Abstract
Duchenne Muscular Dystrophy (DMD) is a fatal skeletal muscle wasting disease presenting with excessive myofibre necrosis and increased inflammation and oxidative stress. In the mdx mouse model of DMD, homeostasis of the amino acid taurine is altered, and taurine administration drastically decreases muscle necrosis, dystropathology, inflammation and protein thiol oxidation. Since the severe pathology of the Golden Retriever Muscular Dystrophy (GRMD) dog model more closely resembles the human DMD condition, we aimed to assess the generation of oxidants by inflammatory cells and taurine metabolism in this species. In muscles of 8 month GRMD dogs there was an increase in the content of neutrophils and macrophages, and an associated increase in elevated myeloperoxidase, a protein secreted by neutrophils that catalyses production of the highly reactive hypochlorous acid (HOCl). There was also increased chlorination of tyrosines, a marker of HOCl generation, increased thiol oxidation of many proteins and irreversible oxidative protein damage. Taurine, which functions as an antioxidant by trapping HOCl, was reduced in GRMD plasma; however taurine was increased in GRMD muscle tissue, potentially due to increased muscle taurine transport and synthesis. These data indicate a role for HOCl generated by neutrophils in the severe dystropathology of GRMD dogs, which may be exacerbated by decreased availability of taurine in the blood. These novel data support continued research into the precise roles of oxidative stress and taurine in DMD and emphasise the value of the GRMD dogs as a suitable pre-clinical model for testing taurine as a therapeutic intervention for DMD boys.
Abbreviations: (2ME;, 2-mercaptoethanol; ACN, acetonitrile; BSA, bovine serum albumin; CD, cysteine deoxygenase; CSD, cysteine sulfinate decarboxylase; DC, detergent-compatible; DMD, Duchenne Muscular Dystrophy; DNPH, 2,4-dinitrophenylhydrazine; EDTA, ethylene diamine tetra acetic acid; FA, formic acid; FLM, BODIPY FL-N-(2-aminoethyl) maleimide; GAP, glyceraldehyde 3-phosphate dehydrogenase; GRMD, Golden Retriever Muscular Dystrophy; HOCl, hypochlorous acid; HPLC, high performance liquid chromatography; IκB-α, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; MPO, myeloperoxidase; MS, mass spectrometry; MS/MS, tandem mass spectrometry; NAC, N-acetylcysteine; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NO, nitric oxide; NOS, nitric oxide synthase; nNOS, neuronal nitric oxide synthase; OPA, o-phthalaldehyde; OTC, L-2-Oxothiazolidine-4-Carboxylate; PBS, phosphate buffered saline; SDS, sodium dodecyl sulphate; SDS-PAGE, sodium dodecyl sulphate polyacrylamide gel electrophoresis; SERCA, sarcoplasmic/endoplasmic reticulum calcium ATPase; Tau-Cl, taurine chloramine; TauT, taurine transporter protein; TCA, trichloroacetic acid; TCEP, tris(2-carboxyethyl)phosphine; Texas red, Texas Red C2-maleimide; TNF, tumour necrosis factor
Keywords: Golden Retriever Muscular Dystrophy (GRMD), Duchenne Muscular Dystrophy (DMD), Taurine, Inflammation, Oxidative stress, Protein thiol oxidation
Graphical abstract
Highlights
-
•
We investigated oxidative stress in muscle from the GRMD dog model of DMD.
-
•
Excess neutrophils were associated with hypochlorous acid generation in GRMD muscle.
-
•
GRMD muscle exhibited oxidative damage to proteins, and protein thiol oxidation.
-
•
These data imply a role of immune cell generated oxidative stress in GRMD pathology.
1. Introduction
Duchenne Muscular Dystrophy (DMD) is a lethal, X-chromosome linked muscle disease affecting about 1 in 3500–6000 boys worldwide (Reviewed in [1], [2]). DMD is characterised by severe muscle weakness caused by mutations in the dystrophin gene, which result in the loss of functional dystrophin protein. In skeletal muscles this defect increases susceptibility to sarcolemma damage after muscle contraction leading to myofibre necrosis, with inflammation and excessive fibrosis initially associated with muscle regeneration [3], [4], [5]. Repeated cycles of widespread myofibre necrosis and progressive failure of regeneration over time in DMD boys (with replacement of myofibres by fatty and fibrous connective tissue) lead to the loss of muscle mass and function with premature death of DMD boys, often due to respiratory or cardiac failure (Reviewed in [1], [5], [6]).
While the mechanisms for loss of muscle function in DMD (and animal models of DMD) are not fully understood, disturbed intracellular calcium homeostasis, inflammation and oxidative stress are implicated [7], [8]. Proposed sources of various oxidants in dystrophic muscle include mitochondria, inflammatory cells, NAD(P)H oxidase, xanthine oxidase, and decoupling of NOS (via dislocation or translocation of nNOS from the dystroglycan complex of the sarcolemma) (reviewed in [9]). Oxidative damage to muscle proteins has been observed in both DMD boys and the widely studied mdx mouse model of DMD [10], [11], [12], [13], [14]. Another major cellular consequence of oxidant exposure is the reversible oxidation of protein thiol side chains (-SH, in the cysteine residue). Protein thiols can undergo numerous reactions, which are dependent on the species and concentration of oxidants they encounter [15]. For example, oxidants such as hydrogen peroxide, can cause reversible oxidation (disulphide formation) of thiols, for which the reduction/oxidation (redox) state is an important regulator of protein function [16]. We have previously shown that reversible protein thiol oxidation is increased in mdx muscle, and is especially pronounced around areas of necrosis, occurring on muscle proteins such as myosin heavy chain, myosin light chain and tropomyosin, as well as on glycolytic proteins phosphoglycerate mutase and triosephosphate isomerase [14], [17], [18], [19], [20], [21], [22].
Antioxidants that target protein thiol oxidation, such as the cysteine/glutathione precursors n-acetylcysteine (NAC) and L-2-Oxothiazolidine-4-Carboxylate (OTC) have been investigated in mdx mice as therapeutic interventions for DMD [19], [20], [23], [24], [25], [26]. Treatment of mdx mice with NAC or OTC reduces muscle pathology, as shown by decreased myofibre necrosis, inflammatory cells and TNF levels, and improved grip strength [19], [20], [23], [24], [25]. NAC and OTC are derivatives of the amino acid cysteine, and can increase tissue content of both cysteine and GSH, two major cellular thiol antioxidants [27], [28]. However we established that the mechanism of action of NAC and OTC in mdx mice was not via an increase in either cysteine or glutathione in muscle, liver and plasma [19], [20], [23]. Instead, we showed that OTC treatment of mdx mice leads to an increase in the content of the semi-essential amino acid taurine (2-aminoethanesulfonic acid) in muscle, liver and plasma [19], [23].
Taurine is synthesised from cysteine, as a mechanism for removing excess cysteine, which is toxic in mammals [29], [30]. Taurine is found in many tissues and is considered important for the function of skeletal muscle; the concentration of taurine in tissues is regulated by interactions between dietary intake, biosynthetic rate (mainly in the liver) tissue uptake and elimination via the kidney [31]. We recently showed in mdx mice that systemic taurine homeostasis is perturbed and may correlate with the onset of pathology [31], and treatment of adult mdx mice with taurine improves both in vivo and ex vivo muscle strength [23], [32], [33]. Importantly, in young mdx mice aged 22 days, taurine treatment (from day 14) prevents the acute onset of myofibre necrosis [34]. Taurine is hypothesised to modulate ion channel function, membrane stability and calcium homeostasis [35], [36], [37], [38], [39], [40]. However we showed that taurine is also a potent thiol antioxidant in mdx muscle and can dramatically decrease muscle tissue content of neutrophils and myeloperoxidase (MPO) [23], [34]. MPO is a heme protein secreted primarily by neutrophils, the key cells involved in acute inflammation that are phagocytes responsible for microbial killing and generation of various pro-inflammatory mediators that attract macrophages to the site of tissue damage [41]. MPO is also secreted (to a lesser extent) by monocytes and can be secreted by some macrophages [42]. MPO oxidises chloride in the presence of hydrogen peroxide to form the potent oxidant hypochlorous acid (HOCl) that targets proteins by reacting with thiols and by causing oxidative damage [43]. Amino acids such as taurine can function as antioxidants by forming chloramines which can trap HOCl [41]. We propose that a possible mechanism for elevated oxidative stress in dystrophic muscles is the excessive generation of HOCl (by inflammatory cells), combined with a disruption in taurine metabolism, that leaves tissues susceptible to oxidative damage by HOCl.
The golden retriever muscular dystrophy (GRMD) dog model manifests a more severe dystropathology with a rapidly progressing and fatal disease similar to DMD boys, in marked contrast with the mdx mouse model [43]. The disruption in taurine metabolism documented for mdx mice has the potential to be species specific, since the homeostasis of taurine differs greatly between carnivores such as dogs and humans, and mice [44]. Furthermore, compared with GRMD dogs and DMD boys, the mdx mice exhibit a very mild pathology, possibly due to the very short growth phase and lifespan as well as the small size of mdx mice [3], [45]. Like the human DMD condition, persistent muscle necrosis in GRMD dogs results in incomplete muscle repair leading to loss of myofibres and increased fibrosis, with progressive weakness and gait abnormalities around 6–9 weeks of age and contractures by 6 months [46]. Death of GRMD dogs usually occurs around 1 year of age as a result of failure of respiratory muscles as well as feeding difficulties (severe dysphagia) [43], [47], [48]. Whilst dystrophin deficiency has now been identified in many breeds of dogs [49], GRMD dogs are the favoured model for pre-clinical trials in DMD research [46], [51], [52], although the colonies are expensive and hard to maintain and show high variation between individual dogs [50]. There is little information on the role of oxidative stress in GRMD muscles and the role of taurine in GRMD dystropathology has yet to be investigated; however it is known that diet induced taurine deficiency predisposes healthy dogs to cardiomyopathy [51].
