Highlights
-
•
No general treatment guidelines have been established for gastricplasmacytoma.
-
•
Combination therapy with chemotherapy involving bortezomib and autologous peripheral blood stem-cell transplantation after the resection could be one of the useful options for the advanced gastricplasmacytoma.
Abbreviations: Auto-PBSCT, autologous peripheral blood stem-cell transplantation; CT, computed tomography; EMP, extramedullary plasmacytoma; ESD, endoscopic submucosal dissection; FDG, fludeoxyglucose (18F); PET-CT, positron emission tomography-CT; VCD, bortezomib, cyclophosphamide, and dexamethasone; VD, bortezomib and dexamethasone
Keywords: Plasmacytoma, Stomach, Surgical resection, Chemotherapy
Abstract
Introduction
Extramedullary plasmacytoma (EMP) is a plasma cell neoplasm that presents as a solitary tumor. EMP in the gastrointestinal organs are extremely uncommon.
Presentation of case
A 36-year-old man was admitted to our hospital with advanced anemia. He had no specific medical history. Gastroendoscopic findings showed an 8.0-cm submucosal tumor with ulcer on the greater curvature of the gastric body. Fine-needle aspiration was performed, and the pathologic diagnosis of the submucosal tumor was a plasmacytoma. Therefore, the patient was diagnosed with gastric plasmacytoma. A total gastrectomy was performed with lymphadenectomy. The result of intraoperative peritoneal lavage cytology was positive. Histological examination revealed serosa-exposed plasmacytoma of the stomach with lymph nodes metastasis. Additionaly the patient received a three-drug chemotherapy regimen (bortezomib, cyclophosphamide, and dexamethasone [VCD]) from 3 weeks after the operation. After 4 cycles of chemotherapy, the patient received autologous peripheral blood stem-cell transplantation (auto-PBSCT). Eighteen months after diagnosis, the patient is in complete remission with no evidence of local relapse or evolution to multiple myeloma.
Conclusions
This is the first reported case of advanced gastric plasmacytoma using adjuvant chemotherapy involving bortezomib and auto-PBSCT after the resection, and the patient has maintained a good course over a year. This protocol could be a new way to treat these tumors.
1. Introduction
Extramedullary plasmacytoma (EMP) is a rare disease and is histopathologically characterized by infiltrates of plasma cells of diverse maturity and by their monoclonal immunoglobulin products [1]. The disease occurs almost exclusively in the head, neck, and upper respiratory tract. EMPs in the gastrointestinal organs are uncommon [2]. The next most frequent site of lesion occurrence is the stomach; however, this is also extremely rare, accounting for less than 5% of all EMPs [3]. Although plasmacytoma is rare and few cases have been reported before, the adjuvant treatment lacks definitive guidelines. In such a situation we attempted with this unique combination therapy and have achieved satisfactory result. This is the first reported case of gastric plasmacytoma treated by combination chemotherapy (bortezomib, cyclophosphamide, and dexamethasone [VCD]), and autologous peripheral blood stem-cell transplantation (auto-PBSCT). Therefore we are presenting this case highlighting a new way to treat these tumors.
