Visuomotor learning in one limb often confers benefits in performance with the other limb, a phenomenon known as interlimb transfer. In this study, we demonstrate for the first time that transfer of learning across limbs can be dissociated into explicit and implicit components and that the extent to which these distinct components of learning transfer to the opposite limb is constrained by the alignment of the learned compensation in joint-based and extrinsic coordinates.
Keywords: interlimb transfer, visuomotor learning, explicit learning, implicit learning
Abstract
Insights into the neural representation of motor learning can be obtained by investigating how learning transfers to novel task conditions. We recently demonstrated that visuomotor rotation learning transferred strongly between left and right limbs when the task was performed in a sagittal workspace, which afforded a consistent remapping for the two limbs in both extrinsic and joint-based coordinates. In contrast, transfer was absent when performed in horizontal workspace, where the extrinsically defined perturbation required conflicting joint-based remapping for the left and right limbs. Because visuomotor learning is thought to be supported by both implicit and explicit forms of learning, however, it is unclear to what extent these distinct forms of learning contribute to interlimb transfer. In this study, we assessed the degree to which interlimb transfer, following visuomotor rotation training, reflects explicit vs. implicit learning by obtaining verbal reports of participants' aiming direction before each movement. We also determined the extent to which these distinct components of learning are constrained by the compatibility of coordinate systems by comparing transfer between groups of participants who reached to targets arranged in the horizontal and sagittal planes. Both sagittal and horizontal conditions displayed complete transfer of explicit learning to the untrained limb. In contrast, transfer of implicit learning was incomplete, but the sagittal condition showed greater transfer than the horizontal condition. These findings suggest that explicit strategies developed with one limb can be fully implemented in the opposite limb, whereas implicit transfer depends on the degree to which new sensorimotor maps are spatially compatible for the two limbs.
NEW & NOTEWORTHY
Visuomotor learning in one limb often confers benefits in performance with the other limb, a phenomenon known as interlimb transfer. In this study, we demonstrate for the first time that transfer of learning across limbs can be dissociated into explicit and implicit components and that the extent to which these distinct components of learning transfer to the opposite limb is constrained by the alignment of the learned compensation in joint-based and extrinsic coordinates.
interlimb transfer is the process whereby practice on a novel motor task with one limb confers a benefit in performance to the other limb. How the motor system achieves this is of great interest, not only because transfer might have practical importance for developing rehabilitation techniques, but also because it may provide theoretical insights into the neural representations of motor learning (Shadmehr 2004). This issue has been frequently studied through adaptation tasks that involve a distortion of visual feedback during reaching movements; however, there remains controversy regarding the characteristics of transfer between limbs. In visuomotor adaptation studies, the extent of transfer varies widely from 0 to 90% depending on various methodological factors, including the number of reaching targets and which limb is trained first (Carroll et al. 2014; Taylor et al. 2011; Wang et al. 2015; Wang and Sainburg 2003, 2004). Even the apparently asymmetrical nature of interlimb transfer is inconsistent, with conflicting reports that transfer is greater from the nondominant to the dominant limb (Sainburg and Wang 2002; Wang and Sainburg 2004) or vice versa (Balitsky Thompson and Henriques 2010).
One key issue in this controversy is that transfer has previously been inferred from the magnitude of errors when the untrained limb was exposed to the perturbation, rather than by measuring reaching directions with the untrained limb in the absence of feedback. Errors made by the untrained limb under perturbed conditions might reflect a visuomotor remapping transferred from the opposite limb and/or a change in learning rate due to previous exposure to perturbation with the opposite limb (i.e., an interlimb form of “savings”). Thus these contrasts do not distinguish among the multiple components of learning that might contribute to transfer in motor adaptation paradigms (Diedrichsen et al. 2010; Huang et al. 2011; Izawa and Shadmehr 2011; Mazzoni and Krakauer 2006). For instance, when a visuomotor rotation is introduced abruptly, participants can gain awareness of the perturbation and “re-aim” their movements to facilitate performance (Taylor et al. 2011, 2014). This strategy might be applied during performance with the untrained limb, giving a false impression that the learned remapping had transferred. Although previous studies have shown that manipulations of awareness have little effect on interlimb transfer (Taylor et al. 2011; Wang et al. 2011), suggesting that explicit strategies play little role in transfer, the evidence is only indirect. In particular, it is important to note that gaining awareness of the visuomotor rotation does not always ensure that participants will form explicit strategies, especially when the rotation is small (Bond and Taylor 2015; Morehead et al. 2015). Thus it is conceivable that at least some transfer could be driven by explicit strategies; however, this has never been directly measured.
Recently, the effects of explicit strategies on learned compensations to visuomotor rotation have been experimentally isolated by having participants state their aiming direction with reference to a numbered landmark. This aiming strategy constitutes an explicit process that the brain implements in parallel with implicit learning to compensate for the target error. When the direction of aim is known, the difference between actual and intended reach direction should reflect the extent of implicit learning (Bond and Taylor 2015; Taylor et al. 2014). The explicit and implicit components of learning uncovered using this paradigm followed distinct time courses; explicit strategies were highly variable early in training before stabilizing to a direction biased toward the solution, whereas implicit learning progressed monotonically. In the present study, we employed this approach to directly assay, for the first time, the degree to which transfer between limbs is the result of explicit strategies or implicit learning.
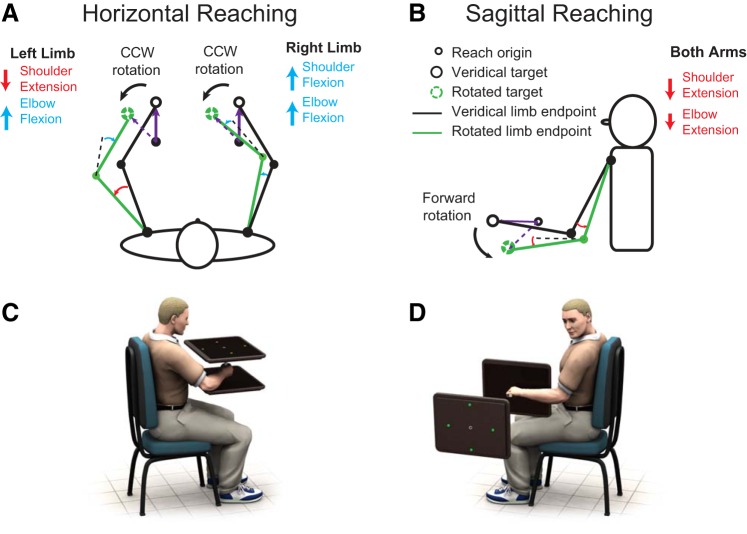
The coordinate system of learning may present additional factors that influence the degree of transfer. Robust transfer of implicit learning would require the motor memory developed in the trained limb to be both accessible and compatible for use with the other limb. In a typical horizontal reaching task, however, because movements of the two limbs are mirror symmetrical, extrinsically defined perturbations require conflicting joint-based remapping for the left and right limbs (Fig. 1A). If the internal representation of motor memory comprises multiple coordinate systems (Berniker et al. 2014; Brayanov et al. 2012), the incompatibility of these coordinate systems might restrict the extent to which the learned mapping is expressed bilaterally, even if the learning is fully accessible to the opposite limb.
Fig. 1.
Schematic illustrations of subjects' posture and position of visual display. A and B: joint angle changes required for each limb to compensate for an extrinsically defined visuomotor rotation. The joint angle changes required for both limbs are similar for reaching in the sagittal but not the horizontal plane. C: horizontal reaching condition in which the joint-based and extrinsic representations of the required remapping are in conflict. D: sagittal reaching condition in which both the joint-based and extrinsic representations of the required remapping are aligned. CCW, counterclockwise.