The present study investigated inflammation, oxidative stress and taurine homeostasis in muscle and blood from GRMD and healthy wild-type dogs, to determine whether taurine deficiency in GRMD dogs may render the dystrophic muscles susceptible to oxidative damage and protein thiol modifications, caused by inflammation. Inflammation was assessed in GRMD and healthy dog muscles by quantifying the presence of neutrophils and macrophages; the contribution of these cells to oxidative stress was assessed by measuring MPO and chlorotyrosines, which are biomarkers of HOCl generation [52]. Oxidative stress was quantified by measuring levels of protein thiol oxidation (including the thiol oxidative status of specific abundant proteins) and protein carbonylation. Taurine can accumulate in cells through two mechanisms; by uptake from the extracellular space by the sodium dependent transporter TauT, and by endogenous local synthesis from cysteine by two enzymes, cysteine deoxygenase and cysteine sulfinate decarboxylase [29], [53]. Since we have previously shown that both mechanisms are perturbed in mdx muscle [31], we measured TauT, cysteine deoxygenase and cysteine sulfinate decarboxylase in GRMD muscle, as well as taurine content of the muscle and plasma (to establish extracellular taurine concentrations). We show that GRMD muscle has an increased content of neutrophils and macrophages with resultant high levels of MPO and HOCl, associated with high levels of protein thiol oxidation and irreversible oxidative damage of both intracellular and extracellular proteins; we also show perturbations of taurine homeostasis in GRMD dogs. These data emphasise the value of using GRMD dogs to evaluate the role of immune cell generated HOCl in dystropathology, and support the use of this dystrophic dog model for pre-clinical trials of interventions that target this pathway.
2. Materials and methods
2.1. Animal procedures
Muscle and plasma samples were obtained from 4 healthy control (unaffected normal) male golden retriever dogs and 5 GRMD male dogs, aged approximately 8 months. These dogs were handled and housed in the Boisbonne Center for Gene Therapy (ONIRIS, Atlantic Gene Therapies, Nantes, France). The Institutional Animal Care and Use Committee of the Region des Pays de la Loire (University of Angers, France) approved all these protocols. Skeletal muscles samples (biceps femoris) were obtained after the dogs were sacrificed, performed by intravenous injection of pentobarbital sodium (Dolethal, Vetoquinol). Muscle samples were placed into sterile microtubes, frozen in liquid nitrogen and subsequently stored at about −80 °C until analysis. Plasma samples were obtained after blood collection in EDTA-coated tubes. Within 15 min, the tubes were centrifuged 8 min at 3000 rpm to obtain plasma. Plasma samples were stored at about −80 °C until analysis.
2.2. Muscle protein extraction and immunoblotting
Frozen muscles were crushed using a mortar and pestle under liquid nitrogen and homogenized in 10 times ice-cold 1% NP40, 1 mM EDTA in phosphate buffered saline (PBS), supplemented with complete EDTA free protease inhibitor tablets and PhosSTOP phosphatase inhibitor tablets (Roche), and centrifuged for 10 min. The protein concentration of supernatants was quantitated using the Detergent Compatible (DC) protein assay (Bio-Rad). Samples were resolved on 4–15% SDS-PAGE (sodium dodecyl sulphate polyacrylamide gel electrophoresis) TGX gels (Bio-Rad) and transferred onto nitrocellulose membrane using a Trans Turbo Blot system (Bio-Rad). Immuno-blotting was performed with antibodies to neutrophil elastase (ab68672, Abcam), macrophage F4/80 (ab74383, Abcam), myeloperoxidase (14,569, Cell Signalling), cysteine dioxygenase type 1 (ab53436, Abcam), cysteine sulfinate decarboxylase (ab101847, Abcam), TauT (TAU11-A, Alpha Diagnostics) and glyceraldehyde 3-phosphate dehydrogenase (GAP, 14C10, Cell Signalling), all dissolved 1:1000 in 5% bovine serum albumin (BSA). Horseradish peroxidase conjugated secondary antibodies were from Thermo Fisher Scientific. Chemiluminescence signal was captured using the ChemiDoc MP Imaging System (Bio-Rad). Resultant images were quantified using ImageJ software [54]. Glyceraldehyde 3-phosphate dehydrogenase (GAP) loading controls were immunoblotted on the same membrane as the immunoblotted protein. All representative immunoblots in figures represent proteins immunoblotted on the same membrane as the loading control GAP.
2.3. Chlorinated tyrosines
Tyrosine and chlorinated tyrosines in proteins from muscle extracts were determined as previously described [55] using stable isotope dilution liquid chromatography with mass spectrometry instead of gas chromatography. In brief, frozen muscles were crushed using a mortar and pestle under liquid nitrogen, homogenized in 20% methane sulfonic acid in acetone and, after centrifugation, supernatants were removed and pellets dried by vacuum centrifugation. 13C6 and 13C9 Tyrosine and 13C6 chlorotyrosine internal standards were added to samples containing 80 μg of protein (as determined by the DC protein assay, Bio-Rad) to allow stable isotope quantification and determine any artificial chlorination. Samples were hydrolysed for 18 h at 110 °C in 6 M hydrogen bromide. The hydrolysates were diluted in 0.1% formic acid and tyrosine derivatives were isolated using a Dionex 3000 high performance liquid chromatography (HPLC) pump with a C18 Gemini column (100×2.00 mm, 3 µm). A reverse phase gradient elution using 0.1% formic acid in water and 0.1% formic acid in acetonitrile was used for elution of analytes. Analytes were detected on a 4000QTRAP mass spectrometry using Multiple Reaction Monitoring for the tyrosine and chlorotyrosine isotopes.
2.4. Quantification of protein thiol oxidation
Reduced and oxidized protein thiols were measured in muscles using the 2 tag technique as described previously [14], [18], [19], [20], [21], [22]. In brief, frozen tissue was crushed under liquid nitrogen, before protein was extracted with 20% trichloroacetic acid (TCA) in acetone. Protein was solubilized in 0.5% SDS with 0.5 M Tris at pH 7.3 (SDS buffer) and protein thiols were labelled with the fluorescent dye BODIPY FL-N-(2-aminoethyl) maleimide (FLM, Invitrogen). Following removal of the unbound dye using ethanol, protein was re-solubilized in SDS buffer, pH 7 and oxidized thiols were reduced with tris(2-carboxyethyl)phosphine (TCEP) before the subsequent unlabeled reduced thiols were labelled with a second fluorescent dye Texas Red C2-maleimide (Texas red, Invitrogen). The sample was washed in ethanol and resuspended in SDS buffer. Samples were read using a fluorescent plate reader (Fluostar Optima) with wavelengths set at excitation 485 nm, emission 520 nm for FLM and excitation 595 nm, emission 610 nm for Texas red. A standard curve for each dye was generated using ovalbumin and results were expressed per mg of protein, quantified using the DC protein assay (Bio-Rad).
Reduced and oxidized thiols of specific proteins were quantified using a 1-dimensional SDS–PAGE, as described previously [19]. Briefly, labelled samples (remaining from plate assay above) were diluted to equivalent protein concentrations. FLM and Texas red labelled BSA standards were combined and both the standards and samples were diluted by the addition of sample buffer (125 mM Tris, pH 6.8, 4% SDS, 30% (v/v) glycerol, 0.02% bromophenol blue). Standards and samples were applied to a 12% polyacrylamide gel. Gel electrophoresis was performed using the Bio-Rad Mini Protean III system. Each fluorescent gel was scanned using the ChemiDoc MP Imaging System (Bio-Rad) for fluorescence, with wavelengths set at excitation 485 nm, emission 520 nm for FLM and excitation 595 nm, emission 610 nm for Texas red. The bands were quantified by densitometry using ImageJ version 1.41 software [54] using the integrated density function, after first removing the background. To assess the reversible protein thiol oxidation state of specific protein bands, dominant bands were compared against FLM and Texas red using in-gel standard curves using polynomial regression. Non-labelled samples were run in parallel in the same gel, and after gel scanning, gels were stained with Coomassie brilliant blue, scanned and specific protein bands of interest were excised for mass spectrometry.
2.5. Mass spectrometry to identify specific proteins
Gel bands were excised for in-gel digestion, and cut into 1 mm cubes. Gel pieces were de-stained 3 times with 100 μl of 25 mM ammonium bicarbonate in 50% acetonitrile (ACN) at 37 °C for 30 min. Gel pieces were then dried by vacuum centrifugation. Protein was digested by addition of 125 ng trypsin in 10 μl of 25 mM ammonium bicarbonate. The digestion reaction proceeded at 37 °C for 16 h. Digested protein was extracted by 3 additions of 20 μl 1% triflouroacetic acid in ACN and incubation at room temperature for 20 min. Extracts were pooled and desiccated by vacuum centrifugation.
