Abstract
Data is presented showing expression of non-conventional (NC) heavy chain forms of B27 in synovial tissues from SpA patients. Data is presented showing the expression patterns of NC-B27 in joint, gastrointestinal and lymphoid tissues from B27 transgenic (TG1) rats with M. tuberculosis-induced SpA. Expression of NC-B27 was determined by immunohistochemistry and flow cytometry using HC10 and HD6 antibodies. These data are the extension of the data presented and discussed in “Non-conventional forms of HLA-B27 are expressed in Spondyloarthritis joints and gut tissue” (O. Rysnik, K. McHugh, L. M. van Duivenvoorde, M. N. van Tok, G. Guggino, J. D. Taurog, S. Kollnberger, F. Ciccia, D. L. Baeten, P. Bowness, 2016) [1].
Keywords: HLA class I free-heavy chains, HLA-B27, HLA-B27 transgenic rat model, Spondyloarthropathies
Specifications Table
| Subject area | Biology |
| More specific subject area | Human and rat spondyloarthritis |
| Type of data | Figures |
| How data was acquired | Histology -AperioCS2 Scanner (Leica Biosystem) Flow cytometry - BD FACS Canto |
| Data format | Analyzed |
| Experimental factors | Human and rat tissue |
| Experimental features | Antibody staining documented by histology and FACS |
| Data source location | Oxford UK |
| Data accessibility | Data is with this article |
Value of the data
-
•
Data presented in this article confirm the role of NC-B27 in SpA pathogenesis in both human and transgenic rats.
-
•
This data serves as a benchmark for future studies on the pathogenic role of NC-B27 in SpA.
-
•
The data is valuable for future studies on development of novel treatment strategies for SpA.
1. Data
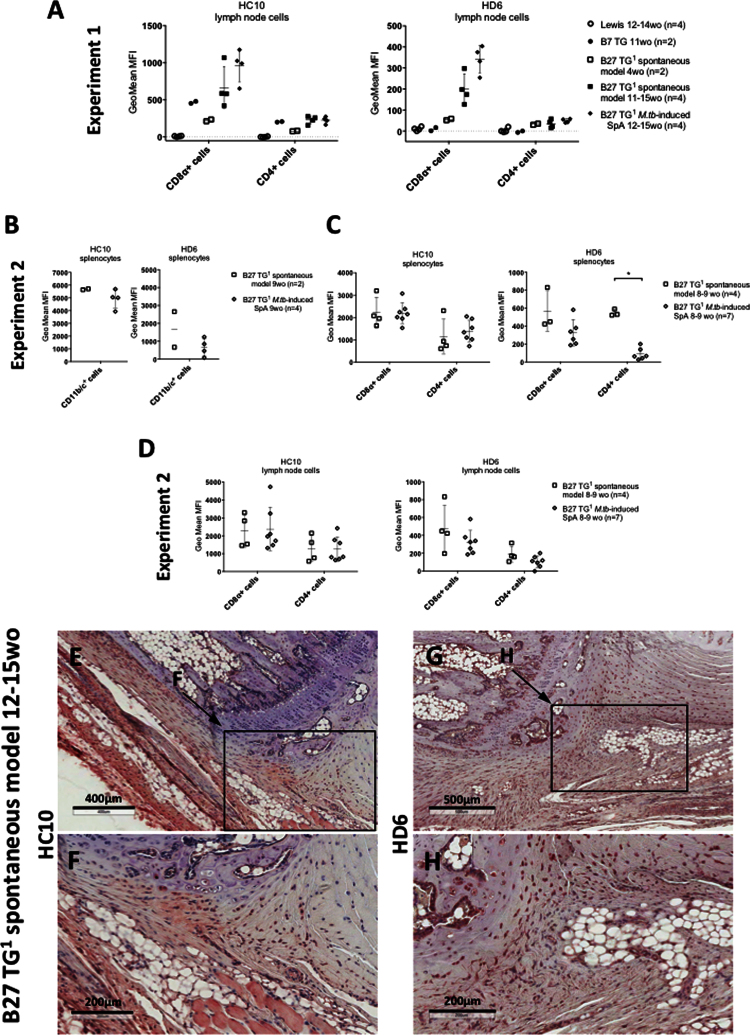
The immunohistochemistry data show expression of NC-B27 forms (HC10 and HD6 staining) in synovial tissues from B27+ve SpA patients (Fig. 1), and in joint and gastrointestinal tissues from B27 TG1 rats with M.tb-induced SpA and in healthy WT and B7 TG controls (Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7). The flow cytometry data describe and quantify the expression of HC10- and HD6-reactive NC-B27 molecules in spleens and lymph nodes from B27 TG1 rats in a spontaneous and M.tb-induced SpA before and after disease onset (Fig. 8, Fig. 9, Fig. 10).
