Abstract
Spinal cord injury (SCI) results in an acute reduction in neuronal and glial cell viability, disruption in axonal tract integrity, and prolonged increases in glial activity and inflammation, all of which can influence regional metabolism and glucose utilization. To date, the understanding of glucose uptake and utilization in the injured spinal cord is limited. Positron emission tomography (PET)-based measurements of glucose uptake may therefore serve as a novel bio-marker for SCI. This study aimed to determine the acute and sub-acute glucose uptake pattern after SCI to determine its potential as a novel non-invasive tool for injury assessment and to begin to understand the glucose uptake pattern following acute SCI. Briefly, adult male Sprague-Dawley rats were subjected to moderate contusion SCI, confirmed by locomotor function and histology. PET imaging with [18F]Fluorodeoxyglucose (FDG) was performed prior to injury and at 6 and 24 hours and 15 days post-injury (dpi). FDG-PET imaging revealed significantly depressed glucose uptake at 6 hours post-injury at the lesion epicenter that returned to sham/naïve levels at 24 hours and 15 dpi after moderate injury. FDG uptake at 15 dpi was likely influenced by a combination of elevated glial presence and reduced neuronal viability. These results show that moderate SCI results in acute depression in glucose uptake followed by an increase in glucose uptake that may be related to neuroinflammation. This acute and sub-acute uptake, which is dependent on cellular responses, may represent a therapeutic target.
Keywords: glucose uptake, spinal cord injury, positron emission tomography, rat
Introduction1
There are approximately 12,000 new cases of spinal cord injury (SCI) each year[1]. SCI results in permanent motor, autonomic, and sensory function loss due to both the initial mechanical insult and secondary tissue damage. SCI is characterized in humans and rats by the development of a lesion that progressively extends from the injury epicenter[2]. This lesion is attributable to acute neuronal and white matter loss continuing for weeks to months following the initial insult[3], and is compounded by continuing neuroinflammation in and surrounding the lesion core[4].
Neurons and glia are known to contribute to glucose uptake and metabolism in the brain and spinal cord. Following brain injury, glucose uptake and metabolism have been shown to follow a pattern of elevation in the first few minutes to hours followed by sub-acute to chronic depression and slow return to baseline [5]. The glucose uptake and metabolism pattern that occurs after SCI is less well known. Autoradiography examination of monkey spinal cord shows an acute increase in glucose metabolism that lasts approximately 1 hour in white matter, followed by depression for the next 8 hours in both white and gray matter at the lesion site, and elevation for the next 8 hours in the perilesion[6]. Chronically, autoradiography studies have shown that glucose uptake and metabolism is depressed in the moderately injured rodent spinal cord[7]. Imaging of glucose uptake and metabolism with positron emission tomography (PET) demonstrated a chronic decrease in 18fluorodeoxyglucose (FDG) uptake in human chronic cervical myelopathy patients[8].
FDG is a glucose analog that reliably simulates glucose uptake in several organs, including within the central nervous system (CNS). We have previously demonstrated that FDG-PET is effective in detecting reductions in glucose uptake after mild traumatic brain injury and correlated this reduction with both cellular and functional alterations[5]. However, to date, only one study, published by Tewarie et al. in 2010, has utilized FDG-PET after severe contusion SCI in rodents, finding significantly increased uptake surrounding the lesion peaking at 7 days post-injury (dpi)[9]. This study did not examine FDG uptake during the acute phase (within the first 24 hours post injury), a crucial period post injury for cellular response and recovery. Further, the Tewarie group failed to use a control region of interest for their uptake values at the injury site, complicating comparison between subjects.
Our study serves to address this gap and determine what changes in FDG uptake occur acutely (6 – 24 hours post-injury) and subacutely (7 – 15 days post-injury). The goal of this study was twofold: a) to examine the acute and sub-acute glucose uptake pattern in the injured spinal cord and b) to determine the potential usefulness of FDG-PET as a non-invasive technique for assessment of cellular changes after SCI. We now show that FDG-PET can detect measurable changes in glucose uptake after SCI, including an acute reduction followed by a return to normal levels by 2 weeks post-injury, and that this return to normal levels is influenced by both inflammation and neuronal loss.