Extracts were reconstituted in 11 μl of 2% ACN, 0.1% formic acid (FA) solution for loading into a Prominence (Shimadzu) HPLC for chromatographic separation, which was directly sprayed into a 5600 TripleTOFTM (Sciex) mass spectrometer. The HPLC mobile phase consisted of 0.1% (FA and 2% ACN in water (A) and 0.1% FA and 2% water in ACN (B). Gradient elution was performed with 2% B for 3 min, increased to 40% B at 15 min, then ramped to 98% B by 16 min and held for 1 min before reduced back to 2% B within 1 min. Column temperature was 40 °C. Positive electrospray ionization mode was operated to acquire MS data by information-dependent acquisition (IDA), where only the top 20 MS peaks between 400 and 1250 m/z were selected for further MS/MS scan. Mass tolerance was 50 mDa. BSA calibration was conducted before a batch of samples was run. Parent mass peaks (mass range m/z 800–3000 from combined MS and MS/MS spectra) were submitted to the MASCOT database for identification of peptides, using the following search conditions: Swissprot database, all mammalian species, trypsin digest with allowance for up to one missed cleavage per peptide, no fixed modifications, variable modification of oxidation on methionine residues, MS tolerance of 1.2 Da, MS/MS tolerance of 0.6 Da. Proteins were identified on the basis of 2 or more peptides with ion scores exceeding the significance threshold.
2.6. Carbonylated protein
Irreversible oxidative damage to proteins in muscle was determined by measuring the carbonyl content with 2,4-dinitrophenylhydrazine (DNPH) as previously described [20], [56], [57]. In brief, frozen muscles were crushed under liquid nitrogen, before protein was extracted with 20% TCA/acetone. The protein pellets were washed in acetone and ethanol, precipitated, dried, re-suspended in 10 mM DNPH in 2 M HCl and incubated for 30 min at room temperature in the dark. Proteins were washed with ethyl acetate/ethanol (1:1), dissolved in 6 M guanidine, and absorbance was measured at 370 nm. Protein concentration (mg/ml) was determined using the Bio-Rad Bradford protein assay. Carbonyl concentrations are expressed as nmol of carbonyl per mg protein.
2.7. HPLC analysis of taurine
Taurine levels in muscle and plasma were measured using reverse phase HPLC as previously described [19]. Frozen tissues were crushed using a mortar and pestle under liquid nitrogen and homogenized in 25 times 5% TCA, plasma samples were precipitated by addition of 10 times 5% TCA and, after centrifugation, supernatants were removed and stored at −80 °C before analysis. Analytes were separated using HPLC with fluorescent detection, with pre-column derivitisation with o-phthalaldehyde (OPA) and 2-mercaptoethanol (2ME). OPA reacts rapidly with amino acids and sulfhydryl groups to yield intensely fluorescent derivatives, and 2ME, a reducing agent, prevents the OPA reagent from oxidising. Supernatants were mixed with iodoacetamide, dissolved in 5% TCA, to a final concentration of 25 mM. An internal standard, o-phospho-dl-serine, dissolved in 5% TCA was added to a final concentration of 5 mM. Sodium borate was used to adjust the pH to 9. Samples were placed in an autosampler, which was maintained at 4 °C. Samples were mixed on a sample loop with a derivatising solution containing 40 mM OPA and 160 mM 2ME in 100 mM sodium borate, pH 12, for 30 s before injection onto the column. Separation was achieved with a C18 column (5 μl, 4.6×150 mm, Phenomenex) using a Dionex Ultimate 3000 HPLC system. Mobile phase A consisted of 50 mM potassium phosphate buffer, methanol and tetrahydrofuran (94:3:3). Mobile phase B consisted of 90% methanol, with a gradient increase in B from 0% to 25%. Fluorescence was set at 360 nm and 455 nm for excitation and emission respectively. The protein content of muscle samples were quantified by solubilising the pellet in 0.5 M sodium hydroxide, before incubation at 80 °C for 15 min. Once fully dissolved, protein concentrations of supernatants were quantified using a Bradford protein assay (Bio-Rad).
2.8. Statistics
Significant differences between groups were determined using t-tests and all data are presented as mean ± standard error of the mean (SEM). Significance was set at p<0.05.
3. Results
3.1. Neutrophil and macrophage quantification
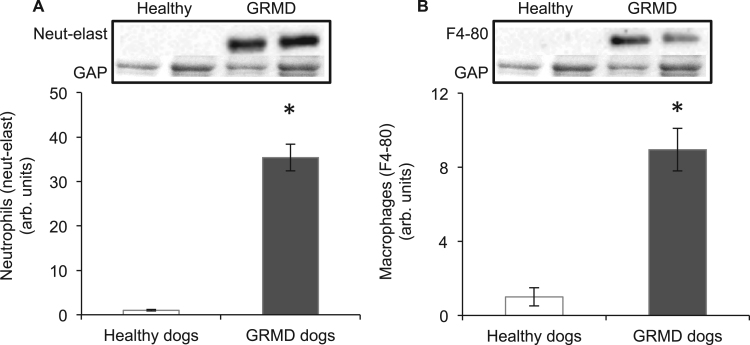
Quantification of neutrophils and macrophages in healthy (normal control) and GRMD dog muscle was measured by western blotting for the proteins neutrophil elastase and F4/80; both considered specific for neutrophils and macrophages, respectively [58]. The levels of neutrophils (Fig. 1A) and macrophages (Fig. 1B) in GRMD muscle were 35 and 9 fold higher (respectively) than for healthy control dog muscle.
Fig. 1.
Neutrophil (A) and macrophage (B) levels in muscles of healthy and GRMD dogs aged 8 months. Asterisks represent significant differences of p<0.05. Data are presented as mean±SEM and n=4 and 5 respectively. Representative blots are shown of neutrophil elastase, F4/80 and the loading control glyceraldehyde 3-phosphate dehydrogenase (GAP), all proteins were blotted on the same membrane.
3.2. Chlorinated tyrosines and myeloperoxidase
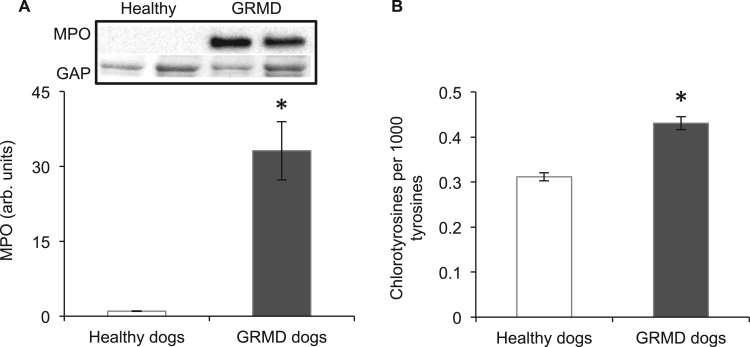
The formation of HOCl is catalysed by MPO (likely produced by neutrophils and macrophages). MPO content of GRMD muscle was 33 fold higher than healthy control dog muscle (Fig. 2A). Evidence of HOCl generation was determined by measuring chlorotyrosines (which are formed when tyrosyl residues of peptides are exposed to HOCl [59]). Chlorinated tyrosines were 1.4 fold higher in GRMD muscle compared with healthy control dog muscle (Fig. 2B).
Fig. 2.
Myeloperoxidase (MPO) (A) and chlorotyrosines (B) in muscles of healthy and GRMD dogs. Asterisks represent significant differences of p<0.05. Data are presented as mean±SEM and n=4 and 5 respectively. Representative blots are shown of MPO and the loading control glyceraldehyde 3-phosphate dehydrogenase (GAP), all proteins were blotted on the same membrane.
3.3. Protein oxidation
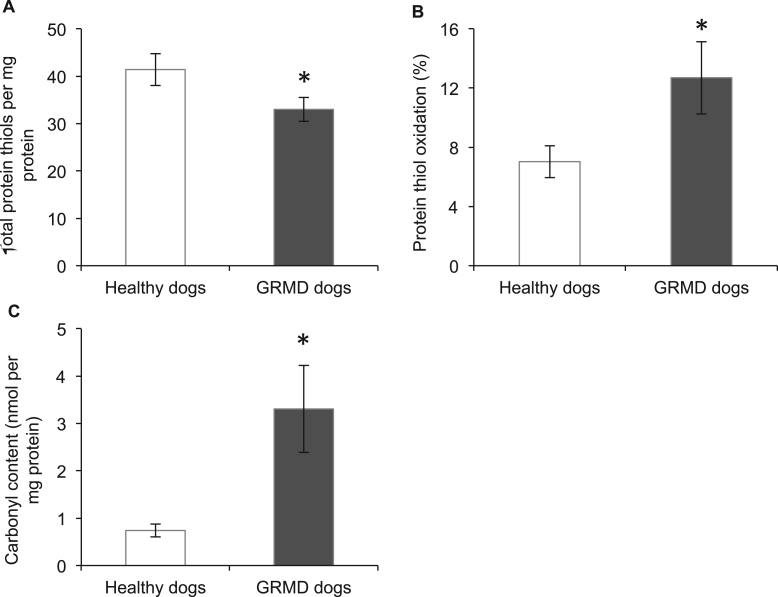
Measurements of (reversible) oxidation of thiol side chains in proteins showed that the total amount of protein thiols was 22% lower (Fig. 3A), whereas total protein thiol oxidation was 1.8 fold higher (Fig. 3B) in GRMD, compared with healthy control, dog muscle. The status of thiol oxidation of specific abundant proteins was also determined. As shown in Table 1, among the 12 proteins identified, 10 had significantly more protein thiol oxidation (from 1.5 to 2.8 fold) in GRMD muscle compared with healthy dog muscle. The identified proteins that underwent thiol oxidation in GRMD muscle included myosin, myosin-binding protein C, myotilin, sarcoplasmic reticulum ATPase, pyruvate kinase, malate dehydrogenase, phosphoglucomutase, glyceraldehyde-3-phosphate dehydrogenase, lactoferrin and albumin.