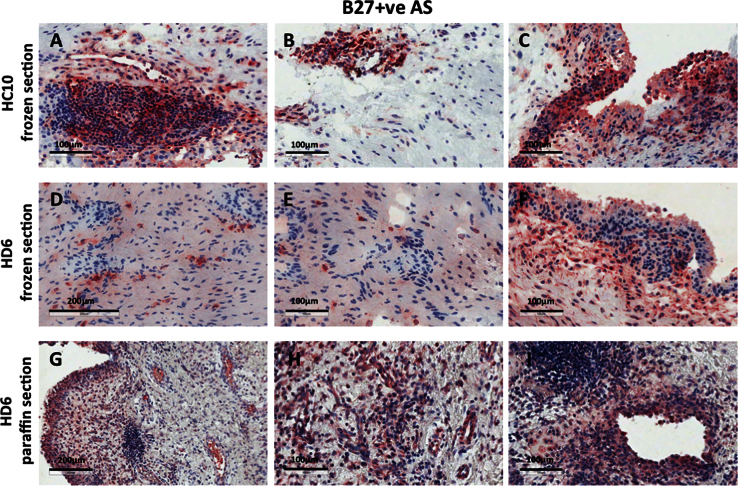
Fig. 1.
shows HC10 and HD6 staining of both frozen and paraffin-fixed synovial tissues from patients with HLA-B27-positive Spondyloarthritis (SpA).
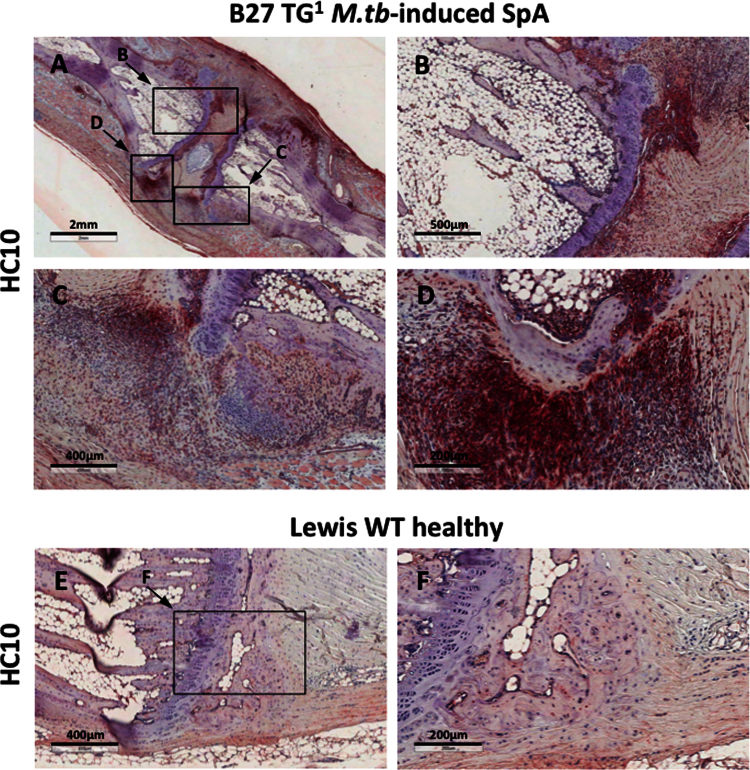
Fig. 2.
(A–D) shows HC10 staining of axial joints from B27 TG1 rats with M.tb-induced SpA. Staining was observed particularly in cell infiltrates at the junction between the vertebrae, connective tissue and annulus fibrosus. We did not observe HC10 staining in ankle or tail joints from healthy Lewis WT rats (Fig. 2E and F).