We have previously addressed this issue by examining the transfer of learning in an isometric wrist force aiming task (Carroll et al. 2014). Because the task was isometric, it enabled effective manipulation of the learned visuomotor map according to specific coordinate systems while maintaining others constant (de Rugy et al. 2012; Kakei et al. 1999). Consistent with the idea that the extent of transfer hinges on the degree to which the joint-based and extrinsic coordinates were aligned for the two limbs, we found that learning transferred strongly in both directions in a sagittal workspace, where the joint-based and extrinsic representations were aligned. In contrast, transfer was absent when performed in a horizontal workspace, where the joint-based and extrinsic representations were in conflict. Although the coordinate system of explicit learning is currently unknown, it is possible that implicit and explicit forms of learning are represented in different coordinate systems. As a consequence, the amount of transfer may vary as a function of the degree to which learning is implicit or explicit because of coordinate system conflicts.
In this study, we sought to quantify the degree to which transfer relies on explicit vs. implicit forms of learning and the extent to which transfer attributable to each of these distinct learning components is constrained by the compatibility of the coordinate systems in a reaching task. We found that whereas explicit transfer was near complete irrespective of coordinate system alignment, the extent of implicit transfer depended on the spatial compatibility of the new sensorimotor mapping for the two limbs. Consistent with our previous findings, implicit transfer was substantially greater when extrinsic and joint-based representations of the required remapping were aligned than when representations were in conflict.
METHODS
Participants.
Forty-eight participants (22 men; 26 women, age range 18–30 yr) were recruited either from the research participation pool of the Department of Psychology at Princeton University or from the university community at The University of Queensland. All participants were right-handed according to the Edinburgh handedness inventory (Oldfield 1971) and provided written informed consent prior to participation. The protocol was approved by Princeton University's Institutional Review Board and the local ethics committee at The University of Queensland, and conformed to the Declaration of Helsinki.
Each participant was randomly allocated to one of four different conditions (n = 12 per group) according to a 2 × 2 factorial design, with two levels for movement plane (horizontal vs. sagittal) and two levels for limb used during rotation training (right vs. left). Participants learned to counter a 45° visuomotor rotation (direction counterbalanced within each condition) while reaching in either the horizontal or sagittal plane. Transfer was examined in the opposite limb in the corresponding plane of movement and with the same direction of visuomotor rotation defined extrinsically.
Task.
Participants made planar reaching movements by sliding their left or right limb across a digitizing tablet while holding onto a pen (Intuos Pro; Wacom, Vancouver, WA). Wrist braces were used on both limbs to restrain accessory movement of the radial-carpal joint and limit motion predominantly to the elbow and shoulder joint. The tablets were aligned vertically or horizontally on an adjustable table to allow operation in sagittal or horizontal workspaces, respectively (see Fig. 1, C and D). All visual stimuli were presented on a 17-in., 1,280 × 1,024-pixel resolution display with 60-Hz refresh rate (Dell, Dallas, TX). The movement of the cursor on the screen was calibrated to the dimensions of the tablet to ensure veridical distance mapping.
In the horizontal condition, a single tablet was positioned 25 cm beneath the visual display (Fig. 1C). Throughout the experiment, participants shared one pen for both limbs, and a visual cue indicating “left” or “right” was presented at the top edge of the monitor to inform the participant which limb to use. Participants had to transfer the pen from one limb to the other when a different limb was required. In the sagittal condition, the two tablets were vertically oriented with their backs against each other and positioned along the midline of the participant. Visual feedback was displayed on a single monitor positioned 65 cm to the left of the participant (see Fig. 1D). In this experiment, participants held a separate pen in each hand. When the visual cue indicating “left” or the “right” was presented, the participant then positioned the corresponding limb on the tablet to start the trial while the other limb rested freely on the participant's lap. To occlude vision of the hand, a black cloth was draped over the workspace. As illustrated in Fig. 1, A and B, the joint rotations required to compensate for an extrinsically defined rotation are comparable for both limbs during sagittal reaching but differ for horizontal reaching. The experiment was implemented with custom software written in MATLAB (The MathWorks, Natick, MA) using the Psychophysics Toolbox extensions (Brainard 1997).
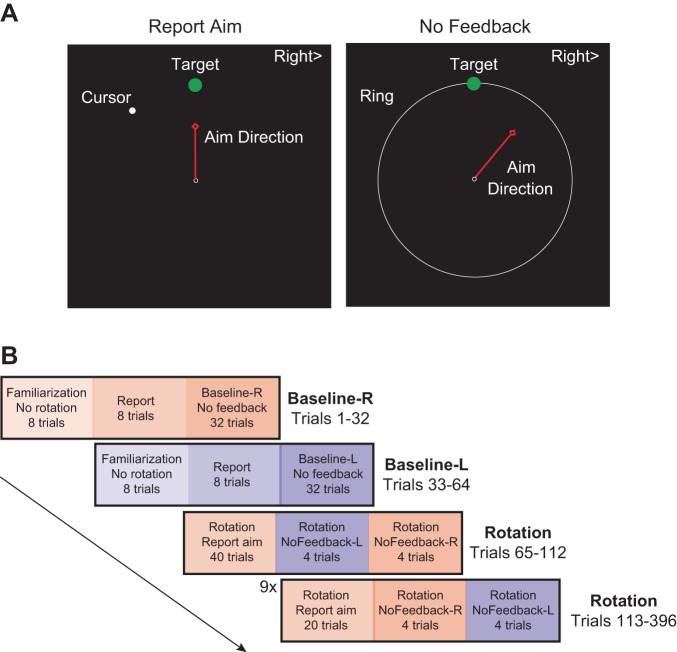
Each trial began when the participant positioned the corresponding limb within a central start location (white empty circle; 3.5-mm diameter). Participants were guided to the start position by a white ring whose radius indicated the radial distance of the limb from the start location. The ring served as a guide to return participants to the start location without providing information of the rotation on the return phase of the movement. When the limb was within 0.5 cm of the start location, the ring turned into the feedback cursor (white filled circle; 2.5-mm diameter). The limb had to remain in the start location for 500 ms before a green target appeared in one of four locations along the cardinal directions (0°, 90°, 180°, and 270°) at a radial distance of 7.5 cm. A red guide line, which started at a random angle on each trial, was also presented when the target appeared. Prior to testing, the participants were provided with the following instructions on the screen “The goal of the task is to get your cursor on the green target. I would like you to tell me, before moving, the direction that you should aim in order to get your cursor on the target. Please align the dial with the direction of your aim.” Participants then had 7 s to verbally instruct the experimenter to rotate the guide line to their intended reach direction to make the cursor hit the target location (Fig. 2A). Participants halted the line by saying “stop,” and the guide line was extinguished. Subsequently, the participant initiated a rapid shooting movement along the intended path to move the cursor through the target. Once the movement amplitude exceeded 7.5 cm, the feedback cursor froze on the screen for 1 s. The discrepancy between the position of the white cursor and the green target provided feedback of the angular error. A pleasant “ding” signaled target acquisition if the center of the cursor was within the target; otherwise, an unpleasant “buzz” sounded. Participants received an auditory warning that the movement was “too slow” when movement from 10% to 100% of target extent exceeded 500 ms. After each trial, the targets were extinguished and subjects were instructed to move the limb back to the start location immediately once the targets were cleared from the screen.
Fig. 2.
