We examined the specificity of primary motor cortex (M1) inputs to specific classes of primary somatosensorty cortex (S1) pyramidal neurons and found that M1 axons synapse on infragranular S1 neurons with greater selectivity than previously appreciated. These results suggest M1 can differentially regulate particular circuits in S1 under conditions of high M1 activity (e.g., whisking). Additionally, each type of pyramidal neuron has a unique set of intrinsic properties that allow them function differently within the circuit.
Keywords: barrel cortex, sensorimotor integration, cortical circuitry
Abstract
The functional role of input from the primary motor cortex (M1) to primary somatosensory cortex (S1) is unclear; one key to understanding this pathway may lie in elucidating the cell-type specific microcircuits that connect S1 and M1. Recently, we discovered that a subset of pyramidal neurons in the infragranular layers of S1 receive especially strong input from M1 (Kinnischtzke AK, Simons DJ, Fanselow EE. Cereb Cortex 24: 2237–2248, 2014), suggesting that M1 may affect specific classes of pyramidal neurons differently. Here, using combined optogenetic and retrograde labeling approaches in the mouse, we examined the strengths of M1 inputs to five classes of infragranular S1 neurons categorized by their projections to particular cortical and subcortical targets. We found that the magnitude of M1 synaptic input to S1 pyramidal neurons varies greatly depending on the projection target of the postsynaptic neuron. Of the populations examined, M1-projecting corticocortical neurons in L6 received the strongest M1 inputs, whereas ventral posterior medial nucleus-projecting corticothalamic neurons, also located in L6, received the weakest. Each population also possessed distinct intrinsic properties. The results suggest that M1 differentially engages specific classes of S1 projection neurons, thereby regulating the motor-related influence S1 exerts over subcortical structures.
NEW & NOTEWORTHY
We examined the specificity of primary motor cortex (M1) inputs to specific classes of primary somatosensorty cortex (S1) pyramidal neurons and found that M1 axons synapse on infragranular S1 neurons with greater selectivity than previously appreciated. These results suggest M1 can differentially regulate particular circuits in S1 under conditions of high M1 activity (e.g., whisking). Additionally, each type of pyramidal neuron has a unique set of intrinsic properties that allow them function differently within the circuit.
during active exploration, sensory and motor systems act in concert in order for sensory feedback to inform ongoing and future movements. Although different regions within the central nervous system process sensory and motor information, the structures are highly interconnected, forming large-scale sensorimotor integration systems (Kleinfeld et al. 2006; Petersen 2007). At the level of the telencephalon, primary somatosensory (S1) and primary motor (M1) cortices are reciprocally connected (Cauller et al. 1998; Miyashita et al. 1994; Porter and White 1983; White and DeAmicis 1977). Both anatomic and physiological studies have demonstrated that S1 sends a robust projection to superficial layers in M1, in particular contacting neurons that project back to S1 (Aronoff et al. 2010; Mao et al. 2011). Recent studies have determined that M1 densely innervates and provides the strongest input to the infragranular layers in S1 (Kinnischtzke et al. 2014; Veinante and Deschenes 2003; Zagha et al. 2013; Zhang and Deschenes 1998), yet the specificity of its connections with excitatory cells in the deep layers remains poorly understood.
The somatic and dendritic morphology of pyramidal neurons is relatively uniform, at least compared with inhibitory interneurons, and consequently they are often considered as a single population. However, accumulating evidence demonstrates that pyramidal neurons differ functionally in terms of their intrinsic properties and their local and extrinsic projections. Recent studies have found that excitatory connections among local S1 pyramidal neurons are not random but instead form sub-circuits based on similarity of physiological properties (Otsuka and Kawaguchi, 2008) receptive field structure (Kampa et al. 2011; Cossell et al. 2015), and/or the projection target of the neurons (Brown and Hestrin, 2009; Otsuka and Kawaguchi, 2011). Our previous study (Kinnischtzke et al. 2014) demonstrated that M1 engages pyramidal cells distributed throughout the cortical layers, consistent with the view that M1 sends a general ‘modulatory’ signal to S1. On the other hand, we also observed significant variation in the strengths of M1 input, with a subset of neurons in the superficial aspect of L6 receiving especially strong inputs. If M1 synapses specifically form stronger connections with pyramidal neurons projecting to a common target, it suggests M1 may regulate S1 communication with long-range cortical and/or subcortical structures in a more precise manner than previously suggested.
Layer (L)5 and L6 of S1 contain a diversity of pyramidal neuron populations that project to a number of cortical and subcortical sites. Here, we investigated synaptic input from M1 onto several types of infragranular S1 pyramidal neurons: pyramidal cells projecting back to M1 (M1 projecting), corticothalamic neurons projecting to either the posteromedial thalamus (PoM projecting) or ventral posterior medial nucleus (VPM projecting), and corticofugal neurons projecting to the spinal trigeminal nucleus (Sp5 projecting). Our results demonstrate that both the intrinsic electrophysiological properties as well as strength and effectiveness of the M1 inputs the neuron receives are strongly related to its projection target.
METHODS
All experiments were carried out in compliance with University of Pittsburgh School of Medicine animal use policies and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Channelrhodopsin and retrograde labeling procedures.
Viral constructs carrying channelrhodopsin (ChR)2 and mCherry genes were injected into the mouse primary motor cortex as previously described (Kinnischtzke et al. 2014). Briefly, mice (10–16 postnatal days of age) were anesthetized with isoflurane (1–2% in oxygen), and a craniotomy was made over the vibrissal area within M1 (1.0 mm anterior to and 0.8 mm lateral from the bregma). The virus was pressure injected at depths of 0.9 and 0.4 mm (corresponding to the deep and superficial layers in M1) within a single penetration. A volume of 0.1–0.2 μl was injected at each depth. We used the adeno-associated virus (AAV) construct AAV2/5.CamKIIα.hChR2(H134R)-mCherry.WPRE.SV40 (University of Pennsylvania Vector Core, permission from Dr. Karl Deisseroth) for all experiments, as this construct results in strong anterograde expression but virtually no retrograde transport (Kinnischtzke et al. 2014).
During the same surgery, mice were also injected with red and green fluorescent retrograde tracers (RetroBeads, Lumafluor). All mice had green retrobeads injected in M1; the undiluted beads were typically coinjected simultaneously with the ChR2 virus (at 1:4 with the virus to achieve 25–50 nl of retrobead injection along with 100–200 nl of virus injection). Mice were then injected with red fluorescent retrobeads in one of the following locations: VPM, PoM, or Sp5. For injections in the thalamus, a small craniotomy was performed dorsal to VPM (1.4 mm posterior and 1.7 mm lateral to the bregma) or PoM (1.6 mm posterior and 1.4 mm lateral to the bregma). Beads were pressure injected using a picospritzer (50–100 nl; depth of 3.20 mm for VPM and 3.10 mm for PoM). The pipette was withdrawn slowly 5–10 min after injection to limit inadvertent leakage in the cortex. For Sp5 injections, the brain stem was exposed directly posterior to the cerebellum, and the pipette was advanced at a 40° angle. Retrobeads were pressure injected using a picospritzer at depths of 0.8 and 0.6 mm (0.1 μl at each depth). Experiments were conducted using both male and female mice from the “GIN” transgenic mouse line (Oliva et al. 2000) to be consistent with our previous study.
Slice preparation.
Electrophysiological experiments were performed 4–8 wk after the injection surgery to allow for transport and full expression of the virus. Mice were between postnatal days 44 and 74 at the time of experiments. This age is not fully adult yet past the stage of major cortical reorganization; therefore, we expect these data to be similar to an adult state. The animal was anesthetized with isoflurane, and the brain was removed and placed in ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM) 126 NaCl, 3 KCl, 1.25 NaH2PO4, 2 MgSO4, 26 NaHCO3, 10 dextrose, and 2 CaCl2 saturated with 95% O2-5% CO2. The tissue was cut into 400-μm slices in the coronal plane using a vibratome. Slices were incubated at 32°C for 30–45 min and then maintained at room temperature until used for recording. Slices containing S1 barrel cortex were identified by the presence of layer IV barrels and a barrel-related pattern of ChR2-mCherry fluorescence, as labeling was dense in deep layers, L5 and L6, and then ascended between the barrels in L5A and L4 (see Fig. 4A). Confirmation of the injection targets was confirmed by examination of fluorescence at the injection site as well as by presence of the expected pattern of laminar cortical labeling. If animals injected with retrobeads in VPM exhibited significant L5B cortical labeling, the injection was considered PoM contamination and the animal was not included in the study.
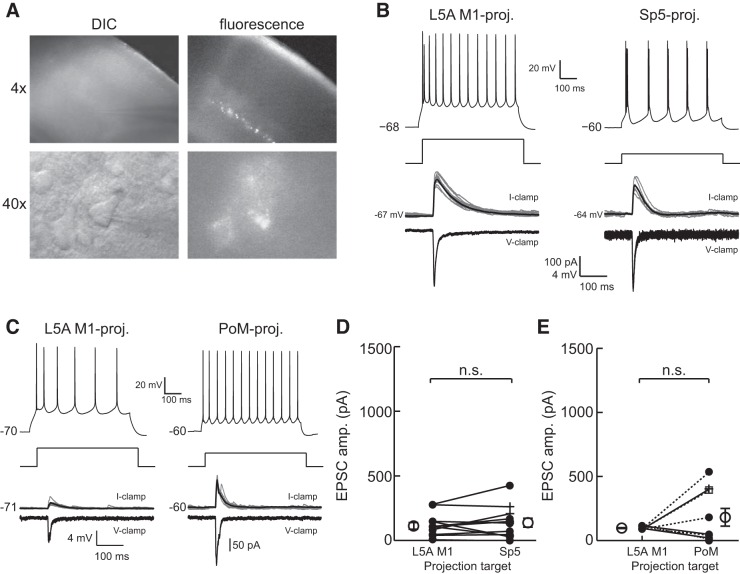
Fig. 4.