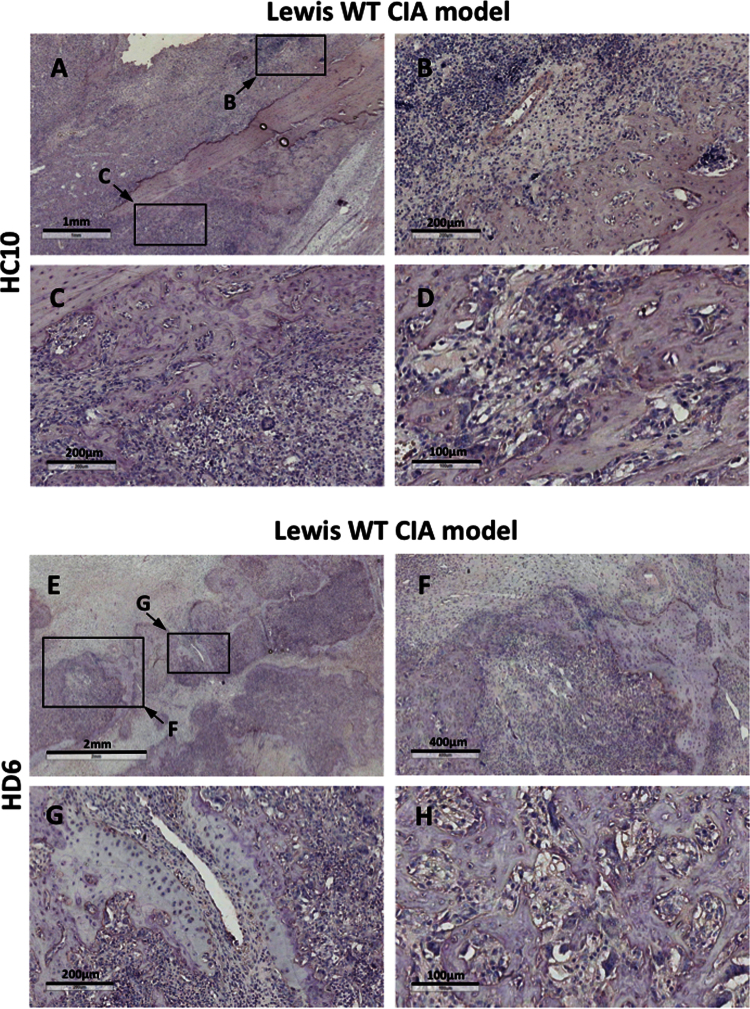
Fig. 3.
We did not observe HC10 or HD6 staining in ankle joints from Lewis WT rats with adjuvant-induced arthritis (AIA).
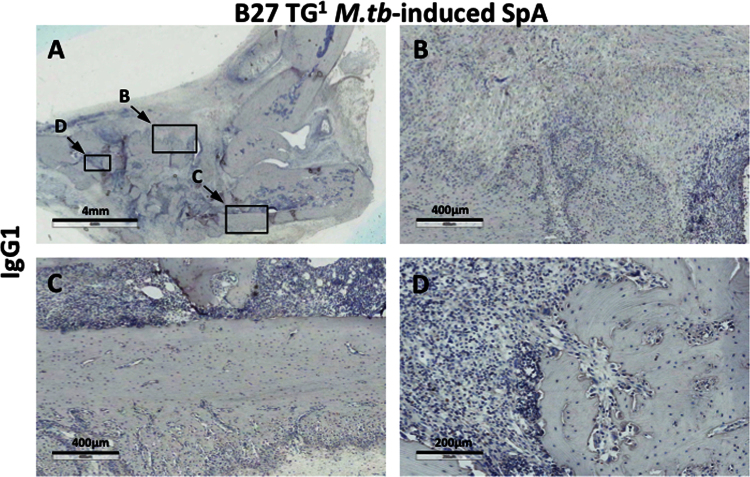
Fig. 4.
Shows that tissue sections from B27 TG1 rats with M.tb-induced arthritis and spondylitis did not stain with IgG1 isotype control antibody.
Fig. 5.
