Abstract
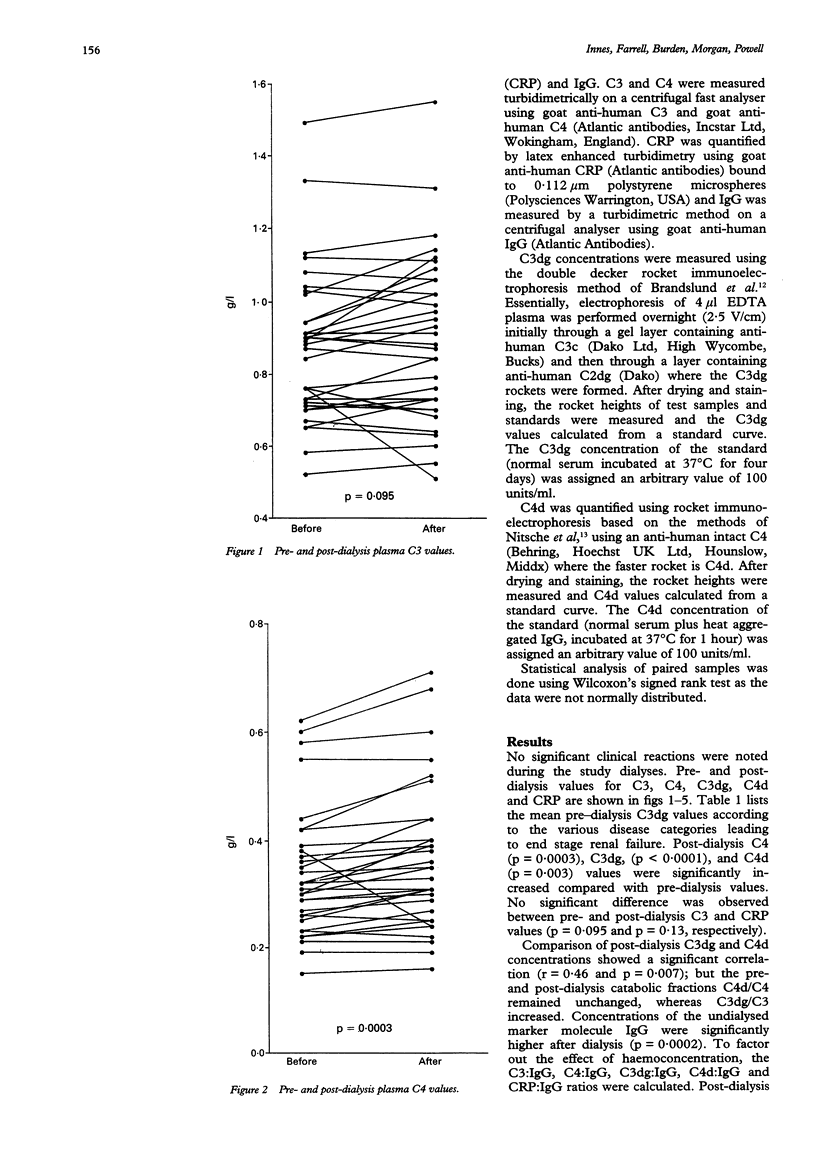
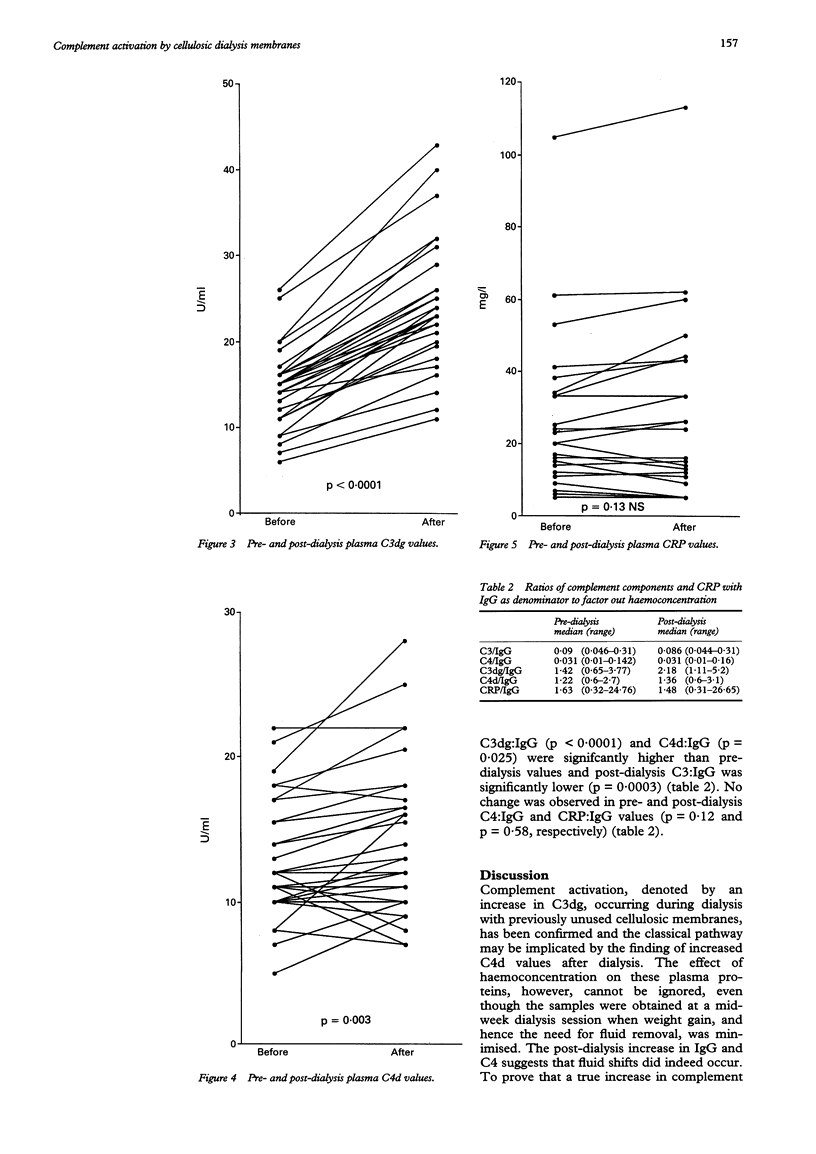
AIMS--To assess the effect of cellulosic dialysis membranes on the production of complement degradation products to determine to the role of the classical pathway. METHOD--Complement activation was studied in 33 patients during a single haemodialysis session using cellulosic membranes. Pre- and post-dialysis plasma EDTA valves of C3, C4, C3dg, C4d and C reactive protein (CRP) were measured. Statistical analysis was done using the Wilcoxon signed rank test. RESULTS--Post-dialysis C4 (p = 0.0003), C3dg (p < 0.0001), and C4d (p = 0.003) concentrations were increased compared with pre-dialysis values. There was no significant change in C3 (p = 0.095) and CRP (p = 0.13) values. Post-dialysis C3dg and C4d concentrations correlated significantly (p = 0.007). IgG, an undialysed molecule, was quantified and post-dialysis valves were significantly higher than those before dialysis (p = 0.0002), indicating a degree of haemoconcentration. To remove this effect, the C3:IgG, C4:IgG, C3dg:IgG, C4d:IgG and CRP:IgG ratios were calculated. Compared with pre-dialysis values, post-dialysis C3dg:IgG and C4d:IgG ratios were increased and C3:IgG decreased significantly. No change was observed in C4:IgG and CRP:IgG ratios. CONCLUSION--This study confirms that significant complement activation takes place following dialysis with cellulosic membranes. This is denoted by an increase in C3dg. This was paralleled by a rise in C4d, implying a contributory role for the classical pathway. Concomitant post-dialysis increases in IgG and C4 indicate a degree of haemoconcentration; but removal of this effect shows that C3dg and C4d are increased following dialysis--suggesting classical, in addition to alternative, pathway activation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Charlesworth J. A., Williams D. G., Sherington E., Lachmann P. J., Peters D. K. Metabolic studies of the third component of complement and the glycine-rich beta glycoprotein in patients with hypocomplementemia. J Clin Invest. 1974 Jun;53(6):1578–1587. doi: 10.1172/JCI107708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth D. E., Cheung A. K., Henderson L. W. Anaphylatoxin formation during hemodialysis: effects of different dialyzer membranes. Kidney Int. 1983 Dec;24(6):764–769. doi: 10.1038/ki.1983.225. [DOI] [PubMed] [Google Scholar]