Materials and Methods
Animals
Adult male Sprague Dawley rats (Taconic, Germantown, MD; 10 weeks of age: 150 – 200g; n = 6 injured, 6 sham for primary study, n = 2 naïve, n = 4 injured for cerebellum assessment) were given free access to food and water and a 12h light/12h dark cycle. Sample sizes were calculated based on our previous work[5]. One rat was removed from the injured group due to baseline FDG measures more than 2 SD away from the mean (final n = 5). All animal procedures were approved by the Uniformed Services University IACUC and complied fully with the principles set forth in the “Guide for the Care and Use of Laboratory Animals” prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Resources, National Research Council (DHEW pub. No. (NIH) 85-23, 2985); this manuscript has been written in accordance with the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines.
Contusion Spinal Cord Injury
Contusion SCI was performed in rats as described previously[10]. Briefly, rats were anesthetized with isoflurane (4% induction, 2.5% maintenance in 50% O2), and a laminectomy was performed at vertebral level T9 to expose the spinal cord. Moderate injury (150 kDynes) was induced with the Infinite Horizons Impactor (Precision Systems Incorporated, Natick, MA). Sham injured rats received laminectomy without impact. Naïve animals received no surgery or anesthesia. Post injury care included dual housing on diamond soft bedding (Harlan Laboratories, Frederick, MD), twice daily bladder expressions until spontaneous micturition returned and analgesic supplementation to drinking water for 48 hours following injury (Children’s Tylenol; acetaminophen, 200mg/kg).
Imaging analysis methods
Prior to injury and at 6 and 24 hours and 15 dpi, PET imaging was performed to assess FDG uptake, as previously described[5]. Animals were anesthetized with isoflurane for tail vein injection (1.5 – 2 mCi FDG) and 70-minute FDG uptake period. After uptake, a static 30-minute PET scan was acquired in list mode using a Siemens Inveon Multimodality scanner (Siemens Medical Solutions USA, Malvern, PA). Immediately prior to the PET scan, rats underwent a micro-computed tomography (CT) scan for anatomical localization and attenuation correction. The field of view center was located at T9 for the spinal cord study and the brain for the cerebellum study. The following parameters were used for PET reconstruction: reconstruction algorithm - OSEM3D/MAP (OSEM3D iterations – 2; MAP iterations – 18); corrections - attenuation and scatter; image size – 256 × 256; requested resolution (smoothing) - 0.5 mm. The intrinsic resolution of the scanner at the center of the field of view was 1.4 mm.
Images were analyzed using Siemens Inveon Research Workplace (IRW) software, version 4.2. Alignment of PET scan with CT ensured that the placement of the ROI was within the spinal column. Additionally, a manual rigid-body registration was used for select subjects in which the scanner transformation matrix produced visibly misaligned images. This resulted in some datasets being coregistered based on the scanner transformation matrix and some by manual registration, which may be attributable as a source of some variation across subjects. Quantification of tracer uptake was obtained in regions of interest (ROIs) standardized in size to the injury site of the spinal cord.
In a separate analysis, 4 animals underwent PET imaging before and at 6 and 24 hours after SCI for assessment of FDG uptake in the cerebellum. The same ROI utilized in the injury study was placed within the cerebellum and compared to whole brain uptake.
Functional Assessment
The Basso–Beattie–Bresnahan (BBB) scale was used to assess neurological function, as detailed previously[11]. Performance of left and right hindlimbs on a scale from 0 (no spontaneous movement) to 21(normal locomotion) was averaged to obtain the total BBB on days 2, 7 and 14 dpi by two independent researchers blinded to treatment group.
Histology methods
At 16 dpi, rats were anesthetized with Euthasol (pentobarbital sodium and phenytoin sodium; 200mg/kg, IP) and intracardially perfused with 10% buffered formalin (Fisher Scientific, Hampton, New Hampshire). A 1 cm block of spinal cord tissue was dissected, extending 5 mm from the lesion site in either direction (or from the T9 vertebral site for naïve and sham animals). Tissue was sectioned in 20μm longitudinal sections, and every other slice was saved for histological analyses. To determine volume of lesion, tissue was processed with a standard H&E stain. Lesion volume from H&E images collected with NanoBrightfield Software were quantified using the Cavalieri method as previously described[12]. Eight sections were analyzed per animal, at even intervals throughout spinal cord to provide analysis of lesion across the spinal cord.
