Abstract
Introduction
Hughes-Stovin syndrome is a life-threatening disorder of unknown etiology. This condition is characterized by vasculitis, deep venous thrombosis and aneurysms that mainly involve the pulmonary arteries resulting in hemoptysis. It has been described in literature less than 40 times. However, we believe it is not very uncommon as it might be diagnosed as pulmonary embolism solely. In such cases, anticoagulation therapy augments the risk of life-threatening hemoptysis.
Materials and methods
We report the case of a 35 years old, Egyptian female patient with Hughes-Stovin syndrome, who initially presented with lower limb deep vein thrombosis and coughing of blood. Anticoagulation regimen for pulmonary embolism was given. This resulted in massive hemoptysis that was successfully controlled by medical therapy.
Conclusion
Adults who present with venous thrombosis and hemoptoic cough, with no predisposing factors of thrombosis, normal platelet count and coagulation, the possibility of Hughes-Stovin syndrome has to be considered.
Keywords: Deep venous thrombosis, Thrombophlebitis, Hemoptysis, Pulmonary embolism, Pulmonary aneurysm
1. Introduction
Hughes-Stovin syndrome (HSS) is a life-threatening disorder of unknown etiology. This condition is usually presented by vasculitis, deep venous thrombosis and aneurysms that usually involve the pulmonary arteries as well as the bronchial arteries resulting in hemoptysis. In such cases, using anticoagulant therapy might be life-threatening as it augments the risk of hemoptysis [1], [4].
2. Case report
A 35 years old female presented at the emergency room with coughing of blood (about half a cup all over the day) associated with bilateral below knee level non-pitting edema of two days' duration, more evident on the right side. She reported that her illness started four years ago by lower limb edema, tense and tender calf muscles and was diagnosed as bilateral acute deep venous thrombosis. She received anticoagulation therapy and stopped by herself after few months. One year ago, she developed dyspnea on moderate exertion, hemoptoic cough, chest pain and erythema nodosum. At that time, she was advised to take anti-inflammatory drugs and steroids to control the erythema nodosum, underwent lower limb duplex scan and multislice CT angiography of the pulmonary arteries that revealed bilateral chronic DVT and bilateral pulmonary embolism respectively. Vascular surgery didn't support the idea of IVC filter placement and advised to keep the patient on LMWH that resulted in partial improvement.
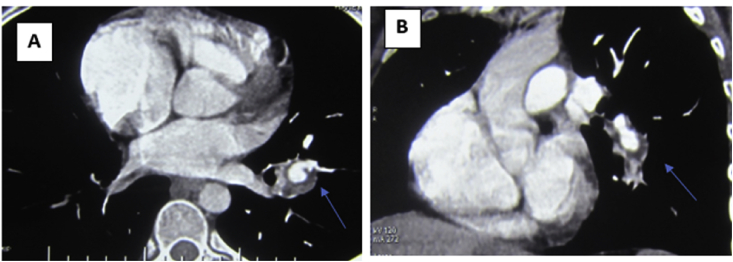
Based on the clinical presentation and patient's medical history she was admitted, kept on LMWH 80 IU twice per day and Warfarin 5mg/day. A new lower limb venous duplex scan and Multislice CT scan of the pulmonary arteries revealed Subacute bilateral DVT with similar findings in the CT pulmonary angiography Which stated the existence of thrombotic filling defects that involved the main right pulmonary artery, extending partially into the segmental lower lobe artery branches with adjacent ectatic bronchial arteries (Fig. 1), also with a smaller thrombotic circumferential filling defect at the left lower lobe artery branch, preserving a centrally patent residual lumen (Fig. 2). Unfortunately, such findings of suspicious pulmonary aneurysms with thrombotic process inside as well as dilated ectatic bronchial arteries could not be correlated at the time of presentation to Hughes Stovin syndrome (see Fig. 3).
Fig. 1.
CT angiography of the pulmonary arteries (a) Axial view (b) Coronal view showing right main pulmonary artery aneurysm with circumferential thrombotic filling defect (Thin arrows) and ectatic adjacent bronchial arteries (Thick arrows).
Fig. 2.
CT angiography of the pulmonary arteries (a) Axial view (b) Coronal view showing left lower lobe pulmonary artery aneurysm with mural thrombotic filling defect (Thin arrows).
Fig. 3.
Catheter angiography of the pulmonary arteries(a) Right pulmonary artery aneurysm (thick arrow) with lower lobe perfusion defect (curved arrow) (b) Left segmental pulmonary artery aneurysm (thick arrow).
