Abstract
Objective
Many breast cancer survivors feel constrained in discussing their cancer experience with others. Limited evidence suggests that social constraints (e.g., avoidance, criticism) from loved ones may negatively impact breast cancer survivors’ global health, but research has yet to examine relationships between social constraints and common physical symptoms. Informed by social cognitive processing theory, this study examined whether perceived social constraints from partners and health care providers (HCPs) were associated with fatigue, sleep disturbance, and attentional functioning among long-term breast cancer survivors (N=1,052). In addition, avoidant coping and self-efficacy for symptom management were examined as potential mediators of these relationships.
Methods
Long-term breast cancer survivors (mean years since diagnosis=6) completed questionnaires assessing social constraints from partners and HCPs, avoidant coping, self-efficacy for symptom management, and symptoms (i.e., fatigue, sleep disturbance, attentional functioning). Structural equation modeling was used to evaluate the hypothesized relationships among variables in two models: one focused on social constraints from partners and one focused on social constraints from HCPs.
Results
Both models demonstrated good fit. Consistent with theory and prior research, greater social constraints from both partners and HCPs were associated with greater symptom burden (i.e., greater fatigue and sleep disturbance, poorer attentional functioning). In addition, all relationships were mediated by avoidant coping and self-efficacy for symptom management.
Conclusions
Findings are consistent with social cognitive processing theory and suggest that symptom management interventions may be enhanced by addressing the impact of social constraints from survivors’ partners and HCPs on their coping and self-efficacy.
Keywords: oncology, breast cancer, social constraints, self-efficacy, coping, symptoms
Background
Many breast cancer survivors experience unwanted changes in their relationships following diagnosis and treatment [1]. For instance, survivors’ loved ones may act in a nervous or uncomfortable manner around them or even avoid them altogether because they do not know how to support them [2]. When breast cancer survivors feel unable to disclose their cancer-related thoughts and feelings because of others’ behavior (e.g., avoidance, criticism), they are experiencing social constraints [1]. Unfortunately, constraints from loved ones are associated with worse mental health outcomes in breast cancer survivors, including greater depressive symptoms, anxiety, and posttraumatic stress disorder (PTSD) symptoms as well as poorer quality of life [3,4].
Social cognitive processing theory (SCPT) provides a framework for understanding the relationship between social constraints and mental health outcomes [5]. Specifically, SCPT posits that a socially constraining environment impedes psychological adjustment by preventing successful cognitive and emotional processing of new information regarding a stressor [1,5]. Thus, SCPT suggests that social constraints negatively impact cancer survivors’ psychological adjustment by decreasing opportunities to engage in healthy processing of cancer-related concerns with others. Prior research with breast and other cancer survivors supports this theory [1,3,6]. For example, studies suggest that social constraints may increase cancer survivors’ use of avoidant coping strategies (e.g., ignoring stressors), which, in turn, is associated with poorer mental health outcomes [6–9]. Furthermore, social constraints have been found to impact breast cancer survivors’ mental health by reducing their self-efficacy or confidence for coping with cancer-related stress [8].
Limited evidence suggests that social constraints from loved ones may also negatively affect breast and other cancer survivors’ physical health outcomes. Specifically, among cancer survivors, greater social constraints have been related to poorer physical quality of life [4,10,11] and greater self-reported physical impairment [12,13]. However, to our knowledge, research has not examined relationships between social constraints and common physical symptoms, including fatigue, sleep disturbance, and poor cognitive functioning, in cancer or other medical populations. These symptoms are a major source of suffering, impairment, and disability in long-term breast cancer survivors [14–16]. Additionally, research has not examined relationships between constraints from health care providers (HCPs) and physical or mental health outcomes in cancer or other medical populations.
Theory and prior research linking social constraints to avoidant coping and self-efficacy suggest that social constraints could impact survivors’ physical symptoms [1,5–9,17]. Specifically, SCPT predicts that survivors experiencing constraints in discussing their illness with HCPs or loved ones may avoid managing their symptoms, which could lead to greater symptom burden [1,5]. For example, if a breast cancer survivor believes her HCP minimized her concerns about treatment-related changes in cognitive functioning, she may avoid discussing cognitive changes with her HCP in the future. As a result, the HCP would not be able to assist with managing this symptom. In addition, greater social constraints have been associated with lower coping self-efficacy [8], which may impact symptom burden via decreased engagement in symptom management strategies. This negative relationship between coping self-efficacy and symptom burden is consistent with the integrated behavioral model (IBM) [17], which theorizes that reduced self-efficacy for behaviors to manage symptoms decreases engagement in those behaviors. Consistent with this theory, research with cancer survivors has found that lower levels of self-efficacy are associated with poorer health behaviors and increased symptom burden [18,19].
