Abstract
BACKGROUND
Pancreatic cancer poses a substantial morbidity and mortality burden in the United States, and predominantly affects older adults. The objective of this study was to estimate the direct medical costs of pancreatic cancer treatment in a population-based cohort of Medicare beneficiaries, and the contribution of different treatment modalities and health care services to the total cost of care and trends in costs over time.
METHODS
In the linked Surveillance, Epidemiology, and End Results (SEER)-Medicare database, pancreatic cancer patients were identified who were aged 66 years or older and who were diagnosed from 2000 to 2007. Total direct medical costs were estimated from Medicare payments overall and within categories of care. Costs attributable to pancreatic cancer were estimated by subtracting the costs of medical care in a matched cohort of cancer-free beneficiaries.
RESULTS
A total of 15,037 patients were identified, of whom 97% were observed from diagnosis until death. Mean total direct medical costs were $65,500. Mean total costs were greater for patients with resectable locoregional disease ($134,700) than for those with unresectable locoregional or distant disease ($65,300 and $49,000, respectively). Hospitalizations and cancer-directed procedures collectively accounted for the largest fraction of health care costs. The total cost of care appeared to increase slightly over the study period (P = .05). The mean costs attributable to pancreatic cancer were $61,700.
CONCLUSIONS
Despite poor prognosis and short survival, the economic burden of pancreatic cancer in the elderly is substantial. Demographic trends, greater use of targeted therapies, and possible implementation of screening strategies are likely to impact treatment patterns and costs in the future.
Keywords: pancreas cancer, adenocarcinoma, treatment, cost, Surveillance, Epidemiology, and End Results, Medicare, health care services
INTRODUCTION
Pancreatic cancer is the fourth leading cause of cancer-related mortality in the United States.1 Surgery is the only potentially curative option, but fewer than 20% of patients are candidates for resection.1,2 Palliative chemotherapy and radiation therapy produce modest improvements in survival, but sustained response rates are low, and attempts to improve outcomes in unresectable patients have been only modestly successful.3–5
In addition to the morbidity and mortality burden, the costs of treating pancreatic cancer are high and increasing.6–11 More than 70% of cases are diagnosed in patients aged 65 years and older.12 Thus, in the United States, the Medicare program pays for a substantial fraction of associated costs. Prior estimates of the costs of treating pancreatic cancer in Medicare beneficiaries have not been reported at a patient level, and population level estimates are now outdated.6 Our objectives were to estimate the direct medical costs of pancreatic cancer treatment in older Americans; to evaluate the contribution of specific treatment modalities and health care services to the total cost of care; and to examine trends in costs over time.
MATERIALS AND METHODS
Data Source
This retrospective, population-based cohort study was conducted using the Surveillance, Epidemiology, and End Results registries linked to Medicare claims data set (SEER-Medicare). The SEER program, sponsored by the National Cancer Institute, is a consortium of cancer registries in selected states and geographic areas covering approximately 28% of the US population.13 The SEER registries collect information on demographic characteristics, site and extent of disease, clinical and pathological stage, and first course of cancer-directed therapy, with active follow-up for date and cause of death. Medicare is the primary health insurer for 97% of the US population aged 65 years and older, covering inpatient hospital care (Part A) and outpatient care and physician services (Part B). The SEER-Medicare files were used in accordance with a data-use agreement between National Cancer Institute and Centers for Medicare & Medicaid Services, and the study was approved by the institutional review board at Memorial Sloan-Kettering Cancer Center.
Study Cohort
We identified Medicare beneficiaries aged 66 years or older with a pathologically confirmed primary diagnosis of pancreatic adenocarcinoma (International Classification of Diseases for Oncology, Third Edition site codes C25.0–C25.3, C25.7–C25.9), from January 1, 2000, through December 31, 2007. Beneficiaries aged 66 and older were included to ensure a full year of Medicare claims prior to diagnosis for identifying comorbid conditions. We excluded patients with neuroendocrine tumors, tumors in situ, and those diagnosed only at the time of death. We also excluded individuals who were enrolled in a Medicare managed care plan and those who did not have continuous coverage with both Parts A and B of Medicare from at least 1 year prior to diagnosis through death or end of follow-up, because these beneficiaries would not have complete claims for the estimation of comorbidity and identification of treatment.
Covariates
Demographic characteristics available in the SEER data set included age, race, sex, geographic location, and marital status. Census tract median income, categorized in quartiles, was used as a measure of socioeconomic status, in the absence of individual-level information. Clinical covariates included tumor location within the pancreas and SEER historic stage. Localized and regional disease were combined as locoregional, but then distinguished on the basis of the resectability of their disease. Patients with locoregional disease who had a claim for a surgical procedure with potentially curative intent were classified as resectable, and all others were classified as unresectable, consistent with prior studies.14,15 Comorbidity was estimated using the Charlson comorbidity index, based on Medicare claims in the year prior to pancreatic cancer diagnosis.16,17
Direct Medical Costs
Costs were defined as the amount reimbursed by Medicare. These were actual payments derived from reimbursement formulas that are intended to reflect the average resource utilization for each good and service.13,18–20 Two separate endpoints were estimated: direct medical costs, expressed as total and monthly costs, and costs attributable to pancreatic cancer, a component of total direct medical costs.
