Abstract
G-protein-coupled receptors (GPCRs) constitute a large group of integral membrane proteins that transduce extracellular signals from a wide range of agonists into targeted intracellular responses. Although the responses can vary depending on the category of G-proteins activated by a particular receptor, responses were also found to be triggered by interactions of the receptor with β-arrestins. It was subsequently discovered that for the same receptor molecule (e.g., the β-adrenergic receptor), some agonists have a propensity to specifically favor responses by G-proteins, others by β-arrestins, as has now been extensively studied. This feature of the GPCR system is known as biased agonism and is subject to various interpretations, including agonist-induced conformational change versus selective stabilization of preexisting active conformations. Here, we explore a complete allosteric framework for biased agonism based on alternative preexisting conformations that bind more strongly, but nonexclusively, either G-proteins or β-arrestins. The framework incorporates reciprocal effects among all interacting molecules. As a result, G-proteins and β-arrestins are in steric competition for binding to the cytoplasmic surface of either the G-protein-favoring or β-arrestin-favoring GPCR conformation. Moreover, through linkage relations, the strength of the interactions of G-proteins or β-arrestins with the corresponding active conformation potentiates the apparent affinity for the agonist, effectively equating these two proteins to allosteric modulators. The balance between response alternatives can also be influenced by the physiological concentrations of either G-proteins or β-arrestins, as well as by phosphorylation or interactions with positive or negative allosteric modulators. The nature of the interactions in the simulations presented suggests novel experimental tests to distinguish more fully among alternative mechanisms.
Main Text
GPCRs are a large and diverse family of seven transmembrane helical receptors to which agonist binding triggers downstream cellular responses via G-proteins or β-arrestin (1, 2, 3). First identified for their role in desensitization, internalization, and recycling (4), β-arrestins were subsequently shown to participate directly in several intracellular signaling pathways (2). Although GPCRs have been studied for many years, with numerous agonists and many active pharmaceutical agents, only relatively recently has attention focused on the different agonists for a particular GPCR molecule that favor either G-protein or β-arrestin signaling (1). Such “biased agonism” reinforces the concept of multiple active conformations for GPCRs (5, 6). However, a fundamental distinction can be made by asking whether their formation reflects “conformations induced” by the particular biased agonist (2) or stabilization of a small number of discrete preexisting conformations consistent with the Monod-Wyman-Changeux (MWC) allosteric model (7). Although particular features of GPCRs have been interpreted using allosteric concepts (8, 9, 10, 11), a global allosteric formalism that explores the full range of allosteric linkages in the original MWC framework (7, 12) is considered here. This formalism includes local steric competition between G-proteins and β-arrestin, as well as reciprocal effects that govern the potentiation of these two proteins on the corresponding agonist affinities. An earlier allosteric model involving two active states was applied to biased agonism, but reciprocal effects associated with binding of G-proteins and β-arrestins were not included (8).
The global allosteric formalism builds on recent developments involving both equilibrium and dynamic formulations of multiple conformational states in proteins (13, 14). The ensemble nature of the states has been emphasized (15, 16, 17), including entropy-driven differences among states showing little or no overt structural changes (18, 19). Changes in quaternary stoichiometry can also play a role, as, for example, in the passage from monomeric to dimeric states for GPCRs (20, 21) and tyrosine kinases (22). Discrete conformational states are stabilized by noncovalent binding interactions of all molecular species (23). These include agonists and antagonists that bind to orthosteric sites, as well as by positive and negative modulators that bind to allosteric modulatory sites.
For GPCRs, the critical G-proteins and β-arrestins that transfer signals to downstream pathways upon binding to the specialized transfer site also influence the distribution of conformations (see Fig. 1). For a conformation that favors binding by a G-protein, the G-protein will also favor binding of the biased agonist. Covalent phosphorylation reactions also modify the stability among conformational states, with particular importance for biased agonism, since phosphorylation by G-protein receptor kinases (GPKs) at critical C-terminal residues of GPCRs can regulate the interactions with β-arrestins (2). Phosphorylation may also bias the coupling to transfer proteins as in the case of 5-HT6 receptors for which Cdk5 phosphorylation changes from a ligand-dependent coupling to Gs to a ligand-independent coupling to Cdc42 (24). Specific agonists may be full or partial, in addition to showing a bias for interactions with G-proteins or β-arrestins. In some cases, ligands can be antagonists, including antagonists with negative intrinsic activity (inverse agonists) for either the G-protein or β-arrestin pathway, but agonists for the other (25, 26). In addition, some antagonists upon close examination may be categorized as very weak partial agonists (27). Constitutively active receptors are a hallmark of preexisting conformational equilibria (12). Mutations that change the side chains of critical amino acid residues can also influence bias for G-proteins versus β-arrestins (28), as well as bias between Gq and Gs proteins (29). Pathologies such as the Kallmann Syndrome can arise from natural missense mutations that alter bias (30). Allosteric modulators also alter the specificity of agonist bias (31), reminiscent of allosteric modulation of enzyme substrate specificity, as in the case of ribonucleotide reductase (32). Complex interactions between agonists and allosteric modulators may occur particularly when their respective binding sites are in close proximity (33).