Present Study
The present study investigated relationships between romantic partner and HCP social constraints and long-term breast cancer survivors’ fatigue, sleep disturbance, and cognitive functioning as well as potential mediators of these relationships. Whereas prior work has largely focused on relationships between partner or family/friend constraints and survivors’ mental health outcomes [1], this study examines the impact of both partner and HCP constraints on survivors’ physical symptoms. Identifying these relationships as well as potential mechanisms underlying them would inform the development of interventions to reduce breast cancer survivors’ symptom burden. Based on SCPT and limited research linking partner constraints to worse global physical health outcomes in cancer survivors [4,5,10–13], we hypothesized that greater social constraints from partners and HCPs would be associated with greater fatigue and sleep disturbance and poorer attentional functioning in long-term breast cancer survivors. In addition, based on SCPT, IBM, and prior literature [5,8,17], we hypothesized that increased avoidant coping and decreased breast cancer self-efficacy (i.e., self-efficacy for managing symptoms and quality-of-life problems related to breast cancer) would mediate relationships between social constraints and each symptom.
Methods
Participants and Procedure
Long-term breast cancer survivors (N = 1,127) were recruited from the Eastern Cooperative Oncology Group-American College of Radiology Imaging Network (ECOG-ACRIN) database of 97 sites as part of a larger, cross-sectional parent study [20]. Descriptions of study procedures have been published [20]. Briefly, eligibility criteria included: (1) having been diagnosed with early-stage breast cancer (i.e., stages I-IIIA) at age 45 or younger or between 55 and 70 years of age; (2) having been treated with a chemotherapy regimen that included Adriamycin, Paclitaxel, or Cyclophosphamide; (3) being 3–8 years post-initial treatment; and (4) not having a cancer recurrence. Enrollment was restricted to survivors diagnosed at age 45 years or younger to obtain a primarily premenopausal sample at diagnosis as well as survivors diagnosed between ages 55 and 70 years to obtain a postmenopausal sample. However, at the time of participation, ages ranged from 28–78 years. Additionally, enrollment was restricted to survivors who received certain chemotherapy regimens in order to reduce treatment-related variance in symptoms and other outcomes.
After obtaining institutional review board approval from the coordinating site (a large Midwestern university) and all 97 participating ECOG-ACRIN sites, the ECOG-ACRIN office contacted the physicians of all eligible survivors. The physicians obtained permission from eligible women to provide their contact information to the coordinating site. Survivors who gave permission were mailed a brochure about the study and called by a research assistant to obtain verbal consent. Of the 1,681 eligible survivors contacted, 1,277 (76%) consented. Subsequently, they were mailed the study questionnaire and consent forms to complete at home as well as a postage-paid envelope for returning the materials. A total of 1,127 (88%) returned the signed consent form and study questionnaires. Survivors who completed study questionnaires were mailed a $25 check to thank them for their participation.
Measures
Demographic and medical characteristics
Age, years of education, total household income, and marital status were self-reported. Clinical variables (e.g., disease stage, cancer treatments received, and time since diagnosis) were collected from survivors’ medical records.
Social constraints
Fourteen items from the Social Constraints Scale [21] were used to assess perceptions of social constraints from a romantic partner during the past four weeks. A modified 13-item version of the measure was developed for the current study to assess perceptions of social constraints from their HCP during their last visit. One of the original items (“Let you down by not showing enough love”) was removed from the HCP version because it was not applicable to providers. Both measures were found to be unidimensional in exploratory factor analyses (results not shown). Each item was rated on a 4-point scale from 1 (never) to 4 (often). A sample item is “Make you feel that you couldn’t talk about your breast cancer because it made them uncomfortable.” Composite scores were calculated by summing the items (after reverse coding, as necessary), with higher scores indicating greater social constraints from partners or HCPs. The scale for partner social constraints has shown adequate validity and internal consistency reliability in studies of breast cancer survivors [3,21]. In this study, internal consistency reliabilities were excellent for both the partner social constraints (α=0.91) and HCP social constraints (α=0.86) measures.
Avoidant coping
Avoidant coping was assessed with a 6-item avoidant coping subscale from the Brief COPE [22]. The items were modified to reflect coping with breast cancer. The avoidant coping items were selected by our research group based on the results of confirmatory factor analyses with the current study population (Rand et al., unpublished manuscript). Each item was rated on a 4-point scale ranging from 0 (I haven’t been doing this at all) to 3 (I’ve been doing this a lot). A sample item is “I’ve been giving up trying to deal with breast cancer.” Good reliability and validity evidence has been reported for the Brief COPE in breast cancer survivors [23]. In this study, internal consistency reliability for the avoidant coping subscale was acceptable (α=0.69).
Breast cancer self-efficacy
Breast cancer self-efficacy was assessed with the 11-item Breast Cancer Self-Efficacy Scale [24], which was designed to measure self-efficacy for symptom management and coping with quality-of-life problems related to breast cancer. Each item was rated on a 5-point scale from 1 (strongly disagree) to 5 (strongly agree). Sample items include “I am able to deal with physical symptoms from having breast cancer” and “I am able to ask for help when I have problems related to my breast cancer.” The composite score was calculated by summing the items, with higher scores indicating greater self-efficacy. The measure was developed as part of the parent study; excellent evidence of validity and internal consistency reliability (α=0.89) were obtained in the current sample [24].