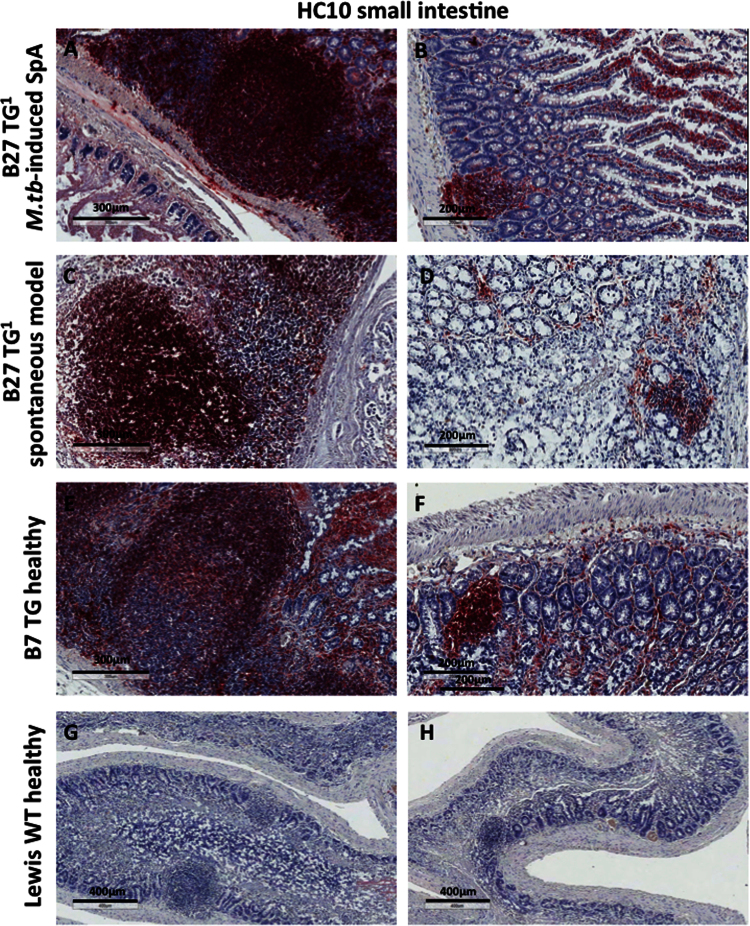
Shows HC10 staining was detectable on mononuclear cells in small intestinal Peyer׳s patches, in lymphoid follicles and in the lamina propria of all transgenic animals. Staining levels were higher for B27 TG1 rats with M.tb-induced arthritis and spondylitis compared to those without M.tb or healthy B7 TG animals.
Fig. 6.
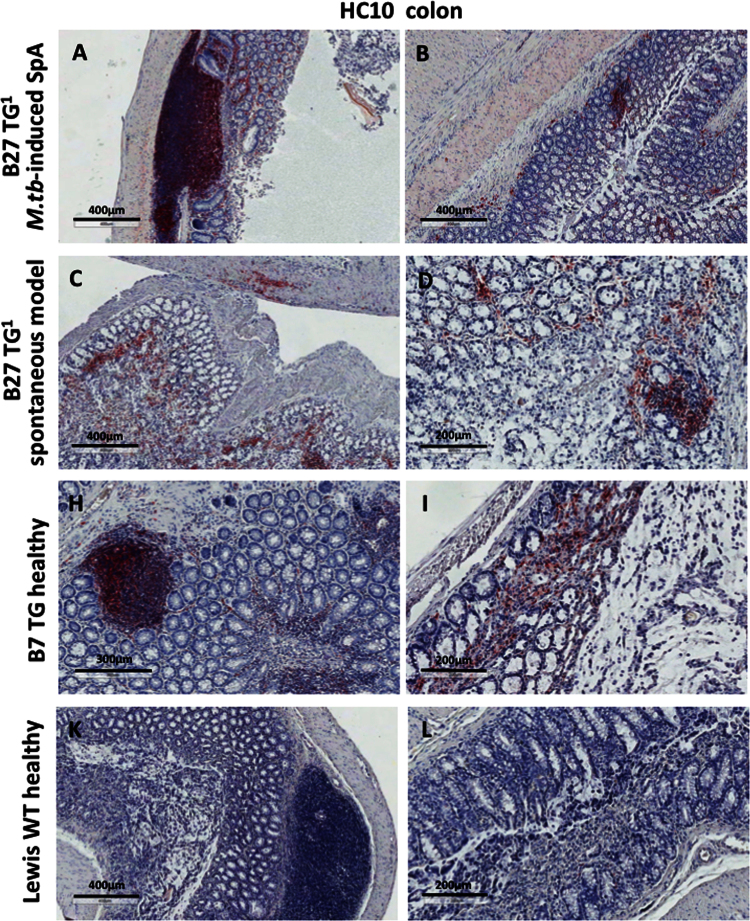
Specific HC10 staining was also seen in the colon.