Fig. 3.
Total protein thiols (A), percentage of protein thiol oxidation (B) and protein carbonylation in muscles of healthy and GRMD dogs. Asterisks represent significant differences of p<0.05. Data are presented as mean±SEM and n=4 and 5 respectively.
Table 1.
Protein thiol oxidation of abundant proteins in healthy and GRMD muscle. Asterisks represent significant differences of p<0.05. Data are presented as mean±SEM and n=4 and 5 for healthy and GRMD dogs respectively.
| Protein name | Accession # | # Sig. peptides | Healthy dogs | GRMD dogs |
|---|---|---|---|---|
| Myosin | Q076A6 | 8 | 11.0±1.5 | 30.8±3.8* |
| Myosin-binding protein C | Q00872 | 18 | 17.5±0.9 | 34.0±2.9* |
| Sarcoplasmic/reticulum calcium ATPase | O14983 | 9 | 15.3±0.8 | 30.5±2.8* |
| Lactoferrin | P02788 | 26 | 27.3±2.6 | 65.7±4.6* |
| Albumin | P49822 | 33 | 13.0±0.8 | 31.7±3.0* |
| Phosphoglucomutase | Q08DP0 | 5 | 12.9±0.8 | 31.0±2.8* |
| Pyruvate kinase | P11980 | 5 | 11.2±0.8 | 24.0±1.8* |
| Myotilin | Q9UBF9 | 8 | 12.8±1.0 | 25.8±2.5* |
| Glyceraldehyde-3-phosphate dehydrogenase | Q28259 | 13 | 10.0±1.3 | 15.2±1.7* |
| Malate dehydrogenase | P08249 | 8 | 8.6±0.9 | 18.7±2.3* |
| Alpha actin | P68138 | 87 | 4.7±0.6 | 5.5±0.6 |
| Creatine kinase | P05123 | 47 | 8.6±0.9 | 8.6±0.9 |
Irreversible protein damage in GRMD muscle (measured by carbonylation of protein), was 4.7 fold higher in GRMD muscle, compared with healthy control dog muscle (Fig. 3C).
3.4. Taurine homeostasis
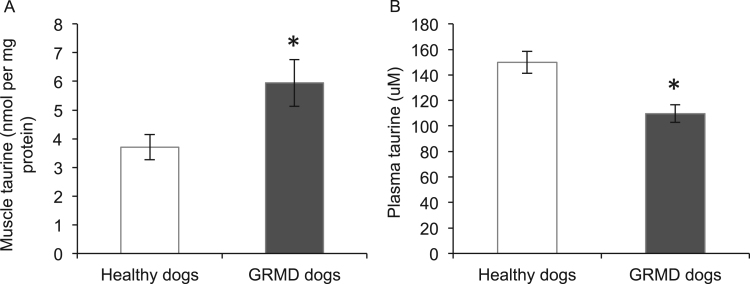
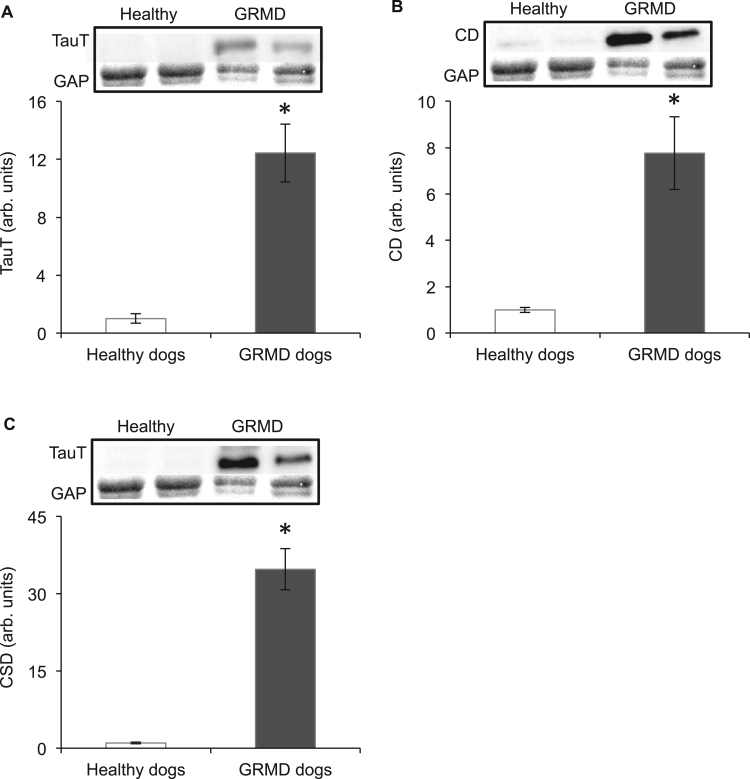
Taurine content of muscle and plasma from healthy control and GRMD dogs was determined using HPLC. Compared with healthy dogs, taurine content was 1.6 fold higher in GRMD muscle (Fig. 4A); however, plasma taurine content was 27% lower in GRMD dogs (Fig. 4B). Since intracellular taurine content depends on transport of taurine into the cells via the transporter protein TauT, combined with the synthesis of taurine from cysteine by the enzymes cysteine deoxygenase and cysteine sulfinate decarboxylase, levels of these proteins in muscle were measured by western blotting. Levels of TauT (Fig. 5A), cysteine deoxygenase (Fig. 5B) and cysteine sulfinate decarboxylase (Fig. 5C) were, respectively, 12, 8 and 35 fold higher in GRMD muscle compared with healthy dog muscle, indicating that both transport and synthesis of taurine are upregulated in GRMD muscle (to likely account for the high levels of taurine that are present).
Fig. 4.
Taurine content of healthy and GRMD muscles (A) and plasma (B). Asterisks represent significant differences of p<0.05. Data are presented as mean±SEM and n=4 and 5 respectively.
Fig. 5.
TauT (A), cysteine deoxygenase (CD) (B) and cysteine decarboxylase (CSD) in muscles of healthy and GRMD dogs. Asterisks represent significant differences of p<0.05. Data are presented as mean±SEM and n=4 and 5 respectively. Representative blots are shown of TauT, CD, CSD and the loading control glyceraldehyde 3-phosphate dehydrogenase (GAP), all proteins were blotted on the same membrane.
4. Discussion
Our key observations (summarised in Table 2) are that, compared with normal control dogs, dystrophic muscles of GRMD dogs contain significantly higher levels of neutrophils and macrophages and associated HOCl, and irreversible and reversible protein oxidation. In addition, taurine homeostasis is perturbed in GRMD muscles and plasma, with the deficiency in GRMD plasma taurine levels potentially rendering dystrophic muscles more susceptible to protein oxidation. These novel data for dystrophic dogs are discussed in detail below.
Table 2.
Summary of differences in GRMD tissues (compared with normal control dogs).
| Parameter measured | Increase ↑ or decrease ↓ | Fold change |
|---|---|---|
| Inflammation in muscle | ||
| Neutrophils | ↑ | 35 |
| Macrophages | ↑ | 9 |
| Oxidative stress in muscle | ||
| MPO | ↑ | 33 |
| HOCL | ↑ | 1.4 |
| Total protein thiols | ↓ | -1.3 |
| % Total protein thiol oxidation | ↑ | 2.8 |
| Thiol oxidation in 10/12 specific proteins | ↑ | ~2 |
| Protein carbonylation | ↑ | 1.4 |
| Taurine metabolism | ||
| Muscle taurine | ↑ | 4.7 |
| Plasma taurine | ↓ | −1.4 |
| Muscle TauT | ↑ | 12 |
| Muscle cysteine deoxygenase | ↑ | 8 |
| Muscle cysteine decarboxylase | ↑ | 35 |
We show increased content of neutrophils and macrophages in GRMD muscle. In skeletal muscle, the neutrophils and macrophages (that invade skeletal muscle after injury such as myonecrosis) phagocytose and remove necrotic cellular debris, are chemotactic, result in modification of the extracellular matrix (ECM) and play key roles in promoting all aspects of myogenesis and the regeneration of the necrotic myofibres [60], [61], [62], [63]. Upon stimulation, neutrophils (that begin accumulating within 30 min of muscle damage) and macrophages undergo a burst of oxygen consumption caused by the NAD(P)H oxidase complex in the phagosomal membrane, which stimulates the generation of superoxide [64]. Superoxide is dismuted to form hydrogen peroxide, which can undergo a Fenton reaction with a metal catalyst to form the highly reactive hydroxyl radical. However, the protein lactoferrin (an iron binding glycoprotein) found in neutrophils, sequesters free iron thus inhibiting production of this damaging hydroxyl radical [65]. Another protein that determines the fate of hydrogen peroxide is MPO, which consumes hydrogen peroxide more rapidly than the Fenton reaction [64]. MPO can also oxidise chloride in the presence of hydrogen peroxide to form HOCl, a highly reactive oxidant. Proteins are major targets for HOCl, and reactions result in thiol modifications as well as protein damage [66]. We show that both MPO content and HOCl generation are increased in GRMD muscle and that this is associated with elevated protein thiol oxidation and protein carbonylation. The carbonyl assay is the most frequently used biomarker of irreversible protein oxidative damage [67], and is elevated in many conditions, including inflammatory disorders such as chronic lung disease, inflammatory bowel disease, rheumatoid arthritis and sepsis [68]. Direct oxidation of proteins by HOCl yields highly reactive carbonyl derivatives, resulting from oxidation of amino acid side chains or from the cleavage of peptide bonds leading to the formation of protein derivatives or peptide fragments containing highly reactive carbonyl groups [69]. HOCl also reacts rapidly with thiol groups, and can cause reversible modifications, however being a strong oxidant, HOCl can also react with thiols to form irreversible products [70], [71]. These modifications were likely evident in the current study, where GRMD muscle showed high reversible thiol oxidation for many proteins, and a reduction in total protein thiols.