2. Presentation of case
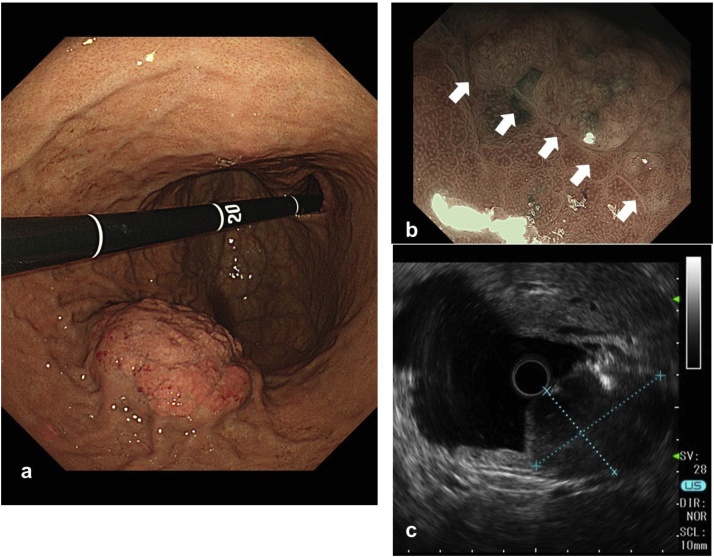
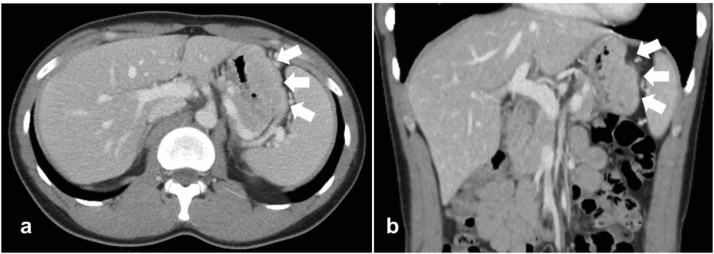
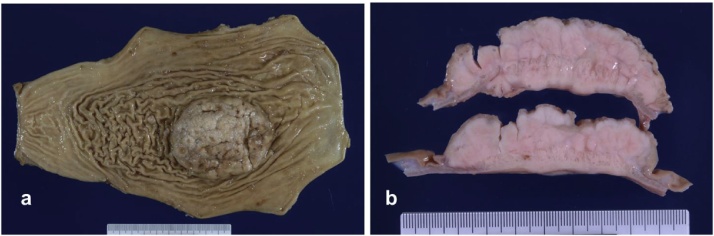
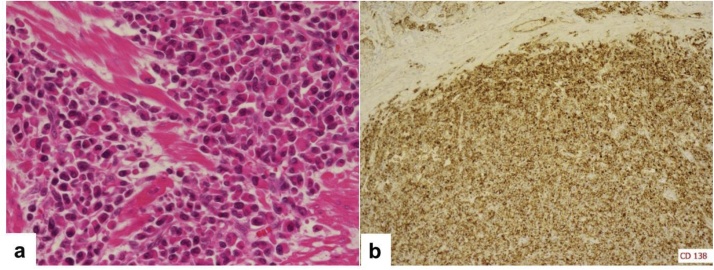
A 36-year-old man with dyspnea and general fatigue visited a local doctor. Blood examination revealed advanced anemia, and he was referred to our hospital for a detailed examination. The results of laboratory examinations were as follows: white blood cell count, 5150/mm3 (normal range 4500–9000); red blood cell count, 234 × 104/mm3 (normal range 435–555); hemoglobin level, 5.7 g/dL (normal range 13.6–17.0); hematocrit, 20.6% (normal range 40.7–50.1); platelet count, 26.8 × 104/mm3 (normal range 14.0–36.0); serum blood urea nitrogen level, 25.0 mg/dL (normal range 8–20); serum creatinine level, 0.73 mg/dL (normal range 0.5–1.2); serum alkaline phosphatase level, 123 U/L (normal range 100–340); serum calcium level, 9.2 mg/dL (normal range 8.2–10.2); and serum Fe level, 14.2 μg/dL (normal range 54–181). The levels of tumor markers were within the normal ranges (carcinoembryonic antigen 1.0 ng/mL and CA 19–9 2.0 U/mL). The patient had no history of serious illness, operations, or hospitalizations. Gastrointestinal endoscopy showed an 8.0-cm submucosal tumor with ulcer on the greater curvature of the gastric body (Fig. 1a). Magnifying narrow-band imaging endoscopy revealed the abnormal mucosal microstructure in the discolored protrusion (Fig. 1b). Examination by color Doppler endoscopic ultrasonography showed the hypoechoic mass with hypervascularity arising from the submucosal layer (Fig. 1c). Fine-needle aspiration was performed, and the pathologic diagnosis of the submucosal tumor was a possible plasmacytoma of the stomach. Abdominal computed tomography (CT) revealed focal wall thickening with hyperenhancement on the greater curvature and no sign of any lymph node swelling (Fig. 2a, b). We did not observe any accumulation of fludeoxyglucose uptake in positron emission tomography (PET)-CT, even at the lesion site of the main stomach tumor. Bone marrow puncture and M protein identification for the evaluation of multiple myeloma were normal. Testing for urinary Bence Jones protein was negative. The patient was diagnosed with primary gastric plasmacytoma, and we performed a total gastrectomy with extended removal of regional lymph nodes (D2) specified in the Japanese classification of gastric cancer [4]. The result of intraoperative peritoneal lavage cytology was positive. Histological examination revealed serosa-exposed plasmacytoma of the stomach with 4 out of 15 lymph nodes metastasis. (Fig. 3a, b). The immunohistological findings showed that the tumor cells were negative for CD20, CD79a, CD3, and cyclin D1, and positive for CD138 (Fig. 4a, b). The Ki-67 labeling index was high. The surface immunoglobulin was the IgA-κ type. The patient received combination therapy with a three-drug chemotherapy (VCD) 3 weeks after the operation. Bortezomib (1.3 mg/m2; days 1, 4, 8, and 11), cyclophosphamide (1.5 g/m2; days 1–14), and dexamethasone (40 mg/day; days 1, 4, 8, and 11) were given in each cycle. After 4 cycles of the three-drug chemotherapy, the patient received high-dose melphalan (140 mg/m2) followed by auto-PBSCT. Eighteen months after diagnosis, the patient is in complete remission with no evidence of local relapse or evolution to multiple myeloma.