Immunohistochemistry
Standard fluorescent immunohistochemistry was performed on tissue sections from animals 16 dpi obtained as described above. Iba1 (microglia; Wako Bioproducts, Richmond, VA), GFAP (astrocytes; Abcam, Cambridge, MA), and NeuN (neurons; Millipore, Temecula, CA) were used as primary antibodies, with visualization by fluorescent secondary antibodies (Alexa-Fluor Secondaries, Molecular Probes, Eugene, OR). Negative controls (sections not stained with primary antibody) were used to confirm specificity of secondary antibodies. Specificity of primary antibodies was confirmed by visualization of appropriate and expected staining of cells. Immunofluorescent images were captured on a NanoZoomer system (Hamamatsu, Bridgewater, NJ) or an Olympus BX43 microscope (Olympus America). Four longitudinal sections were analyzed per animal starting at first sign of lesion in the corticospinal tract between the dorsal horns, then at even intervals through the central gray matter and ventral horns of the spinal cord to provide analysis throughout the lesion. Analysis was performed at 10X magnification, in the center of the lesion site frame with a 1.5mm width and 0.95mm height. Immunofluorescence was quantified using pixel density measurement in Scion Image as previously described[13].
Statistical Analysis
Quantitative data are presented as mean +/− standard deviation. FDG uptake (an average of measures by 2 blinded investigators) and functional tests were analyzed with two-way analysis of variance (ANOVA) and repeated measures between injured and sham groups. Immunohistochemical analyses were analyzed with unpaired t-tests between injured and sham groups at a given day post injury. Correlation between FDG uptake and lesion volume was performed using Spearman Correlation analysis. Statistical tests were performed using the GraphPad Prism Program, Version 6 (GraphPad Software, San Diego, CA). A p value of < 0.05 was considered statistically significant.
Results
Moderate spinal cord injury induces functional impairment and lesion
To ensure that the moderate injury assessed using FDG-PET was consistent with that seen by us and others with a moderate contusion SCI, we completed BBB scoring and H&E lesion volume analysis. BBB score assessments demonstrated impaired performance by rats after injury (Fig. 1A), with a score of 0.333 +/− 0.2 by day 2 and 13.42 +/− 1.8 by day 14, comparable to previous work by us [2,14] and others[11,15].
Figure 1.
Functional recovery and lesion volume analysis show injured animals have decreased functional recovery and lesion volume compared to sham. A) Injured rats demonstrated reduced BBB scores after injury. N=6/injured, 6/sham. B) Representative H&E stained spinal cords from sham and injured rats at 16 dpi; border delineates measured lesion. Four longitudinal sections were analyzed per animal starting at first sign of lesion (in the corticospinal tract between the dorsal horns), then at even intervals through the central gray matter and ventral horn of the spinal cord. Analysis was performed at 10x magnification, in center of lesion site (1.5mm width and 950μm height). C) Lesion volume is significantly greater in injured rats compared to sham (p<0.0001). N=5/injured, 6/sham. Bar represents mean +/− SD.
H&E stained longitudinal spinal cord sections from injured rats were used to assess the size of the lesion at 16 dpi. Sham rats demonstrated no lesion while injured rats demonstrated a conical lesion that spanned the injury site and showed less staining with H&E than the non-injured surrounding tissue (Fig. 1B), similar to that shown previously[2,14]. Lesion volume was significantly greater in injured rats (36.38 mm3 +/− 5.44) than sham (0 mm3) animals (p < 0.0001; Fig. 1C).
FDG uptake is measurable within the injured spinal cord
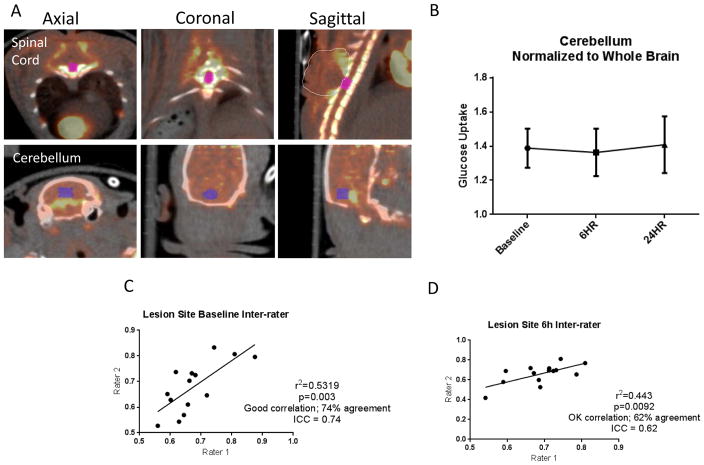
Alignment of the PET scan with CT ensured that the placement of the ROI was within the spinal column (Fig. 2A). FDG uptake in the cerebellum was found to not change at 6 or 24 hours after a moderate SCI (Fig. 2B), demonstrating that this region is a reliable reference region. To confirm that FDG measures in the spinal cord were equally reliable, uptake values within the spinal cord ROI were first normalized to the reference site (cerebellum) and then normalized to baseline uptake values by two blinded investigators. Inter-rater reliability was assessed using InterClass Correlation (ICC score)[16] and showed 74% agreement (p=0.003; r2=0.5319) in baseline measures (Fig. 2C) and 62% agreement (p=0.0092; r2=0.443) in 6 hour post-injury measures (Fig. 2D), due to slight differences in ROI placement.