Experimental task and protocol. A, left: participants are exposed to a 45° visuomotor rotation while reaching to 4 different target locations located on the cardinal directions. A visual cue indicating which limb to use was presented at the top edge of the monitor (“Right>”). Before each movement, participants were asked to verbally instruct the experimenter to rotate the red guide line (Aim Direction) to their intended reach direction to make the cursor hit the target location. Subsequently, the participant initiated a rapid shooting movement to move the cursor through the target, after the guide line was extinguished. When the movement amplitude exceeded 7.5 cm, the feedback cursor froze on the screen for 1 s. Right, for trials with no feedback (probe trials), the visual cursor was replaced by an expanding white ring whose radius corresponded to the radial distance of the limb from the start location. The white ring provided only information about the extent, not the direction, of the movement. B: the baseline phase consisted of 8 Familiarization trials with no rotation, followed by 8 trials with the reporting protocol. Feedback was then removed to assess baseline performance in each hand. In the rotation phase, feedback was rotated by 45° and participants reported their aiming location such that the visual error was minimized. In the Rotation-No Feedback trials, the visual cursor was removed and reported their aiming direction before each movement. L and R, left and right limbs.
Experimental procedures.
Testing began with a baseline phase in which participants completed 12 blocks of trials, with each block consisting of 4 movements (one to each target in a pseudorandomized order) in the absence of any perturbation with each limb (Fig. 2B). The first two blocks for each limb in this phase were performed with continuous visual feedback to familiarize with the reaching task (Fig. 2B, Familiarization-L, Familiarization-R). Subsequently, in the next two blocks, participants provided their aiming direction via the reporting protocol described above to indicate the direction that they intended to reach toward to make the cursor hit the target (Report-L, Report-R). Finally, in the remaining eight blocks of the baseline phase, visual feedback was removed while participants continued to report their aiming direction so that we could measure any systematic biases in their reaches and to get participants accustomed to reaching without visual feedback with either limb (Baseline-L, Baseline-R).
The next rotation phase consisted of 55 blocks of trials in which a 45° visuomotor rotation was introduced. According to group assignment, the first 10 blocks during the rotation phase were performed with the limb first used during the baseline phase. Introduction of the rotation prompted participants to alter their aiming direction to compensate for the visual cursor error, despite not being explicitly informed of the visuomotor rotation. This was followed by two blocks of probe trials, in which visual feedback of the cursor was removed and replaced with an expanding ring that provided only radial distance information. Participants were still instructed to report their aiming direction prior to movement. They experienced eight probe trials in a row, one trial for each target per limb in a pseudorandomized order. Since the untrained limb was never exposed to the visuomotor rotation, this allowed us to obtain a measure of interlimb transfer that is independent of any learning that might have occurred in the untrained limb (Rotation NoFeedback-R, Rotation NoFeedback-L). This procedure was repeated after every fifth block in the remaining 45 blocks of trials. It is important to note that participants were not informed about the onset of probe trials. Thus the probe trials should have proceeded just like the rest of the trials in the experiment, but without visual cursor feedback. This allowed us to continuously track the progress of interlimb transfer of explicit and implicit learning throughout the rotation phase. In total, participants experienced 40 probe trials for each limb to probe the extent of learning and transfer.
Data analysis.
Data analysis was performed offline in MATLAB. For each trial, movement trajectory, irrespective of target location, was rotated to a common reference axis with the target location set at 0°. The angular differences between the direction of target and the direction of the vector from participants' starting location to the point when movements passed a radial distance of 7.5 cm were computed as the terminal reach direction. The terminal reach directions were binned into four trial blocks and averaged across participants in each group to visualize performance during learning and transfer for each limb. Reach directions that were more than 2 standard deviations (SD) away from the mean reach direction errors during each phase of the experiment were discarded as outliers (total proportion of trials discarded = 1.4%). Movement durations were specified as the time taken to travel from 10% to 100% of target extent. It is important to note that because we are examining interlimb transfer, we had to first assess whether participants successfully learned to counter the visuomotor rotation. We performed one sample t-tests for each participant to determine whether the level of implicit learning attained in the last four blocks of the rotation phase was significantly different from zero. Four participants from the sagittal condition and one participant from the horizontal condition were excluded from further analysis because they did not successfully learn to counter the visuomotor rotation. Results from 23 participants in the horizontal condition and 20 participants in the sagittal condition remained for statistical analysis.
To assess baseline performances in the different reaching conditions for each limb, the average reaching direction from the last four blocks of Baseline-L and Baseline-R were submitted to a mixed factorial ANOVA with movement plane (horizontal vs. sagittal) as a between-subjects factor and limb as a within-subjects factor (left vs. right). The average reach direction during the last four blocks of the baseline phase was then subtracted from the reach direction during the rotation phase to remove any baseline directional biases.
To determine whether behavior differed between the different movement and limb conditions during the rotation phase, we compared the average reach direction in the first and final four blocks of this phase. We chose to probe learning at these two intervals because performance in the first four blocks of the rotation phase captures the behavior at the point where learning transpired most quickly in previous studies (Huang et al. 2011; Huberdeau et al. 2015; Kitago et al. 2013), and the last four blocks represents the stage of learning where participants would have reached asymptote if they had fully compensated for the visuomotor rotation. We submitted these measures to a repeated -measures ANOVA with two between-subjects factors [factor 1: limb (left vs. right); factor 2: movement plane (horizontal vs. sagittal)] and one within-subject factor [factor 3: time (first 4 blocks vs. final 4 blocks)].
To examine the contributions of implicit and explicit learning, we compared the average explicit and implicit learning obtained in the first and final four blocks of the rotation phase. Explicit learning was calculated by subtracting participants' reported aiming direction for each trial from the target direction, whereas implicit learning was computed by subtracting the reported aiming direction from the terminal reach direction on each trial. We then performed separate repeated-measures ANOVA with two between-subjects factors [factor 1: limb (left vs. right); factor 2: movement plane (horizontal vs. sagittal)] and one within-subject factor [factor 3: time (first 4 blocks vs. final 4 blocks)] for the explicit and implicit measures.
To quantify the degree of explicit and implicit learning for interlimb transfer, we focused our analyses on the first and last two blocks of probe trials performed by each limb (Rotation NoFeedback-R, Rotation NoFeedback-L). We chose to probe the extent of interlimb transfer at these intervals because the point at which the first and last two blocks of probe trials took place corresponded to the time during initial learning, when there is rapid error correction, and the latter stage of learning, when participants would have reached asymptote if they had fully compensated for the visuomotor rotation. Note that since probe blocks were infrequent, these blocks corresponded to the time periods of interest in our above analyses. The average reach direction, implicit and explicit learning in the first and final two blocks for the untrained limb, was expressed as a percentage of that for the trained limb for each participant. Specifically, the amounts of implicit and explicit transfer were
| 1) |
| 2) |
Similarly, tests for significance across conditions were performed using a repeated-measures ANOVA with two between-subjects factors [factor 1: limb (left vs. right); factor 2: movement plane (horizontal vs. sagittal)] and one within-subject factor [factor 3: time (first 2 blocks vs. final 2 blocks)]. All statistical comparisons were performed using Statistica (StatSoft, Tulsa, OK), and dependent measures are presented as means ± SE in both text and figures.
RESULTS
In this study we examined interlimb transfer of implicit and explicit learning during a visuomotor rotation task. We also sought to determine whether the extent of interlimb transfer is influenced by manipulating the spatial congruence between the coordinate frames of the two limbs. Specifically, we predicted greater interlimb transfer for sagittal movements, because the required visuomotor remapping to compensate for the rotation is identical according to both joint-based and extrinsic coordinates for the two limbs.
Rotation training.
