Abstract
Purpose of Review
Myocardial infarction (MI) leading to heart failure displays an important cause of death worldwide. Adequate restoration of blood flow to prevent this transition is a crucial factor to improve long-term morbidity and mortality. Novel regenerative therapies have been thoroughly investigated within the past decades.
Recent Findings
Increased angiogenesis in infarcted myocardium has shown beneficial effects on the prognosis of MI; therefore, the proangiogenic capacity of currently tested treatments is of specific interest. Molecular imaging to visualize formation of new blood vessels in vivo displays a promising option to monitor proangiogenic effects of regenerative substances.
Summary
Based on encouraging results in preclinical models, molecular angiogenesis imaging has recently been applied in a small set of patients. This article reviews recent literature on noninvasive in vivo molecular imaging of angiogenesis after MI as an integral part of cardiac regeneration.
Keywords: Angiogenesis, Myocardial regeneration, Molecular imaging, Integrins, Radiotracers, Myocardial infarction
Introduction
Heart failure following myocardial infarction (MI) still displays a major cause of death and disability worldwide [1]. Even though a wide range of therapeutic options to prevent or delay transition to chronic heart failure (CHF) after MI are available, its treatment is still unsatisfactory, as CHF is generally not reversible and treatment needs to be continued indefinitely [2]. Angiogenesis, the formation of new blood vessels, is a part of the natural healing process after MI to restore blood flow and discard cellular debris [3]. The extent of angiogenesis is associated with postinfarct remodeling and has implications on prognosis in MI patients [4]. Although a variety of approaches to stimulate myocardial angiogenesis after MI have been explored, including gene therapy as well as the delivery of angiogenic factors and stem cells, results have been controversial and were partly disappointing [5–7]. In many cases, stimulation of angiogenesis was not shown convincingly and only moderate clinical improvement was demonstrated. To reliably assess the therapeutic potential of proangiogenic therapies and monitor myocardial angiogenesis for enabling better preclinical and clinical drug development, noninvasive methods such as molecular imaging are warranted. Molecular imaging of newly built microvessels is a promising strategy which allows direct visualization of vessel formation instead of indirect measurements of efficacy. Thus, it is an important modality for improving risk stratification and for facilitating the development of novel therapeutic interventions in MI patients.
Angiogenesis
Angiogenesis represents the growth of new capillaries from preexisting vessels [8]. It is a complex process involving numerous growth factors and signal cascades [9]. Although vessels are generally quiescent in adults, endothelial cells (ECs) lining the vessel walls retain their ability to respond to angiogenic signals [8]. Proangiogenic signals such as VEGF, ANG-2, FGFs, or chemokines released by hypoxic, inflammatory, or tumor cells activate ECs, and they become motile and invasive [10]. Before ECs can sprout into surrounding tissue, degradation of basement membrane by matrix metalloproteases and detachment of mural cells is necessary in order to loosen activated ECs [8]. VEGF induces increased permeability of the EC layer, and extravasated plasma proteins serve as a provisional extracellular matrix (ECM) scaffold. Migration of ECs into this scaffold is mediated by integrins. To allow blood flow, those newly built vessels need to be connected with other vessels to build branches and become mature and stable. ECs regain their quiescent state and protease inhibitors cease basement membrane degradation [10].
Insufficient vessel maintenance can lead to MI [10]. Intact and functional blood vessels are essential for regeneration of ischemic tissues to enable immune surveillance, supply of oxygen and nutrients to and discarding of waste from the cells of the healing wound [10, 11]. Insufficiently healed MI results in an expanded infarction area and dilation of the heart by left ventricular (LV) remodeling, both resulting in heart failure [12]. However, in some patients, recovery of blood flow after MI is not possible. In those patients, restoration of tissue reperfusion depends on myocardial angiogenesis [1]. Within the first hours after MI, proangiogenic factors are released to compensate ischemia with induced angiogenesis [11]. Restoration of the blood flow in the infarct border zone is essential to alleviate infarct expansion and heart failure [1, 13]. Moreover, the extent of angiogenesis has positive effects on postinfarct remodeling and the prognosis of MI patients [4]. Hence, stimulation of myocardial angiogenesis as a therapeutic option through administering growth factors, stem or progenitor cells, and pharmacological molecules has been thoroughly studied [14]. Due to the increasing amount of research on myocardial angiogenesis as a treatment option, molecular imaging of newly built vessels has a significant potential impact on predicting outcome of MI patients and guiding novel therapies.
Molecular Imaging Tools
Molecular imaging describes in vivo targeted, noninvasive visualization and quantification of various molecular pathways without interfering with them [15–18]. Throughout the past decades, there has been significant advances in molecular imaging techniques used for diagnostic, prognostic, as well as therapeutic purposes [18]. In the field of cardiology, molecular imaging by magnetic resonance imaging (MRI), ultrasound, bioluminescence imaging, positron emission tomography (PET), and SPECT has shown improvements of LV function, myocardial perfusion, viability, scar tissue, inflammatory cells, and indirect signs of angiogenesis, and some of these images are able to directly detect angiogenesis [15].
Nuclear Imaging
PET imaging is a tomographic technique that detects the decay of positron emitters (radiotracers), which can be attached to small molecules for molecular recognition [17, 19]. It is well validated to have superior sensitivity, relatively high resolution, and tissue penetration [19 20•]. Various metabolic and pathophysiological biomarkers have been investigated as targets for PET imaging. The nonspecific metabolic tracer 18F (in form of 18-fluorodeoxyglucose), 18F-FDG, is the most frequently employed PET tracer [21, 22]. Many studies are directed toward incorporation of radiotracers with short half-lives, such as fluorine-18 (18F), which successfully leads to reduced patient exposure of ionizing radiation [23]. Rather low spatial resolution is the main limiting characteristic of this imaging technique [21].
SPECT imaging is well established and offers several advantages over PET. Camera equipment is less expensive and more widely available as compared to PET systems [23]. SPECT imaging performance is based on using single photon emitting radioisotopes, which are easier accessible for the investigation of a wider range of biological processes [15, 24]. Technetium-99m (99mTc) and indium-111 (111In) are frequently used radioactive probes [19]. These radiotracers emit gamma rays with different energies, thus introducing the possibility of simultaneous evaluation of dual or multiple radiotracers. Advantages of SPECT are high sensitivity and tissue penetration depth. However, SPECT imaging does not ensure high-resolution anatomical information of cellular location. Another disadvantage is the inability to track radioisotopes over weeks as the signal rapidly declines [19].
MRI
In contrast to PET and SPECT, MRI offers better spatial resolution, excellent soft tissue contrast and enables concomitant angiography or perfusion acquisition [21]. However, it has a lower sensitivity for detection of molecular contrast agents and application is limited in patients with devices, e.g., cardiac pacemakers or cardioverter defibrillators, and metal implants [15, 25]. Paramagnetic contrast agents (e.g., gadolinium) targeting integrin αvβ3 via antibodies or peptidomimetics as well as gadolinium-based lipid nanoparticles, have been previously used to study tumor angiogenesis [26–28]. A further advance in MR angiogenesis imaging are ultra-small superparamagnetic particles of iron oxide (USPIO) [29]. However, USPIOs have a long blood half-life and show nonspecific extravasation [30]. Microparticles of iron oxide (MPIO) have a higher particle size, and thus a shorter half-life, offering a better contrast to noise ratio [31]. Safety concerns of superparamagnetic iron oxide particles exist [32].
Ultrasound Molecular Imaging
Cardiac ultrasound is a widely used technique that has several advantages over the previously described imaging modalities, e.g., lack of ionizing radiation, routine accessibility, and superior spatial resolution compared to SPECT and PET [33]. Hitherto tissue perfusion assessed by ultrasound has been used as an endpoint reflecting angiogenesis; however, an increase in perfusion does not necessarily reflect angiogenic activity [34]. For more detailed imaging, targeted microbubbles can be used as contrast agents in a technique known as contrast-enhanced ultrasound (CEU) [33]. Microbubbles that target integrins or VEGFRs reflect angiogenesis in a more direct manner than perfusion imaging [34, 35].
