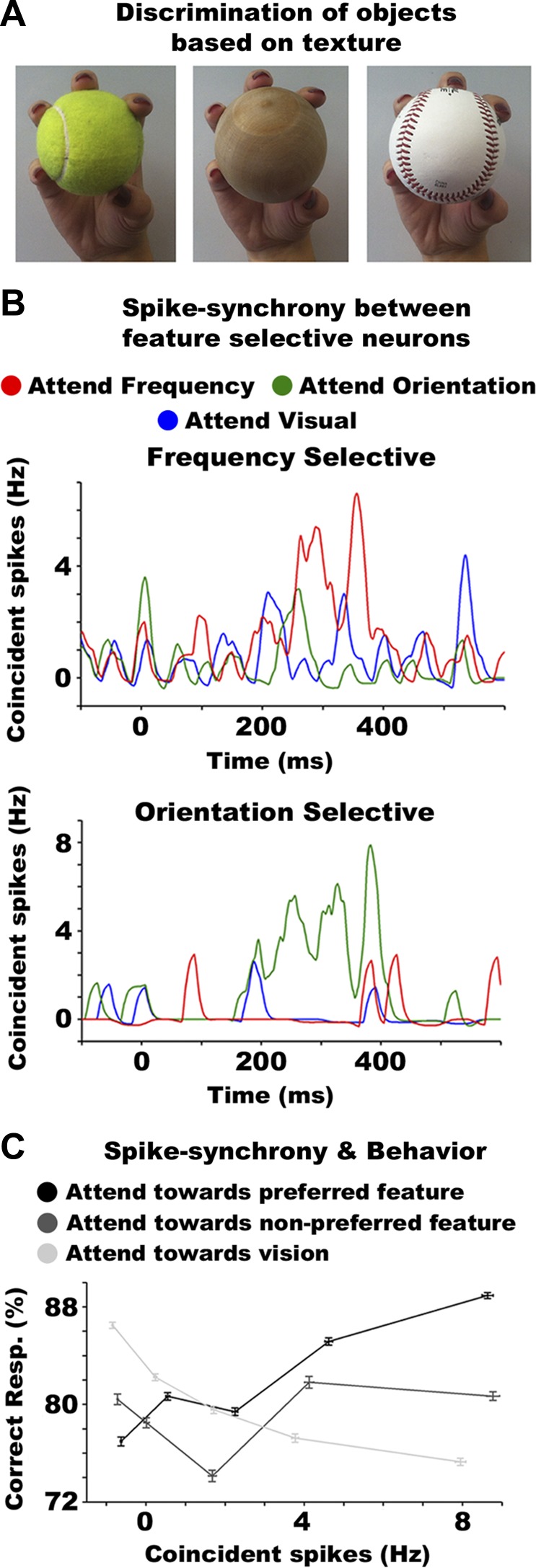
When holding an object with our hands, we effortlessly sense a multitude of tactile features, such as its local curvature, texture, edges, or motion (e.g., when the object is slipping). Often it is necessary to focus on just one (or a set) of these features because recognition of a particular object may rely on identifying a specific feature of the stimulus (e.g., its texture or size). This is shown in Fig. 2A, which shows three objects with similar shape, size, and local curvature. Without assistance from the visual system (e.g., in dark environments), the most effective approach for differentiating between these particular objects is to base discrimination on their texture. This requires selective deployment of attention to textural features while ignoring irrelevant or redundant tactile information (e.g., local curvature). The neural mechanisms mediating this ability were recently investigated in a study (Gomez-Ramirez et al. 2014) in which nonhuman primates were trained to perform tactile orientation and frequency discrimination tasks as well as a visual luminance discrimination task. Neural activity was recorded from SII neurons that were selective for orientation and/or vibrating (i.e., frequency) tactile features. The data revealed increased firing rates when attention was directed toward a neuron's preferred feature (e.g., enhanced activity in orientation selective neurons when performing the orientation task). More strikingly, the frequency of spike-synchrony events between two neurons that were selective for the same tactile feature was also increased when attention was deployed to their preferred stimulus feature (Fig. 2B). Importantly, spike-synchrony attention effects correlated with performance. Specifically, higher accuracy was associated with increased spike synchrony in feature-selective neurons, but only when attention was directed to the preferred feature of the neurons (Fig. 2C). The opposite effect was found when animals performed the visual task, that is, greater spike synchrony between feature-selective somatosensory cells was correlated with decreased performance in the visual task. These findings highlight the effectiveness of attention to enhance the neural circuits that process relevant stimuli but also to disengage the circuits encoding distracting inputs.
Fig. 2.
Feature selection mechanisms in the somatosensory system. A: example of a situation in which tactile feature-based attention is required to recognize and discriminate between objects. All three objects have similar size, shape, and local curvature. To distinguish between them in the absence of visual inputs, one needs to focus on their surface texture. B: instantaneous spike synchrony between two neural pairs selective for frequency (top) and orientation (bottom) tactile features. Frequency of coincident spikes of both neurons is shown when attention is directed toward the orientation (green lines) and frequency (red) of tactile stimuli and toward visual stimuli (blue). Synchrony due to chance for each attention condition was subtracted using the method by Amarasingham et al. (2012), resulting in below-zero synchrony at certain time points. This also applies to C. A synchronous spike was defined as spikes between two neurons occurring within ±2 ms. C: percentage of correct responses as a function of spike synchrony between feature-selective neural pairs, for the “attention toward the preferred feature” (solid black trace), “attention away from the preferred feature” (dark gray trace), and “attention toward vision” (light gray trace). The data reveal systematic, but opposite, relationships between spike-synchrony attention effects and behavior for attending toward the preferred feature of cells and attending toward vision. B and C were adapted with permission from Gomez-Ramirez et al. (2014).
The effects of feature-based attention have been shown to spread across neural populations with different spatial or somatotopic RFs (Martinez-Trujillo and Treue 2004; Schweisfurth et al. 2014; Serences and Boynton 2007) and even across similar features of other sensory systems (e.g., visual and tactile motion) (Konkle et al. 2009). In particular, Schweisfurth and colleagues (2014) had participants perform a tactile task in which they discriminated between bar stimuli with different orientations delivered to their index and middle right fingers. Participants were significantly faster in detecting oriented bars that matched the orientation of the cued stimulus, regardless of whether the stimulus was presented to the cued location or not (i.e., left or right finger). These data indicate that attention biases activity of large numbers of neurons in the population that encodes the relevant features of a task, even neurons whose RF are not colocalized with the spatial spotlight of attention.