Since protein thiol modifications affect protein (and therefore tissue) function, we identified specific proteins that underwent thiol oxidation in GRMD muscles (using SDS-PAGE and mass spectrometry). These diverse proteins were located throughout the cell, and included vital contractile proteins of the sarcomeres (myosin, myosin-binding protein C, myotilin), sarcoplasmic reticulum (sarcoplasmic reticulum ATPase), mitochondria (pyruvate kinase and malate dehydrogenase), cytoplasm (phosphoglucomutase and glyceraldehyde-3-phosphate dehydrogenase) and extracellular fluid (lactoferrin and albumin). In skeletal muscle, contractile function, force production and the development of fatigue are directly influenced by the redox state of thiol side chains of contractile proteins [72], [73], [74], [75], [76], [77], [78]. For example, thiol oxidation of myosin, as we observed in GRMD muscle, affects contraction by decreasing myosin ATPase activity, decreasing Ca2+ sensitivity and modifying kinetics of actin-myosin cross bridge transitions [74], [75], [79], [80], [81]. The myosin-binding protein C contributes to the assembly and stability of thick filaments and regulates the cross-bridge interactions of myosin and actin [82], and myotilin is also important for organisation of the myofibril [83]: both these proteins had increased thiol oxidation in GRMD muscle. While the consequences of thiol oxidation of all these proteins are not fully understood in skeletal muscle, oxidative modification of thiol groups of myosin-binding protein C controls the response of myofilaments to calcium in cardiac tissue [84].
In GRMD muscles, other intracellular proteins with elevated thiol oxidation included: sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) that is a key regulator of muscle contractile activity responsible for the reuptake of cytosolic Ca2+ into the sarcoplasmic reticulum [85]; phosphoglucomutase, a protein with roles in glucose metabolism including glycogenolysis and glycogenesis [86]; pyruvate kinase that catalyzes the last but rate-limiting step of glycolysis [87]; and glyceraldehyde-3-phosphate dehydrogenase- a membrane protein involved in a variety of cellular processes including glycolysis and malate dehydrogenase, which is utilised in numerous metabolic pathways. Previous research shows that thiol oxidation of these proteins can directly effect their functions [88], [89], [90], [91], [92], [93]. While the consequences of high thiol oxidation of these diverse proteins in GRMD muscle are yet to be fully understood and appreciated, functional changes in these proteins and downstream cellular consequences may well contribute to severity of dystropathology in GRMD dogs. Thus there is considerable interest in reversing this high level of protein thiol oxidation as a therapeutic strategy.
Interestingly, we also identified two extracellular proteins, lactoferrin and albumin, with increased protein thiol oxidation in GRMD muscle. A role for lactoferrin in preventing hydroxyl radical production has been identified [65], but the consequences of thiol oxidation on the function of this highly cysteine rich protein has not been described. Lactoferrin contains 33 cysteine residues including many intramolecular disulphide bonds [94]. Albumin contains one reduced cysteine residue and is considered an important circulating thiol antioxidant since it is the most abundant plasma protein (representing about 50–60% of all plasma proteins in rodents and humans) [95], [96]. In healthy humans, abundant albumin is also localised extravascularly in the interstitial fluid, with the amount of albumin being more than double that within the intravascular plasma, suggesting that albumin is likely especially important as a thiol regulator in extracellular fluids in intimate contact with the surface of cells [97]. Our novel GRMD data show that alterations and regulation of oxidative stress occurs both inside and outside the myofibre, supporting the hypothesis that extracellular sources of oxidants (such as generated by immune cells) may significantly increase oxidative stress at the location of the sarcolemma in GRMD.
Investigating taurine homeostasis in GRMD muscle in conjunction with measuring protein oxidation and inflammation is of interest as we have previously shown taurine homeostasis is perturbed in mdx mice [31], and taurine treatment decreased protein thiol oxidation, neutrophil and MPO content in mdx muscle, and prevented myofibre necrosis [23]. The antioxidant and anti-inflammatory properties of taurine are attributed to its ability to interact with HOCl, which reacts with amino acids to form long-lived chloramines such as taurine chloramine; chloramines are much less reactive than HOCl [41]. Unlike HOCl, chloramines discriminate between low molecular weight thiols based on their pKa [98] and thus chloramines cannot oxidise as many molecules as HOCl. Taurine chloramine also exerts anti-inflammatory effects such as inhibiting the production of pro-inflammatory cytokines and nitric oxide (NO), and appears to inhibit NF-κB activation by the oxidation of IκB-α [41], [99].
We and others have observed that taurine content of dystrophic mdx muscle is variable across the age (and stage of disease progression) [31], [34], [100]. Whilst the timing and muscles used in these different mdx studies is variable, early in the disease progression (before 6 weeks) mdx muscles have low taurine content, with taurine content increasing with age. In one study, adult (>6 week) mdx muscle had increased taurine content compared with wild-type muscle [100]. These data suggest that taurine content of mdx muscle increases with stabilisation of pathology. Our study shows that GRMD dogs aged 8 months have increased muscle taurine content, despite already accumulating severe dystropathology. This level of taurine may be a feature of adult dystrophic muscles, since taurine levels are lower in juvenile mdx mice (aged 18 days) during the intensive growth phase, associated with the time when onset of muscle necrosis is pronounced; it may be of interest to examine taurine levels and metabolism in much younger GRMD dogs. Regardless, these data do not support our hypothesis that a taurine deficiency in GRMD muscle may render the muscle more susceptible to oxidative stress. However, since taurine is found in high concentrations in tissues exposed to elevated levels of oxidants [99], the increased taurine content of GRMD muscle aged 8 months may reflect an upregulation of taurine (due to increased taurine requirements). This is reflected in the upregulation of TauT, cysteine deoxygenase and cysteine sulfinate decarboxylase we observed in GRMD muscle. Interestingly, this was not observed in mdx mice, where we have previously described downregulation of TauT in both young (18 day) and adult (6 week) mdx muscle, and a downregulation of cysteine deoxygenase at 4 weeks of age [31].
A further species difference is that while plasma of adult mdx mice has normal levels of taurine [31], GRMD plasma is deficient in taurine. This deficiency in plasma taurine may reflect increased taurine excretion in the kidney or decreased taurine synthesis in the liver, that is observed in juvenile mdx mice [31], however these parameters were not examined in the dystrophic dogs (since liver and kidney tissue was not available). It has also been suggested that after an injury, activated neutrophils sequester taurine from the plasma to the site of injury, leading to a drop in plasma taurine concentrations [101]. Therefore the differences observed (in both muscle and plasma values) between the mdx and GRMD animals may reflect the striking contrast in severity of pathology at the ages examined for these species.
The differences may also relate to striking interspecies differences in neutrophil content of blood, since neutrophils represent only a relatively very small proportion, 10–25%, of leucocytes in mice, whereas neutrophils are far more prevalent in dog and human blood, where they represent about 70% and 50–70% (respectively) of leucocytes [102], [103]. This is an important point since the much higher neutrophil levels in dogs (and humans), and the 35 fold increase of neutrophils in GRMD muscles, implies a much greater role for the adverse effects of neutrophils in the severity of dystropathology in dogs and humans.
5. Conclusions
Our data emphasise the value of the use of GRMD dogs in the evaluation of the role of immune cell-generated HOCl in dystropathology, and suggest that the dystrophic dogs are a particularly useful model for pre-clinical trials of interventions that target this pathway. Our data suggest that taurine homeostasis is regulated in a different manner in dystrophic dogs (compared with mdx mice) and this information, combined with the similar high neutrophil content of dog and human blood emphasises the importance of using the GRMD dog model for pre-clinical testing of therapies such as taurine. However, it is not apparent whether taurine treatment will be beneficial for GRMD dogs; whilst GRMD plasma is deficient in taurine, GRMD muscle exhibits excess taurine, and it not clear whether it is intracellular or vascular taurine that is important for targeting HOCl in GRMD muscle. Future research is required to better describe the metabolism of taurine in GRMD dogs at different stages of dystropthology, to understand the use of taurine in preventing myonecrotic damage caused by inflammatory cell generated oxidants, and to evaluate the potential of taurine as a suitable therapeutic intervention for DMD boys.