Fig. 1.
(a) Endoscopy showed an 8.0-cm protuberant lesion with ulcer on the greater curvature of the gastric body. (b) Magnifying narrow-band imaging endoscopy revealed the abnormal mucosal microstructure in the discolored protrusion (white arrows). (c) Endoscopic ultrasonography showed a hypoechoic mass deriving from the submucosal layer.
Fig. 2.
(a, b) Axial and coronal images of CT showed focal wall thickening with hyperenhancement on the greater curvature of the stomach (white arrows).
Fig. 3.
(a, b) The resected tumor was 8.0 × 6.5 cm in size. The tumor was ash white and elastic hard with the presence of a seroma component.
Fig. 4.
(a) Microscopic examination (hematoxylin-eosin staining, original magnification × 400) revealed numerous plasma cells infiltrating the serosa of the stomach. (b) Immunohistochemical staining of the tumor cells for CD138 showed positive results.
3. Discussion
Plasma cell neoplasms are categorized into four groups; multiple myeloma, plasma cell leukemias, solitary plasmacytomas of the bone, and EMP [3]. The diagnosis of EMP requires demonstration of a histologically evidence of tumor in the bone marrow. In this case, the patient had a biopsy-proven extramedullary plasma cell tumor with lymph node involvement and a monoclonal band on serum protein electrophoresis of IgA. Bone marrow biopsy revealed less than 5% of plasma cells. No bone lesions were found. Therefore, the diagnosis was EMP of the stomach.
All segments of the gastrointestinal tract may be involved by EMPs, with the small intestine being the most common, followed by the stomach, colon, and esophagus. Gastric plasmacytomas are a very rare form of EMP. Gastric tumors account for 2–5% of all EMPs, and tend to be identified at a late stage if an endoscopic examination is not performed [5]. Almost all patients with EMPs are treated with radiation therapy, surgery, or combination therapy. However, no general treatment guidelines have been established for gastric EMPs. Additionally, the invasiveness of these initial therapeutic approaches precludes their recommendation for EMPs of the head and neck [6].
