Abstract
Protothecosis is a rare algal infection, affecting primarily immunocompromised hosts. Optimal management is unclear: in-vitro antimicrobial breakpoints are not established and therapeutic decisions are primarily based on case reports. We present a case of cutaneous Prototheca wickerhamii infection in an immunosuppressed 63 year old male, successfully treated with liposomal amphotericin and prolonged itraconazole. Inoculation may have been through frequent hot-tub use, highlighting hot-tub exposure as an infection risk for the immunocompromised host.
Keywords: Prototheca wickerhamii, Protothecosis, Cutaneous, Liposomal amphotericin, Itraconazole
1. Introduction
Human protothecosis is an algal infection due to environmentally ubiquitous achlorophyllic algae: Prototheca species. Prototheca wickerhamii and Prototheca zopfii are the species reported in human infections, and infection occurs predominantly in the immunocompromised, for whom outcomes are often poor [1]. The optimal management of cases remains unclear with in-vitro minimum inhibitory concentrations (MICs) of antimicrobials difficult to interpret, and no clinical breakpoints established. Published case reports to date are relatively uncommon [2], thus reports of risk factors, clinical presentation and outcomes of potential therapies are valuable for future research into therapy.
2. Case
A 63-year-old male presented with two weeks of right lower limb swelling, erythema and ulceration. The patient recalled frequent use of a home hot-tub over the preceding months before presentation.
His medical history included dermatomyositis with associated pulmonary fibrosis, Type 2 diabetes mellitus, peripheral neuropathy, stage III chronic kidney disease, dilated congestive cardiac failure with moderate systolic dysfunction, atrial fibrillation, and compensated chronic liver disease due to primary biliary cirrhosis. He was not known to have HIV infection but testing was not performed for this. In addition to his chronic co-morbidities, the patient had a history of abdominal wall Mycobacterium chelonae infection requiring debridement and multidrug therapy 3 years previously; and disseminated Mycobacterium haemophilum requiring prolonged antimicrobials 2 years previously. Both mycobacterial infections were considered cured at the time of his presentation.
Medications on presentation included long term prednisolone 30 mg daily, in addition to intermittent higher doses up to 35 mg over the preceding 6 months for presumed flares of his inflammatory lung disease. Other medications included beta-blockers, diuretics, digoxin, ursodeoxycholic acid and subcutaneous insulin.
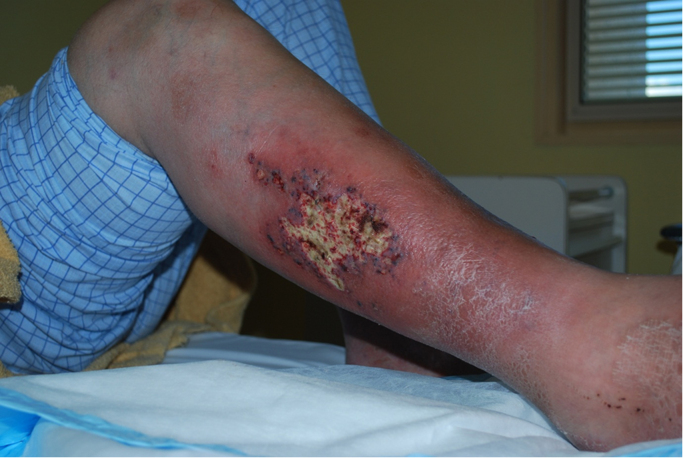
On examination at presentation to hospital (day 0), the patient was febrile at 38.5° Celsius, haemodynamically stable and normoglycemic on presentation. Inspection of the right lower leg (Fig. 1) revealed a 15 cm region of erythema and warmth with central ulceration and several surrounding dark bullae.
Fig. 1.
Right leg ulcer from which Prototheca wickerhamii was subsequently grown.
Laboratory results revealed raised inflammatory markers with a leukocyte count of 12.6×109/L with a left shift, and a C-reactive protein of 32 mg/L. Renal function indices were abnormal with an eGFR of 37 mL/min/1.73 m2.