Conflict of interest statement
All authors have no financial or personal conflict with other people or organisations that could inappropriately influence our work.
Acknowledgments
This research was supported by funding from the National Health and Medical Research Council (NHMRC) (APP1065829) of Australia.
References
- 1.Bushby K., Finkel R., Birnkrant D.J., Case L.E., Clemens P.R., Cripe L., Kaul A., Kinnett K., McDonald C., Pandya S., Poysky J., Shapiro F., Tomezsko J., Constantin C., Group D.M.D.C.C.W. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9(1):77–93. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 2.Emery A.E. The muscular dystrophies. Lancet. 2002;359(9307):687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 3.Grounds M.D. Two-tiered hypotheses for Duchenne muscular dystrophy. Cell. Mol. Life Sci. 2008;65(11):1621–1625. doi: 10.1007/s00018-008-7574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kharraz Y., Guerra J., Pessina P., Serrano A.L., Munoz-Canoves P. Understanding the process of fibrosis in Duchenne muscular dystrophy. Biomed. Res. Int. 2014;2014:965631. doi: 10.1155/2014/965631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falzarano M.S., Scotton C., Passarelli C., Ferlini A. Duchenne muscular dystrophy: from diagnosis to therapy. Molecules. 2015;20(10):18168–18184. doi: 10.3390/molecules201018168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biggar W. Duchenne muscular dystrophy. Pediatr. Rev. 2006;27(3):83–88. doi: 10.1542/pir.27-3-83. [DOI] [PubMed] [Google Scholar]
- 7.Allen D.G., Whitehead N.P., Froehner S.C. Absence of dystrophin disrupts skeletal muscle signaling: roles of Ca2+, reactive oxygen species, and nitric oxide in the development of muscular dystrophy. Physiol. Rev. 2016;96(1):253–305. doi: 10.1152/physrev.00007.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arthur P.G., Grounds M.D., Shavlakadze T. Oxidative stress as a therapeutic target during muscle wasting: considering the complex interactions. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11(4):408–416. doi: 10.1097/MCO.0b013e328302f3fe. [DOI] [PubMed] [Google Scholar]
- 9.Kim J.-H., Kwak H.-B., Thompson L.V., Lawler J.M. Contribution of oxidative stress to pathology in diaphragm and limb muscles with Duchenne muscular dystrophy. J. Muscle Res. Cell Motil. 2013;34(1):1–13. doi: 10.1007/s10974-012-9330-9. [DOI] [PubMed] [Google Scholar]
- 10.Haycock J.W., Neil S.M., Jones P., Harris J.B., Mantle D. Oxidative damage to muscle protein in Duchenne muscular dystrophy. Neuroreport. 1996;8(1):357–361. doi: 10.1097/00001756-199612200-00070. [DOI] [PubMed] [Google Scholar]
- 11.Renjini R., Gayathri N., Nalini A., Srinivas Bharath M. Oxidative damage in muscular dystrophy correlates with the severity of the pathology: role of glutathione metabolism. Neurochem. Res. 2012:1–14. doi: 10.1007/s11064-011-0683-z. [DOI] [PubMed] [Google Scholar]
- 12.Disatnik M.H., Chamberlain J.S., Rando T.A. Dystrophin mutations predict cellular susceptibility to oxidative stress. Muscle Nerve. 2000;23(5):784–792. doi: 10.1002/(sici)1097-4598(200005)23:5<784::aid-mus17>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 13.Kaczor J.J., Hall J.E., Payne E., Tarnopolsky M.A. Low intensity training decreases markers of oxidative stress in skeletal muscle of mdx mice. Free Radic. Biol. Med. 2007;43(1):145–154. doi: 10.1016/j.freeradbiomed.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 14.El-Shafey A., Armstrong A., Terrill J., Grounds M., Arthur P. Screening for increased protein thiol oxidation in oxidatively stress muscle tissue. Free Radic. Res. 2011;45:991–999. doi: 10.3109/10715762.2011.590136. [DOI] [PubMed] [Google Scholar]
- 15.Eaton P. Protein thiol oxidation in health and disease: techniques for measuring disulfides and related modifications in complex protein mixtures. Free Radic. Biol. Med. 2006;40(11):1889–1899. doi: 10.1016/j.freeradbiomed.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 16.Zuo L., Pannell B.K. Redox characterization of functioning skeletal muscle. Front. Physiol. 2015;6:338. doi: 10.3389/fphys.2015.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwasaki T., Terrill J., Shavlakadze T., Grounds M.D., Arthur P.G. Visualizing and quantifying oxidized protein thiols in tissue sections: a comparison of dystrophic mdx and normal skeletal mouse muscles. Free Radic. Biol. Med. 2013;65:1408–1416. doi: 10.1016/j.freeradbiomed.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 18.Radley-Crabb H., Terrill J., Shavlakadze T., Tonkin J., Arthur P., Grounds M.D. A single 30 min treadmill exercise session is suitable for ‘proof-of concept studies’ in adult mdx mice: a comparison of the early consequences of two different treadmill protocols. Neuromuscul. Disord. 2012;22(2):170–182. doi: 10.1016/j.nmd.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Terrill J.R., Boyatzis A., Grounds M.D., Arthur P.G. Treatment with the cysteine precursor l-2-oxothiazolidine-4-carboxylate (OTC) implicates taurine deficiency in severity of dystropathology in mdx mice. Int. J. Biochem. Cell Biol. 2013;45(9):2097–2108. doi: 10.1016/j.biocel.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Terrill J.R., Radley-Crabb H.G., Grounds M.D., Arthur P.G. N-acetylcysteine treatment of dystrophic mdx mice results in protein thiol modifications and inhibition of exercise induced myofibre necrosis. Neuromuscul. Disord. 2012;22:422–434. doi: 10.1016/j.nmd.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Terrill J.R., Radley-Crabb H.G., Iwasaki T., Lemckert F.A., Arthur P.G., Grounds M.D. Oxidative stress and pathology in muscular dystrophies: focus on protein thiol oxidation and dysferlinopathies. FEBS J. 2013;280(17):4149–4164. doi: 10.1111/febs.12142. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong A.E., Zerbes R., Fournier P.A., Arthur P.G. A fluorescent dual labeling technique for the quantitative measurement of reduced and oxidized protein thiols in tissue samples. Free Radic. Biol. Med. 2010;50:510–517. doi: 10.1016/j.freeradbiomed.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Terrill J.R., Pinniger G.J., Graves J.A., Grounds M.D., Arthur P.G. Increasing taurine intake and taurine synthesis improves skeletal muscle function in the mdx mouse model for Duchenne Muscular Dystrophy. J. Physiol. 2016;594(11):3095–3110. doi: 10.1113/JP271418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitehead N.P., Pham C., Gervasio O.L., Allen D.G. N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J. Physiol. 2008;586(7):2003–2014. doi: 10.1113/jphysiol.2007.148338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Senzi Moraes Pinto R., Ferretti R., Moraes L.H.R., Neto H.S., Marques M.J., Minatel E. N-Acetylcysteine treatment reduces TNF-α levels and myonecrosis in diaphragm muscle of mdx mice. Clin. Nutr. 2012 doi: 10.1016/j.clnu.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Rapucci Moraes L.H., Bollineli R.C., Mizobuti D.S., dos Reis Silveira L., Marques M.J., Minatel E. Effect of N-acetylcysteine plus deferoxamine on oxidative stress and inflammation in dystrophic muscle cells. Redox Rep. 2014 doi: 10.1179/1351000214Y.0000000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zafarullah M., Li W.Q., Sylvester J., Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell. Mol. Life Sci. 2003;60(1):6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferreira L.F., Gilliam L.A.A., Reid M.B. L-2-Oxothiazolidine-4-carboxylate reverses glutathione oxidation and delays fatigue of skeletal muscle in vitro. J. Appl. Physiol. 2009;107(1):211. doi: 10.1152/japplphysiol.00001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stipanuk M.H., Dominy J.E., Lee J.I., Coloso R.M. Mammalian cysteine metabolism: New insights into regulation of cysteine metabolism. J. Nutr. 2006;136(6):1652S–1659S. doi: 10.1093/jn/136.6.1652S. [DOI] [PubMed] [Google Scholar]
- 30.Stipanuk M.H. Role of the liver in regulation of body cysteine and taurine levels: a brief review. Neurochem. Res. 2004;29(1):105–110. doi: 10.1023/b:nere.0000010438.40376.c9. [DOI] [PubMed] [Google Scholar]
- 31.Terrill J.R., Grounds M.D., Arthur P.G. Taurine deficiency, synthesis and transport in the mdx mouse model for Duchenne Muscular Dystrophy. Int. J. Biochem. Cell Biol. 2015;66:141–148. doi: 10.1016/j.biocel.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Cozzoli A., Rolland J.F., Capogrosso R.F., Sblendorio V.T., Longo V., Simonetti S., Nico B., De Luca A. Evaluation of potential synergistic action of a combined treatment with alpha-methyl-prednisolone and taurine on the mdx mouse model of Duchenne muscular dystrophy. Neuropathol. Appl. Neurobiol. 2011;37(3):243–256. doi: 10.1111/j.1365-2990.2010.01106.x. [DOI] [PubMed] [Google Scholar]