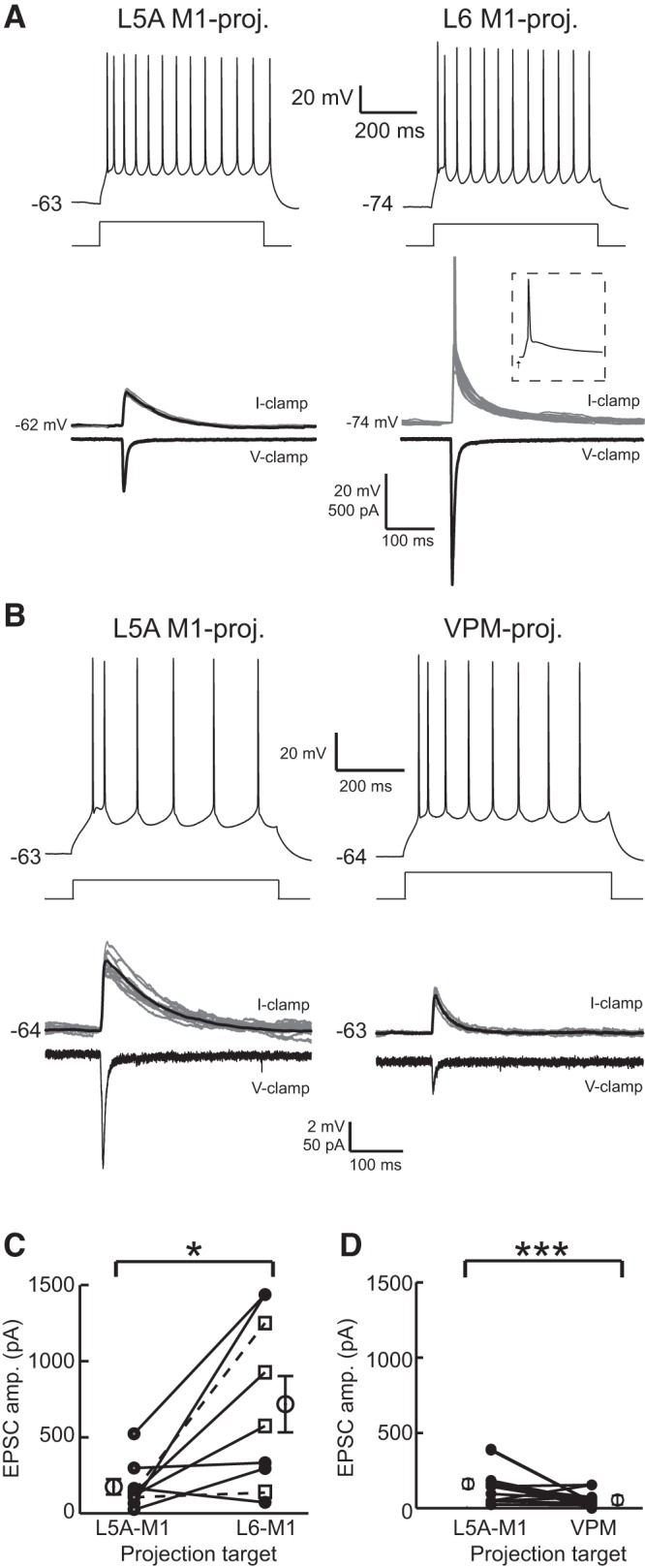
M1 input to L5A M1-, Sp5-, and PoM-projecting neurons is equivalent. A: example of a S1 slice at ×4 magnification under DIC illumination (top left) and epifluorescence (top right) showing bright, retrogradely labeled neurons in L5B that project to Sp5. Diffuse fluorescent labeling was also observed, indicating the presence of ChR2-expressing M1 axon terminals. An example of a recorded pyramidal neuron under DIC optics (bottom left) that expressed fluorescent retrobeads (bottom right) is shown. B: example L5A M1-projecting (left) and Sp5-projecting (right) neurons recorded in the same slice that showed characteristic current step responses (top) and received similar excitatory input from M1 when recorded in current clamp (middle) or voltage clamp (bottom). For current-clamp recordings, multiple traces were overlaid, and the average excitatory postsynaptic potential is shown in black. Excitatory postsynaptic current (EPSC) traces show only the trial-averaged response. C: example L5A M1-projecting and L5B PoM-projecting neurons recorded in the same slice. Conventions are as in B. Cells were held at −80 mV for voltage-clamp recordings in B and C. Population-averaged EPSC responses to M1 stimulation showed that Sp5-projecting neurons (D) and PoM-projecting neurons (E) receive a statistically equivalent magnitude of M1 input as L5A M1-projecting neurons (L5A M1-projecting vs. Sp5-projecting neurons: P = 0.31; L5A M1-projecting vs. PoM-projecting neurons: P = 0.27). In D and E, the closed circles, open squares, and + denote RS cells, B/RS cells, and IB cells, respectively. The open circles and error bars to the left and right of the paired data are the population means and SEs.
We recorded primarily from the larger, more medially situated barrels (rows D and E), as this is where the ChR2-mCherry labeling was typically strongest. We recorded from pyramidal neurons in 1–3 adjacent barrel-related columns/slice.
Recording procedures.
All recorded cells were positive for either red or green retrobeads, which allowed for unambiguous classification of the projection target. Whole cell recordings were performed using glass micropipettes (4–10 MΩ) filled with internal solution containing (in mM) 135 K-gluconate, 4 KCl, 2 NaCl, 10 HEPES, 0.2 EGTA, 4 ATP-Mg, 0.3 GTP-Tris, and 14 phosphocreatine-Tris (pH 7.25, 280–290 mOsm). Biocytin (0.5%) was added to the internal solution in most experiments. Membrane potentials reported here were not corrected for liquid junction potential. Recordings were conducted at 32°C. When patching, cell-attached seal resistances were ≥1 GΩ, and series resistance after achieving whole cell configuration was 5–20 MΩ. After establishing whole cell configuration, a series of current steps was presented in current clamp for use in analysis of the cell's electrophysiological characteristics. Current steps were 600 ms in duration and presented in 20-pA steps ranging from −100 to 300 pA. Steps were presented 5 s apart. In voltage-clamp experiments, series resistances ranged from 10 to 40 MΩ and were compensated for up to 70%. For excitatory postsynaptic current (EPSC) measurements, all cells were held at a potential of −80 mV. Data were collected using a Multiclamp 700B amplifier and pClamp10 software (Molecular Devices) at a sampling rate of 20 kHz.
To reduce between-experiment variability related to differences in ChR expression levels, neurons were recorded in a “paired” manner. For each slice, at least one L5A M1-projecting neuron was recorded in addition to cells having identified projection targets (e.g., Sp5, PoM, or VPM). The amplitude of the M1 input to cells recorded across different animals could then be normalized by the M1 input to a standard population, L5A M1-projecting cells.
Laminar definitions.
Measures of laminar position are reported as normalized distance from the top of L5A (value of 0.0) and the bottom of L6 (value of 1.0). The L4/L5 boundary is distinct, and the normalization procedure accounted for slight variability between experiments in cortical depth due, for example, to section plane. Laminar boundaries were based on Nissl-stained coronal sections in S1. The actual, non-normalized, laminar boundary measurements were similar to previously reported values (Hooks et al. 2011). Borders were identified on the basis of changes in cell densities indicated by Nissl stain. The L4/L5A boundary was distinguishable by an abrupt loss of barrel structure and a decrease in the number and density of cells. The bottom of L5A was typically ∼100 μm below the L4/L5A border. The boundary between L5B and L6, visible as a notable increase in cell density near the top of L6, was typically located approximately halfway between the top of L5A and the subcortical white matter.
Optical stimulation procedures.
All experimental procedures were carried out as previously described (Kinnischtzke et al. 2014). Full-field blue light (470 nm, OptoLED, Cairn Research) was delivered through a ×40 objective; light intensity at the surface of the slice was ∼20 mW/mm2. Each cell recorded was centered in the field of view to maximally activate the synapses onto that particular neuron. The spatial diameter of light through the ×40 objective was ∼250 μm, thereby covering the width of approximately one barrel-related column. A single pulse (duration: 1.0 ms) was delivered per trial, and individual trials were separated by 8 s. Pulse duration (1 ms) was long enough to reliably elicit synaptic responses from postsynaptic neurons yet short enough to limit recruitment of polysynaptic activity.
Data analysis.
Data analyses were performed using custom-written MATLAB code (The MathWorks, Natick, MA; A. K. Kinnischtzke). Intrinsic properties were derived from a series of direct current steps that were presented to the cell (see above). Input resistance was calculated as the slope of the voltage-current relationship for subthreshold voltage deflections that were within ±30 mV of the resting membrane potential. Using hyperpolarizing current steps, sag current magnitude (in mV) was measured as the difference in the minimum voltage during the initial 100 ms and the mean voltage during the last 50 ms in the step. Because sag currents exhibit a voltage dependence, only current steps where the minimum voltage value was between −90 and −75 mV were used.
Action potential (AP) features were calculated from the first AP in the first current step that elicited at least two APs at 10 Hz or greater. The AP threshold was measured as the maximum of the second derivative in the voltage 10 ms preceding the AP peak. AP half-width was calculated as the width halfway between the AP threshold and the AP peak. To measure afterhyperpolarization (AHP) depth, the minimum voltage (Vmin) within 5 ms after the AP threshold was subtracted from the AP threshold (see Fig. 3D). To determine the presence or absence of a depolarizing afterpotential (DAP), the local maximum (Vmax) was found between Vmin and the voltage during the following 10 ms. The voltage 17 ms after Vmin was also calculated (Vend). If Vmax > Vend, the DAP magnitude was calculated as the difference between Vmax and Vmin (see Fig. 3D). If Vmax < Vend, the DAP magnitude was 0.
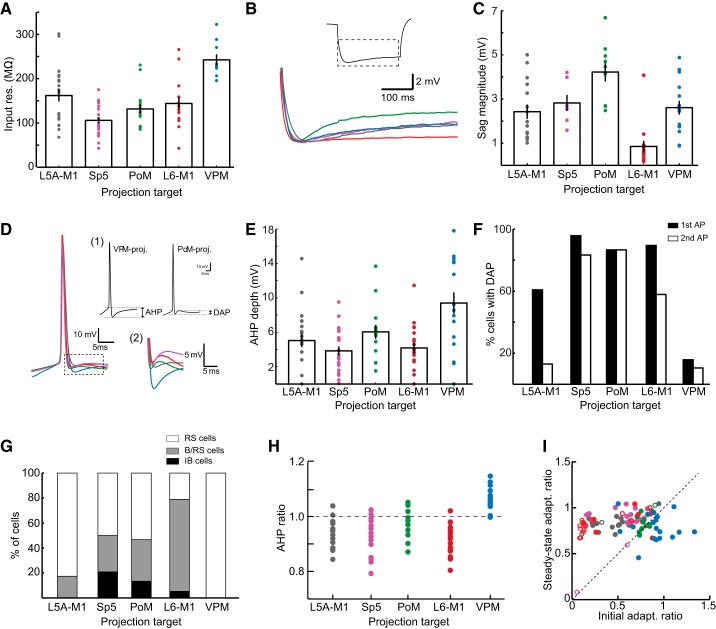
Fig. 3.