The patient was provisionally diagnosed with a right lower limb soft tissue infection and acute on chronic renal failure. Ticarcillin-clavulanate 3.1 g four times daily was commenced. Swabs of the ulcer were sent for general microscopy and culture, with no organisms seen on Gram stain. A biopsy of the ulcer edge was performed and sent for mycobacterial and fungal cultures. Direct microscopy on the biopsy showed no fungal elements, and no acid-fast bacilli were demonstrated on Ziehl-Neelsen stain. Biopsy sample PCR for methicillin-resistant Staphylococcus aureus (MRSA) was positive and renally dose-adjusted vancomycin was commenced.
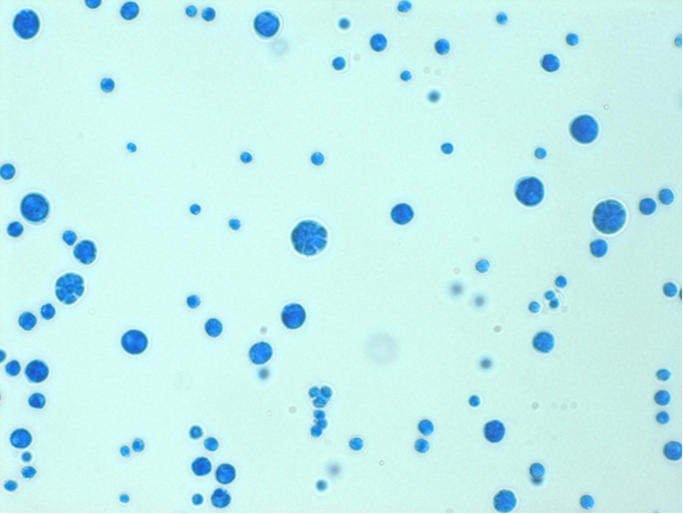
One week following presentation there was no improvement of the right lower limb soft tissue infection. New bullae and ulceration had formed on the medial aspect of the leg. At this time yeast-like organisms were grown from biopsy enrichment cultures (cooked meat broth), along with MRSA, with no growth on primary plates (Horse Blood Columbia Agar and Chocolate Agar). Subcultures of the yeast-like organisms produced creamy colonies on Sabouraud's Dextrose Agar (SDA) and pale purple colonies 1–2 mm in diameter at 2 days’ incubation on Candida CHROMagar™ (CHROMagar, France). These were Gram-positive and had morula forms, characteristic of Prototheca spp. on wet preparation (Fig. 2). RapID™ Yeast Plus (Thermo Fisher Scientific Remel Products, USA) provided an “adequate identification” for Prototheca wickerhamii and API 20C AUX (BioMérieux, France) provided an “excellent identification” for Prototheca wickerhamii. Antifungal susceptibilities were performed by broth microdilution using the Sensititre Yeast-One Test Panel (Trek Diagnostic Systems, USA). The MICs for fluconazole, itraconazole, voriconazole, posaconazole, caspofungin, anidulafungin, micafungin, 5-fluorocytosine, and amphotericin B were 128, 0.5, 0.25, 0.25, >8, >8, >8, >64 and 0.25 mg/liter, respectively. Blood cultures were negative. The Prototheca isolate was later retrieved for isavuconazole susceptibility testing. It was grown on SDA for 24 h at 30 °C. Susceptibility testing for isavuconazole was performed in a 96-well round-bottom plate using standard broth microdilution assay, according to the Clinical and Laboratory Standards Institute (CLSI) document M27-A3 for yeast [3]. The concentration range for isavuconazole was 0.06–32 mg/L. Results were obtained by reading the plates visually after 48 and 72 h incubation at 37 °C. The MIC of isavuconazole was read as the lowest concentration of the drug at which there was 100% inhibition of growth. The MIC obtained for isavuconazole against Prototheca was 0.5 mg/L after both 48 and 72 h incubation periods.
Fig. 2.