Total direct medical costs were estimated from all Medicare claims between time of diagnosis and time of death or end of follow-up. Mean monthly costs were total direct medical costs divided by the number of months patients were alive. In addition to overall total and mean monthly costs, we also estimated total and monthly costs within mutually exclusive categories of care. Cancer-directed procedure costs included Medicare payments for pancreatic resections and biliary drainage procedures. Chemotherapy and radiotherapy costs included Medicare payments for chemotherapy administration, specific chemotherapeutic agents, radiation therapy planning, and administration. Inpatient and hospice care costs included Medicare payments for all hospitalizations and hospice care, respectively. “Other” costs were defined as any additional care reimbursed by Medicare and included Medicare payments for outpatient services unrelated to chemotherapy or radiation therapy, home health care, and durable medical equipment.
In order to estimate costs attributable to pancreatic cancer, costs in a matched group of cancer-free Medicare beneficiaries were subtracted from the costs in the cancer cohort. Cancer-free beneficiaries were matched 1:1 by sex, race, year of birth, and SEER registry to each pancreatic cancer case. The average monthly cost of medical care for each cancer-free beneficiary was calculated based on the 12 months of claims in the calendar year of their matched case’s cancer diagnosis. This average monthly cost was then multiplied by the number of months the matched case was alive. Costs attributable to pancreatic cancer were the total costs of care for each case minus his or her matched cancer-free beneficiary’s medical care costs over the same survival duration. If more than one match was available, a single control was selected at random. Cancer-free matches faced the same exclusion criteria as cases with respect to Medicare enrollment and HMO (health maintenance organization) participation. In addition, eligible controls were required to live at least as long as their matched case.
All costs are reported in 2009 US dollars. We used the Hospital Wage Index21 and the Medicare Economic Index22 to adjust payments for inpatient and outpatient services, respectively, for inflation. We also adjusted for geographic price variability using the Acute Inpatient Prospective Payment System Wage Index23,24 for inpatient services and the Medicare Geographic Practice Cost Index25 for outpatient services.
Statistical Analysis
Mean total direct medical costs and costs by category were estimated for the entire cohort by stage at diagnosis. All cost estimates are presented rounded to the nearest $100. Survival was estimated using Kaplan-Meier methods.26 Trends over time were evaluated using linear regression models with year of diagnosis as the independent variable and costs as the dependent variable.18 For patients whose treatment costs spanned more than 1 calendar year, all costs were assigned to the year of diagnosis.18 Statistical analyses were performed using SAS software (version 9.2; SAS Inc, Cary, NC).
RESULTS
Cohort Characteristics
The study cohort included 15,037 patients diagnosed with pancreatic adenocarcinoma between 2000 and 2007. Most patients (58%) were diagnosed with distant disease. Patients diagnosed with locoregional disease were somewhat more likely to be female and to have cancers in the head of the pancreas. Patients with resectable locoregional disease were younger and healthier than those with unresectable locoregional or distant disease. They were also more likely to be married, white, and reside in urban areas and in census tracts with greater median income (Table 1).
Table 1.
Characteristics of the Cohort by Pancreatic Cancer Stage at Diagnosis
| Characteristic | All Patients | Locoregional Resectable | Locoregional Unresectable | Distant | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | ||
| Total | 15,037 | – | 2,078 | 14% | 4,234 | 28% | 8,725 | 58% | |
| Age at diagnosis, y | <.0001 | ||||||||