Comparison of intrinsic properties between S1 pyramidal neurons populations. A: population average of input resistance for each type of pyramidal neuron. B: illustration of sag currents. Traces are averaged voltage responses for all neurons in each population. The dashed box in the inset illustrates the portion of the current step shown in the averaged traces. C: average sag currents were largest in PoM-projecting neurons and smallest in L6 M1-projecting neurons. D: first spike waveforms (left), averaged across neurons, highlight the differences between populations. Inset 1 shows measurements for AHP depth (left) and DAP magnitude (right). Inset 2 shows the expansion of voltage trajectory in the dashed box and highlights differences in the voltage trajectories for each cell type after an AP. The color scheme is as in B. E: population average of AHP depth. F: percentage of neurons in each population demonstrating DAPs after the first spike in the current step (solid bars) and second spike in the current step (open bars). G: number of cells in each population that showed regular spiking (RS; open bars), initial bursts followed by regular spiking (B/RS; gray bars), or intrinsic bursting (IB; solid bars). H: ratio of the first to second AHP depth in a spike train. I: VPM-projecting neurons (blue dots) displaying distinct adaptation dynamics, as the initial adaptation ratio was higher than the steady-state adaptation ratio. The color scheme for all images with color is as follows: L5A M1-projecting (gray), Sp5-projecting (magenta), PoM-projecting (green), L6 M1-projecting (red), and VPM-projecting (blue). In A–I, statistical relationships are not shown and instead are shown in Table 1. Single dots indicate individual data points, and all bars and error bars indicate means and SEs.
Rheobase current was the first current step amplitude that elicited at least one AP. All spike train properties were calculated from the first current step that resulted in an average of 10 Hz firing or greater. This was typically ∼30 pA (range: 0–80 pA) above the rheobase current. A “burst” was defined as two or more APs with an interspike interval (ISI) of 12 ms or less. An initial adaptation ratio was defined as the ISI between the first and second APs divided by the ISI between the second and third APs. A steady-state adaptation ratio was calculated as the ISI between the third and fourth spikes divided by the ISI of the last two spikes in the train.
For the analysis of M1 input, individual trials were averaged together because we observed little trial-to-trial variability in the responses to optical stimulation. We did not use methods for limiting polysynaptic activity (Petreanu et al. 2009). Although these conditions are ideal for unambiguous detection of the presence of monosynaptic connections, the addition of TTX and 4-aminopyridine to block Na+ and K+ channels, respectively, alters the normal synaptic strength and properties of synaptic connections (Cruikshank et al. 2010) making comparison of synaptic strengths difficult. In addition, blockade of APs with the addition of TTX would not allow for an examination of the intrinsic properties of the cells. Instead, we limited our analysis of postsynaptic responses to an initial time window after the light pulse (10 ms) to minimize the contribution of polysynaptic activity. Activity in this early time window (first 10 ms) also showed very little trial-to-trial variability, consistent with it being monosynaptic in nature; however, it is possible that some of this activity could include polysynaptic events that were reliably evoked by the optical stimulation.
For the analysis of EPSCs, a cell was considered to have received input if the peak of the EPSC exceeded 15 times the SD of the baseline preceding the response. This detected EPSC responses of ∼5 pA and larger. To capture the start of the EPSC, we measured the first of 20 consecutive data points that exceeded 1 SD below the baseline current (i.e., exceeded the noise level). This value was taken as the response onset. EPSC amplitude was calculated as the EPSC peak minus the value at the EPSC onset. In current clamp, spike probability was measured as the number of trials that elicited a spike divided by the total number of trials.
Statistical comparisons of multiple groups were performed using ANOVA followed by multiple pairwise comparison tests that were corrected using the Tukey-Kramer method. Statistical comparison of proportions or probabilities between groups was tested using a Fisher's exact test. Comparison of EPSC amplitudes for within-slice recordings was conducted using a paired t-test.
RESULTS
Anatomic distribution of L5 and L6 pyramidal neurons.
To test for M1 input to pyramidal neurons projecting to a specific cortical or subcortical target, we injected fluorescent retrograde tracers in combination with an AAV containing the gene for ChR2 (see methods). In all animals, a green fluorescent retrograde tracer was coinjected with the ChR2 virus into M1 and a red fluorescent retrograde tracer was injected into either the VPM, PoM, or Sp5 in the brain stem (Fig. 1). As previously described, M1 axons are found throughout L1–L6 in S1 but are especially dense in L1, L5, and L6 (Fig. 1A) (Kinnischtzke et al. 2014).
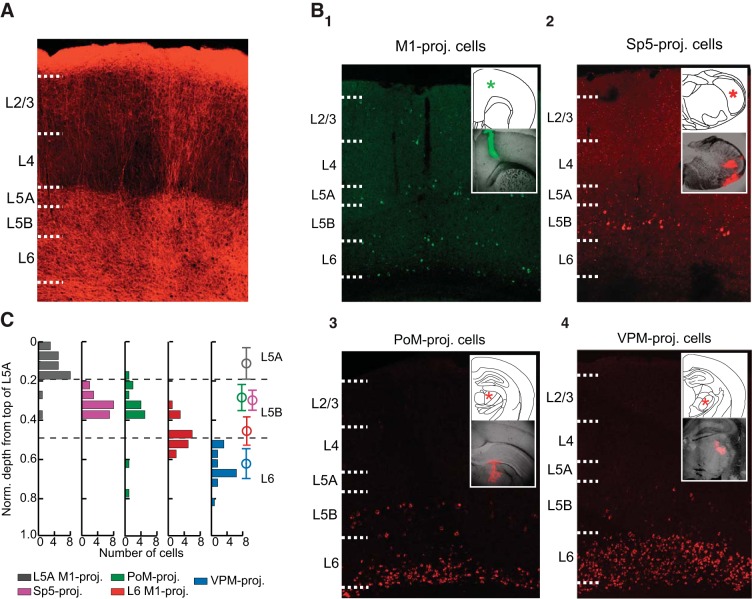
Fig. 1.
Laminar positions of primary somatosensory cortex (S1) pyramidal neurons in layer (L)5 and L6 are organized by projection target. A: confocal image of channelrhodopsin (ChR)2-primary motor cortex (M1) axons within S1 labeled with mCherry showing the typical pattern of innervation within S1. B: retrobead injections into M1 (1) resulted in retrogradely labeled pyramidal neurons throughout L2–L6. Labeled cells were especially numerous in L5A, the top of L6, and the bottom of L6. Red fluorescent retrobeads injected into the spinal trigeminal nucleus (Sp5; 2) exclusively labeled S1 neurons in L5B. Injection of retrobeads into the posteromedial thalamus (PoM; 3) labeled neurons in both L5B and L6, whereas retrobeads in the ventral posterior medial nucleus (VPM; 4) only labeled neurons in L6 of S1. In 1–4, the insets illustrate the injection target (top; *) and an example injection for each target location (bottom). C: laminar depth measurements for each recorded cell were normalized to the top of L5A. Histograms illustrate the distribution of each population within L5 and L6. Open circles and error bars (right) indicate the means and SDs, respectively, for each population.
Although M1 axons diffusely and uniformly terminated in the deep layers (Fig. 1A), pyramidal neurons projecting to specific downstream targets instead tended to cluster in specific laminar locations within L5 or L6 (Fig. 1B). For each retrograde injection target (i.e., M1, PoM, Sp5, or VPM), the position of retrogradely labeled neurons was consistent with known laminar patterns in rodent S1 (Alloway et al. 2004; Chmielowska et al. 1989; Deschenes et al. 1994; Hattox and Nelson 2007; Killackey et al. 1989; Mao et al. 2011; Wise and Jones 1977). M1-projecting neurons were found throughout most layers in S1 but tended to be concentrated in bands within L2/L3, L5A, superficial L6, and deep L6. Neurons projecting to Sp5 were located exclusively in L5B. Corticothalamic neurons projecting to PoM were found in L5B, in a similar position as Sp5-projecting neurons, as well as in deep L6. Finally, corticothalamic neurons projecting to VPM were only found in L6 and tended to be concentrated in the upper half of L6, as previously described (Chmielowska et al. 1989; Zhang and Deschenes 1997).
The retrogradely labeled neurons recorded in this study were grouped into the following five populations for further study: M1-projecting cells in L5A (L5A M1-projecting), M1-projecting neurons at the very top of L6 (L6 M1-projecting), Sp5-projecting neurons, L5B PoM-projecting neurons (PoM-projecting), and VPM-projecting neurons. Because only two L6 PoM-projecting neurons were recorded, they were not included as an additional population (see discussion). Depth measurements, normalized to the L4/L5A border, revealed that each of the studied populations of pyramidal neurons were largely segregated within a layer, e.g., a sublayer, with only a small degree of overlap among populations residing within that layer (Fig. 1C). The exception was that PoM-projecting neurons in L5B and Sp5-projecting neurons were completely overlapping in their depth location (Fig. 1C).
Coexpression of red and green fluorescent retrobeads was observed in only two neurons, both from animals injected in M1 and Sp5 (n = 2; data not shown). These cells were located in the deep part of L5B and were included in both Sp5- and L6 M1-projecting data sets. No other cases of colabeling of green (M1-projecting) and red (PoM-, Sp5-, or VPM-projecting) fluorescent retrobeads were observed in this study.
Examination of the labeled pyramidal neurons revealed morphological differences among them consistent with those of similarly identified populations of S1-projecting neurons (Chagnac-Amitai et al. 1990; Deschenes et al. 1994; Hattox and Nelson 2007; Le Be et al. 2007; Zhang and Deschenes 1997); these anatomic features were therefore were not investigated further.
Intrinsic properties.
We compared the electrophysiological characteristics of L5A M1-projecting neurons (n = 23), L6 M1-projecting neurons (n = 19), Sp5-projecing neurons (n = 24), L5B PoM-projecting neurons (n = 15), and VPM-projecting neurons (n = 19). As seen in juvenile mice (Hattox and Nelson 2007), many subthreshold and AP characteristics were strongly dependent on the projection target of the pyramidal neuron (Table 1 and Figs. 2 and 3). All statistical relationships are shown in Table 1.
Table 1.