Bioluminescence Imaging
Bioluminescence imaging (BLI) represents an indirect cell labeling method particularly used in small animal models [36]. It is greatly valued for its high sensitivity, ease of use, and low cost of instrumentation, but BLI has low spatial resolution and restricted penetration depth, and quantification accuracy is very poor [37, 38]. Most frequently used reporter genes are firefly luciferase (Luc) and herpes simplex virus thymidine kinase (HSV-tk), used for tracking cells with angiogenic capacity [39].
Multimodal Imaging
Advances in molecular imaging, along with identifying drawbacks, have led to the development of multimodal (hybrid) imaging systems such as PET/MR, SPECT/computed tomography (CT), and PET/CT [18]. Hybrid molecular imaging is the focus of many preclinical and clinical studies as it enables simultaneous collection of anatomical and functional information [40, 41].
The addition of CT to SPECT has permitted attenuation correction and better evaluation of SPECT myocardial perfusion [42]. SPECT/CT has proven to be relevant in the characterization of coronary artery calcium, which is a useful method to predict cardiovascular events rate [43, 44]. Even though SPECT/CT has been widely used in cardiology and information gained with this modality are highly valued, exposure of patients to radiation is a major concern [45] and reduction of radiation is the main goal of present studies in nuclear cardiology [46].
PET/CT is a hybrid technology that combines functional molecular imaging modalities with precise anatomical information [47]. This hybrid modality is successful in overcoming low spatial resolution. Many studies indicate that it results in better identification of diseases, and guide management and treatment of patients with stable and unstable coronary artery disease compared to PET imaging alone [48].
Molecular Imaging of Myocardial Angiogenesis
Within the past decade, direct noninvasive evaluation of angiogenesis by molecular imaging has been investigated extensively. With the rapid development of antiangiogenic therapies (e.g., in cancer research) and particularly imaging techniques, tumor angiogenesis has been the focus of attention lately [49]. Although interest has recently increasingly been directed on molecular imaging of myocardial angiogenesis after MI (e.g., to monitor effects of regenerative therapies), it is still rather in its fledgling stage. Table 1 provides a summary of novel studies on molecular imaging of angiogenesis.
Table 1.
A summary of novel studies in molecular imaging of angiogenesis
| Radiotracer | Modality | Target | Therapy | Species | Disease | |
|---|---|---|---|---|---|---|
| Myocardial | ||||||
| Preclinial |
68Ga-NODAGA-RGD 68Ga-TRAP(RGD)3 18F-galacto-RGD [50] |
PET | αvβ3 integrin | None | Rat | MI |
| 68Ga-NOTA-RGD peptidomimetic [51] | PET | αvβ3 integrin | None | Rat | MI | |
| 18F-Alfatide II [20•] | PET | αvβ3 integrin | VEGF, BMSC | Rat | MI | |
| 68Ga-RGD [52•] | PET | αvβ3 integrin | Dissociated HUVECs/cbMSCs or 3D HUVEC/cbMSC aggregates | Rat | MI | |
| 11In-DTPA-cNGR [53] | SPECT | CD13 | None | Mouse | MI | |
| 64Cu-NOTA-TRC105 [54] | PET | CD105 | None | Rat | MI | |
| [11C]ATV-1[9] | PET | VEGFR-2, Tie-2, PDGFα | None | Rat | MI | |
| Clinical | 68Ga-PRGD2 [55••] | PET | αvβ3 integrin | None | Human | MI |
| Hind limb | ||||||
| Preclinical | αv targeted microbubbles [34] | Ultrasound | αv integrins | HIF-1α mutants | Mouse | Ischemic hind limb |
| Tumor | ||||||
| c(RGDyK)-MPIO [56•] | MRI | αvβ3 integrin | None | Mouse | Melanoma, colon carcinoma | |
| 68Ga-aquibeprin [57••] | PET | α5β1 integrin | None | Mouse | melanoma | |
Angiogenesis as a multistep process, orchestrated by a wide range of growth factors, growth factor receptors, cell types, adhesion molecules, integrins, and signaling pathways, all of which offer a multitude of imaging targets. In general, three ways to image myocardial angiogenesis exist: (1) non-EC targets, (2) EC targets, and (3) extracellular matrix proteins and matrix proteases [58]. In particular, integrin αvβ3 has emerged as an interesting target.
Integrins
Integrins are structurally and functionally diverse families of cell adhesion molecules, which regulate cell-cell and cell-ECM interactions and in addition mediate signals for cell growth, proliferation, migration, or apoptosis [59]. They connect the ECM with the cytoskeleton (i.e., the microfilaments) inside the cell and transmit signals of the surrounding into the cell by mediating the downstream consequences of cell adhesion. Therefore, integrins play an important role in cell signaling and can have a relation to cell growth, cell division, cell survival, differentiation, and apoptosis [60]. Several members of the integrin family are overexpressed on ECs under hypoxia [61]. The two main integrins αvβ3 and α5β1 facilitate several mechanisms during angiogenesis in tissue ischemia. In particular, they mediate adhesion to ECM and other cells to initiate building of new capillaries by allowing ECs to bind to provisional ECM scaffold proteins. Furthermore, they mediate interaction of ECs and vascular smooth muscle cells, stimulate vessel growth, and promote vessel maturation [10, 61]. ECM proteins such as fibronectin interact with integrins via the Arg-Gly-Asp (RGD) sequence motif [61]. Multivalent binding is mediated through extracellular integrin clusters, and thus, dimeric and multimeric RGD sequences with improved binding affinity have been developed and are frequently used for integrin imaging.
Integrin αvβ3
Integrin αvβ3-mediated imaging is currently the most frequently applied method to visualize angiogenesis in vivo. Its expression is low in normal tissue, but it becomes highly expressed in activated ECs during angiogenesis in the infarcted myocardium [61, 62]. However, studies in αv- and β3-deficient mice suggest that both integrins are not essentially required for angiogenesis and their absence can be compensated by upregulation of VEGFR-2 expression [63–65]. Additionally, integrin αvβ3 does not seem to be restricted to ECs but is also expressed on macrophages so that results in angiogenesis imaging targeting integrin αvβ3 need to be treated with caution [66].