| 66–69 | 2,576 | 17% | 464 | 22% | 566 | 13% | 1,546 | 18% | |
| 70–74 | 3,807 | 25% | 629 | 30% | 933 | 22% | 2,245 | 26% | |
| 75–79 | 4,087 | 27% | 594 | 29% | 1,137 | 27% | 2,356 | 27% | |
| 80–84 | 2,926 | 19% | 309 | 15% | 914 | 22% | 1,703 | 20% | |
| 85+ | 1,641 | 11% | 82 | 4% | 684 | 16% | 875 | 10% | |
| Sex | <.0001 | ||||||||
| Male | 6,820 | 45% | 889 | 43% | 1,804 | 43% | 4,127 | 47% | |
| Female | 8,217 | 55% | 1,189 | 57% | 2,430 | 57% | 4,598 | 53% | |
| Race | <.0001 | ||||||||
| White | 12,838 | 85% | 1,875 | 90% | 3,550 | 84% | 7,413 | 85% | |
| Black | 1,437 | 10% | 114 | 5% | 438 | 10% | 885 | 10% | |
| Other | 762 | 5% | 89 | 4% | 246 | 6% | 427 | 5% | |
| Census tract median income | <.0001 | ||||||||
| 1st quartile | 3,760 | 25% | 403 | 19% | 1,140 | 27% | 2,217 | 25% | |
| 2nd quartile | 3,769 | 25% | 499 | 24% | 1,074 | 25% | 2,196 | 25% | |
| 3rd quartile | 3,758 | 25% | 541 | 26% | 1,049 | 25% | 2,168 | 25% | |
| 4th quartile | 3,750 | 25% | 635 | 31% | 971 | 23% | 2,144 | 25% | |
| Urban-rural residence | <.05 | ||||||||
| Metro | 12,990 | 86% | 1,830 | 88% | 3,625 | 86% | 7,535 | 86% | |
| Non-metro | 2,047 | 14% | 248 | 12% | 609 | 14% | 1,190 | 14% | |
| Region | <.0001 | ||||||||
| Northeast | 3,963 | 26% | 614 | 30% | 1,019 | 24% | 2,330 | 27% | |
| South | 2,605 | 17% | 379 | 18% | 745 | 18% | 1,481 | 17% | |
| Midwest | 2,195 | 15% | 259 | 12% | 620 | 15% | 1,316 | 15% | |
| West | 6,274 | 42% | 826 | 40% | 1,850 | 44% | 3,598 | 41% | |
| Married | <.0001 | ||||||||
| Yes | 8,137 | 54% | 1,277 | 61% | 2,143 | 51% | 4,717 | 54% | |
| No | 6,421 | 43% | 741 | 36% | 1,945 | 46% | 3,735 | 43% | |
| Unknown | 479 | 3% | 60 | 3% | 146 | 3% | 273 | 3% | |
| Charlson comorbidity score | <.0001 | ||||||||
| 0 | 7,928 | 53% | 1,184 | 57% | 2,177 | 51% | 4,567 | 52% | |
| 1 | 4,209 | 28% | 597 | 29% | 1,175 | 28% | 2,437 | 28% | |
| 2+ | 2,900 | 19% | 297 | 14% | 882 | 21% | 1,721 | 20% | |
| Site | <.0001 | ||||||||
| Head | 7,779 | 52% | 1,558 | 75% | 2,919 | 69% | 3,302 | 38% | |
| Body/tail | 3,278 | 22% | 280 | 13% | 525 | 12% | 2,473 | 28% | |
| Duct/other | 3,980 | 26% | 240 | 12% | 790 | 19% | 2,950 | 34% | |
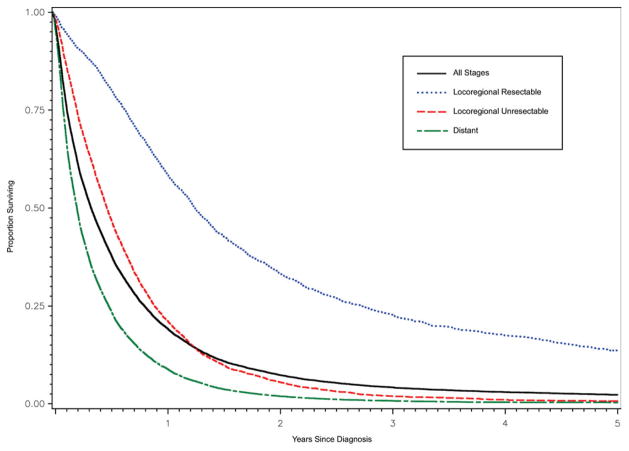
For the entire cohort, median overall survival was 4.1 months (95% CI = 4.0, 4.2 months). Median survival was 15.2 months (95% CI = 14.6, 16.0 months) for resectable locoregional disease, 5.8 months (95% CI = 5.6, 6.0 months) for unresectable locoregional disease, and 2.6 months (95% CI = 2.5, 2.7 months) for distant disease (Fig. 1). The proportions of patients alive at 1 and 5 years were 20% and 2.3%, respectively, and less than 3% of patients were alive at the end of study period.
Figure 1.
Overall survival is shown by stage at diagnosis.
Direct Medical Costs
Mean total direct medical costs for the cohort were $65,500 (standard deviation [SD], $65,400). Total costs were highest for resectable locoregional disease ($134,700; SD, $90,300) and lowest for distant disease ($49,000; SD, $48,800). Costs for unresectable locoregional disease were $65,300 (SD, $58,100). Mean monthly costs were $22,300 (SD, $56,100). Patients diagnosed with distant disease incurred the greatest monthly costs ($25,300; SD, $57,900), followed by resectable ($19,200; SD, $62,800) and unresectable locoregional disease ($17,500; SD, $48,000).
The relative contribution of each health care service category is presented in Table 2. Hospitalizations accounted for the largest fraction of health care costs overall and for patients with unresectable locoregional and distant disease. For patients with resectable locoregional disease, cancer-directed procedures accounted for the largest percentage of costs. Hospice care accounted for the smallest percentage of total costs and was lowest for resectable locoregional patients. The proportion of costs attributable to chemotherapy and radiation therapy was also lowest for resectable locoregional disease, whereas the proportion of costs attributable to “other” health care services was consistent across stages.
Table 2.