Table of intrinsic properties
| Projection Target |
Significance Level |
|||||||
|---|---|---|---|---|---|---|---|---|
| Property | L5A M1 (1) | Sp5 (2) | PoM (3) | L6 M1 (4) | VPM (5) | P < 0.05 | P < 0.01 | P < 0.005 |
| Number | 23 | 24 | 15 | 19 | 19 | |||
| Passive properties | ||||||||
| Input resistance, MΩ | 166 ± 13.7 | 106 ± 6.9 | 131 ± 11.3 | 153 ± 14.5 | 241 ± 9.1 | 2:4 | 1:2 | 5:1,2,3,4 |
| Resting membrane potential, mV | −65.2 ± 0.8 | −62.3 ± 0.9 | −62.4 ± 1.4 | −68.8 ± 1.3 | −61.0 ± 1.1 | 1:5 | 4:2,3,5 | |
| Sag current, mV | 2.43 ± 0.32 | 2.82 ± 0.36 | 4.22 ± 0.42 | 0.85 ± 0.26 | 2.61 ± 0.32 | 3:5 | 4:1,2,3 | 3:1,4 |
| 4:5 | ||||||||
| AP features | ||||||||
| AP half-width, ms | 1.31 ± 0.07 | 1.08 ± 0.05 | 1.05 ± 0.04 | 1.38 ± 0.10 | 1.50 ± 0.06 | 1:2,3 | 4:2,3 | 5:2,3 |
| AP threshold, mV | −37.8 ± 0.7 | −41.5 ± 0.6 | −42.3 ± 1.3 | −39.0 ± 1.3 | −33.8 ± 1.0 | 1:2,3,5 | 5:2,3,4 | |
| AP peak, mV | 43.0 ± 1.0 | 42.7 ± 1.7 | 39.8 ± 2.3 | 43.8 ± 1.7 | 41.6 ± 1.7 | NS | NS | NS |
| Afterhyperpolarization depth, mV | 4.4 ± 0.6 | 3.4 ± 0.5 | 4.9 ± 0.8 | 3.1 ± 0.7 | 8.6 ± 1.0 | 5:3 | 5:1,2,4 | |
| Percentage with depolarizing afterpotential | 59 (13/22) | 92 (22/24) | 87 (13/15) | 94 (15/16) | 5 (1/19) | 1:2 | 1:4 | 5:1,2,3,4 |
| Spiking properties | ||||||||
| Rheobase, pA | 71.0 ± 9.6 | 77.5 ± 7.7 | 60.0 ± 5.5 | 96.3 ± 10.4 | 79.0 ± 7.6 | NS | NS | NS |
| Initial adaptation ratio | 0.52 ± 0.04 | 0.50 ± 0.06 | 0.69 ± 0.07 | 0.38 ± 0.08 | 0.90 ± 0.05 | 3:4 | 5:1,2,4 | |
| Steady-state adaptation ratio | 0.72 ± 0.02 | 0.88 ± 0.03 | 0.79 ± 0.04 | 0.71 ± 0.07 | 0.70 ± 0.04 | 2:1,4,5 | ||
| Percentage with initial burst | 4 (1/23) | 33 (8/24) | 27 (4/15) | 58 (11/19) | 0 (0/19) | 1:2 | 1:4 | |
| 4:2,3 | 5:2,4 | |||||||
| 3:5 | ||||||||
| Percentage with steady-state bursts | 0 (0/23) | 17 (4/24) | 13 (2/15) | 5 (1/19) | 0 (0/19) | NS | NS | NS |
Values are means ± SE or percentages with numbers in parentheses. Primary somatosensory cortex (S1) pyramidal neurons exhibit diverse intrinsic properties based on the projection target. Statistical relationships are shown on the right. See methods for a description of each property. The right side indicates statistical relationships between each pyramidal neuron type. Group numbers (1–5) are indicated at the top and as follows: layer (L5)A primary motor cortex (M1)-projecting neurons (1), spinal trigeminal nucleus (Sp5)-projecting neurons (2), posteromedial thalamus (PoM)-projecting neurons (3), L6 M1-projecting neurons (4), and ventral posterior medial nucleus (VPM)-projecting neurons (5).
AP, action potential; NS, not significant.
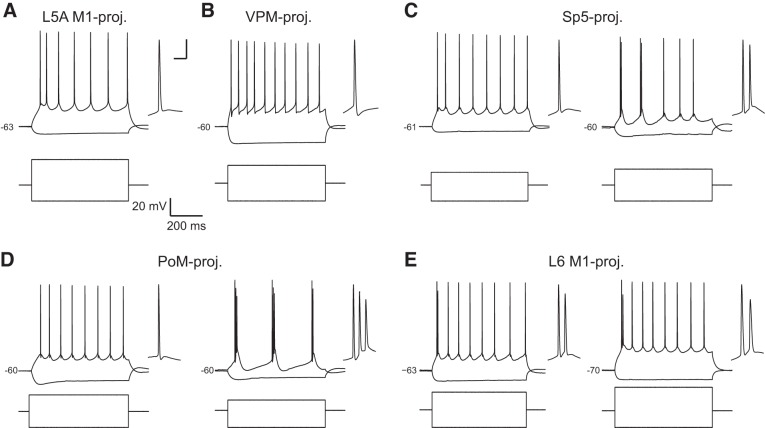
Fig. 2.
Examples of firing properties for S1 pyramidal neuron populations. A: L5A M1-projecting neurons exhibited mostly regular spiking behavior, and the first interspike interval (ISI) tended to be shorter than subsequent ISIs. B: VPM-projecting neurons were also regular spiking with large afterhyperpolarizations (AHPs) after each action potential (AP). Sp5-projecting neurons (C) and PoM-projecting neurons (D) fired either single spikes that were followed by a depolarizing afterpotential (DAP; C and D, left) or a mix of bursting and regular spiking (C, right) or showed intrinsic bursting dynamics (D, right). E: L6 M1-projecting neurons often fired an initial spike “doublet,” and in some cells, subsequent spikes were followed by a DAP (left), whereas others were not (right). In A–E, the first spike in the train was expanded and shown to the right. Scale bars for expanded spike in A–E are 10 ms and 10 mV.
Comparisons of passive properties demonstrated that VPM-projecting neurons had the highest input resistance (241 ± 9.1 MΩ) and PoM- and Sp5-projecting neurons had the lowest input resistance (131 ± 11.3 and 106 ± 6.9 MΩ, respectively; Fig. 3A). These differences are inversely related to the size of the neurons, as Sp5- and PoM-projecting neurons are among the largest neurons in S1 and VPM-projecting neurons are the smallest. L5A M1- and L6 M1-projecting neurons had significantly more hyperpolarized resting membrane potentials (−65.8 ± 0.7 and −68.8 ± 1.2 mV, respectively) than the other three pyramidal neuron types. We also examined the presence of sag potentials, which in pyramidal neurons is mediated by hyperpolarization-activated current (Ih) (Sheets et al. 2011). PoM- and Sp5-projecting neurons exhibited the most sag potential (4.22 ± 0.42 and 2.82 ± 0.36 mV, respectively), and sag potentials in PoM-projecting cells were significantly larger than those of L5A M1-, VPM-, and L6 M1-projecting neurons (Fig. 3, B and C). L6 M1-projecting neurons, in particular, had notably small sag potentials (0.85 ± 0.26 mV).
Each population of pyramidal neurons exhibited distinct AP features as well. Sp5- and PoM-projecting neurons had the narrowest APs (AP half-width of 1.08 ± 0.05 and 1.05 ± 0.04 mV, respectively) as well as the most hyperpolarized threshold for AP initiation (−41.5 ± 0.6 and −42.3 ± 1.3 mV, respectively). By comparison, APs of VPM-projecting neurons were the broadest (AP half-width of 1.50 ± 0.06 ms) and had the most depolarized AP threshold (−33.8 ± 1.0 mV). L5A and L6 M1-projecting neurons had broader APs than Sp5- or PoM-projecting neurons but narrower than VPM-projecting neurons (L5A M1-projecting neurons: 1.32 ± 0.07 ms and L6 M1-projecting neurons: 1.37 ± 0.08 ms). AP thresholds of L5A and L6 neurons were more hyperpolarized than those of VPM-projecting neurons but were more depolarized than those of Sp5- or PoM-projecting neurons (L5A M1-projecting neurons: −38.0 ± 0.7 mV and L6 M1-projecting neurons: −38.9 ± 1.1 mV). As described below, AP thresholds were important determinants of whether the different classes of neurons tended to fire spikes in response to M1 inputs.
Striking differences between pyramidal neuron subtypes were observed in the voltage trajectory after the AP. VPM-projecting neurons were distinct in exhibiting a deep, fast AHP that was not seen in the other cell types (Figs. 2B and 3, D and E). Sp5- and PoM-projecting neurons, on the other hand, displayed a small DAP after a spike (Figs. 2, C and D, and 3F). DAPs were observed in many L6 M1-projecting and some L5A M1-projecting neurons as well (Fig. 3F). Interestingly, in Sp5-, PoM-, and some L6 M1-projecting neurons, a DAP was present after every spike (Fig. 2, C, D, and E, right), whereas for L5A M1-projecting and the remaining L6 M1-projecting neurons, a DAP, if present at all, was only found after the first AP in a train (Figs. 2, A and E, left, and 3F).
DAPs may underlie the generation of bursting behaviors observed in some pyramidal neurons (Llano and Sherman 2009). We therefore compared the proportions of pyramidal neurons that were either completely regular spiking (RS), displayed an initial burst followed by single spikes (B/RS), or exhibited bursting throughout the current step [intrinsic bursting (IB); Fig. 3G]. Consistent with DAPs relating to bursting dynamics, pyramidal neuron types that were most likely to exhibit DAPs, particularly after the second and later APs (e.g., Sp5-, PoM-, and L6M1-projecting neurons; Fig. 3F, solid bars), were also more likely to exhibit initial and steady-state bursting behavior (Fig. 3I). VPM-projecting cells were always RS (19/19 cells; Fig. 2B). L5A-M1 projecting cells were almost always RS cells (19/23 cells; Fig. 2A), with a few cells exhibiting an initial burst followed by regular spiking behavior (B/RS; Fig. 3I). Neurons projecting to Sp5 could be RS, B/RS, or IB cells (RS: 12/24 cells, B/RS: 7/24 cells, and IB: 5/24 cells; Fig. 2C); the same was true for PoM-projecting neurons (RS: 8/15 cells; B/RS: 5/15 cells; and IB: 2/15 cells; Fig. 2D). Interestingly, most L6 M1-projecting neurons were B/RS (14/19 cells) as they often exhibited an initial spike “doublet” (Fig. 2E). Most of the remaining L6 M1-projecting neurons were RS (4/19 cells), although one was IB (1/19 cells).