Wet preparation microscopy of P. wickerhamii isolates demonstrating characteristic morula forms.
The patient was diagnosed with cutaneous protothecosis and intravenous liposomal amphotericin was commenced at 3 mg per kg based on ideal body weight. Other antibiotics were discontinued and prednisone therapy was reduced to 20 mg. By day three of amphotericin (day 10 post admission), however, renal function had deteriorated with an eGFR of 22 mL/min/1.73 m2 and liposomal amphotericin was ceased. Oral itraconazole 200 mg twice daily was commenced with recovery of renal function to baseline over the following week before discharge. Over the next three months, the ulcers improved slowly and a repeat biopsy of the residual lateral ulcer was did not isolate Prototheca species on cultures or microscopy. The patient tolerated the oral therapy well. By 6 months the right lower limb ulceration had healed with a minor residual area of granulation and superficial desquamation.
3. Discussion
Prototheca species are achlorophyllic algae found in soil and water [1]. Human infections due to Prototheca species are rare, with only 160 cases (as described by Todd et al.) up to mid-2011, since the first report in 1964 [2]. Since this report, a further 41 cases have been reported, perhaps suggesting human protothecosis is being increasingly diagnosed. All human cases of protothecosis, where organisms were identified to species level, have been caused by either P. wickerhamii or P. zopfii species. Protothecosis predominantly occurs in adults, although infections in children are described [2]. Inoculation is thought to occur via occult or overt skin trauma, especially in the context of contaminated water [1], [2]. This patient in this case reported frequent hot-tub exposure and this was felt to be the likely environmental exposure, although a sample of the hot-tub water was not available for microbiological testing. Hot tubs have been associated with other cutaneous and systemic infections, particularly with mycobacteria [4], [5] and Pseudomonas aeruginosa [6]. Confirmed or suspected protothecosis acquisition from hot-tub use has not been described before.
Clinical presentations of protothecosis may be broadly divided into the categories of cutaneous and systemic (including disseminated disease). The spectrum of cutaneous protothecosis is broad and constitutes the majority of cases reported, with wide range of findings including ulcers (as with our case), erosions, crusting, papules, plaques and pustules [7]. Olecranon bursitis is a notable manifestation and has been considered a distinct entity [8]. A wide range of systemic manifestations has been reported, including abdominal infections, pneumonia, bloodstream infection and central nervous system infection [2], [9], [10], [11]. Peritoneal catheter and other device infections are also described [2], [12], [13]. Disseminated disease is reported generally in the immunosuppressed, frequently with exogenous steroid use, as with this case [2]. Other immunosuppressants described in association with protothecosis include chemotherapy for malignancy or transplantation [13], [14]. Non-iatrogenic immunosuppressing risk conditions include chronic renal failure and diabetes mellitus [11], [15], the latter which was seen in this case.
Culture and microscopy are essential in the approach to the diagnosis of protothecosis. Prototheca species typically form creamy yeast-like colonies on Sabouraud's media [15], [16], as was seen in this case. On wet preparation, they can be distinguished from yeast by the absence of budding, and presence of small sporangia with endospores forming characteristic ‘spoked wheels’ on microscopy of cultures, as illustrated in Fig. 2 [16]. Diagnosis may also be made in histopathologic sections, with morulae often visible with stains used to detect fungus, such as Periodic Acid Schiff stain or Grocott's modification of Gomori methenamine silver [1]. Commercial systems which may correctly identify Prototheca include the API 20C or API 20C AUX (bio-Mérieux, Marcy L'Etoile, France) as well as Vitek 2 (bio-Mérieux), but the API 32C (bio-Mérieux) does not include these organisms in its database [17]. Molecular techniques have been used to identify Prototheca species in human clinical samples, including ribosomal internal transcribed spacer (ITS) sequencing [18] and 26S rRNA gene sequencing [17]. Successful identification with mass spectrometry has been reported [19] but, as clinical isolates of Prototheca spp. are uncommon, these are not well represented in current databases, leading to potential for failed identification or misidentification [13], [14].