Fig. 7.
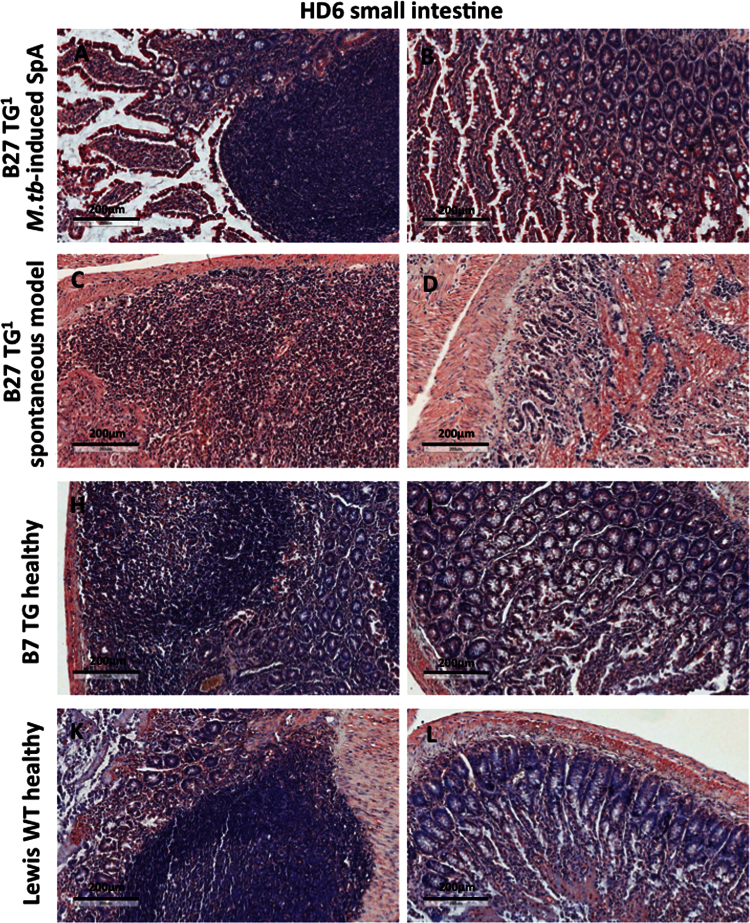
HD6 stained small and large bowel tissues, although with background staining observed (Fig. 7 and data not shown). These data show that NC-B27 are expressed in gut tissue in B27 TG1 rats.
Fig. 8.
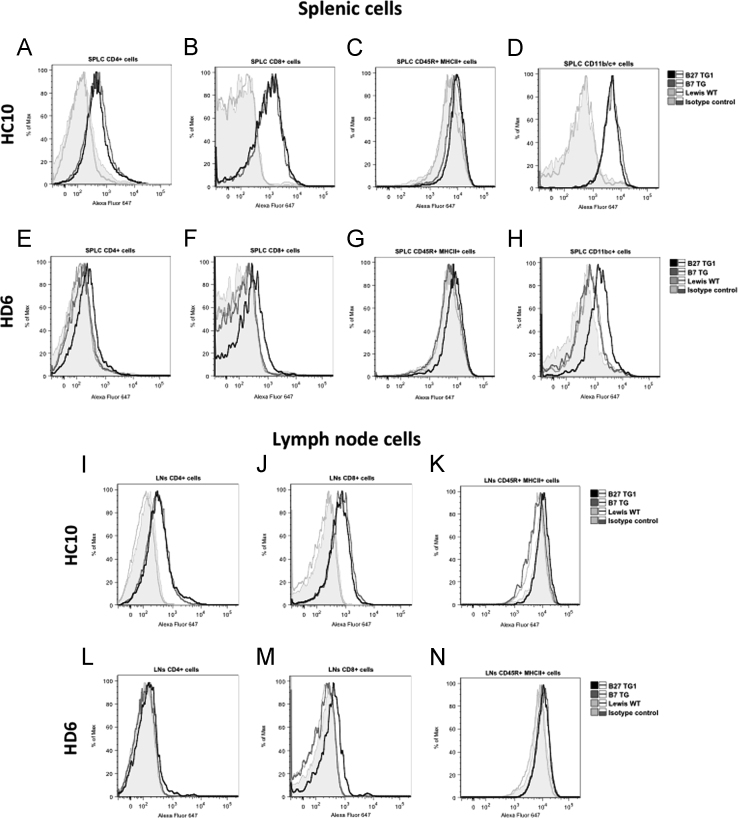
Shows that splenic and lymph node CD45+/MHCII+ leukocytes from B27 TG1 rats with M.tb-induced arthritis and spondylitis expressed very low levels of NC-B27 molecules.