In the baseline phase, participants reached to the targets with no visual feedback while verbally reporting the direction they were aiming through the guide line provided on the display. Baseline reach directions were significantly more accurate for horizontal than sagittal plane movements [average horizontal reach direction −0.4 ± 0.6°; average sagittal reach direction 1.9 ± 0.9°; F(1,82) = 8.5, P = 0.004]. The trial-to-trial variability of reach direction during baseline was also significantly greater for sagittal than horizontal groups [SD of horizontal reach direction 1.5 ± 0.1; SD of sagittal reach direction 2.8 ± 0.33; F(1,82) = 28.9, P < 0.0001]. The differences in baseline performances could be due to the different biomechanical requirements of the task, whereby participants were required to support the weight of the limb in sagittal plane reaching but could partially rest their limb on the tablet in the horizontal plane.
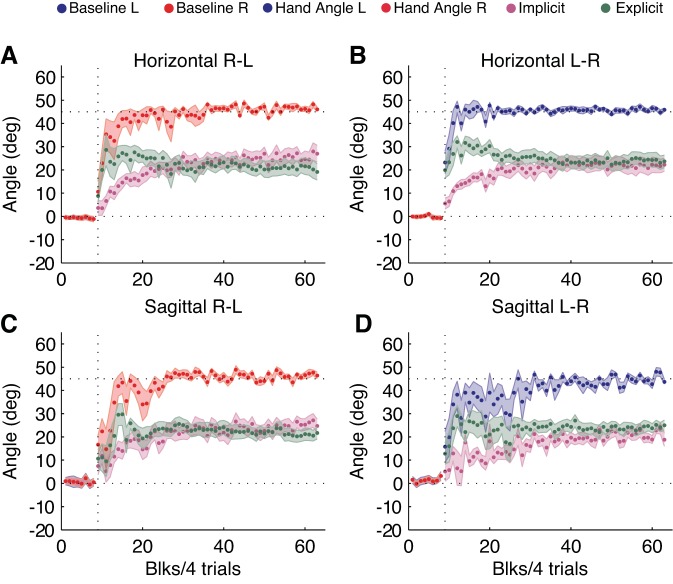
Next, we focused on trials in which feedback was available during the rotation phase. When a 45° visuomotor rotation was abruptly introduced, participants rapidly changed their limb angles to compensate for the rotation (Fig. 3). By the last four blocks of the rotation phase, reach direction for the horizontal and sagittal condition were 46.3 ± 0.6° and 45.5 ± 0.8°, respectively. This was significantly different from the average reach direction in the first four blocks [horizontal 30.5 ± 3.1°; sagittal 23.0 ± 4.0°; F(1,39) = 59.8, P < 0.0001], but there were no main effects of the limb used [F(1,39) = 2.5, P = 0.13] or the movement planes [F(1,39) = 2.5, P = 0.13] and no interaction effects [movement planes × limb: F(1,39) = 0.2, P = 0.64; phase × movement planes × limb: F(1,39) = 0.07, P = 0.78]. This indicates that both left and right limbs were equally effective in correcting for visual errors whether participants were reaching in the horizontal or sagittal plane.
Fig. 3.
Learning time course in the horizontal and sagittal conditions during rotation phase. A and B: average reach direction for the right (A; red) and left (B; blue) limb in the horizontal condition during baseline (blocks 1–8) and rotation phase (blocks 9–63). The green trace represents the average amount of explicit learning measured from the verbally reported aiming direction. The purple trace characterizes the average implicit learning computed from the difference between the actual reach direction and reported aiming location. C and D: average reach direction for the right (C; red) and left (D; blue) limb in the sagittal condition during baseline (blocks 1–8) and rotation phase (blocks 9–63). Green and purple traces represent the average amount of explicit and implicit learning, respectively. Each block represents the average of 4 trials (Blks/4 trials), and shading represents SE of the mean.
During the rotation phase, participants consistently aimed to locations other than the target to make the cursor hit the target. In line with previous studies (Bond and Taylor 2015; McDougle et al. 2015; Morehead et al. 2015; Taylor et al. 2011), the aiming locations rose sharply in the initial stages of learning before appearing to decrease with extended practice (Fig. 3, green trace). The aiming behavior in the initial and later stages of learning was assessed by repeated-measures ANOVA for the first and final four rotation blocks. There was no significant difference between early and late explicit learning [F(1,39) = 1.5, P = 0.22], and there were no main effects of movement plane [F(1,39) = 1.4, P = 0.25] or limb [F(1,39) = 2.9, P = 0.09] and no interactions [movement plane × limb: F(1,39) = 0.01, P = 0.9; time × movement plane × limb: F(1,39) = 0.26, P = 0.6]. This suggests that explicit learning was similar for the first and final four blocks during the rotation phase, regardless of limb used and movement condition (average in final 4 blocks: horizontal explicit 22.6 ± 2.3° vs. sagittal explicit 22.2 ± 1.8°). We also analyzed the stability of the learning by computing the variability of movements in the final four blocks of the rotation phase. The movement variability was significantly different between horizontal and sagittal reaching [F(1,39) = 4.5, P = 0.04], but there was no main effect of the limb used [F(1,39) = 0.01, P = 0.9] and no interaction effects [F(1,39) = 0.77, P = 0.4]. This suggests that learning in the horizontal condition was more stable than in the sagittal condition (SD horizontal 2.9 ± 0.3°; SD sagittal 4.3 ± 0.6°).
To quantify implicit learning, the reported aiming direction was subtracted from the reach direction on every trial. As illustrated in Fig. 3 (purple trace), implicit learning emerged gradually over the course of the rotation phase. To assess the pattern of implicit learning, we performed a repeated-measures ANOVA for the initial and final four blocks of trials during the rotation phase. The extent of implicit learning evident by the final four blocks was significantly greater than in the initial four blocks of trials [F(1,39) = 148.4, P < 0.0001], but there was no main effect of movement plane [F(1,39) = 0.01, P = 0.9] or limb [F(1,39) = 0.23, P = 0.63] and no interaction effects [movement plane × limb: F(1,39) = 0.01, P = 0.89; time × movement plane × limb: F(1,39) = 1.12, P = 0.29]. These results indicate that implicit learning significantly increased after the first four blocks of rotation phase, and the final amount of implicit learning was similar, irrespective of the limb or movement conditions (average in final four blocks: horizontal implicit 23.6 ± 2.4° vs. sagittal implicit 22.9 ± 1.8°).
Transfer to the opposite limb.