Direct Medical Costs of Care by Category and Pancreatic Cancer Stage at Diagnosis
| Cost | All Stages (N = 15,037) | Locoregional Resectable (N = 2078) | Locoregional Unresectable (N = 4234) | Distant (N = 8725) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | % Cost | Mean | % Cost | Mean | % Cost | Mean | % Cost | |
| Total direct cost | $65,500 | 100% | $134,700 | 100% | $65,300 | 100% | $49,000 | 100% |
| Procedures | $14,700 | 22% | $51,000 | 38% | $12,600 | 19% | $7,000 | 14% |
| Chemo/radiotherapy | $8,700 | 13% | $13,600 | 10% | $9,200 | 14% | $7,300 | 15% |
| Inpatient | $21,900 | 33% | $34,100 | 25% | $21,600 | 33% | $19,200 | 39% |
| Hospice | $4,500 | 7% | $4,000 | 3% | $6,300 | 10% | $3,800 | 8% |
| Other | $15,700 | 24% | $32,000 | 24% | $15,600 | 24% | $11,700 | 24% |
| Average monthly cost | $22,300 | 100% | $19,200 | 100% | $17,500 | 100% | $25,300 | 100% |
| Procedures | $5,100 | 23% | $12,300 | 64% | $4,800 | 27% | $3,600 | 14% |
| Chemo/radiotherapy | $1,400 | 6% | $700 | 4% | $1,100 | 6% | $1,800 | 7% |
| Inpatient | $10,300 | 46% | $3,000 | 16% | $7,000 | 40% | $13,500 | 53% |
| Hospice | $1,300 | 6% | $300 | 2% | $1,300 | 7% | $1,500 | 6% |
| Other | $4,200 | 19% | $2,900 | 15% | $3,300 | 19% | $4,900 | 19% |
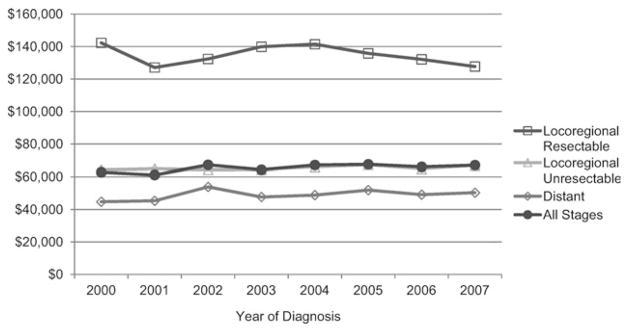
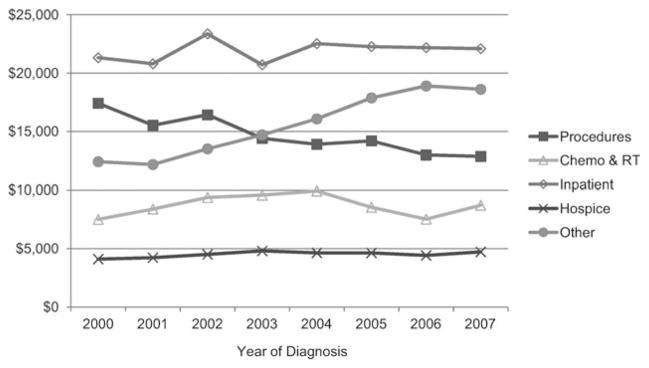
Over the study period, the cost of treating pancreatic cancer appeared to increase, but this trend was only marginally significant (parameter estimate of $692, 95% CI = −$0.42 to $1384; P = .05) (Fig. 2). The costs of “other” health care services increased (parameter estimate of $1090, 95% CI = $843 to $1337, P < .001) (Fig. 3), primarily due to a rise in costs associated with outpatient care and physician services. Costs of cancer-directed procedures decreased during the study period (parameter estimate of −$616, 95% CI = −$854 to −$379, P < .001). Costs associated with chemotherapy and radiation therapy, hospitalizations unrelated to pancreatic cancer surgery, and hospice care did not change significantly.
Figure 2.
Trends in mean total costs are shown by stage at diagnosis. Costs were estimated from Medicare reimbursement for all health services, adjusted for inflation and geographic variability. There appeared to be a marginal increase in mean total costs for the overall cohort between 2000 and 2007 (P = .05).
Figure 3.
Trends in mean costs are shown by category. Costs were estimated from Medicare reimbursement for health services within each category, adjusted for inflation and geographic variability. “Other” costs increased between 2000 and 2007 (P < .001). Procedure costs decreased (P < .001). Chemo indicates chemotherapy; RT, radiation therapy.
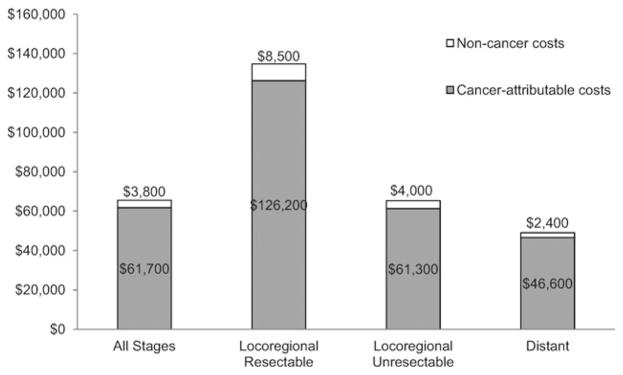
Mean incremental medical costs (costs attributable to pancreatic cancer) were $61,700 (SD, $65,100) (Fig. 4). The lowest incremental costs were incurred by those with distant disease ($46,600; SD, $48,200), and the highest incremental costs were incurred by those with resectable locoregional disease ($126,200; SD, $94,900). For unresectable locoregional disease, incremental costs were $61,300 (SD, $58,000). Costs of care not attributable to pancreatic cancer accounted for approximately 6% of total costs, and this proportion was fairly consistent across stages.