Neural Code Mediating Selection of Somatosensory Stimuli
So far we have established that attention influences the firing rates of neurons and the correlated spiking activity between neurons. A major debate in the literature is whether stimulus selection is encoded in the firing rate of a population or the synchronized (or correlated) activity between neurons. Advantages and disadvantages to both types of coding schemes have been discussed (Mazurek and Shadlen 2002; Niebur et al. 1993, 2002; Shadlen and Movshon 1999; Zohary et al. 1994), and some are addressed below.
A firing rate code for stimulus selection functions by raising the gain of a neural ensemble encoding the relevant stimulus. In contrast, in a synchrony code, the correlation between neurons encoding the relevant stimuli is enhanced. One of the main criticisms of the firing rate code is that it may interfere with other neural codes that rely on gain increases to represent information about sensory stimuli. This is shown in the following example: if attention and stimulus intensity both increase the firing rate of neurons, how do downstream neural populations dissociate between a strong unattended and a weak attended tactile stimulus since both would result in a spike train of intermediate firing rate? This ambiguity places significant limitations to the ability of neural populations to convey the behavioral relevance of their signal to other neural ensembles. This suggests that stimulus selection may rely on a different neural coding scheme.
Theoretical and computational studies indicate that spike synchrony can be a powerful mechanism for stimulus selection and perception. In particular, they show that spikes arriving in synchrony in downstream neural ensembles can aggregate and evoke large postsynaptic potentials compared with asynchronous spikes. Synchronized spiking from a neural cohort (e.g., a feature-selective neural population) will thus be weighted with “higher priority” at the downstream target location (Niebur et al. 2002).
Spike synchrony is a different correlation mechanism than spike-count correlation (Rsc), also called noise correlations (Bair et al. 2001; Cohen and Maunsell 2009, 2011; Ecker et al. 2010; Mitchell et al. 2009; Zohary et al. 1994). Although both quantify correlated spiking activity between neurons, they do so across different temporal windows and seem to be driven by different sources. In particular, spike synchrony refers to the near-simultaneous discharge of spikes between cells, on a scale of a few milliseconds or less. Furthermore, spike synchrony is thought to arise from a source (or sources) that increase(s) a population's signal-to-noise ratio by promoting the temporal alignment of spikes or raising the membrane potential levels across cells to evoke common spikes across the population. In neural correlation models of attention, this serves to enhance spike synchrony across neurons encoding relevant inputs of the task (Gomez-Ramirez et al. 2014; Steinmetz et al. 2000). In contrast, Rsc refers to the trial-by-trial response variability shared between multiple cells to a repeated stimulus. Unlike spike synchrony, Rsc does not depend on the strict alignment of interneuronal spiking. Rather, Rsc is defined as the covariability between neuronal spiking across large time windows (>100 ms to s). Rsc is believed to be deleterious to perception because it makes the readout of a population more difficult by adding common noise and/or redundant information. Thus, as expected, Rsc between neurons with a spatial or somatotopic RF over the attended area has been shown to be reduced (Cohen and Maunsell 2009; Gomez-Ramirez et al. 2014; Mitchell et al. 2009; Ruff and Cohen 2014). The opposite is the case for spike synchrony. The relationship between these two correlation mechanisms is not fully understood, but studies show a linear correlation between spike synchrony and Rsc based on the stimulus selectivity of neurons (Bair et al. 2001; Gomez-Ramirez et al. 2014).
Conclusions
We reviewed the neural mechanisms of attention in the somatosensory system based on psychophysical and neurophysiological data from humans and nonhuman primates. An overwhelming number of studies show that attention to a particular part of the body enhances the likelihood that stimuli delivered to that location are detected more accurately and faster compared with stimuli presented to other locations. Similar to the visual and auditory modalities, the presence of distracters impairs discrimination of target stimuli at attended locations. This performance deficit decreases as the distance between distracters and targets increases.
Attention effects on somatosensory cells emerge during the early phases of stimulus processing, commencing as early as 55 ms after stimulus onset. The magnitude and prevalence of these effects increase across the somatosensory hierarchy, having greater impact on neuronal responses in SII compared with SI. Spatial selection of tactile stimuli is a neural process that likely precedes feature-based attention mechanisms.
Spatial selection of somatosensory stimuli is based on the interplay between proprioceptive, visual-spatial, and anatomically defined coordinates. Feature-based selection is mediated by modulations of neural populations encoding the relevant features of a task. Stimulus selection (both spatial and feature) appears to be driven by neural mechanisms that leverage the correlated activity within neural populations. In particular, somatotopic selection is mediated, at least in part, by reducing spike count correlations across neurons with similar RFs, whereas feature-based attention in the somatosensory system increases spike synchrony between neurons selective for the attended feature.
These spatial- and feature-based attention mechanisms are controlled by a cross-cortical neural network that is located over frontal and parietal cortexes. However, recent studies provide strong evidence that SC neurons (Krauzlis et al. 2013, 2014; Lovejoy et al. 2009; Zenon and Krauzlis 2012) may also play a significant role in this process. Future studies are needed to understand how these higher-order and subcortical areas modulate neural activity in sensory cortexes.
GRANTS
This work was supported by ONR-MURI Grant No. N000141010278 (E. Niebur).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.G.-R. and E.N. conception and design of research; M.G.-R. and E.N. performed experiments; M.G.-R. and E.N. analyzed data; M.G.-R. and E.N. interpreted results of experiments; M.G.-R. and K.H. prepared figures; M.G.-R. and E.N. drafted manuscript; M.G.-R., K.H., and E.N. edited and revised manuscript; M.G.-R., K.H., and E.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the work of Steven S. Hsiao in the field of selective attention in the somatosensory system. During the latter part of his career, Dr. Hsiao devoted much of his efforts towards understanding how selective attention mechanisms regulated cutaneous and proprioceptive cortical populations that facilitate tactile perceptual judgments and, ultimately, tactile object perception. He regularly shared his insights with us.