Fatigue
The 13-item Fatigue subscale of the Functional Assessment of Cancer Therapy (FACT) [25] was used to assess survivors’ fatigue. Each item was rated on a 5-point scale ranging from 0 (not at all) to 4 (very much so). The composite score was calculated by summing the items (after reverse-scoring, as necessary), with higher scores indicating greater fatigue. Excellent validity and internal consistency reliability evidence is available for the subscale [25]. In this study, internal consistency reliability was excellent (α=0.94).
Sleep disturbance
Sleep disturbance was assessed with the global score of the 19-item Pittsburgh Sleep Quality Index (PSQI) [26]. The global score is the sum of all 7 component scores. Total scores range from 0–21, with higher scores indicating greater sleep disturbance. The PSQI has demonstrated good validity and reliability across patient populations, including breast cancer patients [26,27]. In this study, internal consistency reliability for the 7 component scores was acceptable (α=0.75).
Cognitive functioning
Cognitive functioning was assessed with the 16-item total score of the Attentional Function Index [28,29]. Each item was rated on a 10-point scale from 0 (not at all) to 10 (extremely well). The composite score was calculated by averaging all of the items, with higher scores indicating better attentional functioning. The Attentional Functioning Index has shown excellent validity and reliability among breast cancer patients [28,29]. In this study, internal consistency reliability was excellent (α=0.91).
Statistical Methods
Descriptive statistics for study variables were computed using SPSS statistical software (version 20.0; SPSS, Chicago, IL, USA). Zero-order correlations between study variables also were computed. Two path models were examined: the first model focused on the impact of partner social constraints on physical symptoms (i.e., partner constraints model) and the second model focused on the impact of HCP social constraints on these symptoms (i.e., HCP constraints model). The hypothesized path models were tested using structural equation modeling (SEM) with bootstrapping and a robust maximum likelihood estimator in Mplus statistical software. Full information maximum likelihood data imputation was used to address missing data. Endogenous variables (i.e., mediators and dependent variables) in both models included avoidant coping, breast cancer self-efficacy, fatigue, sleep disturbance, and attentional functioning. The error variances of the endogenous variables were allowed to correlate. Exogenous variables (i.e., independent variables and covariates) included social constraints (i.e., partner social constraints in one model and HCP social constraints in the other model) and demographic and medical factors (i.e., age, years of education, income, marital status, and time since diagnosis). The symptom outcomes were regressed on social constraints and demographic and medical factors, whereas the mediators (i.e., avoidant coping, breast cancer self-efficacy) were only regressed on social constraints. The demographic and medical covariates included in the models were selected based on significant associations (p<0.05) with at least one study variable in preliminary analyses. The final sample sizes for the partner and HCP models were 802 and 1,052, respectively.
To evaluate the models’ fit, we examined the goodness-of-fit χ2 statistics, the root mean square error of approximation (RMSEA) statistics, the comparative fit indices (CFI), and the standardized root mean square residual (SRMR) statistics. Adequate model fit was defined as: (1) a non-significant χ2 statistic indicating no difference between the modeled and observed patterns of relationships; (2) RMSEA <0.06; (3) CFI >0.95; and (4) SRMR<.08, as suggested by Hu and Bentler [30]. The correlation matrices were based on all available data. The final models for our data, excluding demographic and medical covariates, are shown in Figures 1a (partner constraints model) and 1b (HCP constraints model). The only differences between the initial hypothesized models and the final models were that modification indices suggested adding paths between some of the demographic and medical covariates and certain mediators. These paths were added because they were also conceptually relevant. Specifically, in the partner constraints model, paths from age to both mediators, education to breast cancer self-efficacy, and income to avoidant coping were added. In addition, in the HCP constraints model, paths from age to both mediators, education to breast cancer self-efficacy, and income to both mediators were added. The final partner constraints model included 25 pathways and the final HCP constraints model included 24 pathways.
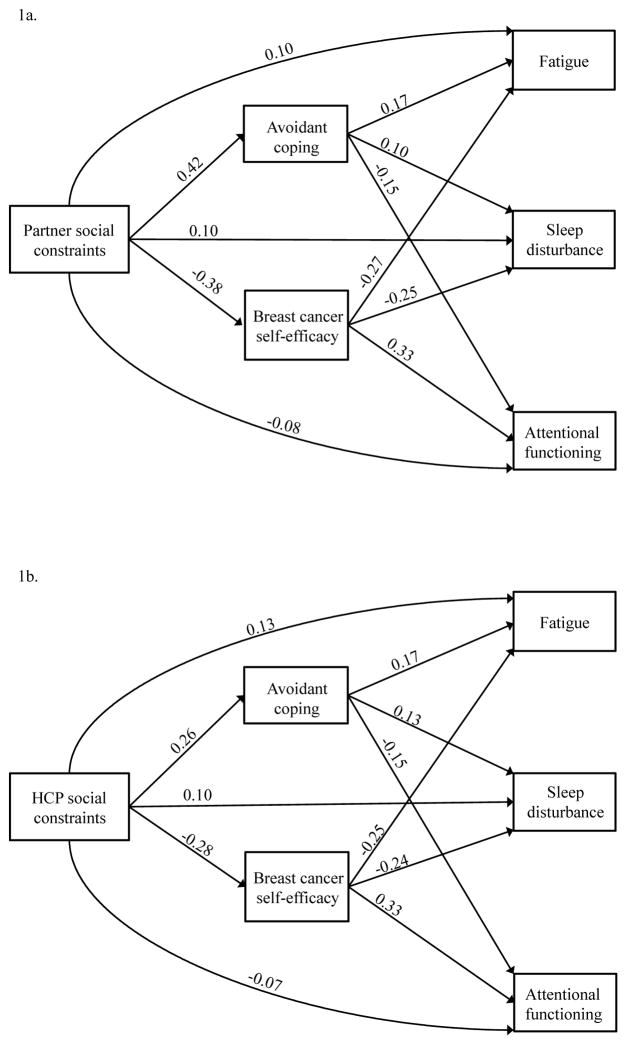
Figure 1.