Finally, we examined changes in spike properties across the spike train. Again, VPM-projecting neurons were distinctive as AHP depth decreased from the first spike to subsequent spikes, resulting in an AHP ratio that was larger than 1 (Fig. 3H). In the other cell types, the first AHP was shallower than subsequent AHPs and AHP ratios were <1 (Fig. 3H). VPM-projecting neurons also displayed different adaptation dynamics, with the ISI gradually increasing across the spike train (Fig. 2B). This resulted in initial adaptation ratios larger than the steady-state adaptation ratios (0.90 ± 0.05 vs. 0.79 ± 0.03; Fig. 3I). For each of the other cell types, the initial adaptation ratio was typically smaller than the steady-state adaptation ratio. This was particularly evident for the neurons that exhibited an initial burst (Fig. 3I, open circles). IB cells were not included in analysis of adaptation dynamics as the irregular spike patterns within and between bursts yielded highly variable adaptation values.
Motor cortex inputs.
To account for possible differences in ChR2 expression between slices, all animals were injected with ChR2 and green retrobeads in M1 as well as red retrobeads in a second target (either VPM, PoM, or Sp5). We were therefore able to use a paired design in which L5A M1-projecting neurons were recorded in the same slice as cells from one of the other pyramidal neuron populations: L6 M1-, Sp5-, PoM-, or VPM-projecting neurons (Figs. 4 and 5). Paired comparisons were used to determine the strength of M1 inputs relative to a standard population (L5A M1-projecting neurons). Comparison between L5A M1-projecting neurons and unlabeled L5A neurons confirmed that this population was representative of the amount of M1 input received by all L5A neurons (data not shown).
Fig. 5.
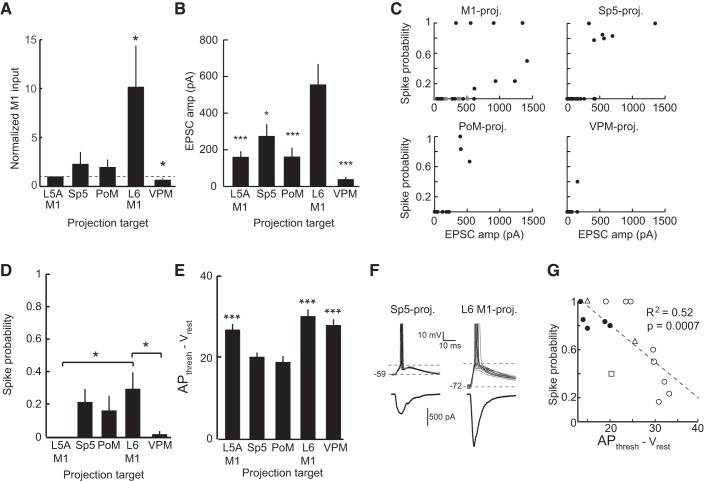
M1 provides strong input to L6 M1-projecting neurons and weak input to VPM-projecting neurons. A: example L5A M1-projecting (left) and L6 M1-projecting (right) neuron responses to current steps (top). In this pair, the L6 M1-projecting neuron received larger M1 input, as shown in current clamp (middle) and voltage clamp (bottom). Note that the L6 M1-projecting neuron fired spikes on several trials (one trial is shown in the dashed box inset). Cells were recorded in the same slice. B: same as in A but for a L5A M1-projecting neuron (left) and a VPM-projecting neuron (right) recorded in the same slice. C: on average, L6 M1-projecting neurons received significantly larger input than paired L5A M1-projecting cells (P < 0.05). D: VPM-projecting neurons received significantly less M1 input than paired L5A M1-projecting neurons (P < 0.005). Conventions for C and D are the same as in Fig. 4, D and E.
The recording from a L5A M1-projecting neuron and a Sp5-projecting neuron within the same slice (Fig. 4A) demonstrated that optical stimulation of ChR2-M1 axons evoked a short-latency EPSC that was similar in amplitude for both cells (Fig. 4B). Comparisons between the populations of recorded pairs (n = 12 pairs) indicated no significant difference in M1 input between these populations (L5A M1-projecting neurons: 113.32 ± 25.97 pA and Sp5-projecting neurons: 138.09 ± 34.51 pA, P = 0.31; Fig. 4D). Similar results were found for L5A M1- and PoM-projecting neurons (n = 9 pairs), indicating that L5A M1-projecting and PoM-projecting neurons also receive inputs of equivalent strength from M1 (L5A M1-projecting neurons: 97.59 ± 3.44 pA and PoM-projecting neurons: 181.55 ± 69.21 pA, P = 0.27; Fig. 4, C and E). Sp5- and PoM-projecting cells that exhibited either initial bursts (Fig. 4, D and E, open circles) or were intrinsically bursting (Fig. 4, D and E, +) received a comparable magnitude of M1 input as the cells that were regular spiking (Fig. 4, D and E, closed circles). Thus, Sp5- and PoM-projecting neurons, which, as noted above, have similar sublaminar locations and intrinsic properties, were similar with respect to the strengths of the M1 inputs they receive.
Unlike L5A M1-, Sp5-, and PoM-projecting neurons, many L6 M1-projecting neurons had large-amplitude M1-evoked responses (Fig. 5A). Indeed, M1 input was often strong enough to evoke APs in L6 M1-projecting neurons; in contrast, APs were never observed in L5A M1-projecting neurons (Fig. 5A; see also below). Across the population (n = 9 pairs), L6 M1-projecting neurons on average received significantly stronger input from M1 than L5A M1-projecting neurons (158.62 ± 50.14 vs. 700.62 ± 184.66 pA, P = 0.01; Fig. 5C). Many of the L6 M1-projecting neurons that received strong M1 input exhibited an initial spike doublet (Fig. 5C, open squares); large-amplitude M1 inputs were observed in regular spiking cells as well (Fig. 5C, closed circles).
M1 input to VPM-projecting neurons was notably small, and these cells received on average the smallest M1 inputs (n = 16 pairs; Fig. 5, B and D). In the sample of paired cells, the amplitude of M1 input to VPM-projecting neurons was significantly smaller than of L5A M1-projecting cells recorded in the same slice (161.55 ± 31.58 vs. 54.55 ± 10.88 pA, P = 0.008). The two L6 PoM-projecting cells we recorded had similar intrinsic properties and received comparable amounts of M1 input as VPM-projecting neurons (data not shown).
Comparisons normalized to within-slice M1 inputs confirmed that L6 M1-projecting cells received significantly stronger and VPM-projecting cells received significantly weaker synaptic input from M1 than L5A M1-projecting neurons (P < 0.05; Fig. 6A). Because each sample of paired L5A M1-projecting neurons was similar (P = 0.43 by ANOVA), we pooled the data to compare all five groups directly (Fig. 6B). The pooled data showed similar results as the data from individual pairs, confirming that synaptic inputs from M1 differentially targeted populations of pyramidal neurons based on their axonal projections (P < 0.00001 by ANOVA; Fig. 6B), with L6 M1-projecting neurons receiving significantly larger inputs than any other population and VPM-projecting neurons receiving the smallest (Fig. 6B).
Fig. 6.
Optical stimulation of ChR2-M1 terminals evoked suprathreshold responses in multiple types of S1 pyramidal neurons. A: paired recordings were normalized to the magnitude of M1 input to the L5A M1-projecting cell. M1 input was significantly greater to L6 M1-projecting cells (P < 0.05) and less to VPM-projecting cells (P < 0.05) compared with the L5A M1-projecting population. B: population average of EPSC amplitude evoked by M1 stimulation showed that L6 M1-projecting neurons received significantly larger M1 input than the other pyramidal neuron populations (P < 0.005). C: pyramidal neurons in each population with the largest M1-evoked EPSC often showed nonzero spike probabilities when recorded in current clamp. Note that the top left image shows both L5A M1-projecting (gray circles) and L6 M1-projecting (solid circles) neurons. D: average spike probability in L6 M1-projecting neurons was significantly greater than in L5A M1- and VPM-projecting neurons (P < 0.05) but not Sp5- and PoM-projecting neurons (P > 0.05). E: the average voltage difference from the resting membrane potential to AP threshold was significantly smaller in Sp5- and PoM-projecting neurons than other pyramidal neuron classes (P < 0.005). F: example Sp5-projecting neuron (left) that spiked on each trial (black) and a L6 M1-projecting neuron (right) that fired on some trials (black) but not others (gray). G: scatterplot of the distance between rest and AP threshold and the M1-evoked spike probability for all neurons that spiked. Symbol conventions are as follows: Sp5-projecting neurons (solid circles), PoM-projecting neurons (open triangles), L6 M1-projecting neurons (open circles), and VPM-projecting neurons (open square). The dashed line shows the linear least-squares fit.
M1-evoked suprathreshold responses.
Because these pyramidal neurons display a range of intrinsic properties, we also recorded in current-clamp to provide a more physiological measure of the cell's response to activation of M1 given the intrinsic properties of the neuron and amount of excitatory and inhibitory synaptic input produced by M1 activation. Analysis of the M1-evoked EPSP amplitude, rather than EPSC, illustrated a similar effect as described above, in that L6 M1-projecting neurons received significantly more M1 input than the other populations we recorded (data not shown).
Many pyramidal neurons responded by spiking, usually those in populations that received the largest amplitude of excitatory input from M1 (Fig. 6C). For example, the L6 M1-projecting population had the highest proportion of neurons exhibiting suprathreshold responses (n = 9/19). Interestingly, although L5A M1-, Sp5-, and PoM-projecting neurons received similar mean amplitudes of input from M1 (Fig. 6, A and B), L5A M1-projecting neurons never spiked (n = 0/23), whereas several Sp5- and PoM-projecting neurons did (n = 6/24 and n = 3/15, respectively). VPM-projecting neurons received notably small excitatory inputs from M1 (Fig. 6, A and B), and, despite having the largest input resistance (Fig. 3A), they rarely responded by firing APs (n = 1/20). Average spiking probability was highest in L6 M1-projecting neurons (0.30 ± 0.09; Fig. 6D); however, spiking probabilities in Sp5- (0.23 ± 0.08) and PoM-projecting (0.19 ± 0.10) neurons were statistically equivalent to L6 M1-projecting cells even though they received significantly smaller excitatory currents from M1. For example, Sp5- and PoM-projecting neurons reliably fired APs for EPSC amplitudes around 500 pA, as did a few L6 M1-projecting neurons. However, other L6 M1-projecting neurons spiked only occasionally despite receiving excitatory inputs of 1000 pA or greater (Fig. 6C). Of the populations where two or more cells had a nonzero spike probability, the amplitude of M1 input alone was not a reliable predictor of whether or not the cell would spike in response to photostimulation of M1 axons (L6 M1: R2 = 0.29, Sp5: R2 = 0.59, and PoM: R2 = 0.74, P > 0.05 for all three).