In this case, we performed a total gastrectomy with D2 lymphadenectomy under the diagnosis of primary solid plasmacytoma of the stomach. The pathological diagnosis was serosa-exposed plasmacytoma (8.0 × 6.5 cm) of the stomach with multiple lymph nodes metastasis. The tumor was large, with lymph nodes metastasis and presence of a serosa component, accompanying a positive cytodiagnosis of ascites. In solitary plasmacytomas treated with radiation therapy, large tumor bulk (≥50 mm) indicates a more progressive course and suggests a poor prognosis [7]. To our knowledge, there is no report in English about the relation between tumor progression and overall survival rate. However, one report in Japanese from Kawata et al. [8] summarized 71 cases of primary solid plasmacytoma of the stomach in Japan. The report detailed disease progression in 43 patients, 15 of whom had died by the time of publication. The invasion depth of the 15 cases had been pT4 (SE), indicating that patients with gastric plasmacytoma with invasion to the serosa have a markedly poorer prognosis than the others. Most of the cases reported in the literature are early stage of gastric plasmacytom, and these cases have been treated with endscopic submucosal dissection [9], [10], [11]. Until now, there has been few evidence that additional chemotherapy is beneficial. Wiltshaw et al. [12], [13] reported on the effectiveness of additional chemotherapy with melphalan, prednisone, cyclophosphamide, or vincristine alone or in combination with complete remission rates of 50–88%. Preud'Homme et al. reported that 20-year-old female of huge gastric plasmacytoma had been treated with chemotherapy (Rubidazone, procarbazine, and vinblastine) for two years [14]. The patient had been free of metastasis for three years after the finishing chemotherapy. Katodritou et al. reported that 68-year-old male with 5-cm gastric plasmacytoma had been treated with 4 cycle of chemotherapy (Bortezomib and dexamethasone) [15]. Thirteen months after the diagnosis the patient had been in complete remission with no evidence of local relapse or evolution to multiple myeloma. Zhao et al. reported that 79-year-old male with 16-cm gastric plasmacytoma infiltrating the lamina propria received surgery consisted of total gastrectomy with a subtotal pancreatectomy, splenectomy, and oesophageal and jejunum Roux-en-Y anastomosis [16]. There was no indication of recurrence or metastasis at eight momths after the operation. Some EMP cases are known to develop multiple myeloma. After treatment of EMP in non-upper aerodigestive regions, 21.2% of patients had recurrence and 14.1% of them converted to multiple myeloma [3]. High-dose chemotherapy (bortezomib) with auto-PBSCT is a standard treatment for young patients with multiple myeloma. The condition typically follows a relapsing course, and many patients require multiple lines of therapy. In this case, this patient was so young and the tumor was large, with lymph nodes metastasis and presence of a serosa component, accompanying a positive cytodiagnosis of ascites which indicated high risk to develop multiple myeloma. From the above reasons, we attempted with high-dose chemotherapy including bortezomib and auto-PBSCT after the resection. The combination of bortezomib and dexamethasone (VD), alone or with cyclophosphamide (VCD), has shown good efficacy and tolerability in patients with relapsed or refractory multiple myeloma in non-randomized trials [17], [18]. We performed combination therapy with VCD and auto-PBSCT after the operation because of the advanced pathological results. This combination was well tolerated and induced complete and sustained remission. The only documented side effect was transient mild neutropenia and thrombocytopenia. The use of bortezomib, with or without dexamethasone, could be a reliable, safe, and effective alternative for treating extramedullary plasmacytomas. Sixteen months after diagnosis, the patient is in complete remission with no evidence of local relapse or evolution to multiple myeloma. This is the first reported case of successful management of advanced EMP of the stomach with the combination of surgical resection and chemotherapy (VCD and auto-PBSCT).
4. Conclusion
No general treatment guidelines have been established for primary gastric plasmacytoma. We performed combination therapy for the advanced gastric plasmacytoma using adjuvant chemotherapy involving bortezomib and auto-PBSCT after the resection, and the patient has maintained a good course over a year. This protocol could be a new way to treat these tumors.
Conflicts of interest
None of the authors has anything to disclose.
Funding
None of the authors has anything to disclose.
Ethical approval
All procedures used in this research were approved by the Ethical Committee of Chugoku Rosai Hospital.
Consent
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. A copy of the written consent form is available for review by the Editor-in-Chief of this journal.
Author contribution
Fukuhara, Tazawa, Sakimoto and Ohdan wrote the manuscript. Okanobu diagnosed this case. Tazawa and Sakimoto performed the operation. Kida, Kido, and Takafuta performed the chemotherapy. Nishida diagnosed this disease pathologically. All authors conceived of the study and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Guarantor
Hirofumi Tazawa has accepted full responsibility for this work and the decision to publish it.
Acknowledgment
None.