- 33.De Luca A., Pierno S., Liantonio A., Cetrone M., Camerino C., Fraysse B., Mirabella M., Servidei S., Ruegg U.T., Conte Camerino D. Enhanced dystrophic progression in mdx mice by exercise and beneficial effects of taurine and insulin-like growth factor-1. J. Pharmacol. Exp. Ther. 2003;304(1):453–463. doi: 10.1124/jpet.102.041343. [DOI] [PubMed] [Google Scholar]
- 34.Terrill J.R., Grounds M.D., Arthur P.G. Increased taurine in pre-weaned juvenile mdx mice greatly reduces the acute onset of myofibre necrosis and dystropathology and prevents inflammation. PLoS Curr. 2016 doi: 10.1371/currents.md.77be6ec30e8caf19529a00417614a072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bakker A.J., Berg H.M. Effect of taurine on sarcoplasmic reticulum function and force in skinned fast-twitch skeletal muscle fibres of the rat. J. Physiol. 2004;538(1):185–194. doi: 10.1113/jphysiol.2001.012872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamilton E.J., Berg H.M., Easton C.J., Bakker A.J. The effect of taurine depletion on the contractile properties and fatigue in fast-twitch skeletal muscle of the mouse. Amino Acids. 2006;31(3):273–278. doi: 10.1007/s00726-006-0291-4. [DOI] [PubMed] [Google Scholar]
- 37.Huxtable R.J. Physiological actions of taurine. Physiol. Rev. 1992;72(1):101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 38.Warskulat U., Flogel U., Jacoby C., Hartwig H.G., Thewissen M., Merx M.W., Molojavyi A., Heller-Stilb B., Schrader J., Haussinger D. Taurine transporter knockout depletes muscle taurine levels and results in severe skeletal muscle impairment but leaves cardiac function uncompromised. FASEB J. 2004;18(3):577–579. doi: 10.1096/fj.03-0496fje. [DOI] [PubMed] [Google Scholar]
- 39.Warskulat U., Heller-Stilb B., Oermann E., Zilles K., Haas H., Lang F., Haussinger D. Phenotype of the taurine transporter knockout mouse. Methods Enzym. 2007;428:439–458. doi: 10.1016/S0076-6879(07)28025-5. [DOI] [PubMed] [Google Scholar]
- 40.Camerino D.C., Tricarico D., Pierno S., Desaphy J.F., Liantonio A., Pusch M., Burdi R., Camerino C., Fraysse B., De Luca A. Taurine and skeletal muscle disorders. Neurochem. Res. 2004;29(1):135–142. doi: 10.1023/b:nere.0000010442.89826.9c. [DOI] [PubMed] [Google Scholar]
- 41.Marcinkiewicz J., Kontny E. Taurine and inflammatory diseases. Amino Acids. 2014;46(1):7–20. doi: 10.1007/s00726-012-1361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugiyama S., Okada Y., Sukhova G.K., Virmani R., Heinecke J.W., Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am. J. Pathol. 2001;158(3):879–891. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kornegay J.N., Bogan J.R., Bogan D.J., Childers M.K., Li J., Nghiem P., Detwiler D.A., Larsen C.A., Grange R.W., Bhavaraju-Sanka R.K. Canine models of Duchenne muscular dystrophy and their use in therapeutic strategies. Mamm. Genome. 2012:1–24. doi: 10.1007/s00335-011-9382-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huxtable R. Expanding the circle 1975–1999: sulfur biochemistry and insights on the biological functions of taurine. Taurine. 2002;4:1–25. [PubMed] [Google Scholar]
- 45.Partridge T.A. The mdx mouse model as a surrogate for Duchenne muscular dystrophy. FEBS J. 2013;280(17):4177–4186. doi: 10.1111/febs.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharp N.J., Kornegay J.N., Van Camp S.D., Herbstreith M.H., Secore S.L., Kettle S., Hung W.Y., Constantinou C.D., Dykstra M.J., Roses A.D. An error in dystrophin mRNA processing in golden retriever muscular dystrophy, an animal homologue of Duchenne muscular dystrophy. Genomics. 1992;13(1):115–121. doi: 10.1016/0888-7543(92)90210-j. [DOI] [PubMed] [Google Scholar]
- 47.Sampaolesi M., Blot S., D'Antona G., Granger N., Tonlorenzi R., Innocenzi A., Mognol P., Thibaud J.L., Galvez B.G., Barthelemy I., Perani L., Mantero S., Guttinger M., Pansarasa O., Rinaldi C., Cusella De Angelis M.G., Torrente Y., Bordignon C., Bottinelli R., Cossu G. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444(7119):574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 48.McGreevy J.W., Hakim C.H., McIntosh M.A., Duan D. Animal models of Duchenne muscular dystrophy: from basic mechanisms to gene therapy. Dis. Models Mech. 2015;8(3):195–213. doi: 10.1242/dmm.018424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu X., Bao B., Echigoya Y., Yokota T. Dystrophin-deficient large animal models: translational research and exon skipping. Am. J. Transl. Res. 2015;7(8):1314. [PMC free article] [PubMed] [Google Scholar]
- 50.Willmann R., Possekel S., Dubach-Powell J., Meier T., Ruegg M.A. Mammalian animal models for Duchenne muscular dystrophy. Neuromuscul. Disord. 2009;19(4):241–249. doi: 10.1016/j.nmd.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 51.Ko K.S., Backus R.C., Berg J.R., Lame M.W., Rogers Q.R. Differences in taurine synthesis rate among dogs relate to differences in their maintenance energy requirement. J. Nutr. 2007;137(5):1171–1175. doi: 10.1093/jn/137.5.1171. [DOI] [PubMed] [Google Scholar]
- 52.Winterbourn C.C., Kettle A.J. Biomarkers of myeloperoxidase-derived hypochlorous acid. Free Radic. Biol. Med. 2000;29(5):403–409. doi: 10.1016/s0891-5849(00)00204-5. [DOI] [PubMed] [Google Scholar]
- 53.Ito T., Oishi S., Takai M., Kimura Y., Uozumi Y., Fujio Y., Schaffer S.W., Azuma J. Cardiac and skeletal muscle abnormality in taurine transporter-knockout mice. J. Biomed. Sci. 2010;17(Suppl 1):S1–S5. doi: 10.1186/1423-0127-17-S1-S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kettle A.J., Chan T., Osberg I., Senthilmohan R., Chapman A.L., Mocatta T.J., Wagener J.S. Myeloperoxidase and protein oxidation in the airways of young children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2004;170(12):1317–1323. doi: 10.1164/rccm.200311-1516OC. [DOI] [PubMed] [Google Scholar]
- 56.Levine R.L., Garland D., Oliver C.N., Amici A., Climent I., Lenz A.G., Ahn B.W., Shaltiel S., Stadtman E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzym. 1990;186:464. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 57.Hawkins C.L., Morgan P.E., Davies M.J. Quantification of protein modification by oxidants. Free Radic. Biol. Med. 2009;46(8):965–988. doi: 10.1016/j.freeradbiomed.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 58.Inoue T., Plieth D., Venkov C.D., Xu C., Neilson E.G. Antibodies against macrophages that overlap in specificity with fibroblasts. Kidney Int. 2005;67(6):2488–2493. doi: 10.1111/j.1523-1755.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- 59.Kettle A.J. Neutrophils convert tyrosyl residues in albumin to chlorotyrosine. FEBS Lett. 1996;379(1):103–106. doi: 10.1016/0014-5793(95)01494-2. [DOI] [PubMed] [Google Scholar]
- 60.Tidball J.G. Inflammatory processes in muscle injury and repair. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288(2):R345–R353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 61.Brunelli S., Rovere-Querini P. The immune system and the repair of skeletal muscle. Pharmacol. Res. 2008;58(2):117–121. doi: 10.1016/j.phrs.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 62.Tidball J.G., Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J. Physiol. 2007;578(Pt 1):327–336. doi: 10.1113/jphysiol.2006.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grounds M.D. The need to more precisely define aspects of skeletal muscle regeneration. Int. J. Biochem. Cell Biol. 2014;56:56–65. doi: 10.1016/j.biocel.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 64.Hampton M.B., Kettle A.J., Winterbourn C.C. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92(9):3007–3017. [PubMed] [Google Scholar]
- 65.Farnaud S., Evans R.W. Lactoferrin—a multifunctional protein with antimicrobial properties. Mol. Immunol. 2003;40(7):395–405. doi: 10.1016/s0161-5890(03)00152-4. [DOI] [PubMed] [Google Scholar]
- 66.Winterbourn C.C. Biological reactivity and biomarkers of the neutrophil oxidant, hypochlorous acid. Toxicology. 2002;181–182:223–227. doi: 10.1016/s0300-483x(02)00286-x. [DOI] [PubMed] [Google Scholar]