Associated clinical findings included few primary erythema nodosum for which methylprednisolone 8mg 1 tablet/day and colchicine 0.5 mg two tablets twice daily were given. Co-existing vaginal bleeding was also investigated by transvaginal ultrasound that revealed right-sided hemorrhagic ovarian cyst.
Vital data and general laboratory work up like CBC, liver and kidney functions, T3, T4 and TSH were all fine. ESR was 74, ANA, ANCA, Antiphospholipid antibody, lupus anticoagulant, anticardiolipin, PCR for factor V and sputum culture and pathergy tests were all negative. Patient was diagnosed as DVT with PE and received the classical anticoagulation therapy, stayed in the hospital for few days.
After few days, hemoptoic cough stopped with remarkable improvement of the limb swelling and tense calf muscles. A decision was made to discharge the patient on anticoagulation therapy and monitor the coagulation profile. One week later, she presented with massive hemoptysis and hypovolemic shock that was medically corrected.
Bronchoscopy revealed dilated tortuous submucosal vessels on the bronchus intermedius and lower lobe bronchi on the right side as well as the anterior and posterior basal segments on the left side. Moreover, the lateral wall of the lower lobe bronchus showed submucosal pulsating bulge with free overlying mucosa. Such findings of vascular malformation and aneurysmal dilatation were highly consistent with the CT angiography findings, and biopsy was avoided.
Conventional pulmonary angiography done on the next day for detailed evaluation of the aneurysms and possibility of embolization.
A multidisciplinary discussion between the pulmonologists, thoracic surgeons and interventional radiologists finally decided that either this case is a cardiovascular form of Behcet or Hughes Stovin syndrome, anticoagulation therapy as well as any surgical or interventional therapy must be primarily avoided. We also decided to combine pulse therapy with methylprednisolone (1 g for three days) and cyclophosphamide (1 g per monthly session) for initial management and stabilization of the aneurysms. No attacks of hemoptysis recurred and the patient was discharged. She did not show up in the reconsultation dates. One year later, she got pregnant despite contraception yet spontaneous abortion occurred at about 8 weeks. Her family reported she was not totally compliant to the given therapy and refuses the idea of trans-catheter embolization. Fortunately, she did not develop another attack of hemoptysis.
3. Discussion
Hughes-Stovin syndrome is a life-threatening disorder of unknown etiology. It has been described to be a variant of Behçet's disease. Men aged between 12 and 40 years are the most likely affected, and usually present with hemoptoic cough, dyspnea, chest pain and signs of pulmonary hypertension following history of deep venous thrombosis [1]. Prevalence is unknown but less than 35 cases have been published in literature since its first description in 1959 by Hughes and Stovin [2].
Hughes-Stovin syndrome clinically passes into three phases: a first phase involving symptoms of thrombophlebitis, a second phase involving formation of pulmonary artery aneurysms, and a third phase of aneurysmal rupture that results in massive hemoptysis that is usually fatal [3]. Accordingly, we do think that this condition is not very uncommon. Patients presenting with thrombophlebitis with or without chest pain, particularly those who did not develop sizeable aneurysms, would mostly direct the diagnosis and management towards pulmonary embolism and the traditional anticoagulation therapy.
Aneurysms usually affect both the pulmonary and the bronchial arteries resulting in hemoptysis. However, they might occur anywhere in systemic circulation [4]. Recurrent phlebitis is a common associated finding that usually involves the large vessels resulting in thrombus formation. Thrombosis of the vena cava and the right atrium has also been reported [1], [2], [3], [4], [5]. Histologic studies showed destruction of the arterial wall and perivascular lymphomonocytic infiltration of capillaries and venules [6].
The existence of septic emboli and undetected organisms of low-grade virulence has been suggested in the pathogenesis of the pulmonary aneurysms. (Table 1). However, infectious agents, being one of the etiological factors for HSS, have not been supported for two reasons. Firstly, many antibiotic regimens that were tried during the treatment of HSS were not effective. Secondly, no positive blood cultures have been obtained during the evaluation of patients with HSS [4].
Table 1.
