Abstract
The legionnaires' disease bacterium, Legionella pneumophila, is a facultative intracellular parasite. Its interaction with phagocytes has characteristics in common with several other intracellular parasites. Critical aspects of L. pneumophila intracellular multiplication are evasion of lysosomal host cell defenses and the presence of a nutritionally appropriate environment. Following phagocytosis, wild-type L. pneumophila multiply within a specialized phagosome which does not fuse with secondary lysosomes. Mutants which have lost the ability to grow within phagocytes no longer cause disease in animals, indicating that the capacity to multiply intracellularly is important for pathogenesis. One such mutant, 25D, has been shown to be defective in inhibiting phagosome-lysosome fusion. This phagolysosomal environment is not conducive to Legionella growth. We report the isolation of a region of the L. pneumophila genome (icm, intracellular multiplication) which restores the capacity of 25D to multiply in human macrophages. The complemented mutants also regain the capacity to interfere with phagosome-lysosome fusion and to cause lethal pneumonia in guinea pigs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhardwaj N., Nash T. W., Horwitz M. A. Interferon-gamma-activated human monocytes inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1986 Oct 15;137(8):2662–2669. [PubMed] [Google Scholar]
- Blander S. J., Breiman R. F., Horwitz M. A. A live avirulent mutant Legionella pneumophila vaccine induces protective immunity against lethal aerosol challenge. J Clin Invest. 1989 Mar;83(3):810–815. doi: 10.1172/JCI113962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander S. J., Szeto L., Shuman H. A., Horwitz M. A. An immunoprotective molecule, the major secretory protein of Legionella pneumophila, is not a virulence factor in a guinea pig model of Legionnaires' disease. J Clin Invest. 1990 Sep;86(3):817–824. doi: 10.1172/JCI114779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman R. F., Horwitz M. A. Guinea pigs sublethally infected with aerosolized Legionella pneumophila develop humoral and cell-mediated immune responses and are protected against lethal aerosol challenge. A model for studying host defense against lung infections caused by intracellular pathogens. J Exp Med. 1987 Mar 1;165(3):799–811. doi: 10.1084/jem.165.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catrenich C. E., Johnson W. Characterization of the selective inhibition of growth of virulent Legionella pneumophila by supplemented Mueller-Hinton medium. Infect Immun. 1989 Jun;57(6):1862–1864. doi: 10.1128/iai.57.6.1862-1864.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeley J. C., Gibson R. J., Gorman G. W., Langford N. C., Rasheed J. K., Mackel D. C., Baine W. B. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol. 1979 Oct;10(4):437–441. doi: 10.1128/jcm.10.4.437-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey J., Bagdasarian M., Feiss D., Franklin F. C., Deshusses J. Stable cosmid vectors that enable the introduction of cloned fragments into a wide range of gram-negative bacteria. Gene. 1983 Oct;24(2-3):299–308. doi: 10.1016/0378-1119(83)90090-2. [DOI] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A. Characterization of avirulent mutant Legionella pneumophila that survive but do not multiply within human monocytes. J Exp Med. 1987 Nov 1;166(5):1310–1328. doi: 10.1084/jem.166.5.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
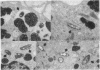
- Horwitz M. A. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983 Oct 1;158(4):1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Maxfield F. R. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J Cell Biol. 1984 Dec;99(6):1936–1943. doi: 10.1083/jcb.99.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. Phagocytosis of the Legionnaires' disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell. 1984 Jan;36(1):27–33. doi: 10.1016/0092-8674(84)90070-9. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Intracellular multiplication of Legionnaires' disease bacteria (Legionella pneumophila) in human monocytes is reversibly inhibited by erythromycin and rifampin. J Clin Invest. 1983 Jan;71(1):15–26. doi: 10.1172/JCI110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980 Sep;66(3):441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983 Dec 1;158(6):2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
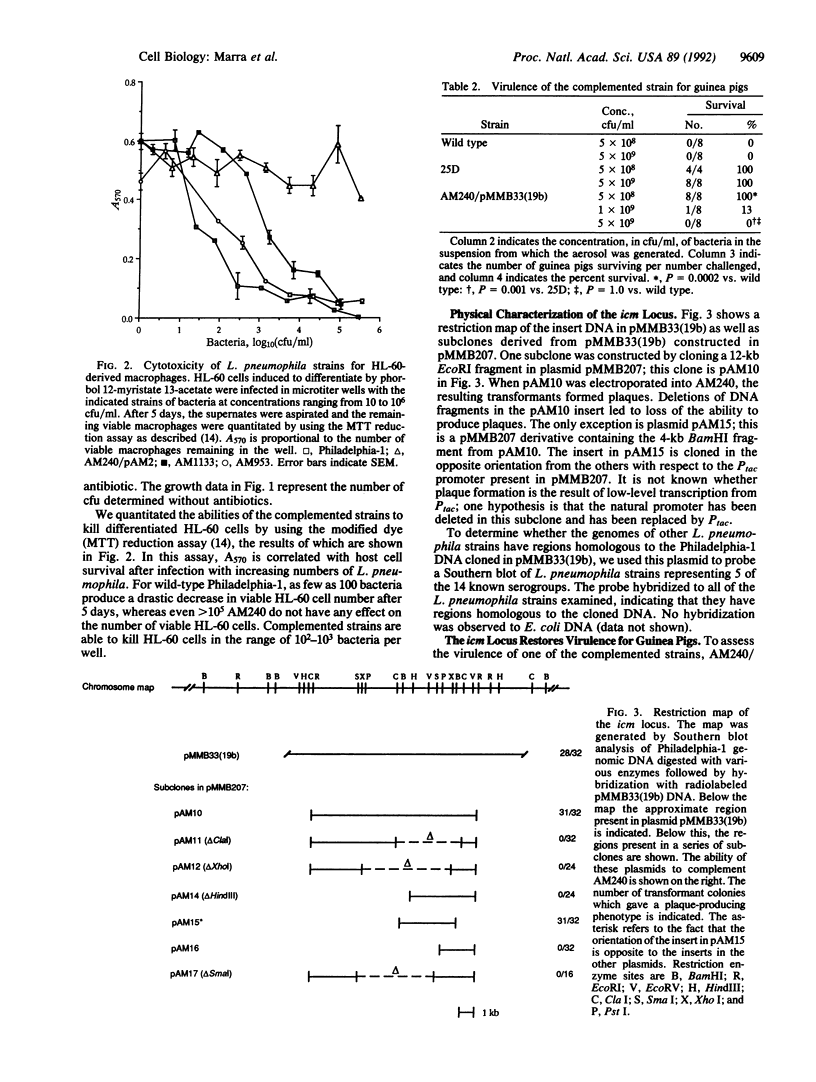
- Marra A., Horwitz M. A., Shuman H. A. The HL-60 model for the interaction of human macrophages with the Legionnaires' disease bacterium. J Immunol. 1990 Apr 1;144(7):2738–2744. [PubMed] [Google Scholar]
- Marra A., Shuman H. A. Isolation of a Legionella pneumophila restriction mutant with increased ability to act as a recipient in heterospecific matings. J Bacteriol. 1989 Apr;171(4):2238–2240. doi: 10.1128/jb.171.4.2238-2240.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R., Figurski D., Helinski D. R. Physical and genetic studies with restriction endonucleases on the broad host-range plasmid RK2. Mol Gen Genet. 1977 Apr 29;152(3):129–135. doi: 10.1007/BF00268809. [DOI] [PubMed] [Google Scholar]
- Mintz C. S., Chen J. X., Shuman H. A. Isolation and characterization of auxotrophic mutants of Legionella pneumophila that fail to multiply in human monocytes. Infect Immun. 1988 Jun;56(6):1449–1455. doi: 10.1128/iai.56.6.1449-1455.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne N. R., Horwitz M. A. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J Exp Med. 1987 Nov 1;166(5):1377–1389. doi: 10.1084/jem.166.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger L. S., Horwitz M. A. Phagocytosis of Mycobacterium leprae by human monocyte-derived macrophages is mediated by complement receptors CR1 (CD35), CR3 (CD11b/CD18), and CR4 (CD11c/CD18) and IFN-gamma activation inhibits complement receptor function and phagocytosis of this bacterium. J Immunol. 1991 Sep 15;147(6):1983–1994. [PubMed] [Google Scholar]