Fig. 9.
Similar results were observed with cells isolated from B27 TG1 lymph nodes +/− M.tb (Fig. 9A), noting that the CD11b/c+ cell population was absent (see Fig. 10J). We also investigated HC10 and HD6 staining of splenocytes taken from 8–9 weeks old B27 TG1 animals with and without M.tb-induced SpA before the appearance of clinical manifestations. HC10 was not significantly altered in splenic CD11b/c+, CD8α+ or CD4+ cells, or on cell populations from LNs (Fig. 9B–D). However, we observed an increase in HD6 staining on splenic CD4+ cells after M.tb treatment (Fig. 9C right-hand panel). No HC10 or HD6 staining was observed in splenic and LN cells from age-matched Lewis WT rats (data not shown). Splenic and LN cells from age-matched B7 TG rats stained with HC10, but not HD6, to a similar degree compared with B27 TG1 animals (spontaneous model) (Fig. 9A).
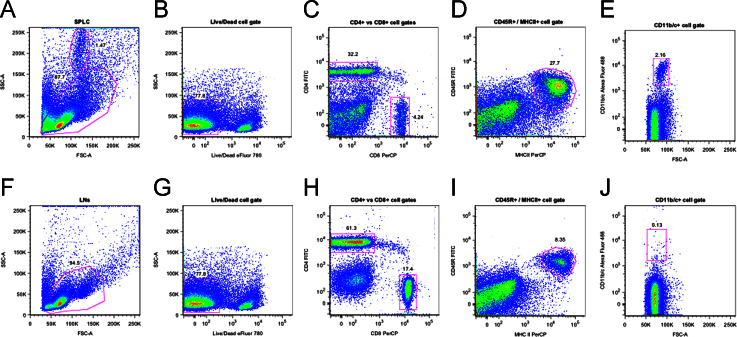
Fig. 10.
Shows the gating strategy for analysis of spleen and lymph node cell populations studied by FACS.
2. Experimental design, materials and methods
2.1. Patients
Human synovial tissue samples were obtained with informed consent and appropriate ethical permission, from B27+ SpA patients, including 1 with Ankylosing Spondylitis (AS) fulfilling the New York classification criteria [2], and patients with Rheumatoid Arthritis (RA) fulfilling the EULAR/ACR criteria [3].
2.2. Rat-derived cells and tissues
B27 transgenic (TG) rats first generated by Hammer and colleagues spontaneously develop inflammatory gut and joint disease [4]. More recently additional human β2m was introduced, i.e. (21-3×283-2) F1 HLA-B27/Huβ2m [5]. We term this model, studied here, as B27 TG1. A higher proportion of these B27 TG1 male rats spontaneously develop arthritis (~70%, 4–6 months of age) and spondylitis (30–50%, 7–9 months of age) without symptoms of gut inflammation [5], [6], [7]. Early and coordinated onset of these SpA-like disease manifestations can be triggered by immunization with low doses of M. tuberculosis (hereafter referred to as “M.tb-induced arthritis and spondylitis”) [8], [9]. Splenocytes, lymph node cells (LNs), ankle, tail joints and GI tissues were isolated from B27 TG1 rats with spontaneous or induced SpA at age 4–15 weeks. For M.tb-induced arthritis and spondylitis [8], 6 week-old B27 TG1 rats were immunized with 30–45 μg of heat-inactivated M.tb in incomplete Freund׳s adjuvant [8], [9]. (120-4×283-2)F1 HLA-B7/Huβ2m TG (B7 TG) and Lewis wild type (WT) animals +/− 200 μg of heat-inactivated M.tb in IFA (adjuvant-induced arthritis, AIA model) were used as controls. All animals were bred and housed at the animal facility of the AMC, University of Amsterdam, Netherlands. All animal procedures were carried out in compliance with Institutional Standards for Human Care and Use of Laboratory Animals.