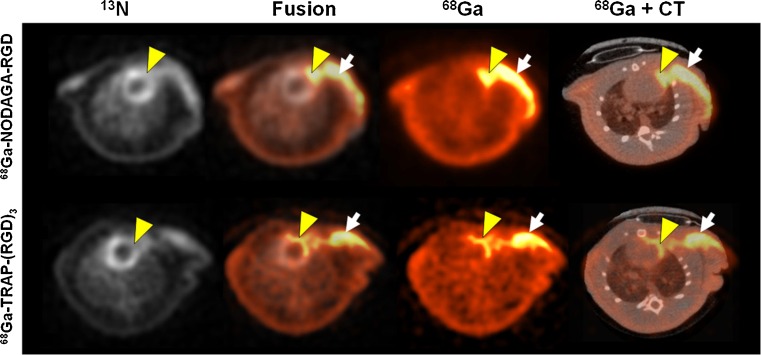
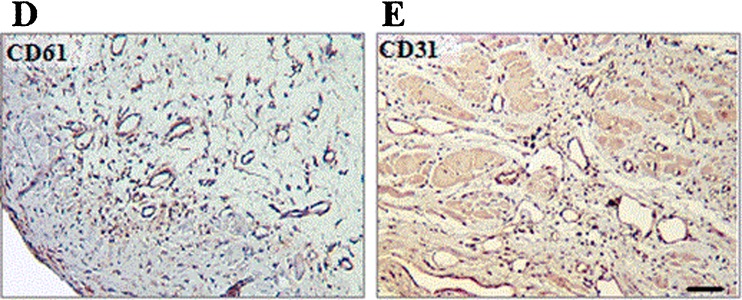
For integrin αvβ3 molecular imaging, cyclic RGD dimers with polyethylene glycol spacers radiolabeled with 18F [67, 68], 68Ga [69, 70], 64Cu [71], 76Br [72], and 89Zr [73] for PET imaging and 99mTc [74, 75] and 111In [76] for SPECT imaging were used in several disease entities. Within the past years, the use of these and other tracer probes that had been investigated predominantly in tumor angiogenesis has been translated to angiogenesis imaging after MI for evaluating proangiogenic effects of regenerative therapies. In a previous study, 18F-galacto-RGD injected in a rat MI model predicted improved healing [77]. The usage of this tracer, however, might be limited as the production of 18F-galacto-RGD is complex and time-consuming. 68Ga tracers, on the other hand, are easy to handle and fast in production. 68Ga-NODAGA-RGD and 68Ga-TRAP-(RGD)3 have been previously tested for angiogenesis imaging in tumor models [78, 79]. Both 68Ga-RGD tracers were compared to 18F-galacto-RGD in postinfarct myocardial angiogenesis, and uptake was similar in all three groups (Fig. 1), indicating that 68Ga-RGD tracers may represent a more easily clinically translatable alternative [50]. Another 68Ga-labeled tracer, a 68Ga-NOTA-RGD peptidomimetic, was used for angiogenesis imaging in a rat MI model. 68Ga-NOTA-RGD uptake was increased in regions of reduced myocardial perfusion and correlated with immunohistochemical staining of CD31 and β3 integrin (Fig. 2) [51].
Fig. 1.
In vivo PET/CT images of rat MI. Representative transaxial sections show hypoperfused myocardium area (13N) and corresponding RGD uptake (68Ga, % ID/g). The focal uptake is seen in infarct (yellow arrowheads) and the operation scar (white arrows), as verified by CT scan (reprinted from [50] under the terms of the Creative Commons Attribution License 2.0)
Fig. 2.
Macrosections and microsections of rat hearts. […] d, e Representative immunostaining for CD61 (d) and CD31 (e) from the border region of infarcted heart at low magnification (×10, calibration bar 50 μm) (reprinted from [51] with permission from Springer/European Journal of Nuclear Medicine)
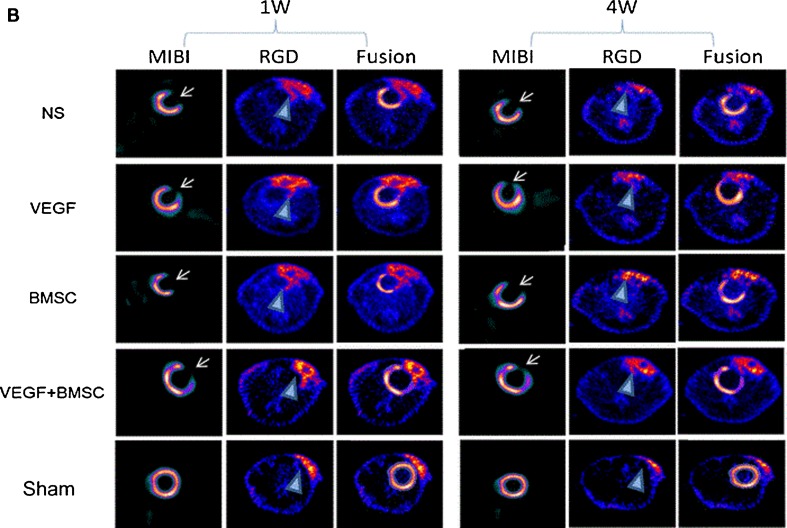
Lately, 18F-Alfatide II (18F-AlF-NOTA-PRGD2) has been developed as a new promising PET tracer. Taking advantage of the preformation of an aluminum-fluoride complex with consequent attachment of the RGD peptide, time for preparation was significantly reduced and HPLC purification was avoided, while receiving radiochemical purity of over 97 % [80]. The 18F-Alfatide II tracer was used to characterize angiogenesis in a rat MI model after treatment with vascular endothelial growth factor (VEGF) gene and/or bone marrow mesenchymal stem cells (BMSCs). In this study, 18F-Alfatide II provided a strong contrast between infarcted and noninfarcted myocardium and uptake was significantly higher in rats treated with VEGF and BMSCs (Fig. 3). Increased uptake of 18F-Alfatide II correlated with the area of 99mTc-MIBI uptake defect [20•].
Fig. 3.
[…] b In vivo PET images of 18F-Alfatide II and representative SPECT myocardial short axis slice images using 99mTc-MIBI at different times after myocardial infarction. Infarcted myocardium showed obvious 99mTc-MIBI uptake defect in the anterior and lateral wall of left ventricle (arrows), which matched the focal RGD peptide tracer uptake region (triangle). c The infarct area/remote area ratio of 18F-Alfatide II uptake as measured by PET (reprinted from [20•] with permission from Springer)
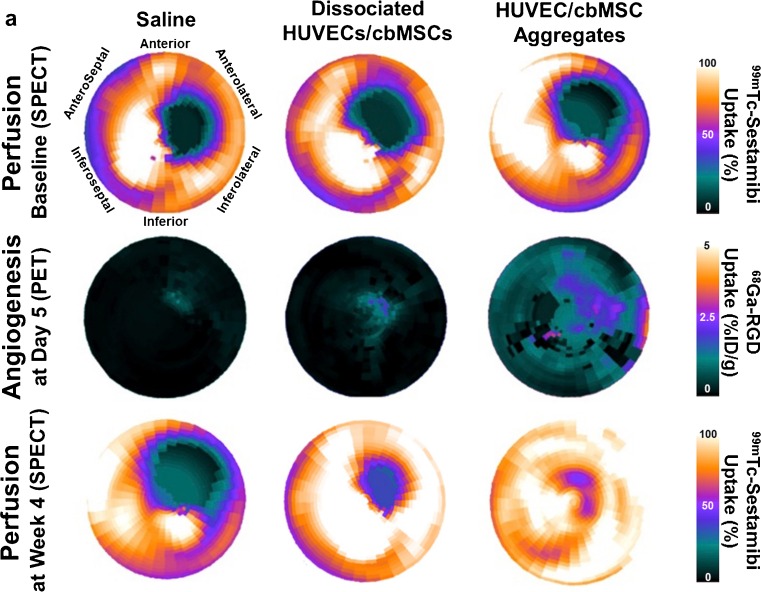
In another study in a rat MI model, the angiogenic potential of 3D HUVEC/cbMSC aggregates was assessed by 68Ga-RGD. Injection of 3D HUVEC/cbMSC aggregates resulted in locally increased 68Ga-RGD uptake suggesting increased angiogenesis and reduction in defect size [52•]. Using SPECT tracers, an increased uptake of 99mTc-labeled RGD peptides (99mTc-RAFT-RGD and 99mTc-NC100692), similar to data of respective 18F-PET tracers, was found in infarcted myocardium tissue and the border zone of infarction, indicating increased integrin αvβ3 expression (Fig. 4) [52•, 81, 82].
Fig. 4.