Infectious agents involved in the pathogenesis of Behcet's disease (adopted from Umair K. and Taimur S [4].
| Agents | Pertinent rationale or refutation for involvement in Behcet's disease |
|---|---|
| Hepatitis A, B, C, E viruses |
|
| Herpes simplex virus (HSV) |
|
| Parvovirus B19 | Parvovirus B19 IgG antibodies were reported more in patients with Behcet's disease as compared to controls |
| Helicobacter pylori | Almost the same proportion of patients with Behcet's disease and controls were found to have H. pylori infection following eradication therapy. |
| Chlamydia pneumoniae |
|
| Streptococcus sanguis, Streptococcus mitis and Streptococcus salivarius |
|
| Saccharomyces cerevisiae | Unclear role, distribution and pathogenetic relationship of ASCA antibodies in patients with Behcet's disease |
| Heat shock proteins |
|
Currently the most supported opinion about the pathogenesis of this condition is that Hughes-Stovin syndrome results from a vasculitis similar to that occurring in Behçet's disease [1]. Vasculitis in Behçet's disease particularly results in arterial occlusions, arterial aneurysms, venous occlusions, and varices [7], denoting systemic vessel involvement. In our case, Vasculitis of the peripheral veins and the pulmonary arteries was the first presenting sign and preceded the attack of hemoptysis by about four years. Furthermore, it is very likely that Hughes-Stovin syndrome represents a cardiovascular manifestation of Behçet's disease, as in both clinical entities, there is co-existence of pulmonary aneurysms [8]. (Table 2) However, most of the reported cases of Hughes-Stovin syndrome, including ours, were not found to fulfil the clinical manifestations of Behçet's syndrome —oral ulceration, recurrent genital ulcers, eye and skin lesions [9].
Table 2.
Similarities in pulmonary involvement between Behcet's disease and HSS (adopted from Umair K. and Taimur S) [4].
| Characteristic | Details |
|---|---|
| Gender | Predominantly young males |
| Triad of clinical findings | Fever, arthralgia's, thrombosis |
| Occurrence of thrombosis with pulmonary artery aneurysms | HSS – 100%; Behcet's disease - 80% |
| Overlapping histopathologic features | Destruction of arterial walls, perivascular infiltrates |
| Therapy | Cytotoxic drugs and corticosteroids |
| Most common cause of death | Rupture of pulmonary artery aneurysm |
Hemoptysis is the main cause of death in patients with Behçet's disease. However, Hughes-Stovin syndrome is mostly fatal as a result of large volumes of hemoptoic cough due to pulmonary/bronchial arterial aneurysm rupture. Moreover, systemic bronchial artery hypertrophy secondary to ischemia that occurs on top of pulmonary artery occlusion could also be the origin of bleeding. Nevertheless, it has been suggested that bronchial to pulmonary shunting might occur secondary to pulmonary artery thrombosis where existence of an iso-flow and hypertrophy could result in aneurysm formation as well [10]. In our case, ectatic bronchial arteries were seen close to the sites of pulmonary artery aneurysms, a finding that goes with the commonly described pathogenic factors of the disease.
Mahlo et al. and Herb et al. also performed digital subtraction angiography of the bronchial arteries and described the existence of distorted and dilated bronchial arteries with convoluted small branches. Mahlo et al. reported that the cause of death in many of the previously encountered Hughes-Stovin cases is likely due to ruptured angiodysplastic bronchial arteries rather than ruptured pulmonary artery aneurysms [10]. Bronchial artery embolization was performed in both cases and was considered an effective therapeutic approach [11]. Finally, it has been suggested that hemoptysis is likely occurring secondary to both pre-mentioned pathogenic mechanisms [12].
Although conventional pulmonary angiography can be used for better anatomical evaluation of the pulmonary aneurysms [13]. It has been reported that multi-detector row helical CT angiography could provide more precise and accurate visualization of bronchial and non-bronchial systemic arteries than does conventional angiography [14].
In our case CT angiography highlighted the existence of thrombotic filling defects that involved the right main pulmonary artery, extending partially into the segmental lower lobe artery branches, also with a smaller thrombotic circumferential filling defect at the left lower lobe artery branch with a centrally patent residual lumen. Thus, the case was interpreted as pulmonary thromboembolism with suspected pulmonary aneurysms and adjacent ectatic bronchial arteries. However, the pulmonary aneurysms were strongly suggested during bronchoscopy performed after an aggressive attack of hemoptysis. Conventional pulmonary angiography was then performed to give a detailed description of the pulmonary artery pathology and to assess for possibility of embolization.