The natural history of isolated skin disease is an indolent progressive course [7], though there are reports of spontaneous resolution [2], [20]. In the immunosuppressed, cutaneous lesions may disseminate and this form of protothecosis has high mortality [2]. Such prognoses underscore the need for prompt diagnosis and rapid treatment of even apparently isolated skin disease, particularly in the context of immunosuppression [17], [21].
Medical treatment for protothecosis most commonly includes antifungal agents and/or tetracyclines [1], [2]. Reported treatment success rates are higher for cutaneous disease (73%) and olecranon bursitis (83%) and low for disseminated disease (33%) [2]. There are no established antimicrobial breakpoints for susceptibility or resistance but Prototheca species generally appear to be susceptible to amphotericin in-vitro [1], with variable susceptibility to triazoles. In-vitro synergy has been reported for tetracycline [1], and recently in-vitro susceptibility to miltefosine and terbinafine, with resistance to echinocandins reported [13]. Intravenous amphotericin, either alone or in combination with other agents, has the highest reported treatment success rates to date: 77% and 86%, respectively [2]. Triazoles, especially itraconazole, have been reported as successful treatments, but failure with these is commonly reported, including with cutaneous disease, which prompted our induction with amphotericin therapy for this case. To our knowledge, there are no reports of isavuconazole susceptibility testing for Prototheca spp. We found a relatively low MIC of 0.5 mg/L in our clinical isolate, suggesting this agent may merit further study as a treatment option for protothecosis. Consideration may be given to surgery in isolated cutaneous disease or olecranon bursitis. Reduction of immunosuppression is also a consideration, as corticosteroids in particular appear to increase susceptibility to Prototheca infection [2].
In our case, we believe prompt microbiological diagnosis, delivery of amphotericin followed by itraconazole therapy and reduction of immunosuppression contributed to the successful outcome. The patient was cautioned about hot-tub use as a potential source of protothecosis or mycobacterial disease.
Conflict of interest
There are none.
Acknowledgments
Dr. Shrada Subedi, Westmead Hospital, for assistance with acquisition of clinical data. Dr. Catriona Halliday, Clinical Mycology Reference Laboratory, Westmead Hospital, for assistance with isavuconazole susceptibility testing.
References
- 1.Lass-Flörl C., Mayr A. Human protothecosis. Clin. Microbiol. Rev. 2007;20:230–242. doi: 10.1128/CMR.00032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Todd J.R., King J.W., Oberle A., Matsumoto T., Odaka Y., Fowler M. Protothecosis: report of a case with 20-year follow-up, and review of previously published cases. Med. Mycol. 2012;50:673–689. doi: 10.3109/13693786.2012.677862. [DOI] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute, M27-A3. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard—Third Edition, Wayne, PA, USA, 2008.
- 4.Edson R.S., Terrell C.L., Brutinel W.M., Wengenack N.L. Mycobacterium intermedium granulomatous dermatitis from hot tub exposure. Emerg. Infect. Dis. 2006;12:821–823. doi: 10.3201/eid1205.051281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piersimoni C. Extrapulmonary infections associated with nontuberculous mycobacteria in immunocompetent persons. Emerg. Infect. Dis. 2009:1351–1358. doi: 10.3201/eid1509.081259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Y., Cheng A.S., Wang L., Dunne W.M., Bayliss S.J. Hot tub folliculitis or hot hand-foot syndrome caused by Pseudomonas aeruginosa. J. Am. Acad. Dermatol. 2007;57:596–600. doi: 10.1016/j.jaad.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Mayorga J., Barba-Gómez J.F., Verduzco-Martínez A.P., Muñoz-Estrada V.F., Welsh O. Protothecosis. Clin. Dermatol. 2012;30:432–436. doi: 10.1016/j.clindermatol.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Pednekar M., Chandra P.A., Margulis Y., Chandra A.B., Schiff C. Protothecal olecranon bursitis: an unusual algal infection. Am. J. Med. Sci. 2011;342:424. doi: 10.1097/MAJ.0b013e31822aefc0. [DOI] [PubMed] [Google Scholar]