Multimodality noninvasive imaging by SPECT and PET, showing myocardial perfusion and angiogenesis, respectively. a SPECT and PET images in polar-map format, showing perfusion defects and angiogenesis of infarcted hearts that were treated with saline, dissociated cells, or cell aggregates. […] (reprinted from [52•] with permission from Elsevier)
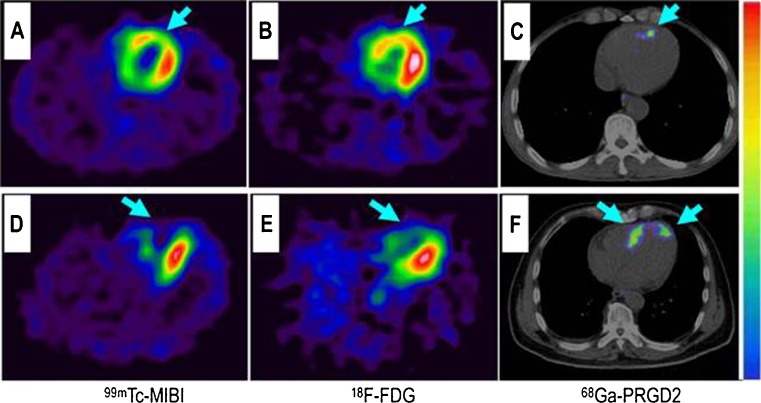
Recently, a 68Ga-labeled cyclic RGD dimer with a PEG spacer (68Ga-PRGD2) was studied for the first time in a small set of patients post-MI. 68Ga-PRGD2 uptake was found in 20 of 23 patients around the ischemic regions. Increased uptake was found 1 week after MI and remained high until 2.5 months after MI. 68Ga-PRGD2 uptake correlated with size and severity of the infarction (Fig. 5). Three patients who did not show any 68Ga-PRGD2 uptake were identified with a very recent MI and events dated back 1–2 years. Nevertheless, 68Ga-PRGD2 uptake showed a patchy pattern, which may be attributable to an uptake not only by angiogenic ECs but also interstitial myofibroblasts contributing to myocardial remodeling [55••]. Although the application of integrin αvβ3 imaging has been previously translated into clinical trials to assess tumor angiogenesis [83], only a single clinical trial imaging angiogenesis after MI has been conducted so far, with two studies currently recruiting (NCT01813045, NCT01542073).
Fig. 5.
Comparison of a patient with slight myocardial infarction (MI) and a patient with severe MI. Upper row: In a 58-year-old man at the fifth day after the event, a small apical region with decreased 99mTc-MIBI perfusion (a, arrow) and 18F-FDG metabolism (b, arrow) showed mild 68Ga-PRGD2 accumulation (c, arrow), with a pSUV of 0.62. Lower row: In a 45-year-old woman on the seventh day after the event, an apical defect on 99mTc-MIBI perfusion images (d, arrow) and 18F-FDG metabolism images (e, arrow) corresponded with moderate 68Ga-PRGD2 uptake (F, arrows), with a pSUV of 2.02 (reprinted from [55••] with permission from Theranostics)
Even though recent work in integrin αvβ3 imaging with PET or SPECT registered substantial improvements, this method is still afflicted with several limitations. Recently, a cyclic RGD moiety conjugated to MPIO (c(RGDyK)-MPIO) for MR angiogenesis visualization in a colorectal carcinoma and melanoma mouse model was studied. c(RGDyK)-MPIO specifically binds to integrin αvβ3 expressing vessels, while unbound particles are rapidly cleared from circulation. Specific binding was verified by ex vivo immunolabeling [56•]. Further optimization of MR-based angiogenesis imaging tracers may enable integrated molecular and anatomical imaging.
CEU imaging with targeted microbubbles displays another radiation-free molecular imaging technique. Microbubbles binding to integrin αvβ3 and other angiogenesis specific targets have been extensively applied for studying angiogenesis and the response to antiangiogenic therapies in several tumor entities [35, 84–86]. However, only limited data exist regarding cardiovascular diseases and treatments [87]. Xie et al. investigated the effect of HIF-1α mutant on angiogenesis in a mouse ischemic hind limb model; CEU imaging using αv-integrin-coated microbubbles has been applied to visualize angiogenesis. Video intensity obtained by αv imaging positively correlated with ultrasound perfusion imaging data, indicating that CEU imaging may also provide quantitative data on angiogenesis. This suggests that αv-integrin imaging via ultrasound can be a reliable method to visualize angiogenesis in vivo [34]. Moreover, ultrasound is not only limited to imaging angiogenesis to monitor cardiac regeneration but offers therapeutic options. Microbubbles loaded with therapeutic agents can be dissolved by high acoustic pressures after accumulation at the region of interest, thus enabling targeted drug delivery. Additionally, it is hypothesized that a combination of targeted imaging and drug release via microbubbles is possible [33, 88, 89].
Integrin α5β1
Integrin α5β1 expression is suggested to be completely restricted to ECs as deletion of the β1 chain leads to full inhibition of angiogenesis [90]. Analogous to integrin αvβ3, expression of integrin α5β1 is low in quiescent ECs and upregulated in angiogenic ECs [91, 92]. These results suggest that integrin α5β1 could be a more reliable biomarker for angiogenesis compared to αvβ3. To assess integrin α5β1 imaging in tumor angiogenesis, Notni et al. developed 68Ga-aquibeprin (a pseudopeptide targeting integrin α5β1) and compared it to 68Ga-avebetrin (targeting integrin αvβ3). In vitro data showed high affinity for integrin α5β1, and no decrease in specificity compared to a previously used 68Ga-labeled monomer selectively targeting integrin α5β1 was detected. In vivo data showed a higher tumor-to-organ ratio of 68Ga-aquibeprin and suggest it to be sufficiently stable. Immunohistochemical stainings further propose integrin α5β1 as a more EC specific marker [57••].
Other targets for molecular imaging
CD13
CD13 is a membrane-bound aminopeptidase, which is upregulated on activated ECs [93]. It is considered an important regulator of EC morphogenesis during angiogenesis [94]. The cyclic tripeptide Asn-Gly-Arg (cNGR) binds to CD13 on activated ECs in infarcted myocardium, but not to CD13-positive macrophages in hypoxic myocardium [53, 95]. Comparative studies with RGD and NGR of tumor angiogenesis revealed a threefold higher target homing ratio for NGR [96]. A recent study investigated CD13-targeted angiogenesis imaging in a mouse model of MI with 111In-DTPA-cNGR by SPECT. Increased uptake of 111In-DTPA-cNGR at day 7 after MI correlated with areas of decreased 99mTc-sestamibi [53].
CD105
CD105 (endoglin) is a transmembrane protein that is solely expressed on activated ECs [97]. Several PET probes based on TRC105, a monoclonal antibody that binds to CD105 with high avidity, have been tested for tumor angiogenesis imaging [98, 99]. 64Cu-NOTA-TRC105 was recently tested to assess angiogenesis in a rat MI model via PET. Tracer uptake was increased in infarcted myocardium. Expression of CD105 was confirmed by immunofluorescence. However, 64Cu-NOTA-TRC105 exhibits a long half-life and its intense background signal acted as a confounder [54].
VEGF
VEGF is commonly considered as the most potent mediator of angiogenesis. Consequently, VEGF and its receptors (VEGFRs) are frequently used for angiogenesis imaging. Due to splice variants, several isoforms of VEGF-A exist, of which some are proangiogenic and other antiangiogenic [100]. Monoclonal human anti-VEGF labeled with 123I and 124I have been employed for PET/SPECT imaging [101]. In a rat model of myocardial infarction, recombinant radiolabeled VEGF (64Cu-DOTA-VEGF121) was used for PET imaging of VEGFRs. An increased radiotracer uptake was reported in a period of up to 2 weeks after induction of MI [101].
Several tyrosine kinases are upregulated in heart tissue undergoing angiogenesis and remodeling after MI. Immunohistochemical analyses of MI samples revealed increased levels of VEGFR-2, Tie-2, and PDGFα suggesting its use as an angiogenesis marker in non-invasive molecular imaging. ATV-1 can act as an inhibitor of those kinases. PET imaging with [11C]ATV-1 was assessed in a rat model of MI. Standard uptake values of [11C]ATV-1 correlated with immunohistochemical staining of VEGFR-2, Tie-2, and PDGFα [9].