Final mediation models
Note. Standardized coefficients for the final model. All paths are statistically significant. HCP = health care provider. Select demographic and medical factors were also included: age, education, income, time since diagnosis, and marital status. Specifically, in Figure 1a, there are paths from age to avoidant coping, self-efficacy, and each symptom. In addition, in Figure 1a, there are paths from education to self-efficacy and each symptom. Finally, in Figure 1a, there are paths from income to avoidant coping and each symptom. In Figure 1b, there are paths from age to avoidant coping, self-efficacy, and each symptom. In addition, in Figure 1b, there are paths from education to self-efficacy and each symptom. Finally, in Figure 1b, there are paths from income to avoidant coping, self-efficacy, and each symptom. Parameter estimates for these pathways are included in online supporting information 2 and 3.
Results
Descriptive Statistics
As shown in Table 1, breast cancer survivors were primarily Caucasian (92%) and married (74%) with a mean age of 57 years (SD=12.0 years). Forty-one percent of survivors’ household incomes fell between $30,000 and $75,000 with a broad range that represented the entire scale. On average, survivors had completed 14.5 years of education (SD=2.7 years). The average time since the breast cancer diagnosis was 5.9 years (SD=1.5 years). On average, participants reported low levels of both partner (M=21.0, SD=8.0) and HCP (M=15.3, SD=4.2) social constraints. Partner and HCP social constraints were moderately correlated (r=0.33, p<0.001). On average, survivors reported having fatigue (M=40.0, SD=10.1), which was comparable to that found in prior literature with long-term breast cancer survivors [31]. On average, survivors’ reported high levels of sleep disturbance (M=6.4, SD=3.7), consistent with prior studies of breast cancer survivors [15]. Lastly, the average score on the Attentional Functioning Index (M=6.9, SD=1.8) was comparable to that found in prior research with breast cancer survivors [29]. Zero-order correlations between all study variables are found in online supporting information 1.
Table 1.
Sample characteristics (N = 1,127)
| Characteristic | n (%) | M (SD) | Range |
|---|---|---|---|
| Average age (years) | 57.1 (11.6) | 28.0–78.0 | |
| Race/ethnicity | |||
| Caucasian | 1041 (92.4) | ||
| Black or African American | 43 (3.8) | ||
| Other | 32 (2.8) | ||
| Asian | 4 (0.9) | ||
| American Indian or Alaskan Native | 1 (0.1) | ||
| Marital status | |||
| Married | 836 (74.2) | ||
| Widowed | 104 (9.2) | ||
| Single | 89 (7.9) | ||
| Divorced | 80 (7.1) | ||
| Missing | 18 (1.6) | ||
| Average years of education (n = 1,115) | 14.5 (2.7) | 7.0–20.0 | |
| Annual Household Income | |||
| <$30,000 | 194 (17.2) | ||
| $30,000 to $75,000 | 457 (40.5) | ||
| >$75,000 to $150,000 | 325 (28.8) | ||
| >$150,000 | 113 (10.0) | ||
| Don’t know or missing | 38 (3.4) | ||
| Average years since diagnosis | 5.9 (1.5) | 3.0–9.0 | |
Main Findings
Figure 1a shows the results for the partner constraints model, adjusted for demographic and medical covariates. The final modified model showed excellent fit as determined by the non-significant χ2 statistic (χ2=7.86, df=6, p=0.25) and goodness of fit indices (i.e., RMSEA=0.02, CFI=1.00, and SRMR=.01). As hypothesized, greater partner social constraints were associated with greater fatigue (β=0.10, p=0.017), greater sleep disturbance (β=0.10, p=0.011), and poorer cognitive functioning (β=−0.08, p=0.040). In addition, both avoidant coping and breast cancer self-efficacy mediated relationships between partner social constraints and each symptom (see Table 2 for indirect effects). Specifically, higher levels of partner social constraints were associated with more avoidant coping (β=0.42, p<0.0001), which in turn was associated with greater fatigue (β=0.17, p<0.0001), greater sleep disturbance (β=0.10, p=0.016), and poorer attentional functioning (β=−0.15, p<0.0001). Furthermore, higher levels of partner social constraints were associated with reduced breast cancer self-efficacy (β=−0.38, p<0.0001), which in turn was associated with greater fatigue (β=−0.27, p<0.0001), greater sleep disturbance (β =−0.25, p<0.0001), and poorer attentional functioning (β=0.33, p<0.0001). Parameter estimates for relationships between demographic and medical covariates and endogenous study variables in the partner constraints model are found in online supporting information 2.