Figure 2.
Methodology for FDG-PET assessment after SCI. A) ROI placement in spinal cord and cerebellum in axial, coronal and sagittal pseudocolored PET images and overlayed on CT images. ROI size=26mm3 (x=2.956mm, y=4.548mm, z=5.003mm). Localization of lesion site was established utilizing CT scans, which demonstrated vertebral processes. Dotted line in sagittal plane highlights uptake caused by surgery outside the injury site, not included in quantification. B) FDG uptake in the cerebellum prior to injury and at 6 and 24 hours after injury showed no significant changes (n=4). C) At baseline, ICC score indicates 74% agreement and significant positive correlation between 2 blinded investigators (p=0.003). D) At 6 hours post injury, ICC score indicates 62% agreement and significant positive correlation between 2 blinded investigators (p=0.0092).
FDG uptake pattern as recorded by PET imaging at 6 hours, 24 hours and 15 days post spinal contusion injury
At 6 and 24 hours and 15 dpi, the site of skin and muscle incision and laminectomy (Fig. 2A – dotted line), dorsal to the spinal cord, demonstrated an increase in glucose uptake in both sham and injured rats (Fig 3A). This FDG uptake is most likely due to inflammation of the surrounding muscle tissue, as has been shown previously[17,18], and was therefore not quantified or included within the ROI.
Figure 3.
SCI results in acute reduction in FDG uptake at the lesion epicenter. A) Representative pseudocolor FDG-PET sagittal images of sham and injured rats at baseline, 6 and 24 hours and 15 dpi. Increased FDG uptake is observed at the surgical site dorsal to the T9 vertebra (beneath arrows). Arrows indicate the lesion epicenter. PET image intensity was normalized to the cerebellum and the Min/Max window was set to 0–4 nCi. At 6 hours post injury, there is reduction in color intensity at the lesion epicenter in injured rats compared to sham. B) Injured rats showed significantly depressed glucose uptake 6 hours post-injury compared to sham (*p=.0260), returning to similar levels as sham by 24 hours and holding steady to 15 dpi. C) Baseline values showed no significant variability between animals (Fig. 2B; SD=0.086). D) Volume of lesion correlated significantly with FDG-PET uptake at 6 hours post-injury (p=0.0059). N=5/injured, 6/sham, 2/naïve. Points represent mean +/− SD.
FDG uptake at 6 hours post injury was slightly elevated above baseline (dotted line, Fig. 3B) to 1.096 +/− 0.01 in the naïve group and 1.108 +/− 0.05 in the sham group (Fig. 3B). In contrast, uptake in the injured group was approximately 16% lower, at 0.928 +/− 0.04, a significant reduction in comparison to the sham group (Fig. 3B; p = 0.0260). By 24 hours, FDG uptake in all groups was above baseline, with no significant difference between groups (sham = 1.262 +/− 0.2544, injured = 1.179 +/− 0.2091). At 15 dpi, the naïve group had returned to baseline values, while the injured and sham groups remained slightly above baseline, although without any significant difference between groups. Overall, glucose uptake in sham group showed a slight overall reduction from 6 hours to 15 dpi, whereas the injured group showed an overall increase in uptake, due to the acute reduction in FDG uptake at 6 hours post injury. The difference in overall uptake was significant between the two groups (p=.0290).
To verify that these findings were not influenced by individual animal variability and FDG injection variability, all FDG uptake values were normalized first to an internal control (cerebellum), followed by normalization to baseline values. Baseline values showed no significant variability within or between groups before injury (Fig. 3C).