Conclusions
Even though proangiogenic therapies have so far largely failed as an effective treatment of MI, targeting angiogenesis after MI to mitigate heart failure is still considered a promising strategy. In order to assess the success of such therapeutic interventions in the clinic or in preclinical development, reliable and sensitive noninvasive imaging modalities are needed. Molecular imaging of angiogenesis via PET, SPECT, MRI, and CEU has been investigated intensively within the past decade. A variety of tracers have been translated from tumor angiogenesis models to MI models, and promising results were achieved. These methods offer the unique opportunity to study in vivo molecular mechanisms characterizing myocardial healing after infarction and to evaluate angiogenic effects of regenerative treatments. Combination of high sensitivity PET and SPECT with high-resolution X-ray CT images allows better identification and quantification of tracer uptake within the region of interest. Multimodal imaging with highly specific tracers yields reliable and detailed data of cardiac angiogenesis in small and large animal models and also in humans. Because PET and SPECT imaging use ionizing radiation, these imaging modalities might also expose patients to a risk of growing neoplastic lesions. For clinical applications, further research is warranted to develop radiotracers with a reasonable level of ionizing radiation or even replacing PET and SPECT with MRI or CEU while still featuring high affinity for visualizing growing blood vessels. Continuing development of noninvasive imaging modalities for future clinical applications may enable improved patient risk stratification and pave the way for personalized therapy.
Acknowledgments
Open access funding provided by Medical University of Vienna.
Compliance with Ethics Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Molecular Imaging
References
Papers of particular interest, published recently, have been highlighted as: • Of importance, •• Of major importance
- 1.Cochain C, Channon KM, Silvestre JS. Angiogenesis in the infarcted myocardium. Antioxid Redox Signal. 2013;18(9):1100–13. doi: 10.1089/ars.2012.4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah AM, Mann DL. In search of new therapeutic targets and strategies for heart failure: recent advances in basic science. Lancet. 2011;378(9792):704–12. doi: 10.1016/S0140-6736(11)60894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battegay EJ. Angiogenesis: mechanistic insights, neovascular diseases, and therapeutic prospects. J Mol Med. 1995;73(7):333–46. doi: 10.1007/BF00192885. [DOI] [PubMed] [Google Scholar]
- 4.Meoli DF, Sadeghi MM, Krassilnikova S, Bourke BN, Giordano FJ, Dione DP, et al. Noninvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J Clin Invest. 2004;113(12):1684–91. doi: 10.1172/JCI20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uchida Y, Yanagisawa-Miwa A, Nakamura F, Yamada K, Tomaru T, Kimura K, et al. Angiogenic therapy of acute myocardial infarction by intrapericardial injection of basic fibroblast growth factor and heparin sulfate: an experimental study. Am Heart J. 1995;130(6):1182–8. doi: 10.1016/0002-8703(95)90140-x. [DOI] [PubMed] [Google Scholar]
- 6.Gyöngyösi M, Wojakowski W, Lemarchand P, Lunde K, Tendera M, Bartunek J, et al. Meta-analysis of cell-based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ Res. 2015 doi: 10.1161/CIRCRESAHA.116.304346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scimia MC, Gumpert AM, Koch WJ. Cardiovascular gene therapy for myocardial infarction. Expert Opin Biol Ther. 2014;14(2):183–95. doi: 10.1517/14712598.2014.866085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–87. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Dissoki S, Abourbeh G, Salnikov O, Mishani E, Jacobson O. PET molecular imaging of angiogenesis with a multiple tyrosine kinase receptor-targeted agent in a rat model of myocardial infarction. Mol Imaging Biol. 2015;17(2):222–30. doi: 10.1007/s11307-014-0790-8. [DOI] [PubMed] [Google Scholar]
- 10.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frangogiannis NG. The mechanistic basis of infarct healing. Antioxid Redox Signal. 2006;8(11-12):1907–39. doi: 10.1089/ars.2006.8.1907. [DOI] [PubMed] [Google Scholar]
- 12.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35(3):569–82. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 13.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, et al. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115(8):2108–18. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silvestre JS, Smadja DM, Levy BI. Postischemic revascularization: from cellular and molecular mechanisms to clinical applications. Physiol Rev. 2013;93(4):1743–802. doi: 10.1152/physrev.00006.2013. [DOI] [PubMed] [Google Scholar]
- 15.Jivraj N, Phinikaridou A, Shah AM, Botnar RM. Molecular imaging of myocardial infarction. Basic Res Cardiol. 2014;109(1):397. doi: 10.1007/s00395-013-0397-2. [DOI] [PubMed] [Google Scholar]
- 16.Stacy MR, Sinusas AJ. Emerging imaging modalities in regenerative medicine. Curr Pathobiol Rep. 2015;3(1):27–36. doi: 10.1007/s40139-015-0073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ripa RS, Kjaer A. Imaging atherosclerosis with hybrid positron emission tomography/magnetic resonance imaging. Biomed Res Int. 2015;2015:914516. doi: 10.1155/2015/914516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stacy MR, Maxfield MW, Sinusas AJ. Targeted molecular imaging of angiogenesis in PET and SPECT: a review. Yale J Biol Med. 2012;85(1):75–86. [PMC free article] [PubMed] [Google Scholar]
- 19.Naumova AV, Modo M, Moore A, Murry CE, Frank JA. Clinical imaging in regenerative medicine. Nat Biotechnol. 2014;32(8):804–18. doi: 10.1038/nbt.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.•.Cai M, Ren L, Yin X, Guo Z, Li Y, He T, Tang Y, Long T, Liu Y, Liu G, Zhang X, Hu S. PET monitoring angiogenesis of infarcted myocardium after treatment with vascular endothelial growth factor and bone marrow mesenchymal stem cells. Amino Acids. 2016; 48(3):811-820. doi:10.1007/s00726-015-2129-4. This experimental rat myocardial infarction model introduces a new18F based radiotracer for angiogenesis imaging which offers a variety of advantages over previous radiotracers. Moreover, 18F-Alfatide II was used to demonstrate increased myocardial angiogenesis in rats treated with VEGF and/or BMSCs. [DOI] [PubMed]
- 21.Li X, Heber D, Rausch I, Beitzke D, Mayerhoefer ME, Rasul S, et al. Quantitative assessment of atherosclerotic plaques on F-FDG PET/MRI: comparison with a PET/CT hybrid system. Eur J Nucl Med Mol Imaging. 2016 doi: 10.1007/s00259-016-3308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratib O, Nkoulou R. Potential applications of PET/MR imaging in cardiology. J Nucl Med. 2014;55(Supplement 2):40S–6. doi: 10.2967/jnumed.113.129262. [DOI] [PubMed] [Google Scholar]