Table 2.
Indirect effects from partner social constraints to symptoms
| Effect | |||
|---|---|---|---|
| Z statistic | p-value | 95% CI | |
| Partner social constraints to fatigue | |||
| Total indirect | 0.17 | <0.0001 | 0.13 to 0.21 |
| Partner social constraints → avoidant coping → fatigue | 0.07 | <0.0001 | 0.04 to 0.10 |
| Partner social constraints → self-efficacy → fatigue | 0.10 | <0.0001 | 0.07 to 0.13 |
| Partner social constraints to sleep disturbance | |||
| Total indirect | 0.14 | <0.0001 | 0.10 to 0.18 |
| Partner social constraints → avoidant coping → sleep disturbance | 0.04 | 0.018 | 0.01 to 0.08 |
| Partner social constraints → self-efficacy → sleep disturbance | 0.09 | <0.0001 | 0.06 to 0.12 |
| Partner social constraints to attentional functioning | |||
| Total indirect | −0.19 | <0.0001 | −0.23 to −0.15 |
| Partner social constraints → avoidant coping → attentional functioning | −0.06 | 0.001 | −0.10 to −0.03 |
| Partner social constraints → self-efficacy → attentional functioning | −0.13 | <0.0001 | −0.16 to −0.10 |
Note. N = 802. 95% CI = 95% confidence interval.
Figure 1b shows the results for the HCP constraints model, adjusted for demographic and medical covariates. The final modified model showed excellent fit as determined by the non-significant χ2 statistic (χ2=1.93, df=5, p=0.86) and goodness of fit indices (i.e., RMSEA=0.00, CFI =1.00, and SRMR=.01). As hypothesized, higher levels of HCP social constraints were associated with greater fatigue (β=0.13, p<0.0001), greater sleep disturbance (β=0.10, p=0.003), and poorer attentional functioning (β=−0.07, p=0.010). In addition, both avoidant coping and breast cancer self-efficacy mediated relationships between HCP social constraints and each symptom (see Table 3 for total indirect effects). Specifically, higher levels of HCP social constraints were associated with more avoidant coping (β=0.26, p<0.0001), which in turn was associated with greater fatigue (β=0.17, p<0.0001), greater sleep disturbance (β=0.13, p<0.0001), and poorer attentional functioning (β=−0.15, p<0.0001). Furthermore, higher levels of HCP social constraints were associated with reduced breast cancer self-efficacy (β=−0.28, p<0.0001), which in turn was associated with greater fatigue (β=−0.25, p<0.0001), greater sleep disturbance (β =−0.24, p<0.0001), and poorer attentional functioning (β=0.33, p<0.0001). Parameter estimates for relationships between demographic and medical covariates and endogenous study variables in the HCP constraints model are found in online supporting information 3.
Table 3.
Indirect effects from health care provider social constraints to symptoms
| Effect | |||
|---|---|---|---|
| Z statistic | p-value | 95% CI | |
| HCP social constraints to fatigue | |||
| Total indirect | 0.11 | <0.0001 | 0.09 to 0.14 |
| HCP social constraints → avoidant coping → fatigue | 0.04 | 0.001 | 0.03 to 0.06 |
| HCP social constraints → self-efficacy → fatigue | 0.07 | <0.0001 | 0.05 to 0.09 |
| HCP social constraints to sleep disturbance | |||
| Total indirect | 0.10 | <0.0001 | 0.08 to 0.13 |
| HCP social constraints → avoidant coping → sleep disturbance | 0.04 | 0.003 | 0.02 to 0.05 |
| HCP social constraints → self-efficacy → sleep disturbance | 0.07 | <0.0001 | 0.04 to 0.09 |
| HCP social constraints to attentional functioning | |||
| Total indirect | −0.13 | <0.0001 | −0.16 to −0.10 |
| HCP social constraints → avoidant coping → attentional functioning | −0.04 | 0.001 | −0.06 to −0.02 |
| HCP social constraints → self-efficacy → attentional functioning | −0.09 | <0.0001 | −0.12 to −0.07 |
Note. N = 1,052. HCP = health care provider. 95% CI = 95% confidence interval
Conclusions
As hypothesized, greater social constraints from partners and HCPs were associated with higher levels of fatigue and sleep disturbance and poorer attentional functioning among long-term breast cancer survivors. Whereas prior research has linked increased partner social constraints to worse global physical health in cancer survivors [4,10–13], this is the first study to find relationships between both partner and HCP social constraints and highly prevalent and disabling physical symptoms in breast cancer survivors. Furthermore, results suggest that increased avoidant coping and reduced self-efficacy for symptom management account for relationships between partner and HCP social constraints and physical symptoms. These findings support hypotheses derived from SCPT and the IBM and extend prior research linking increased partner social constraints to greater avoidant coping and reduced coping self-efficacy in cancer survivors [5–9], as well as research linking avoidant coping and self-efficacy for symptom management to survivors’ symptoms [17,18,32].