REFERENCES
- Abraira VE, Ginty DD. The sensory neurons of touch. Neuron 79: 618–639, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison T, McCarthy G, Wood CC. The relationship between human long-latency somatosensory evoked potentials recorded from the cortical surface and from the scalp. Electroencephalogr Clin Neurophysiol 84: 301–314, 1992. [DOI] [PubMed] [Google Scholar]
- Allison T, McCarthy G, Wood CC, Darcey TM, Spencer DD, Williamson PD. Human cortical potentials evoked by stimulation of the median nerve. I. Cytoarchitectonic areas generating short-latency activity. J Neurophysiol 62: 694–710, 1989. [DOI] [PubMed] [Google Scholar]
- Alvarez GA, Cavanagh P. Independent resources for attentional tracking in the left and right visual hemifields. Psychol Sci 16: 637–643, 2005. [DOI] [PubMed] [Google Scholar]
- Azanon E, Camacho K, Soto-Faraco S. Tactile remapping beyond space. Eur J Neurosci 31: 1858–1867, 2010. [DOI] [PubMed] [Google Scholar]
- Azanon E, Soto-Faraco S. Changing reference frames during the encoding of tactile events. Curr Biol 18: 1044–1049, 2008. [DOI] [PubMed] [Google Scholar]
- Bair W, Zohary E, Newsome WT. Correlated firing in macaque visual area MT: time scales and relationship to behavior. J Neurosci 21: 1676–1697, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Snyder AC, Molholm S, Foxe JJ. Oscillatory alpha-band mechanisms and the deployment of spatial attention to anticipated auditory and visual target locations: supramodal or sensory-specific control mechanisms? J Neurosci 31: 9923–9932, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Oostenveld R, Peeters M, Fries P. Tactile spatial attention enhances gamma-band activity in somatosensory cortex and reduces low-frequency activity in parieto-occipital areas. J Neurosci 26: 490–501, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw JL, Howard MJ, Pierson JM, Phillips J, Bradshaw JA. Effects of expectancy and attention in vibrotactile choice reaction time tasks. Q J Exp Psychol 44A: 1992. [Google Scholar]
- Burton H, Abend NS, MacLeod AM, Sinclair RJ, Snyder AZ, Raichle ME. Tactile attention tasks enhance activation in somatosensory regions of parietal cortex: a positron emission tomography study. Cereb Cortex 9: 662–674, 1999. [DOI] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ. Attending to and remembering tactile stimuli: a review of brain imaging data and single-neuron responses. J Clin Neurophysiol 17: 575–591, 2000a. [DOI] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ. Tactile-spatial and cross-modal attention effects in the primary somatosensory cortical areas 3b and 1–2 of rhesus monkeys. Somatosens Mot Res 17: 213–228, 2000b. [DOI] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ, Hong SY, Pruett JR Jr, Whang KC. Tactile-spatial and cross-modal attention effects in the second somatosensory and 7b cortical areas of rhesus monkeys. Somatosens Mot Res 14: 237–267, 1997. [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH. Mechanical response properties of ventroposterior medial thalamic neurons in the alert monkey. Exp Brain Res 67: 603–614, 1987. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459: 663–667, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh P, Alvarez GA. Tracking multiple targets with multifocal attention. Trends Cogn Sci 9: 349–354, 2005. [DOI] [PubMed] [Google Scholar]
- Chen TL, Babiloni C, Ferretti A, Perrucci MG, Romani GL, Rossini PM, Tartaro A, Del Gratta C. Effects of somatosensory stimulation and attention on human somatosensory cortex: an fMRI study. Neuroimage 53: 181–188, 2010. [DOI] [PubMed] [Google Scholar]
- Chen TL, Babiloni C, Ferretti A, Perrucci MG, Romani GL, Rossini PM, Tartaro A, Del Gratta C. Human secondary somatosensory cortex is involved in the processing of somatosensory rare stimuli: an fMRI study. Neuroimage 40: 1765–1771, 2008. [DOI] [PubMed] [Google Scholar]
- Chica AB, Taylor TL, Lupianez J, Klein RM. Two mechanisms underlying inhibition of return. Exp Brain Res 201: 25–35, 2010. [DOI] [PubMed] [Google Scholar]
- Cohen JC, Bolanowski SJ, Verrillo RT. A direct comparison of exogenous and endogenous inhibition of return and selective attention mechanisms in the somatosensory system. Somatosensory Motor Res 22: 269–279, 2005. [DOI] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JH. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci 12: 1594–1600, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JH. When attention wanders: how uncontrolled fluctuations in attention affect performance. J Neurosci 31: 15802–15806, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colavita FB. Human sensory dominance. Perception Psychophys 16: 409–412, 1974. [Google Scholar]
- Connor CE, Hsiao SS, Phillips JR, Johnson KO. Tactile roughness: neural codes that account for psychophysical magnitude estimates. J Neurosci 10: 3823–3836, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor CE, Johnson KO. Neural coding of tactile texture: comparison of spatial and temporal mechanisms for roughness perception. J Neurosci 12: 3414–3426, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Curro Dossi R, Steriade M. Bursting and tonic discharges in two classes of reticular thalamic neurons. J Neurophysiol 68: 973–977, 1992. [DOI] [PubMed] [Google Scholar]
- Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science 274: 771–774, 1996. [DOI] [PubMed] [Google Scholar]
- Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Spatiotemporal patterns of spindle oscillations in cortex and thalamus. J Neurosci 17: 1179–1196, 1997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Destexhe A, Steriade M. Intracellular and computational characterization of the intracortical inhibitory control of synchronized thalamic inputs in vivo. J Neurophysiol 78: 335–350, 1997b. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3: 201–215, 2002. [DOI] [PubMed] [Google Scholar]
- Cordo PJ, Flores-Vieira C, Verschueren SM, Inglis JT, Gurfinkel V. Position sensitivity of human muscle spindles: single afferent and population representations. J Neurophysiol 87: 1186–1195, 2002. [DOI] [PubMed] [Google Scholar]