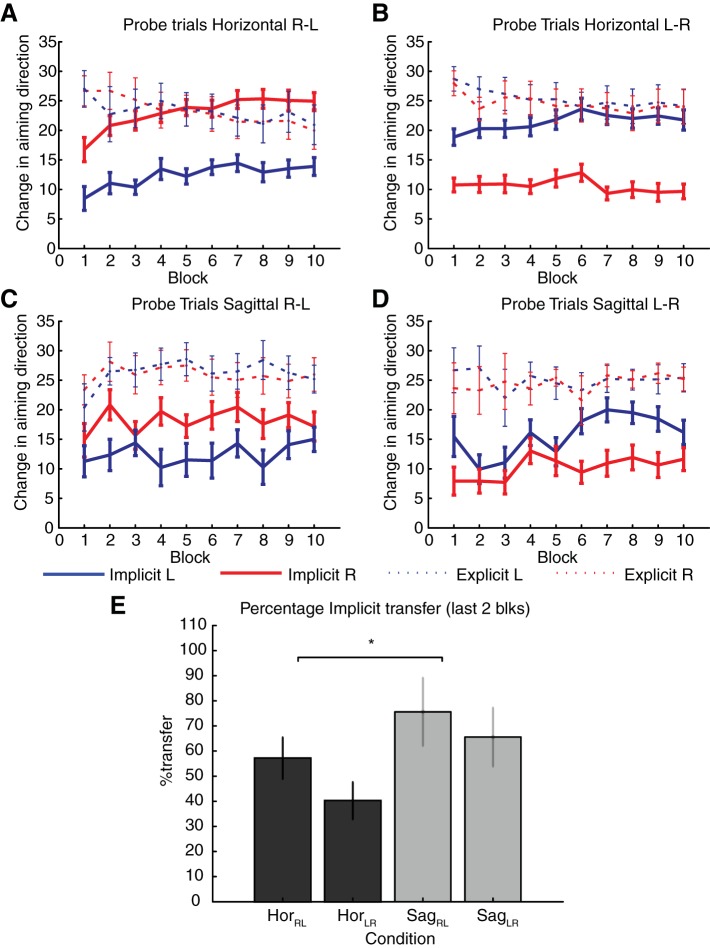
To assess the extent of interlimb transfer, we focused on the reach directions during probe trials with no feedback for both limbs (Adapt NoFeedback-R, Adapt NoFeedback-L). We first compared the absolute extent of transfer using the reach directions during probe trials, which is analogous to the extent of transfer inferred on the basis of the initial errors in response to the perturbation calculated in previous studies. Reach directions for the untrained limb averaged 35.1 ± 2.1° and 38.47 ± 1.9° over the last two blocks for the horizontal and sagittal conditions, respectively. The relative extent of transfer averaged over the last two blocks, when expressed as a percentage of the reach direction in the trained limb, was 74.9 ± 3.7% and 88.1 ± 3.5% for the horizontal and sagittal conditions, respectively. Despite an apparent benefit of transfer in the sagittal condition, a repeated-measures ANOVA yielded no main effect of time [F(1,39) = 0.99, P = 0.32], movement plane [F(1,39) = 1.62, P = 0.2], or limb used [F(1,39) = 0.05, P = 0.81] and no interaction effects [limb × movement plane: F(1,39) = 0.19, P = 0.66; time × limb × movement plane: F(1,39) = 0.12, P = 0.74]. These results demonstrate that the extent of transfer calculated in terms of the average reach direction was similar across movement planes and limbs.
Next, we compared the explicit learning during probe trials for both the trained and untrained limbs. Explicit strategies developed with the trained limb were effectively applied to the untrained limb during interlimb transfer (Fig. 4, dashed lines). This contributed substantially to the transfer measured in the untrained limb during probe trials (average in final 2 blocks: horizontal aim direction 22.79 ± 2.2°, sagittal aim direction 24.3 ± 1.7°). The relative extent of explicit transfer averaged over the last two blocks, when expressed as a percentage of the explicit learning in the trained limb, was 105.01 ± 2.9% and 110.9 ± 9.1% in the horizontal and sagittal conditions, respectively. A repeated-measures ANOVA comparing the percentage of explicit transfer for the first and final two blocks of probe trials showed no effect of time [F(1,39) = 0.51, P = 0.47], movement plane [F(1,39) = 1.19, P = 0.28], or limb [F(1,39) = 1.16, P = 0.29], and there were no interaction effects [movement plane × limb: F(1,39) = 1.4, P = 0.24; time × movement plane × limb: F(1,39) = 0.9, P = 0.36]. These results suggest that modulating the movement plane and limb used did not affect interlimb transfer of explicit learning. Moreover, the lack of difference between the first and final two blocks of probe trials indicated that aiming direction was fairly consistent throughout rotation training.
Fig. 4.
Performance during probe trials. A–D: average implicit and explicit learning for each limb during probe trials with no visual feedback for the horizontal (A and B) and sagittal conditions (C and D). Trials with the right and left limb are indicated by the red and blue lines, respectively. Solid lines reflect implicit learning and dash lines represent explicit learning during each block of probe trials throughout the rotation phase. E: percentage of implicit transfer attained over the last 2 blocks of probe trials. Dark gray bars represent trials with transfer from right to left (HorRL) and from left to right (HorLR) in the horizontal condition, whereas light gray bars represent trials with transfer from right to left (SagRL) and from left to right (SagLR) in the sagittal condition. Error bars indicate SE of the mean. *P < 0.05, significant difference between the amount of implicit transfer for the last 2 blocks of probe trials in the horizontal and sagittal condition following a repeated-measures ANOVA.
In contrast, implicit learning developed by the trained limb transferred only partially to the untrained limb (Fig. 4, solid lines). Implicit learning for the untrained limb averaged 11.5 ± 1.2° and 14.4 ± 1.8° over the last two blocks for the horizontal and sagittal conditions, respectively. When expressed as a percentage of the amount of implicit learning developed in the trained limb, the extent of transfer averaged 48.34 ± 5.7% and 70.6 ± 8.2% in the horizontal and sagittal condition, respectively (Fig. 4E). A repeated-measures ANOVA comparing the percentage of implicit transfer for the first and final two blocks of probe trials showed a main effect of movement plane [F(1,39) = 4.4, P = 0.041] but no effect of limb used [F(1,39) = 0.02, P = 0.88] or time [F(1,39) = 0.16, P = 0.68], and there were no interaction effects [movement plane × limb: F(1,39) = 0.3, P = 0.56; time × movement plane × limb: F(1,39) = 0.19, P = 0.66]. Despite no main effect of limb, there was a limb × time interaction [F(1,39) = 6.1, P = 0.01], suggesting that the time course of implicit transfer to the left and right limbs differed between early and late phases of learning. The main effect of movement plane indicated that implicit transfer in the sagittal condition was significantly greater than in the horizontal condition (average in last 2 blocks: horizontal R-L 57.2 ± 8.3%, horizontal L-R 38.7 ± 7.9%, sagittal R-L, 75 ± 12.1%, sagittal L-R, 65.6 ± 10.1%). The lack of difference in transfer based on the limb used also suggests that the motor memories acquired via implicit learning for the trained limb were accessible for opposite limb use, regardless of whether the dominant or nondominant limb was used during training. This finding is in contrast with the pattern of asymmetrical transfer observed in previous visuomotor rotation studies, in which learning transferred only from nondominant to the dominantly limb (Sainburg and Wang 2002) or only from the dominant to nondominant limb (Balitsky Thompson and Henriques 2010).
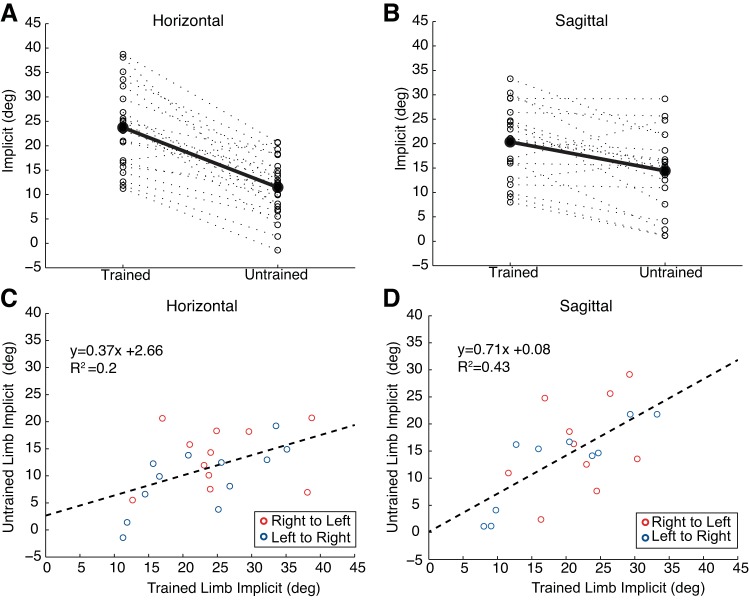
We also tested whether the extent of implicit learning achieved by the trained limb was related to the absolute amount of implicit transfer. Figure 5, A and B, illustrates that the trend for greater implicit transfer is consistent for most subjects in the sagittal condition. We performed a linear regression to predict implicit transfer in the untrained limb based on the level of implicit learning for the trained limb in the last two blocks of probe trials (Fig. 5, C and D). Significant regressions were found for both horizontal [F(1,21) = 5.4, P = 0.03; R2 = 0.2] and sagittal reaching [F(1,18) = 13.7, P = 0.002; R2 = 0.4], but the slope for horizontal groups (0.37) was lower than for sagittal groups (0.71). This estimate of transfer predicts that for each degree of implicit remapping expressed by the trained limb, reach direction with the untrained limb should change by 0.71° for sagittal plane conditions and 0.37° for horizontal plane reaching.