References
- 1.Grogan T.M., Spier C.M. 1st edition. Lippincott Wilkins & Wilkins; 2001. B-Cell Immunoproliferative Disorders, Including Myeloma and Amyloidosis, Neoplastic Hematopathology; pp. 1557–1587. [Google Scholar]
- 2.Weber D.M. Solitary bone and extramedullary plasmacytoma. Hematol. Am. Soc. Hematol. Educ. Program. 2005;1:373–376. doi: 10.1182/asheducation-2005.1.373. [DOI] [PubMed] [Google Scholar]
- 3.Alexiou C., Kau R.J., Dietzfelbinger H., Kremer M. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer. 1999;85:2305–2314. [PubMed] [Google Scholar]
- 4.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma: 3rd english edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 5.Nolan K.D., Mone M.C., Nelson E.W. Plasma cell neoplasms. Review of disease progression and report of a new variant. Surg. Oncol. 2005;14:85–90. doi: 10.1016/j.suronc.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulos M.A., Hamilos G. Solitary bone plasmacytoma and extramedullary plasmacytoma. Curr. Treat. Options Oncol. 2002;3:255–259. doi: 10.1007/s11864-002-0015-2. [DOI] [PubMed] [Google Scholar]
- 7.Tsang R.W., Gospodarowicz M.K., Pintilie M., Bezjak A., Wells W. Solitary plasmacytoma treated with radiotherapy: impact of tumor size on outcome. Int. J. Radiat. Oncol. Biol. Phys. 2001;50:113–120. doi: 10.1016/s0360-3016(00)01572-8. [DOI] [PubMed] [Google Scholar]
- 8.Kawata K., Ikeya T., Tanahashi Y., Aiba S., Shiozaki H. Plasmacytoma of the stomach: a case report. Gan No Rinsho. 1996;42:1706–1710. [Google Scholar]
- 9.Park C.H., Lee S.M., Kim T.O., Kim D.U., Jung W.J. Treatment of solitary extramedullary plasmacytoma of the stomach with endoscopic submucosal dissection. Gut Liver. 2009;3:334–337. doi: 10.5009/gnl.2009.3.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harada S., Fukunishi S., Takeuchi T., Ota K., Kazunori S. Magnifying narrow-band imaging endoscopy for the diagnosis of gastric primary extramedullary plasmacytoma: a first case report. Endoscopy. 2014;46:E435–E436. doi: 10.1055/s-0034-1377428. [DOI] [PubMed] [Google Scholar]
- 11.Park S.Y., Moon H.S., Seong J.K., Jeong H.Y., Yoon B.Y. Successful treatment of a gastric plasmacytoma using a combination of endoscopic submucosal dissection and oral thalidomide. Clin. Endosc. 2014;47:564–567. doi: 10.5946/ce.2014.47.6.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witshaw E. Chemotherapy in the management of extramedullary plasmacytoma. Cancer Chemother. Phamacol. 1978;1:167–175. doi: 10.1007/BF00253117. [DOI] [PubMed] [Google Scholar]
- 13.Soesan M., Paccagnella A., Chiarion-Sileni V., Salvagno L., Fornasiero A. Extramedullary plasmacytoma: clinical behavior and response to treatment. Ann. Oncol. 1992;3:51–57. doi: 10.1093/oxfordjournals.annonc.a058070. [DOI] [PubMed] [Google Scholar]
- 14.Preud'Homme J.L., Galian A., Danon F., Marti R., Rambaud J.C. Extramedullary plasmacytoma with gastric and lymph node involvement: an immunological study. Cancer. 1980;46:1753–1758. doi: 10.1002/1097-0142(19801015)46:8<1753::aid-cncr2820460809>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Katodritou E., Kartsios C., Gastari V., Verrou E., Mihou D. Successful treatment of extramedullary gastric plasmacytoma with the combination of bortezomib and dexamethasone: first reported case. Leuk. Res. 2008;32:339–341. doi: 10.1016/j.leukres.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Z.H., Yang J.F., Wang J.D., Wei J.G., Liu F. Imaging finding of primary gastric plasmacytoma: a case report. World J. Gastroenterol. 2014;20:10202–10207. doi: 10.3748/wjg.v20.i29.10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikhael J.R., Belch A.R., Prince H.M., Lucio M.N., Maiolino A. High response rate to bortezomib with or without dexamethasone in patients with relapsed or refractory multiple myeloma: results of a global phase 3b expanded access program. Br. J. Haematol. 2009;144:169–175. doi: 10.1111/j.1365-2141.2008.07409.x. [DOI] [PubMed] [Google Scholar]
- 18.Kropff M., Bisping G., Schuck E., Liebisch P., Lang N. Bortezomib in combination with intermediate-dose dexamethasone and continuous low-dose oral cyclophosphamide for relapsed multiple myeloma. Br. J. Haematol. 2007;138:330–337. doi: 10.1111/j.1365-2141.2007.06656.x. [DOI] [PubMed] [Google Scholar]