- Craig JC. Attending to two fingers: two hands are better than one. Percept Psychophys 38: 496–511, 1985. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci 18: 193–222, 1995. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Steriade M. Mechanisms underlying the synchronizing action of corticothalamic feedback through inhibition of thalamic relay cells. J Neurophysiol 79: 999–1016, 1998. [DOI] [PubMed] [Google Scholar]
- Doesburg SM, Roggeveen AB, Kitajo K, Ward LM. Large-scale gamma-band phase synchronization and selective attention. Cereb Cortex 18: 386–396, 2008. [DOI] [PubMed] [Google Scholar]
- Dubner R, Hoffman DS, Hayes RL. Neuronal activity in medullary dorsal horn of awake monkeys trained in a thermal discrimination task. III. Task-related responses and their functional role. J Neurophysiol 46: 444–464, 1981. [DOI] [PubMed] [Google Scholar]
- Eardley AF, van Velzen J. Event-related potential evidence for the use of external coordinates in the preparation of tactile attention by the early blind. Eur J Neurosci 33: 1897–1907, 2011. [DOI] [PubMed] [Google Scholar]
- Ecker AS, Berens P, Keliris GA, Bethge M, Logothetis NK, Tolias AS. Decorrelated neuronal firing in cortical microcircuits. Science 327: 584–587, 2010. [DOI] [PubMed] [Google Scholar]
- Edin BB, Abbs JH. Finger movement responses of cutaneous mechanoreceptors in the dorsal skin of the human hand. J Neurophysiol 65: 657–670, 1991. [DOI] [PubMed] [Google Scholar]
- Eimer M, Cockburn D, Smedley B, Driver J. Cross-modal links in endogenous spatial attention are mediated by common external locations: evidence from event-related brain potentials. Exp Brain Res 139: 398–411, 2001. [DOI] [PubMed] [Google Scholar]
- Eimer M, Driver J. Crossmodal links in endogenous and exogenous spatial attention: evidence from event-related brain potential studies. Neurosci Biobehav Rev 25: 497–511, 2001. [DOI] [PubMed] [Google Scholar]
- Eimer M, Forster B. Modulations of early somatosensory ERP components by transient and sustained spatial attention. Exp Brain Res 151: 24–31, 2003a. [DOI] [PubMed] [Google Scholar]
- Eimer M, Forster B. The spatial distribution of attentional selectivity in touch: evidence from somatosensory ERP components. Clin Neurophysiol 114: 1298–1306, 2003b. [DOI] [PubMed] [Google Scholar]
- Eimer M, Forster B, Fieger A, Harbich S. Effects of hand posture on preparatory control processes and sensory modulations in tactile-spatial attention. Clin Neurophysiol 115: 596–608, 2004a. [DOI] [PubMed] [Google Scholar]
- Eimer M, Forster B, Van Velzen J. Anterior and posterior attentional control systems use different spatial reference frames: ERP evidence from covert tactile-spatial orienting. Psychophysiology 40: 924–933, 2003a. [DOI] [PubMed] [Google Scholar]
- Eimer M, Van Velzen J. Crossmodal links in spatial attention are mediated by supramodal control processes: evidence from event-related potentials. Psychophysiology 39: 437–449, 2002. [DOI] [PubMed] [Google Scholar]
- Eimer M, van Velzen J, Driver J. Cross-modal interactions between audition, touch, and vision in endogenous spatial attention: ERP evidence on preparatory states and sensory modulations. J Cogn Neurosci 14: 254–271, 2002. [DOI] [PubMed] [Google Scholar]
- Eimer M, van Velzen J, Driver J. ERP evidence for cross-modal audiovisual effects of endogenous spatial attention within hemifields. J Cogn Neurosci 16: 272–288, 2004b. [DOI] [PubMed] [Google Scholar]
- Eimer M, van Velzen J, Forster B, Driver J. Shifts of attention in light and in darkness: an ERP study of supramodal attentional control and crossmodal links in spatial attention. Brain Res Cogn Brain Res 15: 308–323, 2003b. [DOI] [PubMed] [Google Scholar]
- Eriksen CW, Hoffman JE. Some characteristics of selective attention in visual perception determined by vocal reaction-time. Percept Psychophys 11: 169–171, 1972. [Google Scholar]
- Eriksen CW, St. James JD. Visual attention within and around the field of focal attention: a zoom lens model. Percept Psychophys 40: 225–240, 1986. [DOI] [PubMed] [Google Scholar]
- Ernst MO, Banks MS. Humans integrate visual and haptic information in a statistically optimal fashion. Nature 415: 429–433, 2002. [DOI] [PubMed] [Google Scholar]
- Evans PM, Craig JC, Rinker MA. Perceptual processing of adjacent and nonadjacent tactile nontargets. Percept Psychophys 52: 571–581, 1992. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PJ, Lane JW, Thakur PH, Hsiao SS. Receptive field (RF) properties of the macaque second somatosensory cortex: RF size, shape, and somatotopic organization. J Neurosci 26: 6485–6495, 2006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, Lane JW, Thakur PH, Hsiao SS. Receptive field properties of the macaque second somatosensory cortex: evidence for multiple functional representations. J Neurosci 24: 11193–11204, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, Lane JW, Thakur PH, Hsiao SS. Receptive field properties of the macaque second somatosensory cortex: representation of orientation on different finger pads. J Neurosci 26: 6473–6484, 2006b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster B, Eimer M. The attentional selection of spatial and non-spatial attributes in touch: ERP evidence for parallel and independent processes. Biol Psychol 66: 1–20, 2004. [DOI] [PubMed] [Google Scholar]
- Forster B, Eimer M. Covert attention in touch: behavioral and ERP evidence for costs and benefits. Psychophysiology 42: 171–179, 2005. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV, Ahlfors SP. Parieto-occipital approximately 10 Hz activity reflects anticipatory state of visual attention mechanisms. Neuroreport 9: 3929–3933, 1998. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Snyder AC. The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front Psychol 2: 154, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman AW, Johnson KO. A model accounting for effects of vibratory amplitude on responses of cutaneous mechanoreceptors in macaque monkey. J Physiol 323: 43–64, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291: 1560–1563, 2001. [DOI] [PubMed] [Google Scholar]