Fig. 5.
Correlation between the amount of implicit learning in the trained and untrained limb for the horizontal and sagittal conditions. A and B: extent of implicit learning over the last 2 blocks of probe trials for each participant in the trained and untrained limb. Solid lines indicate the amount of implicit learning in the horizontal (A) and sagittal (B) condition averaged across all participants. C and D: linear regression to predict implicit transfer based on the level of implicit learning for the trained limb in the last 2 blocks of probe trials. The slope for horizontal groups (0.37) was lower than for sagittal groups (0.71). Each point on the graph is indicative of a single participant (red, transfer from right to left; blue, transfer from left to right).
DISCUSSION
Using a visuomotor rotation task, we examined the extent to which both explicit and implicit components of learning can transfer to the opposite limb, and we quantified the influence of the spatial compatibility of the coordinate system representation on the transfer of learning. To assess how the explicit component contributes to interlimb transfer, verbal reports of participants' intended aiming direction were recorded before each movement. We observed that participants developed a strategy to re-aim their movements in a direction opposite to the rotation during the rotation phase. This strategy was also applied during movements with the untrained limb, regardless of whether the required joint-based and extrinsic remappings were aligned or in conflict for the two limbs. On the basis of the difference between explicit aim and actual reach direction, we also directly assayed the contributions of the implicit component of learning. Implicit transfer was incomplete between limbs (<50%), regardless of the direction of transfer when the joint-based and extrinsic representation of the required remapping were in conflict, but this transfer was enhanced (∼70%) when the required joint-based and extrinsic remappings were aligned. These findings suggest that whereas explicit strategies developed in one limb can be fully implemented in the other limb, implicit transfer depends on spatial compatibility of new sensorimotor maps for both limbs.
Separating explicit strategies from implicit learning during interlimb transfer.
The extent to which explicit strategies and implicit learning contribute to interlimb transfer has been studied by comparing the behavior of the untrained limb following exposure to gradual/abrupt perturbations or provision of an explicit strategy during training with the opposite limb (Taylor et al. 2011; Wang et al. 2011). In the study by Taylor et al. (2011), participants were presented with a landmark representing the correct solution for compensating for the rotation and given explicit instructions to reach toward it. This approach canceled out initial performance errors (i.e., target error) but subsequently resulted in overcompensation for the rotation. This overcompensation, referred to as “drift,” is thought to reflect continued implicit learning via sensory prediction error, despite negligible target error (Mazzoni and Krakauer 2006; Taylor and Ivry 2011). When transfer was probed with suppressed visual feedback in the opposite limb, the drift emerged in the untrained limb, as well, but the extent was less than 50% of that measured in the trained limb. This suggests that provision of an explicit strategy does not preclude the transfer of implicit learning to the opposite limb. Furthermore, both Wang et al. (2011) and Taylor et al. (2011) also showed that the amount of transfer, inferred from the performance of the opposite limb when the rotation was present, was similar regardless of whether the rotation was introduced gradually or abruptly or when participants were informed of the presence of a rotation during training. Although these results were interpreted to indicate that interlimb transfer was not influenced by awareness of the rotation, manipulating participants' awareness of a rotation through instructions or the gradual/abrupt dichotomy prevented direct quantification of about how and when explicit strategies were used during learning.
Our approach differs from previous studies that attempted to isolate transfer of implicit learning components, in that we probed explicit strategies directly using verbal reports for each limb. This enabled us to directly assay the contributions of implicit and explicit components of learning in a visuomotor adaptation task and subsequent interlimb transfer. Unlike the previous studies, we found that performance of the untrained limb relied on the relative contributions of both explicit and implicit components of learning. Notably, performance was dominated by an explicit component and was complemented by a smaller but significant implicit component. The apparent discrepancy between the contributions of the explicit component to interlimb transfer may be attributed to differences in rotation size. We note that in the studies by Wang et al. (2011) and Taylor et al. (2011), the sizes of the rotations employed were 32° and 22.5°, respectively. Given that implicit learning typically accounts for ∼15–25° of the overall learning in the time frame of training employed by these studies (Bond and Taylor 2015; Taylor et al. 2014), the use of explicit strategies might not have been necessary by the time transfer was probed in the opposite limb, since implicit learning alone would have been expected to account for ∼90% of the rotation. If the explicit strategy had been maintained, it would have led to a systematic increase in target error (Mazzoni and Krakauer 2006). Furthermore, Morehead et al. (2015) also showed that savings, which appear at least partially attributable to the explicit component of learning (Haith et al. 2015), were not observed with 30° rotations. Taken together, these results indicate that when the rotation is small, explicit strategies may have a negligible role in interlimb transfer, especially when probed at the end of training.
We interpreted aiming directions as manifestations of explicit learning during a visuomotor rotation task, with the difference in the extent of aiming between nondominant and dominant limb reflecting transfer of explicit learning. An alternative explanation is that the aiming strategies developed by the untrained limb do not constitute “transfer” of explicit learning per se, but rather a recall of previously learned strategy in the training context. As such, the extent to which explicit strategies are expressed in the other limb depends on the similarity of the task demands for the trained and untrained limb. By this view, asking participants to report on each trial might reinforce their explicit strategy and warrants its application with the untrained limb, thus contributing to the complete transfer of explicit learning across limbs. It is possible that without the added reporting component of the task, which would presumably reduce the similarity in the task demands between movements with both limbs, it would simply be the prerogative of the participant to choose whether to apply the strategy or not. This would lead to inconsistent application of the strategy during transfer. Currently, this will remain an open question, since an alternative method of probing the direction of aim during training without verbal reports has yet to be developed. Nevertheless, our results highlight the need to consider the role of explicit processes in future interlimb transfer studies.
This also raises the question whether the nature of learning in our task setting might be qualitatively different from that obtained in more traditional adaptation paradigms. It has been shown that provision of a strategy during a visuomotor rotation task reduced the size of aftereffects, despite having similar overall task performance during training compared with a naive group (Benson et al. 2011). This suggests a fundamental difference in the learning processes underlying performance improvements when one is provided with an explicit strategy to compensate for the rotation. Thus it is clearly possible that the progression of implicit learning can be modulated by performing verbal reports, which promotes the use of an explicit strategy. However, inconsistent with such a theory, we have reported that the use of verbal reports of explicit strategies did not substantially alter the canonical time course for learning in terms of reach direction measured. Importantly, the extent of implicit learning, as measured by the size of aftereffects, was only minimally affected by the reporting task (McDougle et al. 2015; Taylor et al. 2014). Thus we believe that learning in adaptation paradigms can be effectively dissociated into explicit and implicit subcomponents. Furthermore, this approach should have had little impact on the “true transfer” of implicit learning developed in the trained limb.
Symmetry of transfer.