- Cheung A. K., Parker C. J., Janatova J. Analysis of the complement C3 fragments associated with hemodialysis membranes. Kidney Int. 1989 Feb;35(2):576–588. doi: 10.1038/ki.1989.26. [DOI] [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Brigham K. L., Kronenberg R. S., Jacob H. S. Complement and leukocyte-mediated pulmonary dysfunction in hemodialysis. N Engl J Med. 1977 Apr 7;296(14):769–774. doi: 10.1056/NEJM197704072961401. [DOI] [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Dalmasso A. P., Brighan K. L., Jacob H. S. Hemodialysis leukopenia. Pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J Clin Invest. 1977 May;59(5):879–888. doi: 10.1172/JCI108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E. T., Nasaruddin B. A., Alhaq A., Senaldi G., Vergani D. Clinical application of new technique that measures C4d for assessment of activation of classical complement pathway. J Clin Pathol. 1988 Feb;41(2):143–147. doi: 10.1136/jcp.41.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim R. M., Breillatt J., Lazarus J. M., Port F. K. Complement activation and hypersensitivity reactions to dialysis membranes. N Engl J Med. 1984 Oct 4;311(14):878–882. doi: 10.1056/NEJM198410043111403. [DOI] [PubMed] [Google Scholar]
- Hakim R. M., Fearon D. T., Lazarus J. M. Biocompatibility of dialysis membranes: effects of chronic complement activation. Kidney Int. 1984 Aug;26(2):194–200. doi: 10.1038/ki.1984.155. [DOI] [PubMed] [Google Scholar]
- Hauser A. C., Derfler K., Stockenhuber F., Janata O., Balcke P. Generation of the membrane attack complex during haemodialysis: impact of classical and alternative pathway components. Clin Sci (Lond) 1990 Nov;79(5):471–476. doi: 10.1042/cs0790471. [DOI] [PubMed] [Google Scholar]
- Petersen N. E., Elmgreen J., Teisner B., Svehag S. E. Activation of classical pathway complement in chronic inflammation. Elevated levels of circulating C3d and C4d split products in rheumatoid arthritis and Crohn's disease. Acta Med Scand. 1988;223(6):557–560. [PubMed] [Google Scholar]
- Walker F., Lindsay R., Sibbald W., Linton A. Changes in pulmonary vascular tone during early hemodialysis. Trans Am Soc Artif Intern Organs. 1984;30:168–172. [PubMed] [Google Scholar]
- Zvaifler N. J. The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol. 1973;16(0):265–336. doi: 10.1016/s0065-2776(08)60299-0. [DOI] [PubMed] [Google Scholar]