- Fu KM, Foxe JJ, Murray MM, Higgins BA, Javitt DC, Schroeder CE. Attention-dependent suppression of distracter visual input can be cross-modally cued as indexed by anticipatory parieto-occipital alpha-band oscillations. Brain Res Cogn Brain Res 12: 145–152, 2001. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Lukaszewicz AC, Mauguiere F. Somatosensory responses during selective spatial attention: The N120-to-N140 transition. Psychophysiology 32: 526–537, 1995. [DOI] [PubMed] [Google Scholar]
- Gherri E, Forster B. Attention to the body depends on eye-in-orbit position. Front Psychol 5: 683, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherri E, Forster B. Crossing the hands disrupts tactile spatial attention but not motor attention: evidence from event-related potentials. Neuropsychologia 50: 2303–2316, 2012a. [DOI] [PubMed] [Google Scholar]
- Gherri E, Forster B. The orienting of attention during eye and hand movements: ERP evidence for similar frame of reference but different spatially specific modulations of tactile processing. Biol Psychol 91: 172–184, 2012b. [DOI] [PubMed] [Google Scholar]
- Goltz D, Gundlach C, Nierhaus T, Villringer A, Muller M, Pleger B. Connections between intraparietal sulcus and a sensorimotor network underpin sustained tactile attention. J Neurosci 35: 7938–7949, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goltz D, Pleger B, Thiel SD, Villringer A, Muller MM. Sustained spatial attention to vibrotactile stimulation in the flutter range: relevant brain regions and their interaction. PLoS One 8: e84196, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Ramirez M, Kelly SP, Molholm S, Sehatpour P, Schwartz TH, Foxe JJ. Oscillatory sensory selection mechanisms during intersensory attention to rhythmic auditory and visual inputs: a human electrocorticographic investigation. J Neurosci 31: 18556–18567, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Ramirez M, Trzcinski NK, Mihalas S, Niebur E, Hsiao SS. Temporal correlation mechanisms and their role in feature selection: a single-unit study in primate somatosensory cortex. PLoS Biol 12: e1002004, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin AW, Macefield VG, Bisley JW. Encoding of object curvature by tactile afferents from human fingers. J Neurophysiol 78: 2881–2888, 1997. [DOI] [PubMed] [Google Scholar]
- Guest S, Spence C. Tactile dominance in speeded discrimination of textures. Exp Brain Res 150: 201–207, 2003. [DOI] [PubMed] [Google Scholar]
- Haegens S, Handel BF, Jensen O. Top-down controlled alpha band activity in somatosensory areas determines behavioral performance in a discrimination task. J Neurosci 31: 5197–5204, 2011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Luther L, Jensen O. Somatosensory anticipatory alpha activity increases to suppress distracting input. J Cogn Neurosci 24: 677–685, 2012. [DOI] [PubMed] [Google Scholar]
- Haegens S, Nacher V, Luna R, Romo R, Jensen O. alpha-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc Natl Acad Sci USA 108: 19377–19382, 2011b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallin RG, Carlstedt T, Wu G. Population behaviour of human cutaneous mechanoreceptive units. Behav Brain Res 135: 19–26, 2002. [DOI] [PubMed] [Google Scholar]
- Hamalainen H, Hiltunen J, Titievskaja I. Activation of somatosensory cortical areas varies with attentional state: an fMRI study. Behav Brain Res 135: 159–165, 2002. [DOI] [PubMed] [Google Scholar]
- Harter MR, Miller SL, Price NJ, Lalonde ME, Keyes AL. Neural processes involved in directing attention. J Cogn Neurosci 1: 223–237, 1989. [DOI] [PubMed] [Google Scholar]
- Harvey MA, Saal HP, Dammann JF 3rd, Bensmaia SJ. Multiplexing stimulus information through rate and temporal codes in primate somatosensory cortex. PLoS Biol 11: e1001558, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes RL, Dubner R, Hoffman DS. Neuronal activity in medullary dorsal horn of awake monkeys trained in a thermal discrimination task. II. Behavioral modulation of responses to thermal and mechanical stimuli. J Neurophysiol 46: 428–443, 1981. [DOI] [PubMed] [Google Scholar]
- Hoffman DS, Dubner R, Hayes RL, Medlin TP. Neuronal activity in medullary dorsal horn of awake monkeys trained in a thermal discrimination task. I. Responses to innocuous and noxious thermal stimuli. J Neurophysiol 46: 409–427, 1981. [DOI] [PubMed] [Google Scholar]
- Hopf JM, Mangun GR. Shifting visual attention in space: an electrophysiological analysis using high spatial resolution mapping. Clin Neurophysiol 111: 1241–1257, 2000. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci 3: 284–291, 2000. [DOI] [PubMed] [Google Scholar]
- Houk J, Henneman E. Responses of Golgi tendon organs to active contractions of the soleus muscle of the cat. J Neurophysiol 30: 466–481, 1967. [DOI] [PubMed] [Google Scholar]
- Hsiao S, Gomez-Ramirez M. Touch. In: Neurobiology of Sensation and Reward. Boca Raton, FL: CRC, 2011. [Google Scholar]
- Hsiao SS, O'Shaughnessy DM, Johnson KO. Effects of selective attention on spatial form processing in monkey primary and secondary somatosensory cortex. J Neurophysiol 70: 444–447, 1993. [DOI] [PubMed] [Google Scholar]
- Hunt AR, Kingstone A. Inhibition of return: dissociating attentional and oculomotor components. J Exp Psychol Hum Percept Perform 29: 1068–1074, 2003. [DOI] [PubMed] [Google Scholar]
- Inui K, Wang X, Tamura Y, Kaneoke Y, Kakigi R. Serial processing in the human somatosensory system. Cereb Cortex 14: 851–857, 2004. [DOI] [PubMed] [Google Scholar]
- Itti L, Koch C, Niebur E. A model of saliency-based fast visual attention for rapid scene analysis. IEEE Trans Pattern Anal Machine Intell 20: 1254–1259, 1998. [Google Scholar]
- Johansen-Berg H, Lloyd DM. The physiology and psychology of selective attention to touch. Front Biosci 5: D894–D904, 2000. [DOI] [PubMed] [Google Scholar]
- Johnson KO. Neural mechanisms of tactual form and texture discrimination. Fed Proc 42: 2542–2547, 1983. [PubMed] [Google Scholar]
- Johnson KO, Yoshioka T, Vega-Bermudez F. Tactile functions of mechanoreceptive afferents innervating the hand. J Clin Neurophysiol 17: 539–558, 2000. [DOI] [PubMed] [Google Scholar]