In our case, the circumferential filling defect at the left lower lobe artery branch with the centrally patent residual lumen found on the CT images confirms the co-existing thrombosis and wall inflammation of the pulmonary artery aneurysm – such a finding is not usually seen in isolated acute or chronic pulmonary embolism [15]. Accordingly, the pathogenesis of pulmonary artery aneurysms in Hughes-Stovin syndrome was attributed to weakening of the vessel wall due to inflammation, which matches what Ketchum et al. reported; that the aneurysms usually develop at the locations of prior thrombus and abnormal enhancement [15].
In our case, the conventional angiography provided superior images compared to those obtained by CT angiography as regards the number, size and location of the pulmonary aneurysms, as well as the accuracy in depicting the adjacent pathological bronchial arteries. This agrees with what Remy-Jardin et al. mentioned [14]. However, we suggest that conventional angiography would be the procedure of choice if lifesaving embolization is going to be carried out.
Conventional angiography would not be applicable in all patients with venous thromboses. Contrast-Enhanced MRA and Contrast-Enhanced MDCTA may provide an alternative [16], [17]. Moreover, 3D volume rendering images can ideally visualize the pathological bronchial arteries even before aneurysm formation [15].
As only few cases were reported and no controlled trials have been obtained, there is no standard therapy for Hughes-Stovin syndrome. However, similar to the treatment regimen applied in Behcet's disease, treatment of Hughes-Stovin syndrome with steroids alone or in combination with immunosuppressant drugs, has been suggested [18]. It has been found that the use of immunosuppressant drugs, either systemic corticosteroids or cytotoxic agents (a combination of cyclophosphamide and glucocorticoids), in patients with Hughes Stovin syndrome without or with small amount of hemoptysis would positively impact the stability of the pulmonary artery aneurysm walls, or even make them disappear [1] If pulmonary embolism is present a therapeutic dilemma often occurs as regards the use of anticoagulants. This is what we faced in our case as there was co-existing thromboembolism with right lower lobe perfusion defect. Anticoagulation may prevent the progression of pulmonary embolism and resolve vein thrombi, but since it increases the risk and severity of hemoptysis, it is not recommended [4].
In our case, we aimed at stabilizing the wall of the aneurysms and reducing the risk of rebleeding so we decided to avoid anticoagulation therapy as well as any surgical or interventional therapy and to combine pulse therapy with methylprednisolone and cyclophosphamide as an initial management. This also agrees with the initial management protocol implemented by Umair K. and Taimur S [4].
Surgical resection of the affected segments of the lung has also been considered in cases of high risk rupture aneurysms limited to one segment or one lung [8].
However, the high morbidity and mortality associated with surgery, and the likelihood of multifocality and bilaterality of the pulmonary artery aneurysms at the time of diagnosis, makes trans-catheter embolization an alternative to surgery as a life saving measure in many cases [1]. Identification of aneurysms in the bronchial arteries could be treated by bronchial artery embolization [11]. Surgical or interventional management was also planned in our case after 6 months of medical therapy aiming to stabilize the aneurysm walls first. However, as previously mentioned, the patient was not totally compliant to the given regimen and refused any further investigations and possible intervention.
4. Conclusion
Hughes Stovin syndrome could be described as an isolated cardiovascular form of Behcet's disease. Patients who present with DVT and PE with no predisposing factor of thrombosis, whether associated with hemoptysis or not, should be meticulously investigated for the coexistence of pulmonary artery aneurysms. Early diagnosis and prompt treatment of HSS would greatly improve the prognosis.