- 23.Stacy MR, Paeng JC, Sinusas AJ. The role of molecular imaging in the evaluation of myocardial and peripheral angiogenesis. Ann Nucl Med. 2015;29(3):217–23. doi: 10.1007/s12149-015-0961-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JS, Kim JH. Recent advances in hybrid molecular imaging systems. Semin Musculoskelet Radiol. 2014;18(2):103–22. doi: 10.1055/s-0034-1371014. [DOI] [PubMed] [Google Scholar]
- 25.Constantine G, Shan K, Flamm SD, Sivananthan MU. Role of MRI in clinical cardiology. Lancet. 2004;363(9427):2162–71. doi: 10.1016/S0140-6736(04)16509-4. [DOI] [PubMed] [Google Scholar]
- 26.Mulder WJ, Strijkers GJ, Habets JW, Bleeker EJ, van der Schaft DW, Storm G, et al. MR molecular imaging and fluorescence microscopy for identification of activated tumor endothelium using a bimodal lipidic nanoparticle. FASEB J. 2005;19(14):2008–10. doi: 10.1096/fj.05-4145fje. [DOI] [PubMed] [Google Scholar]
- 27.Schmieder AH, Winter PM, Caruthers SD, Harris TD, Williams TA, Allen JS, et al. Molecular MR imaging of melanoma angiogenesis with alphanubeta3-targeted paramagnetic nanoparticles. Magn Reson Med. 2005;53(3):621–7. doi: 10.1002/mrm.20391. [DOI] [PubMed] [Google Scholar]
- 28.Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC. Detection of tumor angiogenesis in vivo by alphaVbeta3-targeted magnetic resonance imaging. Nat Med. 1998;4(5):623–6. doi: 10.1038/nm0598-623. [DOI] [PubMed] [Google Scholar]
- 29.Zhang C, Jugold M, Woenne EC, Lammers T, Morgenstern B, Mueller MM, et al. Specific targeting of tumor angiogenesis by RGD-conjugated ultrasmall superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner. Cancer Res. 2007;67(4):1555–62. doi: 10.1158/0008-5472.CAN-06-1668. [DOI] [PubMed] [Google Scholar]
- 30.Leung K. Cyclo(Arg-Gly-Asp-D-Try-Glu) conjugated to ultrasmall superparamagnetic iron oxide nanoparticles. Bethesda (MD): Molecular Imaging and Contrast Agent Database (MICAD); 2004. [PubMed]
- 31.Yang Y, Yang Y, Yanasak N, Schumacher A, Hu TC. Temporal and noninvasive monitoring of inflammatory-cell infiltration to myocardial infarction sites using micrometer-sized iron oxide particles. Magn Reson Med. 2010;63(1):33–40. doi: 10.1002/mrm.22175. [DOI] [PubMed] [Google Scholar]
- 32.Singh N, Jenkins GJS, Asadi R, Doak SH. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Reviews. 2010; 1:10.3402/nano.v3401i3400.5358. doi:10.3402/nano.v1i0.5358. [DOI] [PMC free article] [PubMed]
- 33.Smith AH, Fujii H, Kuliszewski MA, Leong-Poi H. Contrast ultrasound and targeted microbubbles: diagnostic and therapeutic applications for angiogenesis. J Cardiovasc Transl Res. 2011;4(4):404–15. doi: 10.1007/s12265-011-9282-2. [DOI] [PubMed] [Google Scholar]
- 34.Xie J, Liao Y, Yang L, Wu J, Liu C, Xuan W, et al. Ultrasound molecular imaging of angiogenesis induced by mutant forms of hypoxia-inducible factor-1alpha. Cardiovasc Res. 2011;92(2):256–66. doi: 10.1093/cvr/cvr229. [DOI] [PubMed] [Google Scholar]
- 35.Payen T, Dizeux A, Baldini C, Le Guillou-Buffello D, Lamuraglia M, Comperat E, et al. VEGFR2-targeted contrast-enhanced ultrasound to distinguish between two anti-angiogenic treatments. Ultrasound Med Biol. 2015;41(8):2202–11. doi: 10.1016/j.ultrasmedbio.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Kim JE, Kalimuthu S, Ahn BC. In vivo cell tracking with bioluminescence imaging. Nucl Med Mol Imaging. 2015;49(1):3–10. doi: 10.1007/s13139-014-0309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker M. Whole-animal imaging: the whole picture. Nature. 2010;463(7283):977–80. doi: 10.1038/463977a. [DOI] [PubMed] [Google Scholar]
- 38.Ahn BC. Applications of molecular imaging in drug discovery and development process. Curr Pharm Biotechnol. 2011;12(4):459–68. doi: 10.2174/138920111795163904. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen PK, Lan F, Wang Y, Wu JC. Imaging: guiding the clinical translation of cardiac stem cell therapy. Circ Res. 2011;109(8):962–79. doi: 10.1161/CIRCRESAHA.111.242909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blankstein R, Di Carli MF. Integration of coronary anatomy and myocardial perfusion imaging. Nat Rev Cardiol. 2010;7(4):226–36. doi: 10.1038/nrcardio.2010.15. [DOI] [PubMed] [Google Scholar]
- 41.Gaemperli O, Kaufmann PA, Alkadhi H. Cardiac hybrid imaging. Eur J Nucl Med Mol Imaging. 2014;41(Suppl 1):S91–103. doi: 10.1007/s00259-013-2566-9. [DOI] [PubMed] [Google Scholar]
- 42.Fricke E, Fricke H, Weise R, Kammeier A, Hagedorn R, Lotz N, et al. Attenuation correction of myocardial SPECT perfusion images with low-dose CT: evaluation of the method by comparison with perfusion PET. J Nucl Med. 2005;46(5):736–44. [PubMed] [Google Scholar]
- 43.Brown ER, Kronmal RA, Bluemke DA, Guerci AD, Carr JJ, Goldin J, et al. Coronary calcium coverage score: determination, correlates, and predictive accuracy in the Multi-Ethnic Study of Atherosclerosis. Radiology. 2008;247(3):669–75. doi: 10.1148/radiol.2473071469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49(18):1860–70. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 45.Henzlova MJ, Duvall WL. The future of SPECT MPI: time and dose reduction. J Nucl Cardiol. 2011;18(4):580–7. doi: 10.1007/s12350-011-9401-0. [DOI] [PubMed] [Google Scholar]
- 46.Palyo R, Sinusas A, Liu YH. High-sensitivity and high-resolution SPECT/CT systems provide substantial dose reduction without compromising quantitative precision for assessment of myocardial perfusion or function. J Nucl Med. 2016 doi: 10.2967/jnumed.115.164632. [DOI] [PubMed] [Google Scholar]
- 47.Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA, Craighead FH, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383(9918):705–13. doi: 10.1016/S0140-6736(13)61754-7. [DOI] [PubMed] [Google Scholar]
- 48.Mazurek T, Kobylecka M, Zielenkiewicz M, Kurek A, Kochman J, Filipiak KJ, et al. PET/CT evaluation of F-FDG uptake in pericoronary adipose tissue in patients with stable coronary artery disease: Independent predictor of atherosclerotic lesions’ formation? J Nucl Cardiol. 2016 doi: 10.1007/s12350-015-0370-6. [DOI] [PubMed] [Google Scholar]
- 49.Iagaru A, Gambhir SS. Imaging tumor angiogenesis: the road to clinical utility. AJR Am J Roentgenol. 2013;201(2):W183–91. doi: 10.2214/AJR.12.8568. [DOI] [PubMed] [Google Scholar]
- 50.Laitinen I, Notni J, Pohle K, Rudelius M, Farrell E, Nekolla SG, et al. Comparison of cyclic RGD peptides for alphavbeta3 integrin detection in a rat model of myocardial infarction. EJNMMI Res. 2013;3(1):38. doi: 10.1186/2191-219X-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menichetti L, Kusmic C, Panetta D, Arosio D, Petroni D, Matteucci M, et al. MicroPET/CT imaging of alphavbeta(3) integrin via a novel (6)(8)Ga-NOTA-RGD peptidomimetic conjugate in rat myocardial infarction. Eur J Nucl Med Mol Imaging. 2013;40(8):1265–74. doi: 10.1007/s00259-013-2432-9. [DOI] [PubMed] [Google Scholar]