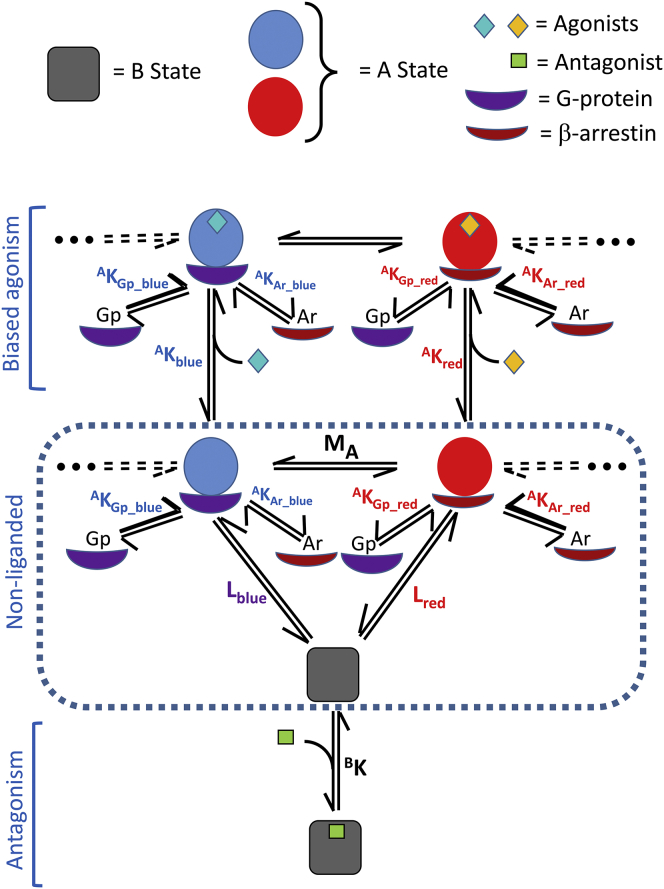
Figure 1.
Minimal reactions for a GPCR molecule with two active states. The two active conformational A states are indicated by red and blue circles, with the basal B state as a gray square. (For the print version, the red and blue colors are replaced by shades of gray.) The central rectangular box presents the GPCR molecules in conformational equilibrium in the absence of agonists or transfer molecules. Above the rectangle are presented reactions involving binding by agonists, G-proteins, and β-arrestins. The agonists are indicated by turquoise or orange diamonds that preferentially bind to the Ablue or Ared states, respectively. All of these molecules can bind to both the Ablue and Ared states (as well as the B state) according to the principle of nonexclusive binding, but for simplicity, only binding to the A state with the highest affinity is shown. Similarly, for the two classes of agonists depicted, binding to the less favored state is not shown. Antagonists may also bind to each state, but are only shown for the B state. Allosteric modulators (not shown, but see Fig. 2D) can also bind to each state. The conformational equilibrium constants are indicated by Lblue and Lred for the interactions of the respective states with the B state. The constant MA defines the equilibrium between the Ablue and Ared states. Other constants are defined in Table 1. To see this figure in color, go online.
Recent progress in structural studies on GPCRs and rhodopsins have provided considerable insights into functional features of these proteins (34, 35), including complexes with arrestin (36, 37). The structural studies reinforce the evidence that G-proteins and β-arrestin cannot bind simultaneously and their binding is competitive. Other structural studies have addressed the issues of conformational switching (6, 38), as well as allosteric modifications, including “bitopic” ligands active at both agonist and allosteric sites (39). Insights into the structure and dynamics of heterotrimeric G-proteins trimers have also been recently obtained (40).
Historically, modeling of GPCRs has been placed in the classical context of the empirical approach of Black and Leff for competitive steric antagonism (41). The critical factors in their model are the apparent agonist affinity for the receptor (KA) and the operational “transducer ratio” (τ), where τ = [R0]/KE (41, 42). Here, R0 is defined as the total receptor concentration and KE is a virtual equilibrium constant for the physiological response, defined as the concentration of the agonist-receptor complex that elicits half-maximal response. Hence, τ is a composite constant with two components that cannot readily be determined independently, but τ remains a popular parameter for characterizing GPCR (43). In practice, τ is related to a given response for a specific GPCR agonist by the relationship EC50 = KA/(1 + τ). When normalized to a reference full agonist, a particular agonist under consideration is characterized by its value of Δlog(τ/KA). Although the Black-Leff model is not based on intrinsic parameters of chemical reactions, it has endured as the favored model for quantifying biased agonism. For two agonists (Ag1 and Ag2) with bias toward G-protein or β-arrestin signaling, their comparative bias is given by ΔΔlog(τ/KA)Ag1-Ag2 (26).
In contrast to the phenomenological formulation of Black and Leff (41), we develop an explicit molecular mechanism based on defined chemical reactions within an allosteric context of multiple conformational transitions. The same principles were applied to extended networks of conformations and specific interactions (27), but for simplicity, here we consider only two preexisting active GPCR conformations (represented schematically as red and blue in Figs. 1 and 2, or with shades of gray in the black-and-white print versions). Both conformations can bind G-proteins and β-arrestin molecules nonexclusively at their transfer site, but where their affinities are not identical, we assign the blue conformation to the higher affinity for G-proteins and the red conformation to the higher affinity for β-arrestin. In the minimal form of this model, as examined here, we assume a single discrete active conformation stabilized by agonist binding and available for G-protein or β-arrestin binding without additional conformational change. As a result, under all conditions, reciprocal effects arise from competition between G-protein and β-arrestin binding, as well as their synergistic linkage effects on the apparent agonist affinities.