Our findings demonstrate that “true transfer” of implicit learning is equally strong regardless of whether it is from nondominant to dominant limb or vice versa, although it evolves differently from early to late phases of learning, whereby transfer to the nondominant limb was significantly greater than transfer to the dominant limb during the latter phase of learning. This finding contradicts previous studies, which claimed that interlimb transfer is asymmetrical and that the extent to which performance of the untrained limb is facilitated depends on the features of movement measured (Sainburg and Wang 2002; Wang and Sainburg 2006a). Specifically, Sainburg and Wang (2002) reported that initial direction accuracy transferred from nondominant to dominant limb, whereas endpoint position accuracy transferred from the dominant to the nondominantly only. These observations led the authors to hypothesize that the two limb controllers might specialize in controlling for different aspects of movement, and the nature and extent of information transfer across limbs would depend on the properties of the controller used during learning. Interestingly, contrary to the pattern of interlimb transfer predicted by this hypothesis, Balitsky Thompson and Henriques (2010) found that when subjects countered a visuomotor rotation while provided with a cursor or an outline of their hand, initial direction accuracy transferred from dominant to nondominant limb, but not vice versa. These conflicting results, together with our current data, seem unlikely to be accounted for by a mechanism based on specialized visuomotor control for each hemisphere, because such a mechanism would predict interlimb transfer in one direction only.
It has been noted previously that the pattern of interlimb transfer depends on the spatial locations of the visual and motor workspaces for both limbs (Wang 2008; Wang and Sainburg 2006b). Whereas interlimb transfer was asymmetrical when examined in a shared midline workspace where the visual and motor workspace overlapped (Sainburg and Wang 2002), transfer occurred in both directions equally when the visual and motor workspaces were separated for both limbs (Wang and Sainburg 2006b). This result suggests that the symmetry of transfer depends on the concordance of the visual and motor workspaces for each limb. However, this cannot explain our data, since we found no asymmetries in the pattern of implicit transfer, regardless of whether the visual and motor workspaces were overlapping or minimally separated in our horizontal and sagittal conditions, respectively. It is important to note that implicit and explicit components of learning were not dissociated in the previous studies, and thus it is unclear how much of the transfer to the opposite limb was due to “true” implicit recalibration of the sensorimotor map rather than an application of an explicit strategy. Taking these findings together, we believe that the symmetry of transfer we observed in both sagittal and horizontal conditions cannot be explained by differences in the spatial locations of the visual and motor workspaces.
An alternative explanation regarding the asymmetries in the pattern of interlimb transfer reported in previous studies may have to do with differences in task demands. An important consideration is the type of feedback provided during training, which can affect the extent to which different learning processes are recruited and consequently alter the pattern of transfer to novel conditions (Taylor et al. 2013). The work by Balitsky Thompson and Henriques (2010) and Sainburg and Wang (2002) provided online feedback throughout movement and required feedback corrections for the cursor to acquire the target. As a consequence, learning involved not only feedforward modifications of the sensorimotor maps but also feedback modifications to correct for the observed error. In contrast, we instructed participants not to make feedback modifications to correct the target errors, which placed all the emphasis on feedforward processes during learning. Thus one could argue that the difference in the pattern of interlimb transfer could be attributed to how feedforward and feedback processes are differentially recruited depending on the instructions of the task. However, feedback corrections were mandatory in the study by Balitsky Thompson and Henriques (2010), yet they saw the opposite pattern for transfer between limbs compared with Sainburg and Wang (2002); therefore, this hypothesis is unlikely to fully explain the contradicting results.
Another point for consideration is that the discrepancies in the symmetry of transfer observed in previous studies may be due to differences in the magnitude of visuomotor rotation. In fact, Balitsky Thompson and Henriques (2010) showed that when relatively large rotation sizes were used (45° and 75°), learning transferred from the dominant to the nondominant limb only. In contrast, when a smaller rotation size was employed (30°), Sainburg and Wang (2002) found the opposite pattern of transfer, whereby learning transferred from nondominant to dominantly limb only. This could suggest that the discrepancies in the symmetry of transfer may be due to the use of different rotation sizes. However, it is difficult to pinpoint rotation magnitude as the only underlying factor because there are other differences between the studies, such as the number of training targets (8 vs. 1) and type of feedback (endpoint vs. continuous online feedback). Testing this hypothesis would require comparison of the pattern of transfer in a similar task design across different rotation magnitudes.
A key issue in the experimental design of Sainburg and Wang (2002), as well as that of other studies on interlimb transfer, is that transfer to the untrained limb was measured over several trials of exposure with the rotation present. This approach effectively tested the combined effects of interlimb transfer of learning as well as the rate of learning with the untrained limb. In fact, when interlimb transfer was measured on the very first transfer trial, neither initial direction nor endpoint position accuracy showed any improvement relative to naive performance measured on the same limb in a separate session (Wang and Sainburg 2004). It is also important to note that because the untrained limb was also exposed to the perturbation for a number of trials, it is conceivable that at least some of the transfer observed was driven by learning occurring in the untrained limb (Huberdeau et al. 2015), independently of any “transfer” of learning driven by the trained limb. Taken together, this combination of factors could mask subtle interlimb transfer. Our experiment was designed such that the untrained limb was never exposed to the visuomotor rotation and transfer performance was probed continuously throughout learning. This approach effectively distinguished the effects of direct interlimb transfer of the learned compensation to the rotation from any mechanisms related to the actions performed by the untrained limb.
Effect of coordinate frames alignment on implicit transfer.
The greater implicit transfer to the opposite limb after sagittal versus horizontal plane training suggests that transfer of learning is attenuated when the coordinate system representation of the learned compensation is in conflict for the two limbs. This result is consistent with our previous studies, which showed greater transfer of force field (Carroll et al. 2016) and isometric visuomotor rotation learning (Carroll et al. 2014) when the joint-based and extrinsic representation of the required remapping were aligned for both limbs. These studies demonstrate that both joint-based and extrinsic coordinate systems contribute to the internal representation of motor learning (Berniker et al. 2014; Brayanov et al. 2012). In this framework, the degree of transfer across limbs in a given context will hinge on the spatial compatibility of the learned compensation according to multiple coordinate systems for the trained and untrained limb.
However, given that the arrangement of the projections from the visual to motor workspace in the sagittal condition was orthogonal to the horizontal condition, different processing stages of the visuomotor transformation may have been affected by our training procedure. As such, the greater extent of implicit transfer in the sagittal than horizontal condition may be related to differences in the fundamental visuomotor transformation required in the two tasks (Buneo and Andersen 2006; Sabes 2011), instead of the congruity of joint-based and extrinsic coordinates. Despite this, we showed previously that a reversal in orientation of the visual display resulted in less transfer of isometric visuomotor rotation learning when the coordinate representation conflicted for the right and left limbs, but near complete transfer when the coordinate representations were aligned (see control experiment 2 of Carroll et al. 2014). This result showed that coordinate system alignment between limbs, rather than the degree to which the visual and motor workspaces aligned for each limb, was the key determinant of transfer for visuomotor rotation. Consistent with this view, we also found a transfer benefit of aligned coordinates (for sagittal workspaces) over conflicting coordinates (for horizontal workspaces) for force field learning when the feedback was spatially veridical (i.e., via 3-dimensional projections) in both cases (Carroll et al. 2016).
It is important to note that comparisons between arms within each condition showed that reaching performance in the sagittal plane was less accurate and more variable than horizontal plane reaching movements during the baseline phase. Although the amount of learning, as measured during the last 10 blocks in the rotation phase, was comparable between horizontal and sagittal conditions, it was also significantly more variable for sagittal reaching movements. It is possible that the variability in sagittal movements during baseline and during the rotation phase might have contributed to the improved implicit transfer we observed. For example, Wu et al. (2014) showed that higher levels of baseline motor variability resulted in faster learning. As such, participants might have reached asymptotic performance quicker, resulting in a longer period of training at plateau. It has been shown that period of time spent at plateau can lead to greater transfer of learning across limbs (Block and Celnik 2013). Additionally, it has also been shown that variability of movements during learning can predict the amount of transfer, with greater variability leading to greater transfer (Lefumat et al. 2015). Although we favor the conclusion that the improvement in transfer was due to the alignment of the joint-based and extrinsic coordinate frames, we are cognizant of the possibility that differences in motor variability in the horizontal and sagittal conditions might have contributed to the improved transfer.