- 52.•.Huang CC, Wei HJ, Lin KJ, Lin WW, Wang CW, Pan WY, Hwang SM, Chang Y, Sung HW. Multimodality noninvasive imaging for assessing therapeutic effects of exogenously transplanted cell aggregates capable of angiogenesis on acute myocardial infarction. Biomaterials. 2015; 73:12-22. doi:10.1016/j.biomaterials.2015.09.009. This is an experimental rat MI study showing that the application of 3D aggregates of HUVECs/cbMSCs improves blood perfusion and global/regional ventricular function. Additionally those aggregates induced increased angiogenesis which was shown in vivo using non-invasive angiogenesis imaging by PET targeting integrin αvβ3and immunohistochemistical stainings. [DOI] [PubMed]
- 53.Hendrikx G, De Saint-Hubert M, Dijkgraaf I, Bauwens M, Douma K, Wierts R, et al. Molecular imaging of angiogenesis after myocardial infarction by (111)In-DTPA-cNGR and (99m)Tc-sestamibi dual-isotope myocardial SPECT. EJNMMI Res. 2015;5:2. doi: 10.1186/s13550-015-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orbay H, Zhang Y, Valdovinos HF, Song G, Hernandez R, Theuer CP, et al. Positron emission tomography imaging of CD105 expression in a rat myocardial infarction model with (64)Cu-NOTA-TRC105. Am J Nucl Med Mol Imaging. 2013;4(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- 55.••.Sun Y, Zeng Y, Zhu Y, Feng F, Xu W, Wu C, Xing B, Zhang W, Wu P, Cui L, Wang R, Li F, Chen X, Zhu Z. Application of (68)Ga-PRGD2 PET/CT for alphavbeta3-integrin imaging of myocardial infarction and stroke. Theranostics. 2014; 4(8):778-786. doi:10.7150/thno.8809. This trial is the first larger trial in humans on angiogenesis after myocardial infarction. It offers an insight into the role of angiogenesis throught myocardial healing after infarction and provides evidence that PET imaging of integrin αvβ3is feasable. [DOI] [PMC free article] [PubMed]
- 56.•.Melemenidis S, Jefferson A, Ruparelia N, Akhtar AM, Xie J, Allen D, et al. Molecular magnetic resonance imaging of angiogenesis in vivo using polyvalent cyclic RGD-iron oxide microparticle conjugates. Theranostics. 2015;5(5):515–29. doi: 10.7150/thno.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.••.Notni J, Steiger K, Hoffmann F, Reich D, Kapp TG, Rechenmacher F, Neubauer S, Kessler H, Wester HJ. Complementary, selective PET imaging of integrin subtypes alpha5beta1 and alphavbeta3 using 68Ga-aquibeprin and 68Ga-avebetrin. J Nucl Med. 2016; 57(3):460-466. doi:10.2967/jnumed.115.165720. This trial compares for the first time molecular imaging of myocardial angiogenesis in rat with MI two integrin subtypes. The so far rather neglected integrin α5β1was demonstrated to be more specific for activated endothelial cells with however no decline in affinity. [DOI] [PubMed]
- 58.Dobrucki LW, de Muinck ED, Lindner JR, Sinusas AJ. Approaches to multimodality imaging of angiogenesis. J Nucl Med. 2010 doi: 10.2967/jnumed.110.074963. [DOI] [PubMed] [Google Scholar]
- 59.Luo BH, Springer TA. Integrin structures and conformational signaling. Curr Opin Cell Biol. 2006;18(5):579–86. doi: 10.1016/j.ceb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Humphries MJ. Integrin cell adhesion receptors and the concept of agonism. Trends Pharmacol Sci. 2000;21(1):29–32. doi: 10.1016/s0165-6147(99)01410-8. [DOI] [PubMed] [Google Scholar]
- 61.Horton MA. The alpha v beta 3 integrin “vitronectin receptor”. Int J Biochem Cell Biol. 1997;29(5):721–5. doi: 10.1016/s1357-2725(96)00155-0. [DOI] [PubMed] [Google Scholar]
- 62.Higuchi T, Bengel FM, Seidl S, Watzlowik P, Kessler H, Hegenloh R, et al. Assessment of alphavbeta3 integrin expression after myocardial infarction by positron emission tomography. Cardiovasc Res. 2008;78(2):395–403. doi: 10.1093/cvr/cvn033. [DOI] [PubMed] [Google Scholar]
- 63.Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95(4):507–19. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- 64.Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, et al. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8(1):27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- 65.Reynolds AR, Reynolds LE, Nagel TE, Lively JC, Robinson SD, Hicklin DJ, et al. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in beta3-integrin-deficient mice. Cancer Res. 2004;64(23):8643–50. doi: 10.1158/0008-5472.CAN-04-2760. [DOI] [PubMed] [Google Scholar]
- 66.Atkinson SJ, Ellison TS, Steri V, Gould E, Robinson SD. Redefining the role(s) of endothelial alphavbeta3-integrin in angiogenesis. Biochem Soc Trans. 2014;42(6):1590–5. doi: 10.1042/BST20140206. [DOI] [PubMed] [Google Scholar]
- 67.Mittra ES, Goris ML, Iagaru AH, Kardan A, Burton L, Berganos R, et al. Pilot pharmacokinetic and dosimetric studies of (18)F-FPPRGD2: a PET radiopharmaceutical agent for imaging alpha(v)beta(3) integrin levels. Radiology. 2011;260(1):182–91. doi: 10.1148/radiol.11101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beer AJ, Lorenzen S, Metz S, Herrmann K, Watzlowik P, Wester HJ, et al. Comparison of integrin alphaVbeta3 expression and glucose metabolism in primary and metastatic lesions in cancer patients: a PET study using 18F-galacto-RGD and 18F-FDG. J Nucl Med. 2008;49(1):22–9. doi: 10.2967/jnumed.107.045864. [DOI] [PubMed] [Google Scholar]
- 69.Lang L, Li W, Guo N, Ma Y, Zhu L, Kiesewetter DO, et al. Comparison study of [18F]FAl-NOTA-PRGD2, [18F]FPPRGD2, and [68Ga]Ga-NOTA-PRGD2 for PET imaging of U87MG tumors in mice. Bioconjug Chem. 2011;22(12):2415–22. doi: 10.1021/bc200197h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu Z, Yin Y, Zheng K, Li F, Chen X, Zhang F, et al. Evaluation of synovial angiogenesis in patients with rheumatoid arthritis using (6)(8)Ga-PRGD2 PET/CT: a prospective proof-of-concept cohort study. Ann Rheum Dis. 2014;73(6):1269–72. doi: 10.1136/annrheumdis-2013-204820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen X, Liu S, Hou Y, Tohme M, Park R, Bading JR, et al. MicroPET imaging of breast cancer alphav-integrin expression with 64Cu-labeled dimeric RGD peptides. Mol Imaging Biol. 2004;6(5):350–9. doi: 10.1016/j.mibio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 72.Lang L, Li W, Jia HM, Fang DC, Zhang S, Sun X, et al. New methods for labeling RGD peptides with bromine-76. Theranostics. 2011;1:341–53. doi: 10.7150/thno/v01p0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jacobson O, Zhu L, Niu G, Weiss ID, Szajek LP, Ma Y, et al. MicroPET imaging of integrin alphavbeta3 expressing tumors using 89Zr-RGD peptides. Mol Imaging Biol. 2011;13(6):1224–33. doi: 10.1007/s11307-010-0458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Axelsson R, Bach-Gansmo T, Castell-Conesa J, McParland BJ, Study G An open-label, multicenter, phase 2a study to assess the feasibility of imaging metastases in late-stage cancer patients with the alpha v beta 3-selective angiogenesis imaging agent 99mTc-NC100692. Acta Radiol. 2010;51(1):40–6. doi: 10.3109/02841850903273974. [DOI] [PubMed] [Google Scholar]
- 75.Jia B, Liu Z, Zhu Z, Shi J, Jin X, Zhao H, et al. Blood clearance kinetics, biodistribution, and radiation dosimetry of a kit-formulated integrin alphavbeta3-selective radiotracer 99mTc-3PRGD 2 in non-human primates. Mol Imaging Biol. 2011;13(4):730–6. doi: 10.1007/s11307-010-0385-y. [DOI] [PubMed] [Google Scholar]