Although our findings are consistent with theory, alternative explanations for the results may be considered. For instance, although we hypothesized that social constraints contribute to lower self-efficacy, greater avoidant coping, and greater symptom burden, it is possible that these factors and related constructs (e.g., distress) lead to greater social constraints. Specifically, a breast cancer survivor who avoids managing stressors and feels less confident in her problem-solving abilities may perceive others as more critical or elicit more critical responses from others. Additionally, fatigue, sleep disturbance, and attentional functioning are common symptoms of depression, and a depressed individual may elicit more negative interactions from others [33–35]. Longitudinal research is needed to explore the direction of these relationships. In addition, alternative behavioral, psychological, and physiological mechanisms underlying relations between social constraints and physical symptoms warrant exploration. For example, poorer health behaviors may help explain relationships between social constraints and symptoms, as a socially constraining environment may affect determinants of these behaviors (e.g., self-efficacy, coping processes). Loneliness is another potential mediator of the relationship between social constraints and symptoms that deserves examination. Theory and prior research suggest that social constraints are associated with survivors’ feelings of loneliness [5,36]. Furthermore, loneliness has been associated with reduced immune functioning, which in turn has predicted greater symptom burden among breast cancer survivors [37].
This study has important implications for future intervention research. Findings suggest that reducing HCP and partner social constraints may decrease breast cancer survivors’ symptom burden, yet social constraints have not been studied as an intervention outcome in the cancer literature. To date, couple-based interventions to improve communication skills have shown positive effects on other relational outcomes in breast cancer survivors [38]. In addition, interventions to improve breast cancer survivors’ assertive communication with medical providers, which is likely hindered in socially constraining HCP interactions, have been found to decrease their post-treatment symptom burden [39]. Further research is needed to examine whether such intervention approaches may also impact social constraints.
The present findings also suggest that interventions targeting survivors’ use of avoidant coping and their self-efficacy for symptom management may mitigate the negative impact of social constraints on their symptom burden. A number of efficacious interventions have focused on improving breast cancer survivors’ coping skills and self-efficacy for behavior changes (e.g., exercise) that are related to their symptom burden [40,41]. But these intervention trials have not been conducted within a SCPT framework that would address the impact of the socially constraining environment on survivors’ coping skills and self-efficacy. Specifically, psychosocial interventions have tended to focus on increasing positive social interactions (e.g., social support) rather than decreasing negative social interactions (e.g., social constraints), although theory and research suggest that negative social interactions may have a greater impact on health outcomes than positive social interactions [42]. Further, symptom management interventions for cancer survivors have rarely targeted social interactions [39]. Reducing negative social interactions, such as criticism, between survivors and their partners and HCPs would be a novel intervention approach.
Limitations of this study and directions for future research should be noted. First, the sample primarily consisted of Caucasian, middle-class women. Future studies should include survivors with greater diversity with respect to demographic characteristics. Second, the main study variables were self-reported; future research may include behavioral or observer assessments of study variables. For instance, social constraints are defined as both objective and subjective experiences (i.e., others’ behaviors and survivors’ interpretations of them) [1]. Assessing social constraints by recording and coding social interactions as positive, neutral, or negative is rarely done [44] and might yield different results. Finally, the cross-sectional design precluded an examination of directionality, and a limited number of mediators and outcomes were examined. Longitudinal research is needed to investigate processes through which social constraints may impact a range of physical health outcomes in breast cancer survivors.
The current findings have implications for clinical practice with breast cancer survivors. Specifically, findings suggest that HCPs’ communication style may impact patients’ symptom management; thus, HCPs should communicate in a manner that validates patients’ thoughts and feelings and increases their self-confidence for symptom management. For example, HCPs could use motivational interviewing, an empathic, evidence-based method of interacting with patients that aims to enhance health behavior change (e.g., symptom management) [43]. Motivational interviewing addresses two factors in this study (i.e., greater avoidant coping, decreased self-efficacy) that accounted for relationships between social constraints and physical symptoms. HCPs may also assess patients’ satisfaction with their support network. If patients report distress related to social constraints, referral to individual, couple, or family counseling may be warranted. Additionally, HCPs could provide educational materials to partners and family members that include communication tips. Furthermore, practitioners providing psychotherapy should be attentive to patient reports of negative social interactions with HCPs and family members and intervene by promoting open, empathic communication. Consideration of social factors contributing to suboptimal symptom management among long-term breast cancer survivors may be key to reducing their symptom burden.