FDG-PET imaging at 6 hours post injury correlates with lesion volume in injured rats
Correlation analysis was performed between H&E stained longitudinal spinal cord sections obtained at 16 dpi and FDG-PET glucose uptake. Lesion volume demonstrated a significant negative correlation with 6 hours post injury glucose uptake measures (Spearman r = −0.7165, p = 0.0059; Fig. 3D). No significant correlations were found between H&E sections and glucose uptake at either 24 hours (Spearman r = −0.1629; p= 0.3259) or 15 dpi (Spearman r = 0.0094; p=0.6897).
Glial cell activation is increased and neuronal viability is decreased at 15 days post-injury
Immunohistochemical staining with the microglial marker Iba1 and the astrocyte marker GFAP indicated increased glial activation, particularly within or near the lesion site, in injured animals compared to sham and naïve (Fig. 4A). Pixel density above threshold measurement of Iba1 indicated a significant increase in microglial activation in injured animals (p=0.0045; Fig. 4B), while assessment of GFAP showed a trend toward increased expression in injured animals (p=0.0764; Fig. 4B).
Figure 4.
Glial cell activation is increased and neuronal health is decreased in grey matter after injury. Quantification performed in the central gray matter lying beneath the corticospinal tract. A) Immunohistochemical staining with microglial marker Iba1 and astrocyte marker GFAP indicate increased glial activity in injured rats compared to sham and naïve demonstrated by increased fluorescent staining. Immunohistochemical staining with neuronal marker NeuN show decreased neuronal viability in injured rats compared to sham and naïve at injury site, demonstrated by decreased fluorescent staining. Size bar=800 μm. B) Pixel density measurement of Iba1 show a significant increase (p=0.0045) and of GFAP show a trend toward increased activation in injured rats compared to sham. Pixel density measurement of NeuN show a trend towards decreased neuronal viability in injured rats compared to sham. N=6/group sham and injured, 2/group naïve. Bars represent mean +/− SD.
Qualitative analysis of tissue stained with the neuronal marker NeuN demonstrated decreased staining in injured animals compared to sham and naïve, though quantitatively no significant difference was observed between injured and sham groups (p=0.1196; Fig. 4B).
Discussion
The current study demonstrates that moderate SCI, confirmed by histology and functional outcome, leads to a significant decrease in glucose uptake 6 hours after injury at the injury site that returns to at or above baseline levels by 24 hours and 15 dpi. As SCI causes an acute reduction of cellular viability, decreases in glucose uptake may be attributable to acute results of primary injury, including neuronal damage and death. The return of glucose uptake to baseline levels at later time points suggests a degree of compensation in glucose uptake in the region, most likely due to the elevated glial cell response as histology demonstrates a persistent loss of neurons and cavity development at the lesion epicenter, although further research on this is needed.
Currently, the glucose uptake and metabolism profiles after SCI are not clearly defined. Examination of macaque monkey spinal cord demonstrated an acute increase in glucose metabolism that lasted approximately 1 hour in white matter, followed by depression for the next 8 hours in both white and gray matter at the lesion site, and elevation for the next 8 hours in the perilesion spinal cord[6]. Chronically, autoradiography studies have shown that glucose uptake and metabolism is depressed in the moderately injured rat spinal cord [7]. Previous work by Tewarie et al.[9] set the groundwork for utilizing PET to visualize glucose uptake after traumatic contusion SCI in rodents. However, although they demonstrated that FDG standardized uptake value was significantly increased after a severe spinal cord contusion at 3, 7 and 21 dpi, they did not examine acute changes. Therefore, in order to understand the acute effects of SCI and compare rodent spinal cord with rodent brain injury findings, we assessed FDG uptake at the acute stage (6 hours post-injury). We now show a significant reduction in FDG uptake at 6 hours, which is in agreement with the findings of Rawe et al. in macaque monkeys [6] and with the acute pattern after a mild brain injury in rats[5].