Figure 4.
Mean total and incremental costs are shown by stage at diagnosis. Costs were estimated from Medicare reimbursement for health services within each category, adjusted for inflation and geographic variability. Non-cancer costs were estimated from cancer-free Medicare beneficiaries matched 1:1 by sex, race, year of birth, and Surveillance, Epidemiology, and End Results (SEER) registry to each pancreatic cancer case. The mean monthly costs of medical care for each cancer-free beneficiary was based on the 12 months of claims in the year of diagnosis of their matched case. This average monthly cost was then multiplied by the number of months the matched case was alive. Cancer-attributable costs were the total costs in pancreatic cancer cases minus total costs in the matched cancer-free cohort over the same survival duration.
DISCUSSION
More than a decade ago, investigators noted a paucity of literature on the economics of pancreatic cancer, concluding that future studies should take advantage of the administrative data from large populations.10 Since then, few subsequent studies have been published, and most research in this area remains focused on the cost or cost-effectiveness of specific interventions, such as surgical procedures,27–35 chemotherapy, and radiation therapy.36–40 Using a large, population-based data set, we estimated total direct medical costs of more than $65,000 per pancreatic cancer patient, and we observed a marginal increase in costs over time.
Our estimate of the total direct medical cost of care for pancreatic cancer patients ranks at the lower end of the range of cost estimates reported for other cancers in the Medicare population.19 However, survival for other cancers, such as breast, prostate, colorectal, bladder, and even lung, is typically much longer.19 Considering their limited life expectancy, pancreatic cancer patients incur substantial costs in a very short time period.
A prior payer-based study of the economic impact of pancreatic cancer in working-age adults found incremental monthly and lifetime costs of $7279 and $40,233, respectively.7 An analysis conducted from a hospital perspective reported 6-month and lifetime costs of $37,327 and $48,803 in patients with a median survival of 7 months.8 It is not surprising that our estimates were higher, because we included all costs reimbursed by Medicare and studied exclusively an older population.9
Within specific categories of care, hospitalizations were a substantial driver of costs. These results are consistent with previous studies of pancreatic cancer7–9,41,42 and other cancers.18,19,43 The proportion of costs attributable to inpatient care unrelated to cancer-directed procedures was highest for those diagnosed with distant disease, possibly due to extensive hospitalization over a limited time period.8,41,44 In addition, inpatient costs were relatively stable over time, although we did observe an increase in costs of outpatient care and physician services. In recent years, across a range of cancer types and stages of disease, treatment costs have shifted away from the inpatient setting toward outpatient care.45
Not surprisingly, cancer-directed procedure costs were highest for patients with resectable locoregional disease and accounted for the largest proportion of costs among this group. Prior reports of the underutilization of surgery in potentially resectable patients, accompanied by our finding that white patients and those residing in urban and more affluent areas were more likely to have a resection, suggest potentially higher costs if all eligible candidates received a resection.46,47 Our estimate of costs associated with cancer-directed procedures also included the costs of endoscopically placed biliary and enteral stents. In recent years, these interventions have emerged as less expensive alternatives to palliative surgery for malignant biliary and intestinal obstruction, respectively, but a lack of randomization and variations in research methods have impaired cost comparisons.31,34,48,49 It is possible that, because we did not note any significant change in the cost of resectable locoregional disease over the study period, a trend toward the performance of less costly stenting may be contributing to lower costs for cancer-directed procedures.
The proportion of costs attributable to hospice use was lower for patients with resectable locoregional disease than for those with unresectable locoregional or distant disease. A recent study evaluating end-of-life care in Medicare beneficiaries dying of pancreatic cancer found that patients with locoregional disease who had a surgical resection were less likely to enroll in hospice before death and had a lower odds of hospice use.44 This study also reported that hospice use increased over time, but early enrollment decreased, which may explain the consistency in hospice costs we observed over the study period.44 Given the low proportion of overall costs attributable to hospice care, our findings suggest an underutilization of this service among the pancreatic cancer population. A previous study reported that 43% of patients do not use these services,44 even though patients who receive early palliative care have been shown to live longer than those who delay treatment.50
Chemotherapy and radiation therapy accounted for a greater proportion of costs in patients with unresectable locoregional and distant disease compared with those who had resectable locoregional disease. Recent evidence suggests that patients presenting with distant or unresectable locoregional pancreatic cancer are more likely to receive chemotherapy within the last month of life.44 Average chemotherapy costs were highest in patients with resectable locoregional disease, who may receive chemotherapy in the adjuvant setting and later with palliative intent at the time of disease progression. Although there has been a steady increase in adjuvant chemotherapy use and a growing interest in neoadjuvant therapy for the resected population, we did not observe any significant increase in the cost of chemotherapy and radiation therapy over the study period.51,52
Several limitations should be noted. First, we defined pancreatic cancer-related costs as the difference between total costs among pancreatic cancer patients and a cohort of patients who were cancer-free. This approach provides an estimate of the costs attributable to pancreatic cancer, but it does not assess which health services were specifically related to the disease. Thus, if patients with pancreatic cancer are using general, non-cancer specific medical care to a greater extent than patients without cancer, costs of pancreatic cancer care may be overestimated.
