Abstract
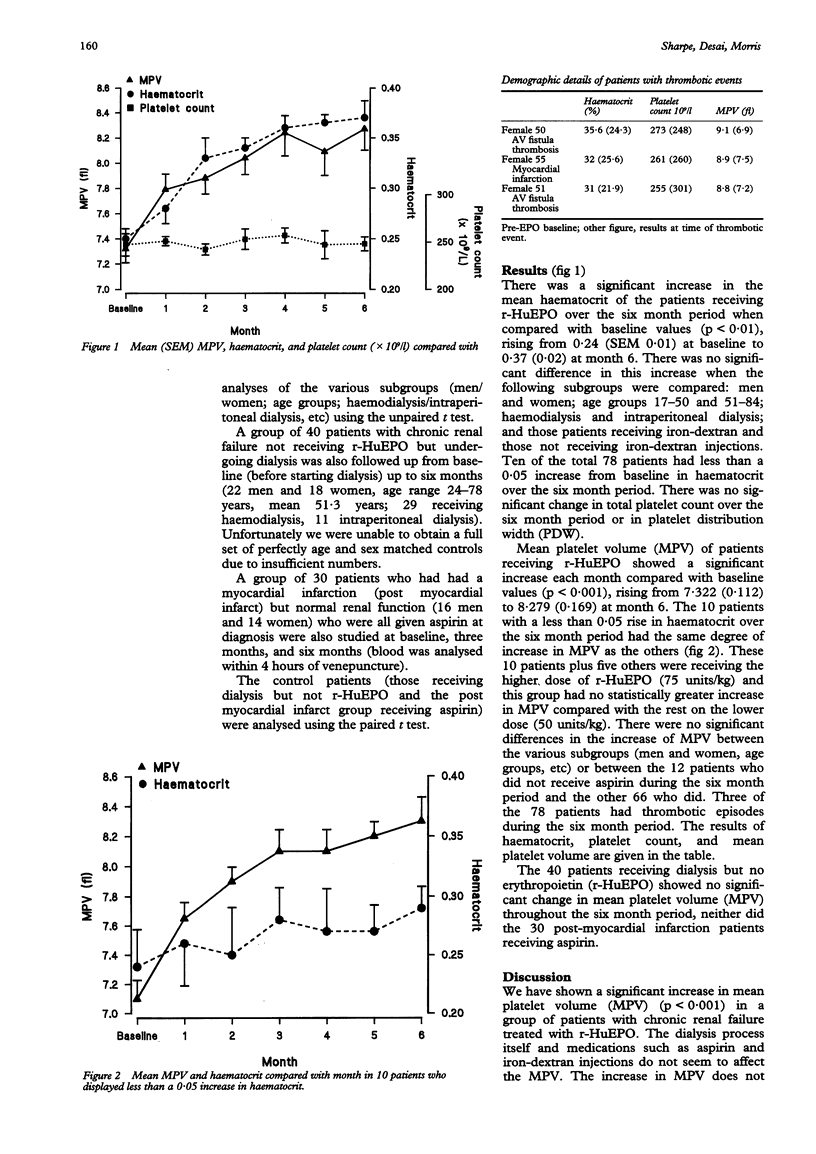
AIMS--To assess whether r-HuEPO (recombinant human erythropoietin) has any effect on thrombopoiesis in patients with chronic renal failure. METHODS--This was a retrospective study of 78 patients with chronic renal failure undergoing either haemodialysis (n = 57) or intraperitoneal dialysis (n = 21). All patients had a full blood count (in EDTA) measured before starting r-HuEPO and at monthly intervals thereafter up to six months. Variables studied were haematocrit, platelet count, mean platelet volume (MPV) and platelet distribution width (PDW). Other groups of control patients were also studied--patients with chronic renal failure receiving dialysis but not r-HuEPO (n = 40) and a group of patients with normal renal function who were receiving aspirin (n = 30). RESULTS--There was a significant increase in mean haematocrit (p < 0.01) and in mean platelet volume (p < 0.001) over the six month period, but no change in either total platelet count or platelet distribution width in the patients with chronic renal failure receiving r-HuEPO. In contrast, both the control groups showed no significant change in MPV. CONCLUSIONS--The results suggest that r-HuEPO affects thrombopoiesis and may be part of a group of humoral factors contributing to megakaryocyte development and maturation. Larger platelets are more reactive and may contribute to the increased risk of thrombosis associated with r-HuEPO.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akizawa T., Kinugasa E., Kitaoka T., Koshikawa S. Effects of recombinant human erythropoietin and correction of anemia on platelet function in hemodialysis patients. Nephron. 1991;58(4):400–406. doi: 10.1159/000186470. [DOI] [PubMed] [Google Scholar]
- Berridge M. V., Fraser J. K., Carter J. M., Lin F. K. Effects of recombinant human erythropoietin on megakaryocytes and on platelet production in the rat. Blood. 1988 Sep;72(3):970–977. [PubMed] [Google Scholar]
- Berridge M. V., Fraser J. K., Carter J. M., Lin F. K. Effects of recombinant human erythropoietin on megakaryocytes and on platelet production in the rat. Blood. 1988 Sep;72(3):970–977. [PubMed] [Google Scholar]
- Burstein S. A. Interleukin 3 promotes maturation of murine megakaryocytes in vitro. Blood Cells. 1986;11(3):469–484. [PubMed] [Google Scholar]
- D'Erasmo E., Aliberti G., Celi F. S., Romagnoli E., Vecci E., Mazzuoli G. F. Platelet count, mean platelet volume and their relation to prognosis in cerebral infarction. J Intern Med. 1990 Jan;227(1):11–14. doi: 10.1111/j.1365-2796.1990.tb00111.x. [DOI] [PubMed] [Google Scholar]
- D'Erasmo E., Aliberti G., Celi F. S., Vecci E., Mazzuoli G. Valutazione sequenziale del numero e del volume medio piastrinico nel decorso dell'infarto del miocardio. Medicina (Firenze) 1988 Jan-Mar;8(1):58–60. [PubMed] [Google Scholar]
- Deykin D. Uremic bleeding. Kidney Int. 1983 Nov;24(5):698–705. doi: 10.1038/ki.1983.214. [DOI] [PubMed] [Google Scholar]