- 76.Terry SY, Abiraj K, Frielink C, van Dijk LK, Bussink J, Oyen WJ, et al. Imaging integrin alphavbeta3 on blood vessels with 111In-RGD2 in head and neck tumor xenografts. J Nucl Med. 2014;55(2):281–6. doi: 10.2967/jnumed.113.129668. [DOI] [PubMed] [Google Scholar]
- 77.Sherif HM, Saraste A, Nekolla SG, Weidl E, Reder S, Tapfer A, et al. Molecular imaging of early alphavbeta3 integrin expression predicts long-term left-ventricle remodeling after myocardial infarction in rats. J Nucl Med. 2012;53(2):318–23. doi: 10.2967/jnumed.111.091652. [DOI] [PubMed] [Google Scholar]
- 78.Notni J, Pohle K, Wester HJ. Be spoilt for choice with radiolabelled RGD peptides: preclinical evaluation of (6)(8)Ga-TRAP(RGD)(3) Nucl Med Biol. 2013;40(1):33–41. doi: 10.1016/j.nucmedbio.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 79.Pohle K, Notni J, Bussemer J, Kessler H, Schwaiger M, Beer AJ. 68Ga-NODAGA-RGD is a suitable substitute for (18)F-Galacto-RGD and can be produced with high specific activity in a cGMP/GRP compliant automated process. Nucl Med Biol. 2012;39(6):777–84. doi: 10.1016/j.nucmedbio.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 80.Gao H, Lang L, Guo N, Cao F, Quan Q, Hu S, et al. PET imaging of angiogenesis after myocardial infarction/reperfusion using a one-step labeled integrin targeted tracer (18)F-AlF-NOTA-PRGD2. Eur J Nucl Med Mol Imaging. 2012;39(4):683–92. doi: 10.1007/s00259-011-2052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dimastromatteo J, Riou LM, Ahmadi M, Pons G, Pellegrini E, Broisat A, et al. In vivo molecular imaging of myocardial angiogenesis using the αvβ3 integrin-targeted tracer 99mTc-RAFT-RGD. J Nucl Cardiol. 2010;17(3):435–43. doi: 10.1007/s12350-010-9191-9. [DOI] [PubMed] [Google Scholar]
- 82.Paeng JC, Bregasi A, Sahul Z, Kalinowski L, Dobrucki LW, Brennan M, et al. Serial reference tissue-based quantitative and volumetric analysis of integrin-targeted angiogenesis imaging: chronic canine model of myocardial infarction. J Nucl Med. 2014;2014(55):1710. [Google Scholar]
- 83.Gaertner FC, Kessler H, Wester HJ, Schwaiger M, Beer AJ. Radiolabelled RGD peptides for imaging and therapy. Eur J Nucl Med Mol Imaging. 2012;39(Suppl 1):S126–38. doi: 10.1007/s00259-011-2028-1. [DOI] [PubMed] [Google Scholar]
- 84.Hu Q, Wang XY, Kang LK, Wei HM, Xu CM, Wang T, et al. RGD-targeted ultrasound contrast agent for longitudinal assessment of Hep-2 tumor angiogenesis in vivo. PLoS One. 2016;11(2):e0149075. doi: 10.1371/journal.pone.0149075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shelton SE, Lindsey BD, Tsuruta JK, Foster FS, Dayton PA. Molecular acoustic angiography: a new technique for high-resolution superharmonic ultrasound molecular imaging. Ultrasound Med Biol. 2016;42(3):769–81. doi: 10.1016/j.ultrasmedbio.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yan F, Xu X, Chen Y, Deng Z, Liu H, Xu J, et al. A lipopeptide-based alphavbeta(3) integrin-targeted ultrasound contrast agent for molecular imaging of tumor angiogenesis. Ultrasound Med Biol. 2015;41(10):2765–73. doi: 10.1016/j.ultrasmedbio.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 87.Leong-Poi H. Molecular imaging using contrast-enhanced ultrasound: evaluation of angiogenesis and cell therapy. Cardiovasc Res. 2009;84(2):190–200. doi: 10.1093/cvr/cvp248. [DOI] [PubMed] [Google Scholar]
- 88.Deng Q, Hu B, Cao S, Song HN, Chen JL, Zhou Q. Improving the efficacy of therapeutic angiogenesis by UTMD-mediated Ang-1 gene delivery to the infarcted myocardium. Int J Mol Med. 2015;36(2):335–44. doi: 10.3892/ijmm.2015.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nguyen AT, Wrenn SP. Acoustically active liposome-nanobubble complexes for enhanced ultrasonic imaging and ultrasound-triggered drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6(3):316–25. doi: 10.1002/wnan.1255. [DOI] [PubMed] [Google Scholar]
- 90.Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9(15):1896–908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- 91.Tanjore H, Zeisberg EM, Gerami-Naini B, Kalluri R. Beta1 integrin expression on endothelial cells is required for angiogenesis but not for vasculogenesis. Dev Dyn. 2008;237(1):75–82. doi: 10.1002/dvdy.21385. [DOI] [PubMed] [Google Scholar]
- 92.Kim S, Bell K, Mousa SA, Varner JA. Regulation of angiogenesis in vivo by ligation of integrin alpha5beta1 with the central cell-binding domain of fibronectin. Am J Pathol. 2000;156(4):1345–62. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Corti A, Curnis F, Arap W, Pasqualini R. The neovasculature homing motif NGR: more than meets the eye. Blood. 2008;112(7):2628–35. doi: 10.1182/blood-2008-04-150862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bhagwat SV, Lahdenranta J, Giordano R, Arap W, Pasqualini R, Shapiro LH. CD13/APN is activated by angiogenic signals and is essential for capillary tube formation. Blood. 2001;97(3):652–9. doi: 10.1182/blood.v97.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Buehler A, van Zandvoort MA, Stelt BJ, Hackeng TM, Schrans-Stassen BH, Bennaghmouch A, et al. cNGR: a novel homing sequence for CD13/APN targeted molecular imaging of murine cardiac angiogenesis in vivo. Arterioscler Thromb Vasc Biol. 2006;26(12):2681–7. doi: 10.1161/01.ATV.0000245807.65714.0b. [DOI] [PubMed] [Google Scholar]
- 96.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279(5349):377–80. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 97.Fonsatti E, Sigalotti L, Arslan P, Altomonte M, Maio M. Emerging role of endoglin (CD105) as a marker of angiogenesis with clinical potential in human malignancies. Curr Cancer Drug Targets. 2003;3(6):427–32. doi: 10.2174/1568009033481741. [DOI] [PubMed] [Google Scholar]
- 98.Hong H, Zhang Y, Orbay H, Valdovinos HF, Nayak TR, Bean J, et al. Positron emission tomography imaging of tumor angiogenesis with a (61/64)Cu-labeled F(ab’)(2) antibody fragment. Mol Pharm. 2013;10(2):709–16. doi: 10.1021/mp300507r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Y, Hong H, Severin GW, Engle JW, Yang Y, Goel S, et al. ImmunoPET and near-infrared fluorescence imaging of CD105 expression using a monoclonal antibody dual-labeled with (89)Zr and IRDye 800CW. Am J Transl Res. 2012;4(3):333–46. [PMC free article] [PubMed] [Google Scholar]
- 100.Nowak DG, Woolard J, Amin EM, Konopatskaya O, Saleem MA, Churchill AJ, et al. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J Cell Sci. 2008;121(20):3487–95. doi: 10.1242/jcs.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li S, Peck-Radosavljevic M, Koller E, Koller F, Kaserer K, Kreil A, et al. Characterization of 123I-vascular endothelial growth factor–binding sites expressed on human tumour cells: Possible implication for tumour scintigraphy. Int J Cancer. 2001;91(6):789–96. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1126>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]