Second, the costs of orally administered prescription drugs such as erlotinib were not captured in our analysis, because these costs were not covered by Medicare prior to 2007. Erlotinib was approved by the US Food and Drug Administration in November 2005 for the treatment, in combination with gemcitabine, of patients with locally advanced, unresectable, or metastatic pancreatic carcinoma. The average incremental cost of adding erlotinib to gemcitabine is estimated at $15,194 per patient.38 Thus, our findings likely underestimate the total direct medical costs of care in the most contemporary cohorts of patients with newly diagnosed pancreatic cancer.
Third, our findings only apply to older pancreatic cancer patients covered by Medicare. Costs may differ in a younger population, who comprise approximately 30% of all pancreatic cancer patients, due to differences in their age and insurance coverage. Finally, although Medicare payment amounts were used to reflect the true resource costs of care, our analysis excluded out-of-pocket costs borne directly by patients.
Conclusions
Our analysis of this older, population-based cohort suggests that, given the short survival time, the economic burden of pancreatic cancer is substantial. Hospital services, including cancer-directed procedures and other inpatient care, accounted for the largest proportion of costs per patient. Average total costs increased marginally between 2000 and 2007, perhaps reflecting the minimal progress that has been made in treating this disease. Changes in treatment patterns and increasing costs are likely to be more substantial in the future as a greater understanding of the biology of pancreatic cancer leads to the dissemination of novel therapies and screening techniques.53,54 Any therapeutic advances that can meaningfully extend survival or improve quality of life will be hailed as major achievements in this disease. However, as the total economic burden for the treatment of pancreatic cancer increases with the aging of the population, it will be essential to evaluate the value of expensive new therapies in relation to their expected health benefit.
Acknowledgments
The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare & Medicaid Services; Information Management Services (IMS), Incorporated; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
FUNDING SOURCES
No specific funding was disclosed.
Footnotes
CONFLICT OF INTEREST DISCLOSURE
Ms. O’Neill is supported by the Health Research Board (Ireland) through the HRB PhD Scholars Programme in Health Service Research (grant PHD/2007/16). Dr. Elkin is supported by a Career Development Award from the National Cancer Institute (grant 1K07CA118189).
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. [Accessed Oct 1, 2011];Cancer Facts &; Figures. 2010 http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/cancer-facts-and-figures-2010.
- 3.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–172. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- 4.Chua YJ, Zalcberg JR. Pancreatic cancer–is the wall crumbling? Ann Oncol. 2008;19:1224–1230. doi: 10.1093/annonc/mdn063. [DOI] [PubMed] [Google Scholar]
- 5.Bayraktar S, Bayraktar UD, Rocha-Lima CM. Recent developments in palliative chemotherapy for locally advanced and metastatic pancreas cancer. World J Gastroenterol. 2010;16:673–682. doi: 10.3748/wjg.v16.i6.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown ML, Riley GF, Schussler N, Etzioni R. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002;40(8 suppl):IV-104–117. doi: 10.1097/00005650-200208001-00014. [DOI] [PubMed] [Google Scholar]
- 7.Chang S, Long SR, Kutikova L, Bowman L, Crown WH, Lyman GH. Burden of pancreatic cancer and disease progression: economic analysis in the US. Oncology. 2006;70:71–80. doi: 10.1159/000091312. [DOI] [PubMed] [Google Scholar]
- 8.Du W, Touchette D, Vaitkevicius VK, Peters WP, Shields AF. Cost analysis of pancreatic carcinoma treatment. Cancer. 2000;89:1917–1924. doi: 10.1002/1097-0142(20001101)89:9<1917::aid-cncr7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 9.Wilson LS, Lightwood JM. Pancreatic cancer: total costs and utilization of health services. J Surg Oncol. 1999;71:171–181. doi: 10.1002/(sici)1096-9098(199907)71:3<171::aid-jso7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Elixhauser A, Halpern MT. Economic evaluations of gastric and pancreatic cancer. Hepatogastroenterology. 1999;46:1206–1213. [PubMed] [Google Scholar]
- 11.Brown DM, Everhart JE. Costs of digestive diseases in the United States. In: Everhart JE, editor. Digestive diseases in the United States: epidemiology and impact. US Department of Health and Human Services, National Institutes of Health; Washington, DC: US Government Printing Office; 1994. pp. 55–82. [Google Scholar]
- 12.Gloeckler Ries LA, Reichman ME, Lewis DR, Hankey BF, Edwards BK. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist. 2003;8:541–552. doi: 10.1634/theoncologist.8-6-541. [DOI] [PubMed] [Google Scholar]
- 13.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 14.Simons JP, Ng SC, McDade TP, Zhou Z, Earle CC, Tseng JF. Progress for resectable pancreatic [corrected] cancer?: a population-based assessment of US practices. Cancer. 2010;116:1681–1690. doi: 10.1002/cncr.24918. [DOI] [PubMed] [Google Scholar]
- 15.Krzyzanowska MK, Weeks JC, Earle CC. Treatment of locally advanced pancreatic cancer in the real world: population-based practices and effectiveness. J Clin Oncol. 2003;21:3409–3414. doi: 10.1200/JCO.2003.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Warren JL, Yabroff KR, Meekins A, Topor M, Lamont EB, Brown ML. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100:888–897. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riley GF, Potosky AL, Lubitz JD, Kessler LG. Medicare payments from diagnosis to death for elderly cancer patients by stage at diagnosis. Med Care. 1995;33:828–841. doi: 10.1097/00005650-199508000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Warren JL, Brown ML, Fay MP, Schussler N, Potosky AL, Riley GF. Costs of treatment for elderly women with early-stage breast cancer in fee-for-service settings. J Clin Oncol. 2002;20:307–316. doi: 10.1200/JCO.2002.20.1.307. [DOI] [PubMed] [Google Scholar]
- 21.The Boards of Trustees of the Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds. Table IV. A1. Components of Historical and Projected Increases in HI Inpatient Hospital Payments. [Accessed October 26, 2011];2010 Annual Report of the Boards of Trustees of the Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds. :154. https://www.cms.gov/ReportsTrust-Funds/downloads/tr2010.pdf.