Supplementary Material
Acknowledgments
This research was supported by American Cancer Society grant RSGPB-04-089-01-PBP. This study was coordinated by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and was supported in part by Public Health Service grants UG1CA189828 and U10CA180795 from the National Cancer Institute. Rebecca Adams’s and Andrea Cohee’s work was supported by R25CA117865 (V. Champion, PI) from the National Cancer Institute. Catherine Mosher’s work was supported by K07CA168883 from the National Cancer Institute, and Victoria Champion’s work was supported by K05CA175048 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Authors’ Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Lepore S, Revenson T. Social constraints on disclosure and adjustment to cancer. Soc Personal Psychol Compass. 2007;1:313–333. doi: 10.1111/j.1751-9004.2007.00013.x. [DOI] [Google Scholar]
- 2.Mosher CE, Johnson C, Dickler M, Norton L, Massie MJ, DuHamel K. Living with metastatic breast cancer: A qualitative analysis of physical, psychological, and social sequelae. Breast J. 2013;19:285–292. doi: 10.1111/tbj.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cordova MJ, Cunningham LL, Carlson CR, Andrykowski MA. Social constraints, cognitive processing, and adjustment to breast cancer. J Consult Clin Psychol. 2001;69:706–711. doi: 10.1037//0022-006X.69.4.706. [DOI] [PubMed] [Google Scholar]
- 4.Graves KD, Jensen RE, Cañar J, et al. Through the lens of culture: Quality of life among Latina breast cancer survivors. Breast Cancer Res Treat. 2012;136:603–613. doi: 10.1007/s10549-012-2291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lepore S. A social–cognitive processing model of emotional adjustment to cancer. In: Baum A, Andersen BL, editors. Psychosocial Interventions for Cancer. Washington, DC: American Psychological Association; 2001. pp. 99–118. [Google Scholar]
- 6.Manne S, Ostroff J, Winkel G, Grana G, Fox K. Partner unsupportive responses, avoidant coping, and distress among women with early stage breast cancer: Patient and partner perspectives. Health Psychol. 2005;24:635–641. doi: 10.1037/0278-6133.24.6.635. [DOI] [PubMed] [Google Scholar]
- 7.Manne SL, Pape SJ, Taylor KL, Dougherty J. Spouse support, coping, and mood among individuals with cancer. Ann Behav Med. 1999;21:111–121. doi: 10.1007/BF02908291. [DOI] [PubMed] [Google Scholar]
- 8.Manne S, Glassman M. Perceived control, coping efficacy, and avoidance coping as mediators between spousal unsupportive behaviors and psychological distress. Health Psychol. 2000;19:155–164. doi: 10.1037//0278-6133.19.2.155. [DOI] [PubMed] [Google Scholar]
- 9.Manne S, Ostroff J, Sherman M, et al. Buffering effects of family and friend support on associations between partner unsupportive behaviors and coping among women with breast cancer. J Soc Pers Relat. 2003;20:771–792. doi: 10.1177/0265407503206004. [DOI] [Google Scholar]
- 10.Dunn J, Occhipinti S, Campbell A, Ferguson M, Chambers SK. Benefit finding after cancer: The role of optimism, intrusive thinking and social environment. J Health Psychol. 2011;16:169–177. doi: 10.1177/1359105310371555. [DOI] [PubMed] [Google Scholar]
- 11.Eton DT, Lepore S, Helgeson V. Early quality of life in patients with localized prostate carcinoma. Cancer. 2001;92:1451–1459. doi: 10.1002/1097-0142(20010915)92:6<1451::AID-CNCR1469>3.0.CO;2-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myers SB, Manne S, Kissane DW, et al. Social-cognitive processes associated with fear of recurrence among women newly diagnosed with gynecological cancers. Gynecol Oncol. 2013;128:120–127. doi: 10.1016/j.ygyno.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norton TR, Manne S, Rubin S, et al. Ovarian cancer patients’ psychological distress: The role of physical impairment, perceived unsupportive family and friend behaviors, perceived control, and self-esteem. Health Psychol. 2005;24:143–152. doi: 10.1037/0278-6133.24.2.143. [DOI] [PubMed] [Google Scholar]
- 14.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–743. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 15.Fortner BV, Stepanski EJ, Wang SC, Kasprowicz S, Durrence HH. Sleep and quality of life in breast cancer patients. J Pain Symptom Manage. 2002;24:471–480. doi: 10.1016/S0885-3924(02)00500-6. [DOI] [PubMed] [Google Scholar]
- 16.Von Ah D, Russell KM, Storniolo AM, Carpenter JS. Cognitive dysfunction and its relationship to quality of life in breast cancer survivors. Oncol Nurs Forum. 2009;36:326–336. doi: 10.1188/09.ONF.326-334. [DOI] [PubMed] [Google Scholar]
- 17.Montano DE, Kasprzyk D. Theory of reasoned action, theory of planned behavior, and the integrated behavioral model. In: Glanz K, Rimer BK, Viswanath K, editors. Health Behavior and Health Education: Theory, Research, and Practice. 4. San Francisco, CA: Jossey-Bass; 2008. pp. 67–96. [Google Scholar]
- 18.Servaes P, Verhagen S, Bleijenberg G. Determinants of chronic fatigue in disease-free breast cancer patients: A cross-sectional study. Ann Oncol. 2002;13:589–598. doi: 10.1093/annonc/mdf082. [DOI] [PubMed] [Google Scholar]
- 19.Rogers LQ, McAuley E, Courneya KS, Verhulst SJ. Correlates of physical activity self-efficacy among breast cancer survivors. Am J Health Behav. 2008;32:594–603. doi: 10.5993/AJHB.32.6.4. [DOI] [PubMed] [Google Scholar]