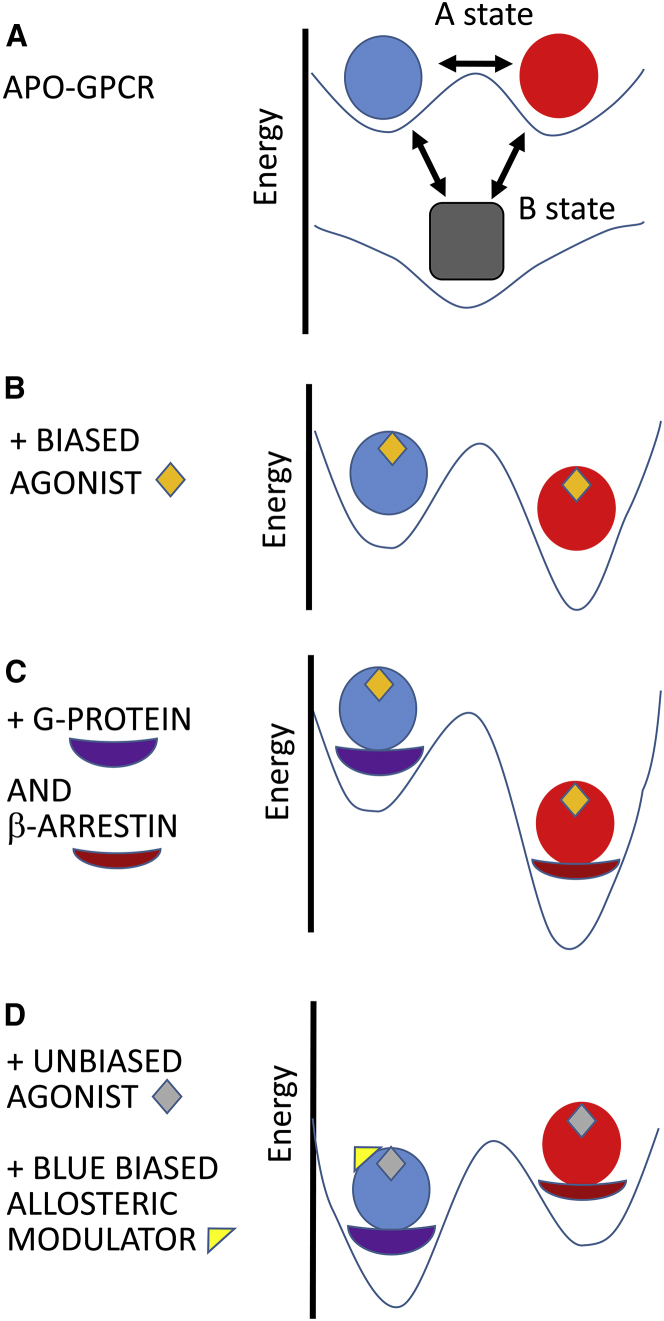
Figure 2.
Energy relationships among the conformational states. (A) Intrinsic equilibrium among the conformations of the GPCR molecules in the absence of agonists, antagonists, transfer proteins, or allosteric modulators. Under these conditions the B state is more stable than the A states (shown here with equal stabilities). (B) The redistribution of Ablue and Ared states in the presence of a biased agonist (in this case favoring the Ared state). Both states are stabilized, but Ared is moreso than Ablue. The B state is not shown. (C) Molecules are shown as in (B), with the addition of transfer proteins, G-protein and β-arrestin. Each transfer protein can bind to both the Ablue and Ared states, but only the preferential binding partners are presented here. An additional stabilization of the Ared state arises from the binding energy provided by β-arrestin. (D) The case of an unbiased agonist (gray diamond) bound in the presence of a biased positive allosteric modulator that favors the Ablue state. To see this figure in color, go online.
A global allosteric formalism for GPCR signaling
The critical parameter for conformational equilibria is the allosteric constant, L, defined by the ratio of the resting or basal (B) and active (A) conformational states and their multiple reactions, as shown in Fig. 1, using the B-A nomenclature as previously defined for membrane receptors (44). Since the A state is subdivided into two classes, distinct L values are defined: Lblue and Lred. In relation to these two parameters, the partition between red and blue active states is defined by the parameter MA, where MA = Lblue/Lred. Following the primary division into two active conformations, all other parameters must be distinguished with respect to these conformations, including agonists, transfer molecules (G-proteins and β-arrestin), and allosteric modulators. The same principles could be applied to any number of additional discrete active states with other distinguishing properties. An antagonist is depicted binding to the B state only in Fig. 1, but such a molecule would also be expected to display a significant, but weaker, affinity for the A states, according to the principle of nonexclusive binding. Although not explicitly represented in Fig. 1, the same principles would apply to any allosteric modulation along the lines depicted for an agonist, in the case of binding more strongly to the A state (a positive effector or a full or partial agonist), or alternatively more strongly to the B state (a negative effector, antagonist, or inverse agonist). The various parameters utilized in the modeling presented here are summarized in Table 1. Full details of the modeling equations are presented in the Supporting Material.
Table 1.