- 22.Centers for Medicare & Medicaid Services. Quarterly Index Levels in the CMS Medicare Economic Index using IHS Global Insight Inc. [Accessed October 26, 2011];(IGI) Forecast Assumptions, by Expense Category:1991–2020. https://www.cms.gov/MedicareProgramRatesStats/downloads/mktbskt-economic-index.pdf.
- 23.Centers for Medicare & Medicaid Services. [Accessed October 26, 2011];Wage Index Files. Table 4A. https://www.cms.gov/AcuteInpatientPPS/WIFN/itemdetail.asp?filterType=none&filterByDID=-99&sortByDID=3&sortOrder=ascending&itemID=CMS059968&intNumPerPage=10.
- 24.Centers for Medicare & Medicaid Services. [Accessed October 26, 2011];Wage Index Files. Table 4B. https://www.cms.gov/AcuteInpatientPPS/WIFN/itemdetail.asp?filterType=none&filterByDID=-99&sortByDID=3&sortOrder=ascending&itemID=CMS059969&intNumPerPage=10.
- 25.Centers for Medicare & Medicaid Services. [Accessed October 26, 2011];Geographic Practice Cost Indices by Medicare Carrier and Locality. https://www.cms.gov/PhysicianFeeSched/PFSRVF/itemdetail.asp?filterType=none&filterByDID=-99&sortByDID=1&sortOrder=ascending&itemID=CMS1186375&intNumPerPage=10.
- 26.Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 27.Lea MS, Stahlgren LH. Is resection appropriate for adenocarcinoma of the pancreas? A cost-benefit analysis. Am J Surg. 1987;154:651–654. doi: 10.1016/0002-9610(87)90236-4. [DOI] [PubMed] [Google Scholar]
- 28.Brandabur JJ, Kozarek RA, Ball TJ, et al. Nonoperative versus operative treatment of obstructive jaundice in pancreatic cancer: cost and survival analysis. Am J Gastroenterol. 1988;83:1132–1139. [PubMed] [Google Scholar]
- 29.Gudjonsson B. Carcinoma of the pancreas: critical analysis of costs, results of resections, and the need for standardized reporting. J Am Coll Surg. 1995;181:483–503. [PubMed] [Google Scholar]
- 30.Holbrook RF, Hargrave K, Traverso LW. A prospective cost analysis of pancreatoduodenectomy. Am J Surg. 1996;171:508–511. doi: 10.1016/s0002-9610(96)00016-5. [DOI] [PubMed] [Google Scholar]
- 31.Raikar GV, Melin MM, Ress A, et al. Cost-effective analysis of surgical palliation versus endoscopic stenting in the management of unresectable pancreatic cancer. Ann Surg Oncol. 1996;3:470–475. doi: 10.1007/BF02305765. [DOI] [PubMed] [Google Scholar]
- 32.Topal B, Peeters G, Vandeweyer H, Aerts R, Penninckx F. Hospital cost-categories of pancreaticoduodenectomy. Acta Chir Belg. 2007;107:373–377. doi: 10.1080/00015458.2007.11680078. [DOI] [PubMed] [Google Scholar]
- 33.Enestvedt CK, Mayo SC, Diggs BS, et al. Diagnostic laparoscopy for patients with potentially resectable pancreatic adenocarcinoma: is it cost-effective in the current era? J Gastrointest Surg. 2008;12:1177–1184. doi: 10.1007/s11605-008-0514-y. [DOI] [PubMed] [Google Scholar]
- 34.Jeurnink SM, Polinder S, Steyerberg EW, Kuipers EJ, Siersema PD. Cost comparison of gastrojejunostomy versus duodenal stent placement for malignant gastric outlet obstruction. J Gastroenterol. 2010;45:537–543. doi: 10.1007/s00535-009-0181-0. [DOI] [PubMed] [Google Scholar]
- 35.Waters JA, Canal DF, Wiebke EA, et al. Robotic distal pancreatectomy: cost effective? Surgery. 2010;148:814–823. doi: 10.1016/j.surg.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 36.Glimelius B, Hoffman K, Graf W, et al. Cost-effectiveness of palliative chemotherapy in advanced gastrointestinal cancer. Ann Oncol. 1995;6:267–274. doi: 10.1093/oxfordjournals.annonc.a059157. [DOI] [PubMed] [Google Scholar]
- 37.Ishii H, Furuse J, Kinoshita T, et al. Treatment cost of pancreatic cancer in Japan: analysis of the difference after the introduction of gemcitabine. Jpn J Clin Oncol. 2005;35:526–530. doi: 10.1093/jjco/hyi144. [DOI] [PubMed] [Google Scholar]