- Jones A, Forster B. Lost in vision: ERP correlates of exogenous tactile attention when engaging in a visual task. Neuropsychologia 51: 675–685, 2013. [DOI] [PubMed] [Google Scholar]
- Jones A, Forster B. Neural correlates of endogenous attention, exogenous attention and inhibition of return in touch. Eur J Neurosci 40: 2389–2398, 2014. [DOI] [PubMed] [Google Scholar]
- Jones A, Forster B. Reflexive attention in touch: an investigation of event related potentials and behavioural responses. Biol Psychol 89: 313–322, 2012. [DOI] [PubMed] [Google Scholar]
- Jones SR, Kerr CE, Wan Q, Pritchett DL, Hamalainen M, Moore CI. Cued spatial attention drives functionally relevant modulation of the mu rhythm in primary somatosensory cortex. J Neurosci 30: 13760–13765, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karns CM, Knight RT. Intermodal auditory, visual, and tactile attention modulates early stages of neural processing. J Cogn Neurosci 21: 669–683, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SP, Gomez-Ramirez M, Foxe JJ. The strength of anticipatory spatial biasing predicts target discrimination at attended locations: a high-density EEG study. Eur J Neurosci 30: 2224–2234, 2009. [DOI] [PubMed] [Google Scholar]
- Kennett S, Eimer M, Spence C, Driver J. Tactile-visual links in exogenous spatial attention under different postures: convergent evidence from psychophysics and ERPs. J Cogn Neurosci 13: 462–478, 2001. [DOI] [PubMed] [Google Scholar]
- Kida T, Inui K, Tanaka E, Kakigi R. Dynamics of within-, inter-, and cross-modal attentional modulation. J Neurophysiol 105: 674–686, 2011. [DOI] [PubMed] [Google Scholar]
- Kida T, Kakigi R. High temporal resolution neuroimaging of attentional and somatosensory-motor processing in the human brain. Curr Med Imaging Rev 4: 144–162, 2008. [Google Scholar]
- Kida T, Kakigi R. Neural mechanisms of attention involved in perception and action: From neuronal activity to network. J Phys Fitness Sports Med 4: 161–169, 2015. [Google Scholar]
- Kida T, Kakigi R. Task-related changes in functional properties of the human brain network underlying attentional control. PLoS One 8: e79023, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida T, Nishihira Y, Wasaka T, Nakata H, Sakamoto M. Differential modulation of temporal and frontal components of the somatosensory N140 and the effect of interstimulus interval in a selective attention task. Brain Res Cogn Brain Res 19: 33–39, 2004. [DOI] [PubMed] [Google Scholar]
- Kim SS, Gomez-Ramirez M, Thakur PH, Hsiao SS. Multimodal Interactions between Proprioceptive and Cutaneous Signals in Primary Somatosensory Cortex. Neuron 86: 555–566, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RM. Inhibition of return. Trends Cogn Sci 4: 138–147, 2000. [DOI] [PubMed] [Google Scholar]
- Koch C, Ullman S. Shifts in selective visual attention: towards the underlying neural circuitry. Hum Neurobiol 4: 219–227, 1985. [PubMed] [Google Scholar]
- Konkle T, Wang Q, Hayward V, Moore CI. Motion aftereffects transfer between touch and vision. Curr Biol 19: 745–750, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppen C, Alsius A, Spence C. Semantic congruency and the Colavita visual dominance effect. Exp Brain Res 184: 533–546, 2008. [DOI] [PubMed] [Google Scholar]
- Koppen C, Spence C. Audiovisual asynchrony modulates the Colavita visual dominance effect. Brain Res 1186: 224–232, 2007a. [DOI] [PubMed] [Google Scholar]
- Koppen C, Spence C. Spatial coincidence modulates the Colavita visual dominance effect. Neurosci Lett 417: 107–111, 2007b. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Bollimunta A, Arcizet F, Wang L. Attention as an effect not a cause. Trends Cogn Sci 18: 457–464, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis RJ, Lovejoy LP, Zenon A. Superior colliculus and visual spatial attention. Annu Rev Neurosci 36: 165–182, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos S, Shepard RN. Time-distance relations in shifting attention between locations on one's body. Percept Psychophys 59: 557–566, 1997. [DOI] [PubMed] [Google Scholar]
- Lederman SJ, Thorne G, Jones B. Perception of texture by vision and touch: multidimensionality and intersensory integration. J Exp Psychol Hum Percept Perform 12: 169–180, 1986. [DOI] [PubMed] [Google Scholar]
- Lloyd DM, Bolanowski SJ, Howard L, McGlone F. Mechanisms of attention in touch. Somatosens Motor Res 16: 3–10, 1999. [DOI] [PubMed] [Google Scholar]
- Lovejoy LP, Fowler GA, Krauzlis RJ. Spatial allocation of attention during smooth pursuit eye movements. Vision Res 49: 1275–1285, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol 77: 24–42, 1997. [DOI] [PubMed] [Google Scholar]
- Martinez-Trujillo JC, Treue S. Feature-based attention increases the selectivity of population responses in primate visual cortex. Curr Biol 14: 744–751, 2004. [DOI] [PubMed] [Google Scholar]
- Martinez A, Anllo-Vento L, Sereno MI, Frank LR, Buxton RB, Dubowitz DJ, Wong EC, Hinrichs H, Heinze HJ, Hillyard SA. Involvement of striate and extrastriate visual cortical areas in spatial attention. Nat Neurosci 2: 364–369, 1999. [DOI] [PubMed] [Google Scholar]
- Mauguiere F, Merlet I, Forss N, Vanni S, Jousmaki V, Adeleine P, Hari R. Activation of a distributed somatosensory cortical network in the human brain: a dipole modelling study of magnetic fields evoked by median nerve stimulation. Part II. Effects of stimulus rate, attention and stimulus detection. Electroencephalogr Clin Neurophysiol 104: 290–295, 1997. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Dorflinger JM, Rao SM, Seidenberg M. Neural networks underlying endogenous and exogenous visual-spatial orienting. Neuroimage 23: 534–541, 2004a. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Seidenberg M, Dorflinger JM, Rao SM. An event-related fMRI study of exogenous orienting: supporting evidence for the cortical basis of inhibition of return? J Cogn Neurosci 16: 1262–1271, 2004b. [DOI] [PubMed] [Google Scholar]
- Mazurek ME, Shadlen MN. Limits to the temporal fidelity of cortical spike rate signals. Nat Neurosci 5: 463–471, 2002. [DOI] [PubMed] [Google Scholar]