Parameters for the GPCR Model
| Conformational Transition Parameters | Initial Values | |
|---|---|---|
| Lblue | Intrinsic allosteric constant for the intrinsic equilibrium between resting and G-protein-favored state in the absence of agonist: Lblue = [B]/[Ablue] | 100 |
| Lred | Intrinsic allosteric constant for the intrinsic equilibrium between resting and G-protein favored state in the absence of agonist. Lred = [B]/[Ared] | 100 |
| MA | Ratio of intrinsic stabilities of Ablue and Ared states: MA = [Ablue]/[Ared] | 1 |
| Ablue State Parameters | ||
| AKGp_blue | Dissociation equilibrium constant of the complex between a GPCR molecule in the Ablue state and a G-protein | 2 × 10−6 M |
| AKAr_blue | Dissociation equilibrium constant of the complex between a GPCR molecule in the Ablue state and a β-arrestin | 10−4 M |
| AKAg_blue | Dissociation equilibrium constant of the complex between a GPCR molecule in the Ablue state and an agonist | 5.5 × 10−8 M |
| AKAl_blue | Dissociation equilibrium constant of the complex between a GPCR molecule in the Ablue state and an allosteric modulator | NA |
| Ared State Parameters | ||
| AKGp_red | Dissociation equilibrium constant of the complex between a GPCR molecule in the Ared state and a G-protein | 10−4 M |
| AKAr_red | Dissociation equilibrium constant of the complex between a GPCR molecule in the Ared state and a β-arrestin | 2 × 10−6 M |
| AKAg_red | Dissociation equilibrium constant of the complex between a GPCR molecule in the Ared state and an agonist | 3.5 × 10−8 M |
| AKAl_red | Dissociation equilibrium constant of the complex between a GPCR molecule in the Ared state and an allosteric modulator | NA |
| B State Parameters | ||
| BKGp | Dissociation equilibrium constant of the complex between a GPCR molecule in the B state and a G-protein | 10−4 M |
| BKAr | Dissociation equilibrium constant of the complex between a GPCR molecule in the B state and a β-arrestin | 10−4 M |
| BKAg | Dissociation equilibrium constant of the complex between a GPCR molecule in the B state and an agonist | 10−4 M |
| BKAl | Dissociation equilibrium constant of the complex between a GPCR molecule in the B state and an allosteric modulator | NA |
NA, not applicable.
The effect of biased agonism according to the global allosteric formalism is schematically represented by the energy diagram in Fig. 2. In the absence of other components, the blue and red active conformations are of equivalent stability to, but less energetically favorable than, the weakly active B state (Fig. 2 A). Addition of an agonist biased toward the Ared state is represented in Fig. 2 B. The binding of an agonist enhances the stability of all states, but preferentially one of the A states for a full or partial agonist. In contrast, preferential stabilization of a B state would arise from binding of an antagonist with higher affinity for a B state than for an A state (also referred to as an inverse agonist). In the case of biased agonism, an agonist preferentially enhances the stability of the favored A state, the Ared state in Fig. 2 (for simplicity, the B state is not presented). Additional stability is accrued by binding of the transfer proteins, G-protein or β-arrestin, with quantitative differences related to the Ablue conformation with higher affinity for G-proteins versus the Ared conformation with higher affinity for β-arrestin (Fig. 2 C). An allosteric modulator with nonexclusive binding would enhance the stability of all states, but preferentially A or B, depending on whether the effector is positive or negative. For a nonbiased agonist, an allosteric modulator can produce biased agonism, as presented in Fig. 2 D for a modulator that favors the Ablue state. Weaker binding of the modulator to the Ared state is implicit.
The global allosteric formalism represents a system of fully linked reactions, such that the concentrations and affinities of each component influence the distribution of all other components. If a GPCR molecule is considered in isolation, the fundamental conformational equilibrium is defined by the allosteric constant L, as applied to the relative stability of two states (7). With two preexisting active states, two intrinsic constants apply, Lblue and Lred, but operationally the presence of G-proteins and β-arrestins will necessarily diminish these values due to preferential binding to the active states. Since these interactions are competitive due to their binding to the same region on the intracellular surface of the GPCRs, their relative balance will clearly influence the downstream signaling under physiological conditions. Moreover, the exact shape of the physiological response curve corresponds to the state function for which the shape and dependence on the allosteric constant are distinct from the agonist-binding function (45, 46).
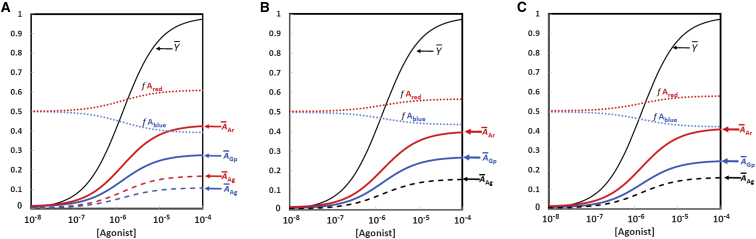
Quantitative features of the global allosteric model can be examined to explore the interactions between all of the components. An example is illustrated in Fig. 3 for clenbuterol, a biased agonist for β1-adrenoceptors (47). In the data reported, clenbuterol produces partial signaling levels compared to the reference agonist for the G-protein response and ∼50% higher for β-arrestin (47). Starting with the simple initial assumption of equal intrinsic stabilities of the red and blue conformations (Lblue = Lred =100), the first consequence of the global allostery formulation is that stronger interactions of G-proteins and β-arrestin molecules with the A conformations compared to the B conformation necessarily reduce the effective L values by preferentially stabilizing the active state. In this simulation in Fig. 3 A, the experimentally observed bias in favor of β-arrestin can be accounted for by 1.5-fold stronger binding of clenbuterol to the red conformation. Therefore, as agonist binding increases (present as the curve), the G-protein (blue) and β-arrestin (red) response curves (initially at the same low but nonzero value due to the effective L values of <100) diverge in favor of the β-arrestin response. However, the Ared and Ablue molecules are only partially saturated with G-proteins or β-arrestin, as presented by the dashed red and blue lines for receptor-agonist complexes in the two states. Concomitantly, the fraction of receptor molecules in the Ablue conformation, f Ablue, diminishes as the corresponding value for the Ared conformation, f Ared, increases. The principle of nonexclusive binding implies that low levels of response could also be generated by binding of G-protein or β-arrestin molecules to the B state, but these responses would be considered background effects and were not included in the simulations presented here.