In addition, it should be noted that Tewarie et al.[9] utilized fasted animals and an awake uptake period. Our previous work demonstrated that awake uptake periods lead to marked increases in variability of FDG uptake[5]. Maintaining animals under isoflurane anesthesia during the uptake period was sufficient to reduce variability and avoid fasting in animals after surgery, which could have detrimental health outcomes. Though anesthesia can influence FDG uptake, maintaining a steady uptake rate allows for less variability in uptake and allows for a more precise comparison between subjects. This difference in study design, including anesthesia, severity of injury, and so forth, may have contributed to the fact that while we did see an elevation in uptake beyond 6 hours post-injury toward baseline, the elevation did not reach statistical significance in comparison to sham.
Our previous work has provided evidence that alterations to glucose uptake after injury are related to the viability and functionality of both glial and neuronal cells[5]. Our findings now show that lesion volume after a moderate SCI significantly correlate with the decreased glucose uptake found in injured rats at 6 hours post injury. This suggests a potential use for FDG-PET as a non-invasive outcome measure or biomarker of SCI. The lesion caused by traumatic SCI is a combination of neuronal, axonal and glial loss at the lesion epicenter. Further work should explore this use further. Previous studies have shown that glial activation can contribute to FDG uptake, such as in multiple sclerosis[19] or traumatic brain injury models[5]. This study demonstrated elevated glial activation, as measured by microglia/macrophage and astrocyte immunolabeling, in the spinal cord at the time point when FDG uptake had returned to baseline levels. At the same time, neuronal labeling was depressed. Therefore, it is possible that the return of FDG uptake to baseline levels is a reflection of glial activation rather than neuronal recovery. And as lesion volume is a measure of tissue loss specifically, it does not account for glial activation, potentially leading to the lack of correlation between lesion volume and FDG uptake at 24 hours and 15 dpi.
Experimentally, manipulation of glucose uptake in the spinal cord has been shown to improve outcome. Administration of a ketogenic diet increases glucose transporter 1 (GLUT1) expression in spinal cord endothelial cells and was associated with reduced lesion volume[20]. The chemical compound FM19G11, which activates the AKT pathway and induces GLUT4 activation, improved motor function by 4 weeks post-injury[21].
Overall, our findings indicate that FDG-PET imaging can successfully demonstrate alterations in glucose uptake caused by SCI. In addition, this work indicates an acute but transient reduction in glucose uptake that may be a useful target for future neurotherapeutics.
Acknowledgments
The authors would like to acknowledge the technical assistance provided by Yujia Zhao. This work was funded by the Uniformed Services University and a generous gift from the Christopher and Dana Reeve Foundation. The opinions or assertions contained herein are the private ones of the author(s) and are not to be construed as official or reflecting the views of the DoD or the USUHS.
Footnotes
Abbreviations: FDG – fluorodeoxyglucose, PET – positron emission tomography, CT – computerized axial tomography, ROI – region of interest, SCI – spinal cord injury, DPI – days post injury
Contributions of Authors:
REV completed all surgeries, functional and tissue analysis, including immunohistochemistry, and drafted the manuscript. RGS, CMW and SJ performed PET and CT scans, reconstructed all scans with attenuation, and assisted with data analysis. GK assisted in surgeries, functional analysis and immunohistochemistry. KRB conceived of the study and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
References
- 1.Spinal Cord Injury: Facts and Figures, TNSS Cent. 2009 www.spinalcord.uab.edu.