- McCormick PA. Orienting attention without awareness. J Exp Psychol Hum Percept Perform 23: 168–180, 1997. [DOI] [PubMed] [Google Scholar]
- McMains SA, Somers DC. Multiple spotlights of attentional selection in human visual cortex. Neuron 42: 677–686, 2004. [DOI] [PubMed] [Google Scholar]
- Meftah el-M, Shenasa J, Chapman CE. Effects of a cross-modal manipulation of attention on somatosensory cortical neuronal responses to tactile stimuli in the monkey. J Neurophysiol 88: 3133–3149, 2002. [DOI] [PubMed] [Google Scholar]
- Michie PT. Selective attention effects on somatosensory event-related potentials. Ann NY Acad Sci 425: 250–255, 1984. [DOI] [PubMed] [Google Scholar]
- Michie PT, Bearpark HM, Crawford JM, Glue LC. The effects of spatial selective attention on the somatosensory event-related potential. Psychophysiology 24: 449–463, 1987. [DOI] [PubMed] [Google Scholar]
- Mima T, Nagamine T, Nakamura K, Shibasaki H. Attention modulates both primary and second somatosensory cortical activities in humans: a magnetoencephalographic study. J Neurophysiol 80: 2215–2221, 1998. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron 63: 879–888, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz C, Holz P, Baudewig J, Treue S, Dechent P. Split of attentional resources in human visual cortex. Vis Neurosci 24: 817–826, 2007. [DOI] [PubMed] [Google Scholar]
- Morrow TJ, Casey KL. Attention-related, cross-modality modulation of somatosensory neurons in primate ventrobasal (VB) thalamus. Somatosens Mot Res 17: 133–144, 2000. [DOI] [PubMed] [Google Scholar]
- Morrow TJ, Casey KL. State-related modulation of thalamic somatosensory responses in the awake monkey. J Neurophysiol 67: 305–317, 1992. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. The Sensory Hand: Neural Mechanisms of Somatic Sensation. Cambridge, MA: Harvard University Press, 2005, p. xiv. [Google Scholar]
- Mountcastle VB, LaMotte RH, Carli G. Detection thresholds for stimuli in humans and monkeys: comparison with threshold events in mechanoreceptive afferent nerve fibers innervating the monkey hand. J Neurophysiol 35: 122–136, 1972. [DOI] [PubMed] [Google Scholar]
- Niebur E, Hsiao SS, Johnson KO. Synchrony: a neuronal mechanism for attentional selection? Curr Opin Neurobiol 12: 190–194, 2002. [DOI] [PubMed] [Google Scholar]
- Niebur E, Koch C, Rosin C. An oscillation-based model for the neuronal basis of attention. Vision Res 33: 2789–2802, 1993. [DOI] [PubMed] [Google Scholar]
- Occelli V, O'Brien JH, Spence C, Zampini M. Assessing the audiotactile Colavita effect in near and rear space. Exp Brain Res 203: 517–532, 2010. [DOI] [PubMed] [Google Scholar]
- Olausson H, Wessberg J, Kakuda N. Tactile directional sensibility: peripheral neural mechanisms in man. Brain Res 866: 178–187, 2000. [DOI] [PubMed] [Google Scholar]
- Pei YC, Denchev PV, Hsiao SS, Craig JC, Bensmaia SJ. Convergence of submodality-specific input onto neurons in primary somatosensory cortex. J Neurophysiol 102: 1843–1853, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci 35: 73–89, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Cohen Y. Components of visual orienting. In: Attention and Performance X: Control of Language Processes, edited by Bouma H and Bouwhuis DG. Hillsdale, NJ: Psychology Press, 1984, p. 531–556. [Google Scholar]
- Posner MI, Cohen Y, Rafal RD. Neural systems control of spatial orienting. Philos Trans R Soc Lond B Biol Sci 298: 187–198, 1982. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rafal RD, Choate LS, Vaughan J. Inhibition of return: neural basis and function. Cognitive Neuropsych 2: 211–228, 1985. [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. J Exp Psychol 109: 160–174, 1980. [PubMed] [Google Scholar]
- Praamstra P, Boutsen L, Humphreys GW. Frontoparietal control of spatial attention and motor intention in human EEG. J Neurophysiol 94: 764–774, 2005. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Kourtis D. An early parietal ERP component of the frontoparietal system: EDAN not = N2pc. Brain Res 1317: 203–210, 2010. [DOI] [PubMed] [Google Scholar]
- Proske U, Gregory JE. Signalling properties of muscle spindles and tendon organs. Adv Exp Med Biol 508: 5–12, 2002. [DOI] [PubMed] [Google Scholar]
- Pylyshyn ZW, Storm RW. Tracking multiple independent targets: evidence for a parallel tracking mechanism. Spat Vis 3: 179–197, 1988. [DOI] [PubMed] [Google Scholar]
- Rafal RD, Calabresi PA, Brennan CW, Sciolto TK. Saccade preparation inhibits reorienting to recently attended locations. J Exp Psychol Hum Percept Perform 15: 673–685, 1989. [DOI] [PubMed] [Google Scholar]
- Ray S, Niebur E, Hsiao SS, Sinai A, Crone NE. High-frequency gamma activity (80-150Hz) is increased in human cortex during selective attention. Clin Neurophysiol 119: 116–133, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci 27: 611–647, 2004. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J Neurosci 19: 1736–1753, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder B, Focker J, Hotting K, Spence C. Spatial coordinate systems for tactile spatial attention depend on developmental vision: evidence from event-related potentials in sighted and congenitally blind adult humans. Eur J Neurosci 28: 475–483, 2008. [DOI] [PubMed] [Google Scholar]
- Roder B, Spence C, Rosler F. Assessing the effect of posture change on tactile inhibition-of-return. Exp Brain Res 143: 453–462, 2002. [DOI] [PubMed] [Google Scholar]
- Roll JP, Vedel JP, Ribot E. Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Exp Brain Res 76: 213–222, 1989. [DOI] [PubMed] [Google Scholar]
- Ruff DA, Cohen MR. Attention can either increase or decrease spike count correlations in visual cortex. Nat Neurosci 17: 1591–1597, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambo CF, Forster B. Sustained spatial attention in touch: modality-specific and multimodal mechanisms. Sci World J 11: 199–213, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schicke T, Roder B. Spatial remapping of touch: confusion of perceived stimulus order across hand and foot. Proc Natl Acad Sci USA 103: 11808–11813, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweisfurth MA, Schweizer R, Treue S. Feature-based attentional modulation of orientation perception in somatosensation. Front Hum Neurosci 8: 519, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiss E, Driver J, Eimer M. Effects of attentional filtering demands on preparatory ERPs elicited in a spatial cueing task. Clin Neurophysiol 120: 1087–1095, 2009. [DOI] [PubMed] [Google Scholar]