2.3. Antibodies
The HC10 antibody stains many or all heavy chain forms (but not beta-2-microglobulin-associated conventional forms) of most human HLA-B and some HLA-A alleles, but does not cross react with rat MHC [10]. HC10 stains HLA-B27 free heavy chains (FHC) including dimers [10], [11]. The HD6 antibody was raised against B27 homodimers using a fully human FAb antibody library (kindly provided by Dynax, MA, USA) as previously described [11], [12], and is more specific for heavy chain forms of HLA-B27. HD6r (same specificity as HD6 but with rat IgG1 Fc region) was used for some stains.
2.4. Immunohistochemistry of human and rat tissue samples
Human SpA and RA, and rat paraffin-embedded synovial tissue samples were prepared as previously described [6], [13], [14]. Paraffin-embedded tissue sections were blocked using Peroxidase Blocking Reagent (EnVision™, Dako), than incubated with PBS/1%FBS/10% goat serum and subsequently stained overnight with HC10 or HD6 primary mAb. HC10-stained sections were incubated with HRP-labeled anti-mouse IgG (EnVision™, Dako). HD6-stained sections were incubated with biotinylated goat anti-mouse IgG1 (Southern Biotech) followed by streptavidin-HRP (Dako). Tissue sections were than incubated with AEC+ substrate-chromogen (EnVision™, Dako) and counterstained using Mayer׳s hematoxylin. Slides were visualized using an LSM Zeiss confocal microscope, scanned using AperioCS2 Scanner and analyzed using Aperio ImageScope software (Leica Biosystems, UK).
2.5. Flow cytometry
Splenocytes and LNs were freshly isolated and immediately stained as described previously [15]. Cells were incubated in blocking buffer, and then stained with primary antibody (HC10, HD6, ME1 or IgG1/IgG2a), followed by incubation with secondary goat anti-mouse antibody (Alexa Fluor 647, Invitrogen). Subsequently, cells were stained for the phenotypic surface markers: CD4 and CD8α or CD45R and MHCII, or CD11b/c. Dead cells were excluded using fixable viability dye eFluor®780 (eBioscience). Flow cytometric analysis was performed with BD FACS Canto and data were analyzed using FlowJo Software (TreeStar). Staining was performed in triplicates. Error bars were calculated based on SD mean of the values if 3≥ animals per group. P values were determined using nonparametric Mann–Whitney test.
Funding
OR was supported by Arthritis Research UK, United Kingdom Grant no. 19,611, and by an EMBO travel award. This work was supported by the Oxford National Institute of Health Research (NIHR) Biomedical Research Center, the Oxford NIHR Biomedical Research Unit (PB).
Acknowledgments
We thank Dr Hidde Ploegh (Massachusetts Institute of Technology, MA, USA) for the HC10 antibody.
Footnotes
Transparency data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.dib.2016.08.046.
Transparency document. Supplementary material
Supplementary material
.
References
- 1.Rysnik O., McHugh K., van Duivenvoorde L.M., van Tok M.N., Guggino G., Taurog J.D., Kollnberger S., Ciccia F., Baeten D.L., Bowness P. Non-conventional forms of HLA-B27 are expressed in Spondyloarthritis joints and gut tissue. J. Autoimmun. 2016 doi: 10.1016/j.jaut.2016.03.009. Mar 29. pii: S0896-8411(16)30022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Linden S., Valkenburg Ha, Cats a. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheumatol. 1984;27(4):361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 3.Aletaha D., Neogi T., Silman A.J., Funovits J., Felson D.T., Bingham C.O., Birnbaum N.S., Burmester G.R., Bykerk V.P., Cohen M.D., Combe B., Costenbader K.H., Dougados M., Emery P., Ferraccioli G., Hazes J.M.W., Hobbs K., Huizinga T.W.J., Kavanaugh A., Kay J., Kvien T.K., Laing T., Mease P., Ménard Ha, Moreland L.W., Naden R.L., Pincus T., Smolen J.S., Stanislawska-Biernat E., Symmons D., Tak P.P., Upchurch K.S., Vencovský J.J., Wolfe F., Hawker G. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 4.Hammer R.E., Maika S.D., Richardson J.A., Tang J.P., Taurog J.D. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: an animal model of HLA-B27-associated human disorders. Cell. 1990;63:1099–1112. doi: 10.1016/0092-8674(90)90512-d. [DOI] [PubMed] [Google Scholar]