- 20.Champion VL, Wagner LI, Monahan PO, et al. Comparison of younger and older breast cancer survivors and age-matched controls on specific and overall quality of life domains. Cancer. 2014;120:2237–2246. doi: 10.1002/cncr.28737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lepore S, Ituarte PH. Optimism about cancer enhances mood by reducing negative social interactions. Cancer Res Ther Control. 1999;8:165–174. [Google Scholar]
- 22.Carver CS. You want to measure coping but your protocol’s too long: Consider the brief COPE. Int J Behav Med. 1997;4:92–100. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- 23.Yusoff N, Low W, Yip C. Reliability and validity of the Brief COPE Scale (English version) among women with breast cancer undergoing treatment of adjuvant chemotherapy: A Malaysian study. Med J Malaysia. 2010;65:41–44. [PubMed] [Google Scholar]
- 24.Champion VL, Ziner KW, Monahan PO, et al. Development and psychometric testing of a breast cancer survivor self-efficacy scale. Oncol Nurs Forum. 2013;40:E403–E410. doi: 10.1188/13.ONF.E403-E410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/S0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 26.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 27.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh sleep quality index. J Psychosom Res. 1998;45:5–13. doi: 10.1016/S0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 28.Cimprich B, Visovatti M, Ronis DL. The Attentional Function Index—a self-report cognitive measure. Psychooncology. 2011;20:194–202. doi: 10.1002/pon.1729. [DOI] [PubMed] [Google Scholar]
- 29.Cimprich B, So H, Ronis DL, Trask C. Pre-treatment factors related to cognitive functioning in women newly diagnosed with breast cancer. Psychooncology. 2005;14:70–78. doi: 10.1002/pon.821. [DOI] [PubMed] [Google Scholar]
- 30.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- 31.Minton O, Stone P. How common is fatigue in disease-free breast cancer survivors? A systematic review of the literature. Breast Cancer Res Treat. 2008;112:5–13. doi: 10.1007/s10549-007-9831-1. [DOI] [PubMed] [Google Scholar]
- 32.Schoulte JC, Lohnberg JA, Tallman B, Altmaier EM. Influence of coping style on symptom interference among adult recipients of hematopoietic stem cell transplantation. Oncol Nurs Forum. 2011;38:582–586. doi: 10.1188/11.ONF.582-586#sthash.x9UxgTqj.dpuf. [DOI] [PubMed] [Google Scholar]
- 33.Coyne JC. Depression and the response of others. J Abnorm Psychol. 1976;85:186–193. doi: 10.1037/0021-843X.85.2.186. [DOI] [PubMed] [Google Scholar]
- 34.Coyne JC. Toward an interactional description of depression. Psychiatry. 1976;39:28–40. doi: 10.1521/00332747.1976.11023874. [DOI] [PubMed] [Google Scholar]
- 35.Passik SD, Lowery AE. Recognition of depression and methods of depression screening in people with cancer. In: Kissane DW, Maj M, Sartorius N, editors. Depression and Cancer. Chichester, UK: John Wiley & Sons, Ltd; 2010. pp. 81–100. [Google Scholar]
- 36.Mosher CE, Lepore S, Wu L, et al. Social correlates of distress following hematopoietic stem cell transplantation: Exploring the role of loneliness and cognitive processing. J Health Psychol. 2012;17:1022–1032. doi: 10.1177/1359105311432490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaremka LM, Fagundes CP, Peng J, et al. Loneliness promotes inflammation during acute stress. Psychol Sci. 2013;24:1089–1097. doi: 10.1177/0956797612464059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kayser K. Enhancing dyadic coping during a time of crisis: A theory-based intervention with breast cancer patients and their partners. In: Revenson TA, Kayser K, Bodenmann G, editors. Couples Coping with Stress: Emerging Perspectives on Dyadic Coping. Washington, DC: American Psychological Association; 2005. pp. 175–194. [Google Scholar]
- 39.Andersen BL, Shelby RA, Golden-Kreutz DM. RCT of a psychological intervention for patients with cancer: I. mechanisms of change. J Consult Clin Psychol. 2007;75:927–938. doi: 10.1037/0022-006X.75.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanton AL, Ganz PA, Kwan L, et al. Outcomes from the Moving Beyond Cancer psychoeducational, randomized, controlled trial with breast cancer patients. J Clin Oncol. 2005;23:6009–6018. doi: 10.1200/JCO.2005.09.101. [DOI] [PubMed] [Google Scholar]
- 41.Graves KD. Social cognitive theory and cancer patients’ quality of life: A meta-analysis of psychosocial intervention components. Health Psychol. 2003;22:210. doi: 10.1037/0278-6133.22.2.210. [DOI] [PubMed] [Google Scholar]
- 42.Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Rev Gen Psychol. 2001;5:323–370. doi: 10.1037/1089-2680.5.4.323. [DOI] [Google Scholar]
- 43.Rollnick S, Miller WR, Butler CC, Aloia MS. Motivational interviewing in health care: Helping patients change behavior. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2008;5:203–203. [Google Scholar]
- 44.Manne S, Sherman M, Ross S, Ostroff J, Heyman RE, Fox K. Couples’ support-related communication, psychological distress, and relationship satisfaction among women with early stage breast cancer. J Consult Clin Psychol. 2004;72:660–670. doi: 10.1037/0022-006X.72.4.660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.