- Serences JT, Boynton GM. Feature-based attentional modulations in the absence of direct visual stimulation. Neuron 55: 301–312, 2007. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Movshon JA. Synchrony unbound: a critical evaluation of the temporal binding hypothesis. Neuron 24: 67–77, 111–125, 1999. [DOI] [PubMed] [Google Scholar]
- Sherman SM. Thalamic relays and cortical functioning. Prog Brain Res 149: 107–126, 2005. [DOI] [PubMed] [Google Scholar]
- Sherman SM. Tonic and burst firing: dual modes of thalamocortical relay. Trends Neurosci 24: 122–126, 2001. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci 357: 1695–1708, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle JH, Pritchett DL, Moore CI. Gamma-range synchronization of fast-spiking interneurons can enhance detection of tactile stimuli. Nat Neurosci 17: 1371–1379, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GV, Dale CL, Luks TL, Miller WL, Ritter W, Foxe JJ. Rapid targeting followed by sustained deployment of visual spatial attention. Neuroreport 17: 1595–1599, 2006. [DOI] [PubMed] [Google Scholar]
- Sinclair RJ, Kuo JJ, Burton H. Effects on discrimination performance of selective attention to tactile features. Somatosens Mot Res 17: 145–157, 2000. [DOI] [PubMed] [Google Scholar]
- Sinclair RJ, Sathian K, Burton H. Neuronal responses in ventroposterolateral nucleus of thalamus in monkeys (Macaca mulatta) during active touch of gratings. Somatosens Mot Res 8: 293–300, 1991. [DOI] [PubMed] [Google Scholar]
- Smith DT, Schenk T, Rorden C. Saccade preparation is required for exogenous attention but not endogenous attention or IOR. J Exp Psychol Hum Percept Perform 38: 1438–1447, 2012. [DOI] [PubMed] [Google Scholar]
- Spence C, Gallace A. Recent developments in the study of tactile attention. Can J Exp Psychol 61: 196–207, 2007. [DOI] [PubMed] [Google Scholar]
- Spence C, Lloyd D, McGlone F, Nicholls MER, Driver J. Inhibition of return is supramodal: a demonstration between all possible pairings of vision, touch, and audition. Exp Brain Res 134: 42–48, 2000a. [DOI] [PubMed] [Google Scholar]
- Spence C, Pavani F, Driver J. Crossmodal links between vision and touch in covert endogenous spatial attention. J Exp Psychol Hum Percept Perform 26: 1298–1319, 2000b. [DOI] [PubMed] [Google Scholar]
- Steinmetz PN, Roy A, Fitzgerald PJ, Hsiao SS, Johnson KO, Niebur E. Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature 404: 187–190, 2000. [DOI] [PubMed] [Google Scholar]
- Tassinari G, Campara D. Consequences of covert orienting to non-informative stimuli of different modalities: a unitary mechanism? Neuropsychologia 34: 235–245, 1996. [DOI] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci 26: 9494–9502, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay N, Bushnell MC, Duncan GH. Thalamic VPM nucleus in the behaving monkey. II. Response to air-puff stimulation during discrimination and attention tasks. J Neurophysiol 69: 753–763, 1993. [DOI] [PubMed] [Google Scholar]
- Turatto M, Benso F, Facoetti A, Galfano G, Mascetti GG, Umilta C. Automatic and voluntary focusing of attention. Percept Psychophys 62: 935–952, 2000. [DOI] [PubMed] [Google Scholar]
- Turatto M, Galfano G, Bridgeman B, Umilta C. Space-independent modality-driven attentional capture in auditory, tactile and visual systems. Exp Brain Res 155: 301–310, 2004a. [DOI] [PubMed] [Google Scholar]
- Turatto M, Galfano G, Gardini S, Mascetti GG. Stimulus-driven attentional capture: an empirical comparison of display-size and distance methods. Q J Exp Psychol A 57: 297–324, 2004b. [DOI] [PubMed] [Google Scholar]
- van Ede F, de Lange F, Jensen O, Maris E. Orienting attention to an upcoming tactile event involves a spatially and temporally specific modulation of sensorimotor alpha- and beta-band oscillations. J Neurosci 31: 2016–2024, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede F, Szebenyi S, Maris E. Attentional modulations of somatosensory alpha, beta and gamma oscillations dissociate between anticipation and stimulus processing. Neuroimage 97: 134–141, 2014. [DOI] [PubMed] [Google Scholar]
- Van Velzen J, Eardley AF, Forster B, Eimer M. Shifts of attention in the early blind: an erp study of attentional control processes in the absence of visual spatial information. Neuropsychologia 44: 2533–2546, 2006. [DOI] [PubMed] [Google Scholar]
- van Velzen J, Eimer M. Early posterior ERP components do not reflect the control of attentional shifts toward expected peripheral events. Psychophysiology 40: 827–831, 2003. [DOI] [PubMed] [Google Scholar]
- van Velzen J, Forster B, Eimer M. Temporal dynamics of lateralized ERP components elicited during endogenous attentional shifts to relevant tactile events. Psychophysiology 39: 874–878, 2002. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci 26: 676–682, 2003. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 373: 612–615, 1995. [DOI] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J Neurosci 20: RC63, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Kitazawa S. Reversal of subjective temporal order due to arm crossing. Nat Neurosci 4: 759–765, 2001. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Gibb B, Dorsch AK, Hsiao SS, Johnson KO. Neural coding mechanisms underlying perceived roughness of finely textured surfaces. J Neurosci 21: 6905–6916, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenon A, Krauzlis RJ. Attention deficits without cortical neuronal deficits. Nature 489: 434–437, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohary E, Shadlen MN, Newsome WT. Correlated neuronal discharge rate and its implications for psychophysical performance. Nature 370: 140–143, 1994. [DOI] [PubMed] [Google Scholar]