- 5.Tran T.M., Dorris M.L., Satumtira N., Richardson J.A., Hammer R.E., Shang J., Taurog J.D. Additional human beta2-microglobulin curbs HLA-B27 misfolding and promotes arthritis and spondylitis without colitis in male HLA-B27-transgenic rats. Arthritis Rheumatol. 2006;54(4):1317–1327. doi: 10.1002/art.21740. Apr. [DOI] [PubMed] [Google Scholar]
- 6.van Duivenvoorde L.M., Dorris M.L., Satumtira N., van Tok M.N., Redlich K., Tak P.P., Taurog J.D., Baeten D.L. Relationship between inflammation, bone destruction, and osteoproliferation in the HLA-B27/human β2 -microglobulin-transgenic rat model of spondylarthritis. Arthritis Rheumatol. 2012;64(10):3210–3219. doi: 10.1002/art.34600. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taurog J.D., Rival C., van Duivenvoorde L.M., Satumtira N., Dorris M.L., Sun M., Shelton J.M., Richardson J.A., Hamra F.K., Hammer R.E., Tung K.S.K. Autoimmune epididymoorchitis is essential to the pathogenesis of male-specific spondylarthritis in HLA-B27-transgenic rats. Arthritis Rheumatol. 2012;64(8):2518–2528. doi: 10.1002/art.34480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vieira-Sousa E., van Duivenvoorde L.M., Fonseca J.E., Lories R.J., Baeten D.L. Animal models as a tool to dissect pivotal pathways driving spondyloarthritis. Arthritis Rheumatol. 2015 doi: 10.1002/art.39282. p. n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 9.van Duivenvoorde L.M., Slobodin G.M., Satumitira N., Dorris M.L., Tak P.P., Baeten D.L., Taurog J.D. Innate immune stimulation triggers early-onset spondyloarthritis in HLA-B27/human beta2 microglobulin transgenic rats. Arthritis Rheumatol. 2011;63(S387) [Google Scholar]
- 10.Fleur Sernee M., Ploegh H.L., Schust D.J. Why certain antibodies cross-react with HLA-A and HLA-G: epitope mapping of two common MHC class I reagents. Mol. Immunol. 1998;35(3):177–188. doi: 10.1016/s0161-5890(98)00026-1. [DOI] [PubMed] [Google Scholar]
- 11.Payeli S.K., Kollnberger S., Belaunzaran O.M., Thiel M., Mchugh K., Giles J., Shaw J., Kleber S., Ridley A., Wong-baeza I., Keidel S., Kuroki K., Maenaka K., Wadle A., Renner C., Bowness P. Inhibiting HLA – B27 homodimer – driven Immune cell inflammation in spondylarthritis. Arthritis Rheumatol. 2012;64(10):3139–3149. doi: 10.1002/art.34538. [DOI] [PubMed] [Google Scholar]
- 12.Iuraşcu M., Marroquin Belaunzaran O., Cozma C., Petraush U., Renner C., Przybylski M. An HLA-B27 homodimer specific antibody recognises a discontinious mixed-disulfide epitope as identified by affinity-mass spectometry. J. Am. Soc. Mass Spectrom. 2016 doi: 10.1007/s13361-016-1361-9. vol. in press. [DOI] [PubMed] [Google Scholar]
- 13.Lories R.J., Baeten D.L.P. Differences in pathophysiology between rheumatoid arthritis and ankylosing spondylitis. Clin. Exp. Rheumatol. 2009;27(Suppl.):S10–S14. [PubMed] [Google Scholar]
- 14.Baeten D., Kruithof E., De Rycke L., Boots A.M., Mielants H., Veys E.M., De Keyser F. Infiltration of the synovial membrane with macrophage subsets and polymorphonuclear cells reflects global disease activity in spondyloarthropathy. Arthritis Res. Ther. 2005;7(2):R359–R369. doi: 10.1186/ar1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McHugh K., Rysnik O., Kollnberger S., Shaw J., Utriainen L., Al-Mossawi M.H., Payeli S., Marroquin O., Milling S., Renner C., Bowness P. Expression of aberrant HLA-B27 molecules is dependent on B27 dosage and peptide supply. Ann. Rheum. Dis. 2014;73(4):763–770. doi: 10.1136/annrheumdis-2012-203080. Apr. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material