- 38.Miksad RA, Schnipper L, Goldstein M. Does a statistically significant survival benefit of erlotinib plus gemcitabine for advanced pancreatic cancer translate into clinical significance and value? J Clin Oncol. 2007;25:4506–4507. doi: 10.1200/JCO.2007.13.0401. [DOI] [PubMed] [Google Scholar]
- 39.Danese MD, Reyes C, Northridge K, Lubeck D, Lin CY, O’Connor P. Budget impact model of adding erlotinib to a regimen of gemcitabine for the treatment of locally advanced, nonresectable or metastatic pancreatic cancer. Clin Ther. 2008;30:775–784. doi: 10.1016/j.clinthera.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 40.Krzyzanowska MK, Earle CC, Kuntz KM, Weeks JC. Using economic analysis to evaluate the potential of multimodality therapy for elderly patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;67:211–218. doi: 10.1016/j.ijrobp.2006.07.1390. [DOI] [PubMed] [Google Scholar]
- 41.Hjelmgren J, Ceberg J, Persson U, Alvegård TA. The cost of treating pancreatic cancer. Acta Oncol. 2003;42:218–226. doi: 10.1080/02841860310000386. [DOI] [PubMed] [Google Scholar]
- 42.Müller-Nordhorn J, Brüggenjürgen B, Böhmig M, et al. Direct and indirect costs in a prospective cohort of patients with pancreatic cancer. Aliment Pharmacol Ther. 2005;22:405–415. doi: 10.1111/j.1365-2036.2005.02570.x. [DOI] [PubMed] [Google Scholar]
- 43.Etzioni R, Urban N, Baker M. Estimating the costs attributable to a disease with application to ovarian cancer. J Clin Epidemiol. 1996;49:95–103. doi: 10.1016/0895-4356(96)89259-6. [DOI] [PubMed] [Google Scholar]
- 44.Sheffield KM, Boyd CA, Benarroch-Gampel J, Kuo YF, Cooksley CD, Riall TS. End-of-life care in medicare beneficiaries dying with pancreatic cancer. Cancer. 2011;117:5003–5012. doi: 10.1002/cncr.26115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tangka FK, Trogdon JG, Richardson LC, Howard D, Sabatino SA, Finkelstein EA. Cancer treatment cost in the United States: has the burden shifted over time? Cancer. 2010;116:3477–3484. doi: 10.1002/cncr.25150. [DOI] [PubMed] [Google Scholar]
- 46.Riall TS, Lillemoe KD. Underutilization of surgical resection in patients with localized pancreatic cancer. Ann Surg. 2007;246:181–182. doi: 10.1097/SLA.0b013e31811eaa2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173–180. doi: 10.1097/SLA.0b013e3180691579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yim HB, Jacobson BC, Saltzman JR, et al. Clinical outcome of the use of enteral stents for palliation of patients with malignant upper GI obstruction. Gastrointest Endosc. 2001;53:329–332. doi: 10.1016/s0016-5107(01)70407-5. [DOI] [PubMed] [Google Scholar]
- 49.Johnsson E, Thune A, Liedman B. Palliation of malignant gastro-duodenal obstruction with open surgical bypass or endoscopic stenting: clinical outcome and health economic evaluation. World J Surg. 2004;28:812–817. doi: 10.1007/s00268-004-7329-0. [DOI] [PubMed] [Google Scholar]
- 50.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 51.Simons JP, Ng SC, McDade TP, Zhou Z, Earle CC, Tseng JF. Progress for resectable cancer? Cancer. 2010;116:1681–1690. doi: 10.1002/cncr.24918. [DOI] [PubMed] [Google Scholar]
- 52.Artinyan A, Anaya DA, McKenzie S, Ellenhorn JD, Kim J. Neoadjuvant therapy is associated with improved survival in resectable pancreatic adenocarcinoma. Cancer. 2011;117:2044–2049. doi: 10.1002/cncr.25763. [DOI] [PubMed] [Google Scholar]
- 53.Almhanna K, Philip P. Defining new paradigms for the treatment of pancreatic cancer. Curr Treat Options Oncol. 2011;12:111–125. doi: 10.1007/s11864-011-0150-8. [DOI] [PubMed] [Google Scholar]
- 54.Huang H, Dong X, Kang MX, et al. Novel blood biomarkers of pancreatic cancer-associated diabetes mellitus identified by peripheral blood-based gene expression profiles. Am J Gastroenterol. 2010;105:1661–1669. doi: 10.1038/ajg.2010.32. [DOI] [PubMed] [Google Scholar]