Figure 3.
Simulated dose-response curves to represent clenbuterol. Each graph shows the fractional separation of the A state into red and blue components, given by f Ared and f Ablue, respectively, along with the binding function, . (A) Ratio of β-arrestin/G-protein = 1 (concentration of 5.0 × 10−6 M for both), but with stronger binding of the agonist to the Ared state corresponding to the parameter values in Table 1. The curves are separated for A-state molecules with agonist and G-protein (Ablue), agonist and β-arrestin (Ared), or agonist alone (AAg) shown separately for the Ablue and Ared states. Not shown are the fraction of A states that are fully nonliganded (negligible, ∼10−4) and the B state in its various liganded forms (∼2% at [agonist] = 10−4 M). (B) Ratio of β-arrestin/G-protein = 1, with stronger binding for β-arrestin to the Ared state than for G-protein to the Ablue state. Parameter values are [G-protein] = [β-arrestin] = 2.0 × 10−5 M; other values are as in Table 1, except for AKGp_blue = 3.0 × 10−6 M and AKAr_red = 2.0 × 10−6 M, agonist affinity for both Ared and Ablue = 4.0 × 10−8. For these conditions agonist alone (AAg) is shown in black since the curves for the Ablue and Ared states overlap. (C) Ratio of β-arrestin/G-protein > 1: [G-protein] = 3.0 × 10−6 M, and [β-arrestin] = 5.0 × 10−6 M, with AKGp_blue = AKAr_red = 2.0 × 10−6 M. Other details are as in (B). To see this figure in color, go online.
An important consequence of placing the GPCR system in a global allosteric context is to emphasize that biased responses can arise from factors other than biased agonism, hence the more appropriate designation “biased allostery.” A schematic example is presented in Fig. 2 D. In addition, under specific physiological conditions, the degree of bias can be affected by differences in the affinities of concentrations of G-proteins and β-arrestins for each active conformation. As shown in Fig. 3 B, a biased response can be achieved with equal affinities of the agonist for the blue and red conformations, but with slightly stronger (1.5-fold) binding by β-arrestin. Biased response can be influenced also by differences in their respective concentrations (Fig. 3 C), with equal affinities of the agonist, as well as G-proteins and β-arrestin, for the red and blue states but a slightly higher (1.7-fold) concentration of β-arrestin (Fig. 3 C).
General considerations
Exploring biased agonism with a global allosteric model involving all components interacting in the complete system reveals that response-specific preexisting active states can readily accommodate the basic observations generated by biased agonists. In addition, incorporating G-proteins and β-arrestins directly into the allosteric framework reveals the reciprocal effects related to the concentrations and affinities of the G-proteins and β-arrestins, as well as their competition for binding to the cytoplasmic surface of GPCRs. Were all components to be manipulated quantitatively in an experimental setting, the global allosteric formalism would predict specific reciprocal effects. For example, increasing concentrations of G-proteins or β-arrestins should produce precise shifts in the agonist dose-response curves to the left.
With respect to the shape of dose-response curves, cooperativity in the binding of agonists may occur, as indicated by a sigmoidal character, although, as noted, the classical Hill coefficient cannot be interpreted in precisely the same manner as applied to ligand-binding equilibria (46). The issue of cooperativity of ligand binding can readily be incorporated into the global allosteric framework by incorporating multiple sites into the basic equations, as needed for a more comprehensive evaluation for oligomeric GPCRs including both dimeric and tetrameric heteroligomers (20, 21, 48). Where data are available, differences between binding curves and response curves (which follow the conformational state function) can provide additional insights, especially with respect to cooperativity (46). In addition, instances of negative cooperativity may reveal indications of homooligomerization, notably for β1-adrenoceptors (49). The kinetic context of various steps in the signal transduction pathway may also influence the impact of agonist bias depending on the timescale of the measurements (50, 51). Ultimately, the distinctions between red and blue conformations as presented here, without considerations of the time domain, may require specific adjustments for nonidentical kinetic properties of the signaling pathways. More generally, models based on equilibrium considerations cannot capture the full dynamics of the GPCR signaling system, particularly the quasi-irreversible step involving GTP hydrolysis, and future modeling efforts should take these features into account.