- 9.Żak I., Jagielski T., Kwiatkowski S., Bielecki J. Prototheca wickerhamii as a cause of neuroinfection in a child with congenital hydrocephalus. First case of human protothecosis in Poland. Diagn. Microbiol. Infect. Dis. 2012;74:186–189. doi: 10.1016/j.diagmicrobio.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Tan R.M.R., Aw M.M., Quak S.H., Chan S.M. Pulmonary protothecosis in a pediatric liver transplant patient. J. Pediatr. Infect. Dis. 2014;3:e31–e34. doi: 10.1093/jpids/pit034. [DOI] [PubMed] [Google Scholar]
- 11.Min Z., Moser S.A., Pappas P.G. Prototheca wickerhamii algaemia presenting as cholestatic hepatitis in a patient with systemic lupus erythematosus – a case report and literature review. Med. Mycol. Case Rep. 2013;2:19–22. doi: 10.1016/j.mmcr.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sykora T., Horakova J., Buzzasyova D., Sladekova M., Poczova M., Sufliarska S. Protothecal peritonitis in child after bone marrow transplantation: case report and literature review of paediatric cases. New Microbes New Infect. 2014;2:156–160. doi: 10.1002/nmi2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macesic N., Fleming S., Kidd S., Madigan V., Chean R., Ritchie D. Protothecosis in hematopoietic stem cell transplantation: case report and review of previous cases. Transpl. Infect. Dis. 2014;16:490–495. doi: 10.1111/tid.12223. [DOI] [PubMed] [Google Scholar]
- 14.Bandaranayake T.D., Paniz Mondolfi A., Peaper D.R., Malinis M.F. Protothecawickerhamii algaemia: an emerging infection in solid organ transplant recipients. Transpl. Infect. Dis. 2015;17:599–604. doi: 10.1111/tid.12407. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q.-Q., Li L., Zhu L.-P., Zhao Y., Wang Y.-R., Zhu J.-H. Cutaneous protothecosis in patient with diabetes mellitus and review of published case reports. Mycopathologia. 2011;173:163–171. doi: 10.1007/s11046-011-9480-0. [DOI] [PubMed] [Google Scholar]
- 16.Okazaki C., Wakusawa C., Chikama R., Murakami K., Hitomi H., Satoh K. A case of cutaneous protothecosis in a Polyarteritis nodosa patient and review of cases reported in Japan. Dermatol. Online J. 2011;17:2. [PubMed] [Google Scholar]
- 17.McMullan B., Muthiah K., Stark D., Lee L., Marriott D. Prototheca wickerhamii mimicking yeast: a cautionary tale. J. Clin. Microbiol. 2011;49:3078–3081. doi: 10.1128/JCM.00487-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirose N., Nishimura K., Inoue-Sakamoto M., Masuda M. Ribosomal internal transcribed spacer of Prototheca wickerhamii has characteristic structure useful for identification and genotyping. PLoS One. 2013;8:e81223. doi: 10.1371/journal.pone.0081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Bergen M., Eidner A., Schmidt F., Murugaiyan J., Wirth H., Binder H. Identification of harmless and pathogenic algae of the genus Prototheca by MALDI-MS. Proteomics Clin. Appl. 2009;3:774–784. doi: 10.1002/prca.200780138. [DOI] [PubMed] [Google Scholar]
- 20.Boyce F., Longcrier D.L., Young J.W., Partlow K. Prototheca as a pathogen. Clin. Microbiol. Newsl. 1979;1:4–5. [Google Scholar]
- 21.Murata M., Ito T., Nagae K., Nakano-Nakamura M., Nishida R., Takei K. Disseminated protothecosis manifesting with multiple, rapidly-progressing skin ulcers: successful treatment with amphotericin B. Eur. J. Dermatol. 2015;25:208–209. doi: 10.1684/ejd.2015.2518. [DOI] [PubMed] [Google Scholar]