Abbreviations
- ANA
Antinuclear antibody
- ANCA
Anti Neutrophil cytoplasmic antibody
- DVT
Deep vein thrombosis
- HSS
Hughes Stovin syndrome
- HSV
Herpes simplex virus
- IVC
Inferior vena cava
- LMWH
Low molecular weight heparin
- MDCTA
Multidetector CT angiography
- MRA
Magnetic Resonance Angiography
References
- 1.Weintraub J.L., DeMayo R., Haskal Z.J., Susman J. SCVIR annual meeting film panel session: diagnosis and discussion of case 1. J. Vasc. Interv. Radiol. 2001;12:531–534. doi: 10.1016/s1051-0443(07)61897-1. [DOI] [PubMed] [Google Scholar]
- 2.Hughes J.P., Stovin P.G.I. Segmental pulmonary artery aneurysms with peripheral venous thrombosis. Br. J. Dis. Chest. 1959;53:19–27. doi: 10.1016/s0007-0971(59)80106-6. [DOI] [PubMed] [Google Scholar]
- 3.Cruz V., Muniz Y., Teixeira P., Torres S., Teixeira K., Rego J., Silva N. Bras. J. Rheumatol. 2009;49(6):747–752. [Google Scholar]
- 4.Khalid Umair, Saleem Taimur. Orphanet J. Rare Dis. 2011;6:15. doi: 10.1186/1750-1172-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khalil A., Parrot A., Fartoukh M., Marsault C., Carette M.F. Large pulmonary artery aneurysms rupture in Hughes Stovin syndrome: multidetector computed tomography pattern and endovascular treatment. Circulation. 2006;114(10):e380–381. doi: 10.1161/CIRCULATIONAHA.106.614636. [DOI] [PubMed] [Google Scholar]
- 6.Davies J.D. Behcet's syndrome with hemoptysis and pulmonary lesions. J. Pathol. 1973;109(4):351–356. doi: 10.1002/path.1711090410. [DOI] [PubMed] [Google Scholar]
- 7.Park J.H., Han M.C., Bettmann M.A. Arterial manifestations of Behcet disease. AJR. 1984;143:821–825. doi: 10.2214/ajr.143.4.821. [DOI] [PubMed] [Google Scholar]
- 8.Durieux P., Bletry O., Huchon G., Wechsler B., Chretien J., Godeau P. Multiple pulmonary arterial aneurysms in Behcet's disease and Hughes-Stovin syndrome. Am. J. Med. 1981;71:736–741. doi: 10.1016/0002-9343(81)90245-x. [DOI] [PubMed] [Google Scholar]
- 9.Erkan F., Gul A., Tasali E. Pulmonary manifestations of Behcet's disease. Thorax. 2001;56:572–578. doi: 10.1136/thorax.56.7.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahlo H.R., Elsner K., Rieber A. New approach in the diagnosis of and therapy for Hughes-Stovin syndrome. Am. J. Roentgenol. 1996;167:817–818. doi: 10.2214/ajr.167.3.8751710. [DOI] [PubMed] [Google Scholar]
- 11.Herb S., Hetzel M., Hetzel J., Friedrich J., Weber J. An unusual case of Hughes-Stovin syndrome. Eur. Respir. J. 1998;11:1191–1193. doi: 10.1183/09031936.98.11051191. [DOI] [PubMed] [Google Scholar]
- 12.Khalil A., Parrot A., Nedelcu C., Fartoukh M., Marsault C., Carette M.F. Severe hemoptysis of pulmonary arterial origin: signs and role of multidetector row CT angiography. Chest. 2008;133(1):212–219. doi: 10.1378/chest.07-1159. [DOI] [PubMed] [Google Scholar]
- 13.Ammann M.E., Karnel F., Olbert F., Mayer K. Radiologic findings in the diagnosis of Hughes-Stovin syndrome. AJR. 1991;157:1353–1354. doi: 10.2214/ajr.157.6.1950888. [DOI] [PubMed] [Google Scholar]
- 14.Remy-Jardin M., Bouaziz N., Dumont P., Brillet P.Y., Bruzzi J., Remy J. Bronchial and nonbronchial systemic arteries at multi-detector row CT angiography: comparison with conventional angiography. Radiology. 2004;233:741–749. doi: 10.1148/radiol.2333040031. [DOI] [PubMed] [Google Scholar]
- 15.Ketchum E., Zamanian R., Fleischmann D. CT angiography of pulmonary artery aneurysms in Hughes-Stovin Syndrome. AJR. 2005;185:330–332. doi: 10.2214/ajr.185.2.01850330. [DOI] [PubMed] [Google Scholar]
- 16.Tsai C.L., Lu T.S., Tsai K.C., Chen W.J. Hemoptysis caused by Hughes-Stovin syndrome. Am. J. Emerg. Med. 2005;23:209–211. doi: 10.1016/j.ajem.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Bowman S., Honey M. Pulmonary arterial occlusions and aneurysms: a forme fruste of Behçet's or Hughes-Stovin syndrome. Br. Heart J. 1990;63:66–68. doi: 10.1136/hrt.63.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J., Noh J.W., Hwang J.W., Kim H., Ahnn J.K., Koh E.M., Cha H.S. Successful cycophsphamide therapy with complete resolution of pulmonary artery aneurysm in Hughes Stovin syndrome patient. Clin. Rheumatol. 2008;27:1455–1458. doi: 10.1007/s10067-008-0951-8. [DOI] [PubMed] [Google Scholar]