Information on the exact stereochemical basis for conformational equilibria and the alterations provoked by various interacting components is emerging from studies combining structural observations with dynamic measurement using powerful optical methods (38, 52, 53, 54). These approaches bring a new level of precision to the classical concept of isosterism concerning the potential of small differences in pharmacological agents to exert novel effects (55). Recent developments now permit characterization at the level of single molecules (56, 57). The equilibrium equations presented here are readily extended to single molecules with a stochastic kinetic model, as developed for nicotinic receptors (58). Experimental observations may therefore be applied to testing the basic assumptions of the general allosteric formalism, particularly the reciprocal effects of G-proteins or β-arrestins on agonist affinities, as well as the minimalist assumption that discrete, relatively rigid active conformations are effectively unchanged by binding of agonist or G-proteins or β-arrestins. There is considerable evidence in the scientific literature for reciprocal effects that involve enhancement of agonist affinity by G-proteins (59, 60, 61), although they have not as yet been interpreted with a quantitative functional model along the lines of that presented here. Evidence has also been presented to indicate that β-arrestin binding favors a conformational state with high agonist affinity (37, 62).
More broadly, new experimental approaches may also examine the question of whether the transfer molecules, G-proteins or β-arrestins, can be formally represented as allosteric modulators, a role previously assigned to smaller druggable molecules (63). Finally, the rapidly expanding field of DREADDs (Designer Receptors Exclusively Activated by Designer Drugs) is clarifying the consequences of conformational equilibria among discrete states of GPCRs, including the presence of constitutively active states (64). Since current progress is in a rapidly accelerating phase, all of these issues merit revisiting in the near future. The general allosteric framework provides a context for potentially integrating various experimental approaches while at the same time subjecting the assumptions of the model to new experimental tests.
Author Contributions
S.J.E. and J.-P.C designed research; S.J.E. performed research; and S.L.E. and J.-P.C wrote the manuscript.
Acknowledgments
We thank the three anonymous reviewers for helpful suggestions and Jean-Philippe Pin for valuable discussions. We dedicate this article to the memory of Howard K. Schachman (December 5, 1918–August 5, 2016).
Editor: Brian Salzberg.
Footnotes
Supporting Materials and Methods are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)30656-7.
Contributor Information
Stuart J. Edelstein, Email: edelstei@biologie.ens.fr.
Jean-Pierre Changeux, Email: changeux@pasteur.fr.
Supporting Material
References
- 1.Galandrin S., Oligny-Longpré G., Bouvier M. The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol. Sci. 2007;28:423–430. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Reiter E., Ahn S., Lefkowitz R.J. Molecular mechanism of β-arrestin-biased agonism at seven-transmembrane receptors. Annu. Rev. Pharmacol. Toxicol. 2012;52:179–197. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenakin T., Christopoulos A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat. Rev. Drug Discov. 2013;12:205–216. doi: 10.1038/nrd3954. [DOI] [PubMed] [Google Scholar]
- 4.Lefkowitz R.J. G protein-coupled receptors. III. New roles for receptor kinases and β-arrestins in receptor signaling and desensitization. J. Biol. Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 5.Kahsai A.W., Xiao K., Lefkowitz R.J. Multiple ligand-specific conformations of the β2-adrenergic receptor. Nat. Chem. Biol. 2011;7:692–700. doi: 10.1038/nchembio.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manglik A., Kim T.H., Kobilka B.K. Structural insights into the dynamic process of β2-adrenergic receptor signaling. Cell. 2015;161:1101–1111. doi: 10.1016/j.cell.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monod J., Wyman J., Changeux J.-P. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 8.Leff P., Scaramellini C., McKechnie K. A three-state receptor model of agonist action. Trends Pharmacol. Sci. 1997;18:355–362. doi: 10.1016/s0165-6147(97)01105-x. [DOI] [PubMed] [Google Scholar]
- 9.Christopoulos A., Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol. Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- 10.Canals M., Lane J.R., Christopoulos A. A Monod-Wyman-Changeux mechanism can explain G protein-coupled receptor (GPCR) allosteric modulation. J. Biol. Chem. 2012;287:650–659. doi: 10.1074/jbc.M111.314278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roth S., Bruggeman F.J. A conformation-equilibrium model captures ligand-ligand interactions and ligand-biased signalling by G-protein coupled receptors. FEBS J. 2014;281:4659–4671. doi: 10.1111/febs.12970. [DOI] [PubMed] [Google Scholar]
- 12.Changeux J.P., Edelstein S.J. Allosteric mechanisms of signal transduction. Science. 2005;308:1424–1428. doi: 10.1126/science.1108595. [DOI] [PubMed] [Google Scholar]