Therefore, we further examined spiking probability as it related to both the magnitude of M1 input currents and the voltage difference between resting potential and AP threshold. The voltage difference from rest to AP threshold was smallest for Sp5- and PoM-projecting pyramidal neurons (P < 0.005; Fig. 6E), reflecting the fact that Sp5- and PoM-projecting neurons have the lowest voltage thresholds for AP generation (Table 1). L5A M1- and L6 M1-projecting pyramidal neurons exhibited both more hyperpolarized resting membrane potentials and higher thresholds for spike initiation (Fig. 6E and Table 1). We hypothesized that the smaller voltage differential in Sp5- and PoM-projecting neurons compared with L5A and L6 M1-projecting neurons accounts for differences in spiking probabilities to M1 input (Fig. 6F). We confirmed this by comparing the voltage difference between resting and AP threshold with spike probability output for all neurons having suprathreshold responses. Neurons with the smallest voltage differences, which tended to be Sp5- and PoM-projecting neurons, had the highest spike probability, whereas those with larger voltage differences had a lower probability of spiking (Fig. 6G), despite many of them receiving larger-amplitude EPSCs.
Relationship between the project target, M1 input, and laminar depth.
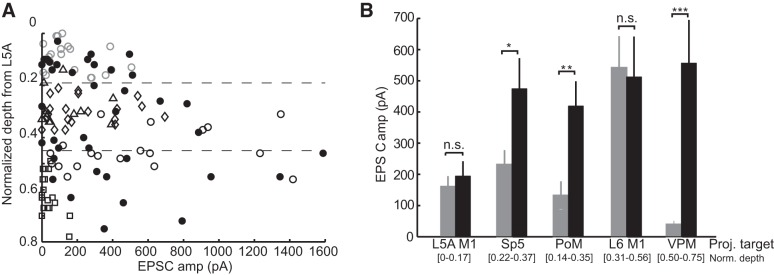
Our findings demonstrate both laminar- and projection target-specific selectivity of M1 projections onto infragranular pyramidal neurons. ANOVA with three factors demonstrated a significant effect of projection target (P = 0.003), a weak effect at trend level of layer (L5, L6: P = 0.08), and no effect of normalized distance from the L4/L5 border (P = 0.99). To disambiguate further the laminar and projection target effects, we compared the present data involving target-identified neurons with data from our previous study (Kinnischtzke et al. 2014) in which unlabeled infragranular pyramidal cells were recorded in unbiased fashion, without projection target indicators. The sample of cells projecting to unknown targets overall had a similar distribution of M1 input amplitude by depth as cells with a known target (Fig. 7A).
Fig. 7.
M1 inputs contact S1 neurons based on the laminar position or projection target. A: population graph of the amplitude of the M1-evoked EPSC as a function of the depth for each neuron indicating that M1 inputs are particularly strong around the border between L5 and L6 for neurons with known projection targets (L5A M1-projecting neurons: gray open circles, Sp5-projecting neurons: open diamonds, PoM-projecting neurons: open triangles, L6 M1-projecting neurons: black open circles, and VPM-projecting neurons: open squares) and unknown projection targets (black filled circles). B: comparison of neurons with known axonal projection (gray bars) with cells at the same laminar position but unknown projection (solid bars) demonstrated that Sp5-, PoM-, and VPM-projecting neurons all received significantly less input than neighboring cells at the same depths (*P < 0.05, **P < 0.01, and ***P < 0.005 by a two-sample t-test).
We compared amplitudes of M1 inputs to projection-identified neurons with unidentified neurons located within the same depth range (Fig. 7B). In some cases, it is possible the unlabeled cells are of the same projection type as those they are being compared with; the unlabeled population as a group, however, serves to provide an indication of the amount of M1 input that depth typically receives from M1. For example, L5A M1-projecting neurons, located in L5A at a normalized depth of 0–0.17 (5–95% percentile), received the same low-amplitude inputs as cells of an unknown projection target located within the same range of depths (162.1 ± 32 vs. 194.5 ± 48 pA, P = 0.56; Fig. 7D). Similarly, M1 inputs to L6 M1-projecting cells, which received on average the strongest M1 input, were equivalent to those of unidentified cells at the same depths (542.1 ± 101.3 vs. 512.5 ± 129 pA, P = 0.86). On the other hand, PoM-, Sp5-, and VPM-projecting populations each received markedly less M1 input than nearby cells having unidentified targets (P = 0.04, P = 0.008, and P = 0.00003, respectively). These findings indicate that the strength of M1 input to reciprocally connected M1-projecting infragranular neurons is governed by their laminar (or sublaminar) location. In contrast, the amount of M1 input to at least three classes of subcortically projecting cells (Sp5, PoM, and VPM) is determined based on the projection target.
DISCUSSION
Here, we combined optogenetic techniques with retrograde tracers to investigate M1 inputs to selected classes of S1 infragranular pyramidal neurons. Retrograde tracers allowed identification of the projection target for each recorded neuron while injection of an AAV containing the gene for ChR2 permitted selective activation of synaptic inputs originating in M1. Determining synaptic connectivity using optogenetic activation of ChR2-expressing terminals is ideal for targeting specifically the synapses of interest, in this case, M1 axons within S1. This approach does result in near-synchronous activation of all ChR2-expressing synapses in the target region and may therefore represent an upper bound on the amount of input received by the postsynaptic neuron. The impact M1 activity has on S1 neurons during natural whisker stimulation will likely be affected by factors not examined here, including the distribution of the M1 synapses across the dendritic arbor of the postsynaptic cell, the timing of the inputs, and ongoing network activity. The contribution of these factors are best considered in an awake, in vivo preparation that is intended to study naturalistic conditions. Nevertheless, the five different populations of infragranular pyramidal neuron we studied displayed a notable degree of specialization and specificity with regards to their sublaminar position, intrinsic properties, and spiking dynamics as well as the strength of their M1 synaptic inputs.
Our results demonstrate a number of important findings. First, pyramidal neuron subtypes that project to a particular target are organized by depth within L5 and L6 such that, in most cases, little spatial overlap occurs among populations. Second, consistent with a previous study in juvenile mice (Hattox and Nelson 2007), S1 pyramidal neurons exhibit a variety of physiological characteristics that correlate with the projection target of the neuron. Third, spiking responses of different projection neurons to optical stimulation of M1 axons reflect the unique combination of their intrinsic properties (i.e., resting membrane potential and AP threshold) and the strength of M1 input they receive. Finally, M1 input strengths are laminar specific in the case of reciprocal M1-projecting neurons and target specific for subcortically projecting neurons. Our results are consistent with findings that local synaptic connections between S1 pyramidal neurons form preferentially based on similarity of physiological properties and/or projection targets (Brown and Hestrin 2009; Otsuka and Kawaguchi, 2008, 2011). Additionally, recent studies have demonstrated that pyramidal neurons in the visual cortex demonstrate unique visual responses as a function of their projection target (Kim et al. 2015; Velez-Fort et al. 2014). Together, the findings emphasize the importance of knowing the projection targets of pyramidal neurons for deciphering their role in the functioning of cortical subcircuits (Chen et al. 2013, 2015; Katz 1987; Katz et al. 1984; Yamashita et al. 2013).
Laminar- and target-related properties of infragranular pyramidal neurons receiving M1 inputs.
Pyramidal neurons in our sample showed marked heterogeneity in their intrinsic properties. Much of this variability could be accounted for on the basis of their axonal target. M1-projecting pyramidal neurons, both L5A and L6, displayed features typically ascribed to pyramidal neurons. Both populations had similar passive and spike properties, with the most notable difference between the groups being that the L6 M1-projecting cells often displayed an initial “spike doublet,” whereas L5A M1-projecting cells did not. PoM- and Sp5-projecting neurons showed bursting (IB type) dynamics that were not observed in any other population. Although many PoM- and Sp5-projecting cells were regular spiking cells that did not burst, even they were distinct from the other groups by the presence of a DAP after each AP. VPM-projecting cells were clearly distinguishable from the other groups by both passive membrane features as well as the dynamics of their AHPs.
We found that neurons projecting to different targets resided, on average, in distinct and largely nonoverlapping sublamina within L5 and L6. Previous studies in the rat also have suggested such a sublaminar organization, with corticospinal and corticotrigeminal neurons residing within L5B at depths distinct from corticotectal neurons or callosal cells (Killackey et al. 1989; Wise and Jones 1977). The relationship between laminar position and projection target is likely an important feature of cortical organization and perhaps reflects a separate developmental time courses (Greig et al. 2013). However, pyramidal neurons often have multiple cortical and/or subcortical targets, which further complicates a classification of neurons simply on the basis of their axonal projection. For example, in our study, we identified two classes of pyramidal neurons (L5A and L6 M1-projecting cells) that were distinct in laminar position, physiology, and strength of M1 input, yet both project back to M1. Perhaps further investigation would demonstrate that each group has additional and different targets (e.g., striatum, contralateral S1, Sp5, etc.). Conversely, as discussed further below, neurons in S1 that project to Sp5 may send an axon collateral to PoM suggesting that the L5B cells labeled here in different animals as Sp5 projecting or PoM projecting could in fact be the same group of neurons. Without complete morphological information, such as can only be obtained through single cell full axonal reconstructions, it is difficult to obtain a clearer understanding of the organization of cortical pyramidal neurons by axonal targeting. Nevertheless, our study confirms that labeling of cells based on a single axon target reveals a notable heterogeneity of pyramidal neurons that reflect a sublaminar organization of deep layers of the mouse barrel cortex.
Comparison of L5A and L6 M1-projecting neurons in S1.