Conclusions.
In summary, our present findings demonstrate that the explicit and implicit components of learning are differentially transferred across limbs during reaching movements. Specifically, explicit learning developed by one limb can be entirely transferred to the opposite limb, whereas implicit learning is only partially available to the opposite limb. Furthermore, we found that implicit transfer can be enhanced when reaching movements are performed in the sagittal plane such that the required remapping is aligned for the two limbs in both joint-based and extrinsic coordinates. These findings reinforce the view that multiple coordinates systems contribute to the internal representation of motor learning, and “true” transfer of the learned remapping depends on the on spatial compatibility of new sensorimotor maps for both limbs.
GRANTS
E. Poh was supported by the University of Queensland Graduate School International Travel Award. J. A. Taylor was supported by National Institute of Neurological Disorders and Stroke Grant R01NS084948 and the Princeton Neuroscience Institute's Innovation Fund. T. J. Carroll was supported by Australian Research Council Future Fellowships Grant FT120100391.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.P., T.J.C., and J.A.T. conception and design of research; E.P. performed experiments; E.P., T.J.C., and J.A.T. analyzed data; E.P., T.J.C., and J.A.T. interpreted results of experiments; E.P. prepared figures; E.P. drafted manuscript; E.P., T.J.C., and J.A.T. edited and revised manuscript; E.P., T.J.C., and J.A.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Richard Ivry for insightful discussions and Krista Bond for assistance in the experiment setup.
REFERENCES
- Balitsky Thompson AK, Henriques DY. Visuomotor adaptation and intermanual transfer under different viewing conditions. Exp Brain Res 202: 543–552, 2010. [DOI] [PubMed] [Google Scholar]
- Benson BL, Anguera JA, Seidler RD. A spatial explicit strategy reduces error but interferes with sensorimotor adaptation. J Neurophysiol 105: 2843–2851, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berniker M, Franklin DW, Flanagan JR, Wolpert DM, Kording K. Motor learning of novel dynamics is not represented in a single global coordinate system: evaluation of mixed coordinate representations and local learning. J Neurophysiol 111: 1165–1182, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block H, Celnik P. Stimulating the cerebellum affects visuomotor adaptation but not intermanual transfer of learning. Cerebellum 12: 781–793, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond KM, Taylor JA. Flexible explicit but rigid implicit learning in a visuomotor adaptation task. J Neurophysiol 113: 3836–3849, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997. [PubMed] [Google Scholar]
- Brayanov JB, Press DZ, Smith MA. Motor memory is encoded as a gain-field combination of intrinsic and extrinsic action representations. J Neurosci 32: 14951–14965, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buneo CA, Andersen RA. The posterior parietal cortex: sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia 44: 2594–2606, 2006. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, de Rugy A, Howard IS, Ingram JN, Wolpert DM. Enhanced crosslimb transfer of force-field learning for dynamics that are identical in extrinsic and joint-based coordinates for both limbs. J Neurophysiol 115: 445–456, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll TJ, Poh E, de Rugy A. New visuomotor maps are immediately available to the opposite limb. J Neurophysiol 111: 2232–2243, 2014. [DOI] [PubMed] [Google Scholar]
- de Rugy A, Davoodi R, Carroll TJ. Changes in wrist muscle activity with forearm posture: implications for the study of sensorimotor transformations. J Neurophysiol 108: 2884–2895, 2012. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, White O, Newman D, Lally N. Use-dependent and error-based learning of motor behaviors. J Neurosci 30: 5159–5166, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haith AM, Huberdeau DM, Krakauer JW. The influence of movement preparation time on the expression of visuomotor learning and savings. J Neurosci 35: 5109–5117, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang VS, Haith A, Mazzoni P, Krakauer JW. Rethinking motor learning and savings in adaptation paradigms: model-free memory for successful actions combines with internal models. Neuron 70: 787–801, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberdeau DM, Haith AM, Krakauer JW. Formation of a long-term memory for visuomotor adaptation following only a few trials of practice. J Neurophysiol 114: 969–977, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa J, Shadmehr R. Learning from sensory and reward prediction errors during motor adaptation. PLoS Comput Biol 7: e1002012, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakei S, Hoffman DS, Strick PL. Muscle and movement representations in the primary motor cortex. Science 285: 2136–2139, 1999. [DOI] [PubMed] [Google Scholar]
- Kitago T, Ryan SL, Mazzoni P, Krakauer JW, Haith AM. Unlearning versus savings in visuomotor adaptation: comparing effects of washout, passage of time, and removal of errors on motor memory. Front Hum Neurosci 7: 307, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefumat HZ, Vercher JL, Miall RC, Cole J, Buloup F, Bringoux L, Bourdin C, Sarlegna FR. To transfer or not to transfer? Kinematics and laterality quotient predict interlimb transfer of motor learning. J Neurophysiol 114: 2764–2774, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26: 3642–3645, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle SD, Bond KM, Taylor JA. Explicit and implicit processes constitute the fast and slow processes of sensorimotor learning. J Neurosci 35: 9568–9579, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morehead JR, Qasim SE, Crossley MJ, Ivry R. Savings upon re-aiming in visuomotor adaptation. J Neurosci 35: 14386–14396, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Sabes PN. Sensory integration for reaching: models of optimality in the context of behavior and the underlying neural circuits. Prog Brain Res 191: 195–209, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Wang J. Interlimb transfer of visuomotor rotations: independence of direction and final position information. Exp Brain Res 145: 437–447, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R. Generalization as a behavioral window to the neural mechanisms of learning internal models. Hum Mov Sci 23: 543–568, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Hieber LL, Ivry RB. Feedback-dependent generalization. J Neurophysiol 109: 202–215, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Ivry RB. Flexible cognitive strategies during motor learning. PLoS Comput Biol 7: e1001096, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Krakauer JW, Ivry RB. Explicit and implicit contributions to learning in a sensorimotor adaptation task. J Neurosci 34: 3023–3032, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Wojaczynski GJ, Ivry RB. Trial-by-trial analysis of intermanual transfer during visuomotor adaptation. J Neurophysiol 106: 3157–3172, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. A dissociation between visual and motor workspace inhibits generalization of visuomotor adaptation across the limbs. Exp Brain Res 187: 483–490, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Joshi M, Lei Y. The extent of interlimb transfer following adaptation to a novel visuomotor condition does not depend on awareness of the condition. J Neurophysiol 106: 259–264, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lei Y, Binder JR. Performing a reaching task with one arm while adapting to a visuomotor rotation with the other can lead to complete transfer of motor learning across the arms. J Neurophysiol 113: 2302–2308, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Interlimb transfer of visuomotor rotations depends on handedness. Exp Brain Res 175: 223–230, 2006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Limitations in interlimb transfer of visuomotor rotations. Exp Brain Res 155: 1–8, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Mechanisms underlying interlimb transfer of visuomotor rotations. Exp Brain Res 149: 520–526, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. The symmetry of interlimb transfer depends on workspace locations. Exp Brain Res 170: 464–471, 2006b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HG, Miyamoto YR, Gonzalez Castro LN, Olveczky BP, Smith MA. Temporal structure of motor variability is dynamically regulated and predicts motor learning ability. Nat Neurosci 17: 312–321, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]