- 13.Cui Q., Karplus M. Allostery and cooperativity revisited. Protein Sci. 2008;17:1295–1307. doi: 10.1110/ps.03259908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Changeux J.P., Edelstein S. Conformational selection or induced fit? 50 years of debate resolved. F1000 Biol. Rep. 2011;3:19. doi: 10.3410/B3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motlagh H.N., Wrabl J.O., Hilser V.J. The ensemble nature of allostery. Nature. 2014;508:331–339. doi: 10.1038/nature13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dror R.O., Arlow D.H., Shaw D.E. Activation mechanism of the β2-adrenergic receptor. Proc. Natl. Acad. Sci. USA. 2011;108:18684–18689. doi: 10.1073/pnas.1110499108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boehr D.D., Nussinov R., Wright P.E. The role of dynamic conformational ensembles in biomolecular recognition. Nat. Chem. Biol. 2009;5:789–796. doi: 10.1038/nchembio.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nussinov R., Tsai C.J. Allostery without a conformational change? Revisiting the paradigm. Curr. Opin. Struct. Biol. 2015;30:17–24. doi: 10.1016/j.sbi.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Tzeng S.R., Kalodimos C.G. Protein activity regulation by conformational entropy. Nature. 2012;488:236–240. doi: 10.1038/nature11271. [DOI] [PubMed] [Google Scholar]
- 20.Vischer H.F., Castro M., Pin J.P. G protein-coupled receptor multimers: a question still open despite the use of novel approaches. Mol. Pharmacol. 2015;88:561–571. doi: 10.1124/mol.115.099440. [DOI] [PubMed] [Google Scholar]
- 21.Ferré S. The GPCR heterotetramer: challenging classical pharmacology. Trends Pharmacol. Sci. 2015;36:145–152. doi: 10.1016/j.tips.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Endres N.F., Barros T., Kuriyan J. Emerging concepts in the regulation of the EGF receptor and other receptor tyrosine kinases. Trends Biochem. Sci. 2014;39:437–446. doi: 10.1016/j.tibs.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Rubin M.M., Changeux J.-P. On the nature of allosteric transitions: implications of non-exclusive ligand binding. J. Mol. Biol. 1966;21:265–274. doi: 10.1016/0022-2836(66)90097-0. [DOI] [PubMed] [Google Scholar]
- 24.Duhr F., Déléris P., Chaumont-Dubel S. Cdk5 induces constitutive activation of 5-HT6 receptors to promote neurite growth. Nat. Chem. Biol. 2014;10:590–597. doi: 10.1038/nchembio.1547. [DOI] [PubMed] [Google Scholar]
- 25.Azzi M., Charest P.G., Piñeyro G. β-Arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc. Natl. Acad. Sci. USA. 2003;100:11406–11411. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenakin T. What is pharmacological “affinity”? Relevance to biased agonism and antagonism. Trends Pharmacol. Sci. 2014;35:434–441. doi: 10.1016/j.tips.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Galzi J.L., Edelstein S.J., Changeux J. The multiple phenotypes of allosteric receptor mutants. Proc. Natl. Acad. Sci. USA. 1996;93:1853–1858. doi: 10.1073/pnas.93.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregory K.J., Hall N.E., Christopoulos A. Identification of orthosteric and allosteric site mutations in M2 muscarinic acetylcholine receptors that contribute to ligand-selective signaling bias. J. Biol. Chem. 2010;285:7459–7474. doi: 10.1074/jbc.M109.094011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valentin-Hansen L., Frimurer T.M., Schwartz T.W. Biased Gs versus Gq proteins and β-arrestin signaling in the NK1 receptor determined by interactions in the water hydrogen bond network. J. Biol. Chem. 2015;290:24495–24508. doi: 10.1074/jbc.M115.641944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sbai O., Monnier C., Rondard P. Biased signaling through G-protein-coupled PROKR2 receptors harboring missense mutations. FASEB J. 2014;28:3734–3744. doi: 10.1096/fj.13-243402. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y., Rodriguez A.L., Conn P.J. Allosteric potentiators of metabotropic glutamate receptor subtype 5 have differential effects on different signaling pathways in cortical astrocytes. J. Pharmacol. Exp. Ther. 2005;315:1212–1219. doi: 10.1124/jpet.105.090308. [DOI] [PubMed] [Google Scholar]
- 32.Eriksson M., Uhlin U., Eklund H. Binding of allosteric effectors to ribonucleotide reductase protein R1: reduction of active-site cysteines promotes substrate binding. Structure. 1997;5:1077–1092. doi: 10.1016/s0969-2126(97)00259-1. [DOI] [PubMed] [Google Scholar]
- 33.Thal D.M., Sun B., Christopoulos A. Crystal structures of the M1 and M4 muscarinic acetylcholine receptors. Nature. 2016;531:335–340. doi: 10.1038/nature17188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manglik A., Kobilka B. The role of protein dynamics in GPCR function: insights from the β2AR and rhodopsin. Curr. Opin. Cell Biol. 2014;27:136–143. doi: 10.1016/j.ceb.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shukla A.K., Singh G., Ghosh E. Emerging structural insights into biased GPCR signaling. Trends Biochem. Sci. 2014;39:594–602. doi: 10.1016/j.tibs.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Shukla A.K., Westfield G.H., Lefkowitz R.J. Visualization of arrestin recruitment by a G-protein-coupled receptor. Nature. 2014;512:218–222. doi: 10.1038/nature13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang Y., Zhou X.E., Xu H.E. Crystal structure of rhodopsin bound to arrestin by femtosecond x-ray laser. Nature. 2015;523:561–567. doi: 10.1038/nature14656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J.J., Horst R., Wüthrich K. Biased signaling pathways in β2-adrenergic receptor characterized by 19F-NMR. Science. 2012;335:1106–1110. doi: 10.1126/science.1215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kruse A.C., Ring A.M., Kobilka B.K. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature. 2013;504:101–106. doi: 10.1038/nature12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dror R.O., Mildorf T.J., Shaw D.E. SIGNAL TRANSDUCTION. Structural basis for nucleotide exchange in heterotrimeric G proteins. Science. 