We distinguished two infragranular populations of M1-projecting neurons on the basis of their nonoverlapping sublaminar locations within L5. One population was restricted to L5A, residing just below the boundary between L4 and L5. L5A M1-projecting cells were relatively homogeneous in their intrinsic properties, exhibiting predominantly regular spiking behavior and passive properties and spike adaptation dynamics similar to those of other corticocortical neurons (Hattox and Nelson 2007). The other population of M1-projecting neurons was located deeper, just below the L6 boundary. The physiological properties of superficial L6 M1-projecting neurons were heterogeneous. Many displayed passive and spike dynamics similar to those of L5A M1-projecting neurons; these cells may therefore also be corticocortical neurons that have M1 as their primary or sole target. However, other L6 M1-projecting neurons resembled Sp5- and PoM-projecting neurons based on the presence of a DAP after each AP and a tendency to burst. Given that we observed a few instances of colabeled neurons in animals injected with retrobeads in M1 and Sp5, perhaps M1-projecting neurons that resemble Sp5-projecting neurons are corticofugal neurons (e.g., Sp5 projecting) that send a collateral to M1.
Based on our previous study (Kinnischtzke et al. 2014), we hypothesized that a specific class of pyramidal neurons near the top of L6 receive particularly strong input from M1. We speculated that these neurons are VPM-projecting cells, largely on the basis of studies that have indicated corticothalamic-mediated modulation of thalamic firing during whisking behavior (Lee et al. 2008). In this regard, the present finding that cells receiving the strongest M1 inputs comprise a subset of M1-projecting neurons was unexpected. Of further interest, L6 M1-projecting neurons receive significantly stronger input than M1-projecting neurons in upper L5. A previous study also found that M1-projecting neurons in L5A, as well as those in L2/L3, are not distinguished by especially strong M1 input (Mao et al. 2011). In contrast to L5A M1 neurons, M1-projecting pyramidal cells in upper L6 were much more likely to fire spikes in response to our photostimulation. Because their intrinsic properties were similar, the higher spiking probability of L6 M1-projecting cells reflects this more robust M1 synaptic input. Both cell types had relatively low excitability, as indicated by low input resistance and large voltage differential to reach AP generation, suggesting that very large and specific sources of synaptic input are likely required to activate these cells in vivo. Here, we observed that L6 M1-projecting neurons do receive sufficiently strong input from M1, but the sources of input that could activate L5A M1-projecting cells is unknown. One possible candidate is the higher-order thalamus PoM, whose axons in S1 densely and specifically target L1 and L5A (Wimmer et al. 2010).
Taken together, studies of S1–M1 connectivity have suggested a model wherein superficial layers of S1 and M1 are strongly connected in the S1 to M1 direction and weakly connected in the reverse direction. Such a pathway may serve to propagate sensory information from S1 to M1 (Ferezou et al. 2007), allowing for sensory guided whisking activity. Conversely, both the present study and previous work suggest that the deep layers are strongly connected from M1 to S1 with a weaker connection in the other direction (Mao et al. 2011; Veinante and Deschenes 2003; Zhang and Deschenes 1998).
Similarities between L5B PoM- and Sp5-projecting pyramidal neurons.
Sp5-projecting neurons and PoM-projecting neurons in L5B were intermixed within the middle of L5B and were equivalent for all intrinsic properties we measured. These neurons thus appear to be more similar to each other than to other types of L5 pyramidal neuron types, such as M1-projecting neurons and VPM-projecting neurons (present study) or callosal and corticostriatal neurons (Hattox and Nelson 2007). Sp5-projecting neurons send a collateral to PoM, giving rise to the possibility that Sp5- and PoM-projecting neurons in L5B may be largely the same population of neurons (Deschenes et al. 1994). However, colabeling within the same animal indicated that only a small percentage of Sp5- and PoM-projecting neurons project to both targets (Hattox and Nelson 2007). Whether the same or different populations, our findings suggest that PoM-projecting and Sp5-projecting neurons in L5B likely have similar or closely related functions.
Many Sp5- and PoM-projecting neurons exhibited bursting properties, either initially or continuously in response to current step injection. Similarly, in the auditory cortex, corticothalamic neurons located in L5B, but not those in L6, exhibit bursting behaviors (Llano and Sherman 2009). Several studies have described IB neurons as large, thick-tufted pyramidal neurons that reside in L5B and project subcortically (Kasper et al. 1994; Rumberger et al. 1998; Tseng and Prince 1993; Wang and McCormick 1993). It therefore appears to be a general finding throughout sensory and motor cortices that neurons exhibiting IB behaviors are pyramidal neurons in L5B that project out of the cortex (e.g., to the thalamus, midbrain, or brain stem).
Interestingly, PoM- and Sp5-projecting neurons that were regular spiking, not bursting, still displayed dynamics that were different from regular spiking M1- or VPM-projecting neurons. In Sp5- and PoM-projecting neurons, every AP was followed by a small depolarization (DAP). DAPs are Ca2+ dependent (Friedman et al. 1992), and the DAP may underlie the bursting behavior in these neurons (Llano and Sherman 2009). Therefore, RS neurons that exhibit DAPs may be capable of firing in bursts to certain types of stimuli and/or under certain conditions (Schwindt and Crill 1999; Schwindt et al. 1997). This is in contrast to early electrophysiological studies, which classified neurons as either RS or IB cells (Agmon and Connors 1989; McCormick et al. 1985). In the case of PoM- and Sp5-projecting neurons, then, RS and IB neurons, possibly possessing the same underlying dynamics and ionic currents, could represent a continuum not two discrete populations (Schwindt et al. 1997).
PoM- and Sp5-projecting neurons received only moderately strong M1 inputs, yet because of their intrinsic properties, they readily fired in response to activation of M1 synapses. L5 neurons also have low spike thresholds in vivo, allowing them to fire robustly to sensory input (Constantinople and Bruno 2013). In a manner similar to our results with M1 input, the intrinsic properties of Sp5- and PoM-projecting neurons may therefore enable them to fire reliably to moderate amounts of synaptic input from several sources, both sensory and motor related, potentially explaining the high firing rates observed in these cells in vivo during both quiescent and whisking states (de Kock and Sakmann 2009; de Kock et al. 2007).
Distinctive properties of VPM-projecting neurons.
VPM-projecting neurons differed notably in a number of respects from the other populations of infragranular neurons. VPM-projecting neurons had high input resistances, likely related to their compact size. They also exhibited deep AHPs and distinct adaptation and regular spiking dynamics, similar to other descriptions of L6 corticothalamic neurons in the rat somatosensory cortex (Yang et al. 2014) and mouse primary auditory cortex (Llano and Sherman 2009). Interestingly, the two L6 PoM-projecting neurons in our sample had intrinsic properties similar to L6 VPM-projecting neurons but not L5B PoM-projecting neurons. As suggested for the auditory cortex (Llano and Sherman 2009), corticothalamic feedback in S1 may be laminar, not projection, specific with corticothalamic neurons in L5B (i.e., PoM projecting only), having a different role than corticothalamic neurons in L6 (i.e., VPM and PoM projecting).
M1 sends a dense projection to L6 of S1 (Zhang and Deschenes 1998), where VPM-projecting neurons comprise about half of all neurons (Zhang and Deschenes 1997). Moreover, activation of M1 in vivo engages VPM-projecting neurons (Lee et al. 2008) and affects the firing and receptive field properties of VPM neurons (Temereanca and Simons 2004). As noted above, it was therefore unexpected that M1 input to VPM-projecting neurons was actually the weakest of all the pyramidal neuron populations we examined. L6 corticothalamic neurons in vivo have extremely low firing rates (Beloozerova et al. 2003; Sirota et al. 2005). Our data indicate that VPM-projecting neurons are relatively excitable (e.g., high input resistance), suggesting that their quiescence in vivo may be attributable more to the weak amount of synaptic input they receive, both long range, as shown here, as well as locally within the cortical column (Hooks et al. 2011; Zarrinpar and Callaway 2006). Engagement of VPM-projecting neurons in vivo may thus require the conjunction of M1 input with one or more other sources, perhaps one that is sensory in nature and another that is highly state dependent.
Functional implications.
Together with our previous study (Kinnischtzke et al. 2014), we have observed that M1 provides input to nearly all S1 pyramidal neurons regardless of projection target, yet most of that input is relatively weak and on its own unlikely to make S1 neurons fire. This weak signal from M1 to most S1 neurons could serve to generally heighten perception of somatosensory stimuli while the whiskers are in motion, as occurs during high arousal states such as environmental exploration. In addition, we report an additional signal from M1 that strongly and specifically activates a subset of L6 S1 neurons, who, in turn, project back to M1. This input could serve to convey more precise information regarding future motor actions to corticocortical S1 neurons that project back to M1, forming cortical “loops” that underlie sensory-guided adjustments in whisking strategies as animals actively sense and explore their environment.
GRANTS
This work was supported in part by the National Institute for Neurological Disorders and Stroke Grant NS-19950 (to D. J. Simons).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.K.K., E.E.F., and D.J.S. conception and design of research; A.K.K. performed experiments; A.K.K. and D.J.S. analyzed data; A.K.K., E.E.F., and D.J.S. interpreted results of experiments; A.K.K. prepared figures; A.K.K. and D.J.S. drafted manuscript; A.K.K. and D.J.S. edited and revised manuscript; A.K.K., E.E.F., and D.J.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Jason Rampelt for excellent technical assistance and Sashi Marella and Chris Rodgers for advice on data analysis.