- 67.Halliwell B., Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br. J. Pharm. 2004;142(2):231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dalle-Donne I., Giustarini D., Colombo R., Rossi R., Milzani A. Protein carbonylation in human diseases. Trends Mol. Med. 2003;9(4):169–176. doi: 10.1016/s1471-4914(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 69.Stadtman E., Levine R. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25(3–4):207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 70.Winterbourn C.C., Hampton M.B. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 2008;45(5):549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 71.Klomsiri C., Karplus P.A., Poole L.B. Cysteine-based redox switches in enzymes. Antioxid. Redox Signal. 2011;14(6):1065–1077. doi: 10.1089/ars.2010.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Andrade F.H., Reid M.B., Allen D.G., Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J. Physiol. 509. 1998;(Pt 2):565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dalle-Donne I., Giustarini D., Rossi R., Colombo R., Milzani A. Reversible S-glutathionylation of Cys374 regulates actin filament formation by inducing structural changes in the actin molecule. Free Radic. Biol. MEd. 2003;34(1):23–32. doi: 10.1016/s0891-5849(02)01182-6. [DOI] [PubMed] [Google Scholar]
- 74.Prochniewicz E., Spakowicz D., Thomas D.D. Changes in actin structural transitions associated with oxidative inhibition of muscle contraction. Biochemistry. 2008;47(45):11811–11817. doi: 10.1021/bi801080x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tiago T., Simao S., Aureliano M., Martin-Romero F.J., Gutierrez-Merino C. Inhibition of skeletal muscle S1-myosin ATPase by peroxynitrite. Biochemistry. 2006;45(11):3794–3804. doi: 10.1021/bi0518500. [DOI] [PubMed] [Google Scholar]
- 76.Pinto J.R., de Sousa V.P., Sorenson M.M. Redox state of troponin C cysteine in the D/E helix alters the C-domain affinity for the thin filament of vertebrate striated muscle. Biochim. Biophys. Acta. 2011;1810(4):391–397. doi: 10.1016/j.bbagen.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 77.Andrade F.H., Reid M.B., Westerblad H. Contractile response of skeletal muscle to low peroxide concentrations: myofibrillar calcium sensitivity as a likely target for redox-modulation. FASEB J. 2001;15(2):309–311. doi: 10.1096/fj.00-0507fje. [DOI] [PubMed] [Google Scholar]
- 78.Mollica J.P., Dutka T.L., Merry T.L., Lamboley C.R., McConell G.K., McKenna M.J., Murphy R.M., Lamb G.D. S-glutathionylation of troponin I (fast) increases contractile apparatus Ca2+ sensitivity in fast-twitch muscle fibres of rats and humans. J. Physiol. 2012;590(Pt 6):1443–1463. doi: 10.1113/jphysiol.2011.224535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moen R.J., Cornea S., Oseid D.E., Binder B.P., Klein J.C., Thomas D.D. Redox-sensitive residue in the actin-binding interface of myosin. Biochem. Biophys. Res. Commun. 2014;453(3):345–349. doi: 10.1016/j.bbrc.2014.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Root D.D., Reisler E. Cooperativity of thiol-modified myosin filaments. ATPase and motility assays of myosin function. Biophys. J. 1992;63(3):730–740. doi: 10.1016/S0006-3495(92)81646-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perkins W.J., Han Y.-S., Sieck G.C. Skeletal muscle force and actomyosin ATPase activity reduced by nitric oxide donor. J. Appl. Physiol. 1997;83(4):1326–1332. doi: 10.1152/jappl.1997.83.4.1326. [DOI] [PubMed] [Google Scholar]
- 82.Ackermann M.A., Kontrogianni-Konstantopoulos A. Myosin binding protein-C: a regulator of actomyosin interaction in striated muscle. J. Biomed. Biotechnol. 2011;2011:636403. doi: 10.1155/2011/636403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salmikangas P., Mykkänen O.-M., Grönholm M., Heiska L., Kere J., Carpén O. Myotilin, a novel sarcomeric protein with two Ig-like domains, is encoded by a candidate gene for limb-girdle muscular dystrophy. Hum. Mol. Genet. 1999;8(7):1329–1336. doi: 10.1093/hmg/8.7.1329. [DOI] [PubMed] [Google Scholar]
- 84.Patel B.G., Wilder T., Solaro R.J. Novel control of cardiac myofilament response to calcium by S-glutathionylation at specific sites of myosin binding protein C. Front. Physiol. 2013;4 doi: 10.3389/fphys.2013.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Toyoshima C. How Ca 2+-ATPase pumps ions across the sarcoplasmic Reticulum Membrane. Biochim. Biophys. Acta – Mol. Cell Res. 2009;1793(6):941–946. doi: 10.1016/j.bbamcr.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 86.Najjar V.A. The isolation and properties of phosphoglucomutase. J. Biol. Chem. 1948;175(1):281–290. [PubMed] [Google Scholar]
- 87.Gupta V., Bamezai R.N. Human pyruvate kinase M2: a multifunctional protein. Protein Sci. 2010;19(11):2031–2044. doi: 10.1002/pro.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reid M.B. Invited Review: redox modulation of skeletal muscle contraction: what we know and what we don't. J. Appl. Physiol. 2001;90(2):724–731. doi: 10.1152/jappl.2001.90.2.724. [DOI] [PubMed] [Google Scholar]
- 89.Anastasiou D., Poulogiannis G., Asara J.M., Boxer M.B., Jiang J.-k, Shen M., Bellinger G., Sasaki A.T., Locasale J.W., Auld D.S. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334(6060):1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tomich J.M., Marti C., Colman R.F. Modification of two essential cysteines in rabbit muscle pyruvate kinase by the guanine nucleotide analog 5'-[p-(fluorosulfonyl) benzoyl] guanosine. Biochemistry. 1981;20(23):6711–6720. doi: 10.1021/bi00526a029. [DOI] [PubMed] [Google Scholar]
- 91.Sirover M.A. Role of the glycolytic protein, glyceraldehyde‐3–phosphate dehydrogenase, in normal cell function and in cell pathology. J. Cell. Biochem. 1997;66(2):133–140. [PubMed] [Google Scholar]
- 92.Bloxham D.P., Cooper G.K. Formation of a polymethylene bis (disulfide) intersubunit crosslink between cysteine-281 residues in rabbit muscle glyceraldehyde-3-phosphate dehydrogenase using octamethylene bis (methane [35S] thiosulfonate) Biochemistry. 1982;21(8):1807–1812. doi: 10.1021/bi00537a016. [DOI] [PubMed] [Google Scholar]
- 93.Eaton P., Shattock M.J. Purification of proteins susceptible to oxidation at cysteine residues: Identification of malate dehydrogenase as a target for S-glutathiolation. Ann. N. Y. Acad. Sci. 2002;973(1):529–532. doi: 10.1111/j.1749-6632.2002.tb04694.x. [DOI] [PubMed] [Google Scholar]
- 94.Metz-Boutigue M.H., Jollés J., Mazurier J., Schoentgen F., Legrand D., Spik G., Montreuil J., Jollès P. Human lactotransferrin: amino acid sequence and structural comparisons with other transferrins. Eur. J. Biochem. 1984;145(3):667–676. doi: 10.1111/j.1432-1033.1984.tb08607.x. [DOI] [PubMed] [Google Scholar]
- 95.Roche M., Rondeau P., Singh N.R., Tarnus E., Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008;582(13):1783–1787. doi: 10.1016/j.febslet.2008.04.057. [DOI] [PubMed] [Google Scholar]
- 96.Zaias J., Mineau M., Cray C., Yoon D., Altman N.H. Reference values for serum proteins of common laboratory rodent strains. J. Am. Assoc. Lab. Anim. Sci. 2009;48(4):387–390. [PMC free article] [PubMed] [Google Scholar]
- 97.Carballal S., Alvarez B., Turell L., Botti H., Freeman B.A., Radi R. Sulfenic acid in human serum albumin. Amino Acids. 2007;32(4):543–551. doi: 10.1007/s00726-006-0430-y. [DOI] [PubMed] [Google Scholar]
- 98.Peskin A.V., Winterbourn C.C. Taurine chloramine is more selective than hypochlorous acid at targeting critical cysteines and inactivating creatine kinase and glyceraldehyde-3-phosphate dehydrogenase. Free Radic. Biol. Med. 2006;40(1):45–53. doi: 10.1016/j.freeradbiomed.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 99.Schuller-Levis G.B., Park E. Taurine and its chloramine: modulators of immunity. Neurochem. Res. 2004;29(1):117–126. doi: 10.1023/b:nere.0000010440.37629.17. [DOI] [PubMed] [Google Scholar]
- 100.McIntosh L., Granberg K.E., Brière K.M., Anderson J.E. Nuclear magnetic resonance spectroscopy study of muscle growth, mdx dystrophy and glucocorticoid treatments: correlation with repair. NMR Biomed. 1998;11(1):1–10. doi: 10.1002/(sici)1099-1492(199802)11:1<1::aid-nbm493>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 101.McLaughlin R., Bowler D., Kelly C.J., Kay E., Bouchier-Hayes D. Taurine protects against early and late skeletal muscle dysfunction secondary to ischaemia reperfusion injury. Eur. J. Surg. 2000;166(5):375–379. doi: 10.1080/110241500750008916. [DOI] [PubMed] [Google Scholar]
- 102.Papasouliotis K., Cue S., Crawford E., Pinches M., Dumont M., Burley K. Comparison of white blood cell differential percentages determined by the in-house LaserCyte hematology analyzer and a manual method. Vet. Clin. Pathol. 2006;35(3):295–302. doi: 10.1111/j.1939-165x.2006.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 103.Mestas J., Hughes C.C. Of mice and not men: differences between mouse and human immunology. J. Immunol. 2004;172(5):2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]