2015;348:1361–1365. doi: 10.1126/science.aaa5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Black J.W., Leff P. Operational models of pharmacological agonism. Proc. R. Soc. Lond. B Biol. Sci. 1983;220:141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- 42.Black J.W., Leff P., Wood J. An operational model of pharmacological agonism: the effect of E/[A] curve shape on agonist dissociation constant estimation. Br. J. Pharmacol. 1985;84:561–571. doi: 10.1111/j.1476-5381.1985.tb12941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kenakin T., Watson C., Novick S. A simple method for quantifying functional selectivity and agonist bias. ACS Chem. Neurosci. 2012;3:193–203. doi: 10.1021/cn200111m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edelstein S.J., Schaad O., Changeux J.P. A kinetic mechanism for nicotinic acetylcholine receptors based on multiple allosteric transitions. Biol. Cybern. 1996;75:361–379. doi: 10.1007/s004220050302. [DOI] [PubMed] [Google Scholar]
- 45.Edelstein S.J., Stefan M.I., Le Novère N. Ligand depletion in vivo modulates the dynamic range and cooperativity of signal transduction. PLoS One. 2010;5:e8449. doi: 10.1371/journal.pone.0008449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edelstein S.J. A novel equation for cooperativity of the allosteric state function. J. Mol. Biol. 2014;426:39–42. doi: 10.1016/j.jmb.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casella I., Ambrosio C., Costa T. Divergent agonist selectivity in activating β1- and β2-adrenoceptors for G-protein and arrestin coupling. Biochem. J. 2011;438:191–202. doi: 10.1042/BJ20110374. [DOI] [PubMed] [Google Scholar]
- 48.Pin J.P., Neubig R., Spedding M. International union of basic and clinical pharmacology. LXVII. Recommendations for the recognition and nomenclature of G protein-coupled receptor heteromultimers. Pharmacol. Rev. 2007;59:5–13. doi: 10.1124/pr.59.1.5. [DOI] [PubMed] [Google Scholar]
- 49.Gherbi K., May L.T., Hill S.J. Negative cooperativity across β1-adrenoceptor homodimers provides insights into the nature of the secondary low-affinity CGP 12177 β1-adrenoceptor binding conformation. FASEB J. 2015;29:2859–2871. doi: 10.1096/fj.14-265199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palanche T., Ilien B., Galzi J.L. The neurokinin A receptor activates calcium and cAMP responses through distinct conformational states. J. Biol. Chem. 2001;276:34853–34861. doi: 10.1074/jbc.M104363200. [DOI] [PubMed] [Google Scholar]
- 51.Klein Herenbrink C., Sykes D.A., Lane J.R. The role of kinetic context in apparent biased agonism at GPCRs. Nat. Commun. 2016;7:10842. doi: 10.1038/ncomms10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sounier R., Mas C., Granier S. Propagation of conformational changes during μ-opioid receptor activation. Nature. 2015;524:375–378. doi: 10.1038/nature14680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nygaard R., Zou Y., Kobilka B.K. The dynamic process of β(2)-adrenergic receptor activation. Cell. 2013;152:532–542. doi: 10.1016/j.cell.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee Y., Choi S., Hyeon C. Communication over the network of binary switches regulates the activation of A2A adenosine receptor. PLOS Comput. Biol. 2015;11:e1004044. doi: 10.1371/journal.pcbi.1004044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bovet D. Physiology or Medicine: 1963–1970 (Nobel lectures) Elsevier; Amsterdam, the Netherlands: 1957. The relationships between isosterism and competitive phenomena in the field of drug therapy of the autonomic nervous system and that of the neuromuscular transmission. [Google Scholar]
- 56.Lamichhane R., Liu J.J., Millar D.P. Single-molecule view of basal activity and activation mechanisms of the G protein-coupled receptor β2AR. Proc. Natl. Acad. Sci. USA. 2015;112:14254–14259. doi: 10.1073/pnas.1519626112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vafabakhsh R., Levitz J., Isacoff E.Y. Conformational dynamics of a class C G-protein-coupled receptor. Nature. 2015;524:497–501. doi: 10.1038/nature14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edelstein S.J., Schaad O., Changeux J.-P. Single binding versus single channel recordings: a new approach to study ionotropic receptors. Biochemistry. 1997;36:13755–13760. doi: 10.1021/bi9718301. [DOI] [PubMed] [Google Scholar]
- 59.Rasmussen S.G., DeVree B.T., Kobilka B.K. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doumazane E., Scholler P., Rondard P. Illuminating the activation mechanisms and allosteric properties of metabotropic glutamate receptors. Proc. Natl. Acad. Sci. USA. 2013;110:E1416–E1425. doi: 10.1073/pnas.1215615110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeVree B.T., Mahoney J.P., Sunahara R.K. Allosteric coupling from G protein to the agonist-binding pocket in GPCRs. Nature. 2016;535:182–186. doi: 10.1038/nature18324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gurevich V.V., Pals-Rylaarsdam R., Onorato J.J. Agonist-receptor-arrestin, an alternative ternary complex with high agonist affinity. J. Biol. Chem. 1997;272:28849–28852. doi: 10.1074/jbc.272.46.28849. [DOI] [PubMed] [Google Scholar]
- 63.Changeux J.P. 50 years of allosteric interactions: the twists and turns of the models. Nat. Rev. Mol. Cell Biol. 2013;14:819–829. doi: 10.1038/nrm3695. [DOI] [PubMed] [Google Scholar]
- 64.Roth B.L. DREADDs for neuroscientists. Neuron. 2016;89:683–694. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.