REFERENCES
- Agmon A, Connors BW. Repetitive burst-firing neurons in the deep layers of mouse somatosensory cortex. Neurosci Lett 99: 137–141, 1989. [DOI] [PubMed] [Google Scholar]
- Alloway KD, Zhang M, Chakrabarti S. Septal columns in rodent barrel cortex: functional circuits for modulating whisking behavior. J Comp Neurol 480: 299–309, 2004. [DOI] [PubMed] [Google Scholar]
- Aronoff R, Matyas F, Mateo C, Ciron C, Schneider B, Petersen CC. Long-range connectivity of mouse primary somatosensory barrel cortex. Eur J Neurosci 31: 2221–2233, 2010. [DOI] [PubMed] [Google Scholar]
- Beloozerova IN, Sirota MG, Swadlow HA. Activity of different classes of neurons of the motor cortex during locomotion. J Neurosci 23: 1087–1097, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SP, Hestrin S. Intracortical circuits of pyramidal neurons reflect their long-range axonal targets. Nature 457: 1133–1136, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauller LJ, Clancy B, Connors BW. Backward cortical projections to primary somatosensory cortex in rats extend long horizontal axons in layer I. J Comp Neurol 390: 297–310, 1998. [PubMed] [Google Scholar]
- Chagnac-Amitai Y, Luhmann HJ, Prince DA. Burst generating and regular spiking layer 5 pyramidal neurons of rat neocortex have different morphological features. J Comp Neurol 296: 598–613, 1990. [DOI] [PubMed] [Google Scholar]
- Chen JL, Carta S, Soldado-Magraner J, Schneider BL, Helmchen F. Behaviour-dependent recruitment of long-range projection neurons in somatosensory cortex. Nature 499: 336–340, 2013. [DOI] [PubMed] [Google Scholar]
- Chen JL, Margolis DJ, Stankov A, Sumanovski LT, Schneider BL, Helmchen F. Pathway-specific reorganization of projection neurons in somatosensory cortex during learning. Nat Neurosci 18: 1101–1108, 2015. [DOI] [PubMed] [Google Scholar]
- Chmielowska J, Carvell GE, Simons DJ. Spatial organization of thalamocortical and corticothalamic projection systems in the rat SmI barrel cortex. J Comp Neurol 285: 325–338, 1989. [DOI] [PubMed] [Google Scholar]
- Constantinople CM, Bruno RM. Deep cortical layers are activated directly by thalamus. Science 340: 1591–1594, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossell L, Iacaruso MF, Muir DR, Houlton R, Sader EN, Ko H, Hofer SB, Mrsic-Flogel TD. Functional organization of excitatory synaptic strength in primary visual cortex. Nature 518: 399–403, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Urabe H, Nurmikko AV, Connors BW. Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron 65: 230–245, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kock CP, Sakmann B. Spiking in primary somatosensory cortex during natural whisking in awake head-restrained rats is cell-type specific. Proc Natl Acad Sci USA 106: 16446–16450, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kock CP, Bruno RM, Spors H, Sakmann B. Layer- and cell-type-specific suprathreshold stimulus representation in rat primary somatosensory cortex. J Physiol 581: 139–154, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes M, Bourassa J, Pinault D. Corticothalamic projections from layer V cells in rat are collaterals of long-range corticofugal axons. Brain Res 664: 215–219, 1994. [DOI] [PubMed] [Google Scholar]
- Ferezou I, Haiss F, Gentet LJ, Aronoff R, Weber B, Petersen CC. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron 56: 907–923, 2007. [DOI] [PubMed] [Google Scholar]
- Friedman A, Arens J, Heinemann U, Gutnick MJ. Slow depolarizing afterpotentials in neocortical neurons are sodium and calcium dependent. Neurosci Lett 135: 13–17, 1992. [DOI] [PubMed] [Google Scholar]
- Greig LC, Woodworth MB, Galazo MJ, Padmanabhan H, Macklis JD. Molecular logic of neocortical projection neuron specification, development and diversity. Nat Rev Neurosci 14: 755–769, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattox AM, Nelson SB. Layer V neurons in mouse cortex projecting to different targets have distinct physiological properties. J Neurophysiol 98: 3330–3340, 2007. [DOI] [PubMed] [Google Scholar]
- Hooks BM, Hires SA, Zhang YX, Huber D, Petreanu L, Svoboda K, Shepherd GM. Laminar analysis of excitatory local circuits in vibrissal motor and sensory cortical areas. PLoS Biol 9: e1000572, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa BM, Roth MM, Gobel W, Helmchen F. Representation of visual scenes by local neuronal populations in layer 2/3 of mouse visual cortex. Front Neural Circuits 5: 18, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper EM, Lubke J, Larkman AU, Blakemore C. Pyramidal neurons in layer 5 of the rat visual cortex. III. Differential maturation of axon targeting, dendritic morphology, and electrophysiological properties. J Comp Neurol 339: 495–518, 1994. [DOI] [PubMed] [Google Scholar]
- Katz LC. Local circuitry of identified projection neurons in cat visual cortex brain slices. J Neurosci 7: 1223–1249, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Burkhalter A, Dreyer WJ. Fluorescent latex microspheres as a retrograde neuronal marker for in vivo and in vitro studies of visual cortex. Nature 310: 498–500, 1984. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Koralek KA, Chiaia NL, Rhodes RW. Laminar and areal differences in the origin of the subcortical projection neurons of the rat somatosensory cortex. J Comp Neurol 282: 428–445, 1989. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Juavinett AL, Kyubwa EM, Jacobs MW, Callaway EM. Three types of cortical layer 5 neurons that differ in brain-wide connectivity and function. Neuron 88: 1253–1267, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnischtzke AK, Simons DJ, Fanselow EE. Motor cortex broadly engages excitatory and inhibitory neurons in somatosensory barrel cortex. Cereb Cortex 24: 2237–2248, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D, Ahissar E, Diamond ME. Active sensation: insights from the rodent vibrissa sensorimotor system. Curr Opin Neurobiol 16: 435–444, 2006. [DOI] [PubMed] [Google Scholar]
- Le Be JV, Silberberg G, Wang Y, Markram H. Morphological, electrophysiological, and synaptic properties of corticocallosal pyramidal cells in the neonatal rat neocortex. Cereb Cortex 17: 2204–2213, 2007. [DOI] [PubMed] [Google Scholar]
- Lee S, Carvell GE, Simons DJ. Motor modulation of afferent somatosensory circuits. Nat Neurosci 11: 1430–1438, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano DA, Sherman SM. Differences in intrinsic properties and local network connectivity of identified layer 5 and layer 6 adult mouse auditory corticothalamic neurons support a dual corticothalamic projection hypothesis. Cereb Cortex 19: 2810–2826, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao T, Kusefoglu D, Hooks BM, Huber D, Petreanu L, Svoboda K. Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron 72: 111–123, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol 54: 782–806, 1985. [DOI] [PubMed] [Google Scholar]
- Miyashita E, Keller A, Asanuma H. Input-output organization of the rat vibrissal motor cortex. Exp Brain Res 99: 223–232, 1994. [DOI] [PubMed] [Google Scholar]
- Oliva AA Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci 20: 3354–3368, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka T, Kawaguchi Y. Firing-pattern-dependent specificity of cortical excitatory feed-forward subnetworks. J Neurosci 28: 11186–11195, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka T, Kawaguchi Y. Cell diversity and connection specificity between callosal projection neurons in the frontal cortex. J Neurosci 31: 3862–3870, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CC. The functional organization of the barrel cortex. Neuron 56: 339–355, 2007. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature 457: 1142–1145, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter LL, White EL. Afferent and efferent pathways of the vibrissal region of primary motor cortex in the mouse. J Comp Neurol 214: 279–289, 1983. [DOI] [PubMed] [Google Scholar]
- Rumberger A, Schmidt M, Lohmann H, Hoffmann KP. Correlation of electrophysiology, morphology, and functions in corticotectal and corticopretectal projection neurons in rat visual cortex. Exp Brain Res 119: 375–390, 1998. [DOI] [PubMed] [Google Scholar]
- Schwindt P, Crill W. Mechanisms underlying burst and regular spiking evoked by dendritic depolarization in layer 5 cortical pyramidal neurons. J Neurophysiol 81: 1341–1354, 1999. [DOI] [PubMed] [Google Scholar]
- Schwindt P, O'Brien JA, Crill W. Quantitative analysis of firing properties of pyramidal neurons from layer 5 of rat sensorimotor cortex. J Neurophysiol 77: 2484–2498, 1997. [DOI] [PubMed] [Google Scholar]
- Sheets PL, Suter BA, Kiritani T, Chan CS, Surmeier DJ, Shepherd GM. Corticospinal-specific HCN expression in mouse motor cortex: Ih-dependent synaptic integration as a candidate microcircuit mechanism involved in motor control. J Neurophysiol 106: 2216–2231, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota MG, Swadlow HA, Beloozerova IN. Three channels of corticothalamic communication during locomotion. J Neurosci 25: 5915–5925, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temereanca S, Simons DJ. Functional topography of corticothalamic feedback enhances thalamic spatial response tuning in the somatosensory whisker/barrel system. Neuron 41: 639–651, 2004. [DOI] [PubMed] [Google Scholar]
- Tseng GF, Prince DA. Heterogeneity of rat corticospinal neurons. J Comp Neurol 335: 92–108, 1993. [DOI] [PubMed] [Google Scholar]
- Veinante P, Deschenes M. Single-cell study of motor cortex projections to the barrel field in rats. J Comp Neurol 464: 98–103, 2003. [DOI] [PubMed] [Google Scholar]
- Velez-Fort M, Rousseau CV, Niedworok CJ, Wickersham IR, Rancz EA, Brown AP, Strom M, Margrie TW. The stimulus selectivity and connectivity of layer six principal cells reveals cortical microcircuits underlying visual processing. Neuron 83: 1431–1443, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, McCormick DA. Control of firing mode of corticotectal and corticopontine layer V burst-generating neurons by norepinephrine, acetylcholine, and 1S,3R-ACPD. J Neurosci 13: 2199–2216, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White EL, DeAmicis RA. Afferent and efferent projections of the region in mouse SmL cortex which contains the posteromedial barrel subfield. J Comp Neurol 175: 455–482, 1977. [DOI] [PubMed] [Google Scholar]
- Wimmer VC, Bruno RM, de Kock CP, Kuner T, Sakmann B. Dimensions of a projection column and architecture of VPM and POm axons in rat vibrissal cortex. Cereb Cortex 20: 2265–2276, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SP, Jones EG. Cells of origin and terminal distribution of descending projections of the rat somatic sensory cortex. J Comp Neurol 175: 129–157, 1977. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Pala A, Pedrido L, Kremer Y, Welker E, Petersen CC. Membrane potential dynamics of neocortical projection neurons driving target-specific signals. Neuron 80: 1477–1490, 2013. [DOI] [PubMed] [Google Scholar]
- Yang Q, Chen CC, Ramos RL, Katz E, Keller A, Brumberg JC. Intrinsic properties of and thalamocortical inputs onto identified corticothalamic-VPM neurons. Somatosens Mot Res 31: 78–93, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagha E, Casale AE, Sachdev RN, McGinley MJ, McCormick DA. Motor cortex feedback influences sensory processing by modulating network state. Neuron 79: 567–578, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrinpar A, Callaway EM. Local connections to specific types of layer 6 neurons in the rat visual cortex. J Neurophysiol 95: 1751–1761, 2006. [DOI] [PubMed] [Google Scholar]
- Zhang ZW, Deschenes M. Intracortical axonal projections of lamina VI cells of the primary somatosensory cortex in the rat: a single-cell labeling study. J Neurosci 17: 6365–6379, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW, Deschenes M. Projections to layer VI of the posteromedial barrel field in the rat: a reappraisal of the role of corticothalamic pathways. Cereb Cortex 8: 428–436, 1998. [DOI] [PubMed] [Google Scholar]