- 2.Byrnes KR, Fricke ST, Faden AI. Neuropathological differences between rats and mice after spinal cord injury. J Magn Reson Imaging JMRI. 2010;32:836–846. doi: 10.1002/jmri.22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ek CJ, Habgood MD, Callaway JK, Dennis R, Dziegielewska KM, Johansson PA, et al. Spatio-temporal progression of grey and white matter damage following contusion injury in rat spinal cord. PloS One. 2010;5:e12021. doi: 10.1371/journal.pone.0012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurother J Am Soc Exp Neurother. 2010;7:366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selwyn R, Hockenbury N, Jaiswal S, Mathur S, Armstrong RC, Byrnes KR. Mild traumatic brain injury results in depressed cerebral glucose uptake: An (18)FDG PET study. J Neurotrauma. 2013;30:1943–1953. doi: 10.1089/neu.2013.2928. [DOI] [PubMed] [Google Scholar]
- 6.Rawe SE, Lee WA, Perot PL. Spinal cord glucose utilization after experimental spinal cord injury. Neurosurgery. 1981;9:40–47. doi: 10.1227/00006123-198107000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Horner PJ, Stokes BT. Fetal transplantation following spinal contusion injury results in chronic alterations in CNS glucose metabolism. Exp Neurol. 1995;133:231–243. doi: 10.1006/exnr.1995.1026. [DOI] [PubMed] [Google Scholar]
- 8.Floeth FW, Stoffels G, Herdmann J, Jansen P, Meyer W, Steiger HJ, et al. Regional impairment of 18F-FDG uptake in the cervical spinal cord in patients with monosegmental chronic cervical myelopathy. Eur Radiol. 2010;20:2925–2932. doi: 10.1007/s00330-010-1877-5. [DOI] [PubMed] [Google Scholar]
- 9.Nandoe Tewarie RDS, Yu J, Seidel J, Rahiem ST, Hurtado A, Tsui BMW, et al. Positron emission tomography for serial imaging of the contused adult rat spinal cord. Mol Imaging. 2010;9:108–116. [PubMed] [Google Scholar]
- 10.Cooney SJ, Bermudez-Sabogal SL, Byrnes KR. Cellular and temporal expression of NADPH oxidase (NOX) isotypes after brain injury. J Neuroinflammation. 2013;10:155. doi: 10.1186/1742-2094-10-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basso DM, Beattie MS, Bresnahan JC. A Sensitive and Reliable Locomotor Rating Scale for Open Field Testing in Rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 12.Kabadi SV, Stoica BA, Byrnes KR, Hanscom M, Loane DJ, Faden AI. Selective CDK inhibitor limits neuroinflammation and progressive neurodegeneration after brain trauma. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2012;32:137–149. doi: 10.1038/jcbfm.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnelly DJ, Gensel JC, Ankeny DP, van Rooijen N, Popovich PG. An efficient and reproducible method for quantifying macrophages in different experimental models of central nervous system pathology. J Neurosci Methods. 2009;181:36–44. doi: 10.1016/j.jneumeth.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrnes KR, Stoica B, Riccio A, Pajoohesh-Ganji A, Loane DJ, Faden AI. Activation of metabotropic glutamate receptor 5 improves recovery after spinal cord injury in rodents. Ann Neurol. 2009;66:63–74. doi: 10.1002/ana.21673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koopmans GC, Deumens R, Honig WMM, Hamers FPT, Steinbusch HWM, Joosten EAJ. The assessment of locomotor function in spinal cord injured rats: the importance of objective analysis of coordination. J Neurotrauma. 2005;22:214–225. doi: 10.1089/neu.2005.22.214. [DOI] [PubMed] [Google Scholar]
- 16.McGraw K, Wong S. Forming inferences about some intraclass correlation coefficients. 1996;1:30–46. [Google Scholar]
- 17.Kiyoto S, Sugawara Y, Inoue T, Takabatake D, Ohsumi S, Nishimura R. False-positive (18)F-fluorodeoxyglucose positron emission tomography/computed tomography caused by incidental injury in a bulky intracystic carcinoma of the breast. Jpn J Radiol. 2010;28:305–308. doi: 10.1007/s11604-009-0417-1. [DOI] [PubMed] [Google Scholar]
- 18.Tateyama M, Fujihara K, Misu T, Arai A, Kaneta T, Aoki M. Clinical values of FDG PET in polymyositis and dermatomyositis syndromes: imaging of skeletal muscle inflammation. BMJ Open. 2015;5:e006763. doi: 10.1136/bmjopen-2014-006763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buck D, Förschler A, Lapa C, Schuster T, Vollmar P, Korn T, et al. 18F-FDG PET detects inflammatory infiltrates in spinal cord experimental autoimmune encephalomyelitis lesions. J Nucl Med Off Publ Soc Nucl Med. 2012;53:1269–1276. doi: 10.2967/jnumed.111.102608. [DOI] [PubMed] [Google Scholar]
- 20.Streijger F, Plunet WT, Lee JHT, Liu J, Lam CK, Park S, et al. Ketogenic diet improves forelimb motor function after spinal cord injury in rodents. PloS One. 2013;8:e78765. doi: 10.1371/journal.pone.0078765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodríguez-Jimnez FJ, Alastrue-Agudo A, Erceg S, Stojkovic M, Moreno-Manzano V. FM19G11 favors spinal cord injury regeneration and stem cell self-renewal by mitochondrial uncoupling and glucose metabolism induction. Stem Cells Dayt Ohio. 2012;30:2221–2233. doi: 10.1002/stem.1189. [DOI] [PubMed] [Google Scholar]