- Fraser J. K., Tan A. S., Lin F. K., Berridge M. V. Expression of specific high-affinity binding sites for erythropoietin on rat and mouse megakaryocytes. Exp Hematol. 1989 Jan;17(1):10–16. [PubMed] [Google Scholar]
- Gordge M. P., Leaker B., Patel A., Oviasu E., Cameron J. S., Neild G. H. Recombinant human erythropoietin shortens the uraemic bleeding time without causing intravascular haemostatic activation. Thromb Res. 1990 Jan 15;57(2):171–182. doi: 10.1016/0049-3848(90)90317-6. [DOI] [PubMed] [Google Scholar]
- Hjelle B., Scalf R., Swenson S. High frequency of human T-cell leukemia-lymphoma virus type II infection in New Mexico blood donors: determination by sequence-specific oligonucleotide hybridization. Blood. 1990 Aug 1;76(3):450–454. [PubMed] [Google Scholar]
- Ishibashi T., Burstein S. A. Interleukin 3 promotes the differentiation of isolated single megakaryocytes. Blood. 1986 May;67(5):1512–1514. [PubMed] [Google Scholar]
- Ishibashi T., Kimura H., Uchida T., Kariyone S., Friese P., Burstein S. A. Human interleukin 6 is a direct promoter of maturation of megakaryocytes in vitro. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5953–5957. doi: 10.1073/pnas.86.15.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T., Koziol J. A., Burstein S. A. Human recombinant erythropoietin promotes differentiation of murine megakaryocytes in vitro. J Clin Invest. 1987 Jan;79(1):286–289. doi: 10.1172/JCI112796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski J. A., Thompson C. B., Vaillancourt R., Valeri C. R., Deykin D. Arachidonic acid metabolism by platelets of differing size. Br J Haematol. 1983 Mar;53(3):503–511. doi: 10.1111/j.1365-2141.1983.tb02052.x. [DOI] [PubMed] [Google Scholar]
- Livio M., Benigni A., Remuzzi G. Coagulation abnormalities in uremia. Semin Nephrol. 1985 Jun;5(2):82–90. [PubMed] [Google Scholar]
- Martin J. F., Bath P. M., Burr M. L. Influence of platelet size on outcome after myocardial infarction. Lancet. 1991 Dec 7;338(8780):1409–1411. doi: 10.1016/0140-6736(91)92719-i. [DOI] [PubMed] [Google Scholar]
- Martin J. F., Slater D. N., Trowbridge E. A. Evidence that platelets are produced in the pulmonary circulation by a physical process. Prog Clin Biol Res. 1986;215:405–416. [PubMed] [Google Scholar]
- Martin J. F., Trowbridge E. A., Salmon G., Plumb J. The biological significance of platelet volume: its relationship to bleeding time, platelet thromboxane B2 production and megakaryocyte nuclear DNA concentration. Thromb Res. 1983 Dec 1;32(5):443–460. doi: 10.1016/0049-3848(83)90255-4. [DOI] [PubMed] [Google Scholar]
- Moia M., Mannucci P. M., Vizzotto L., Casati S., Cattaneo M., Ponticelli C. Improvement in the haemostatic defect of uraemia after treatment with recombinant human erythropoietin. Lancet. 1987 Nov 28;2(8570):1227–1229. doi: 10.1016/s0140-6736(87)91849-6. [DOI] [PubMed] [Google Scholar]
- Taylor J. E., Henderson I. S., Stewart W. K., Belch J. J. Erythropoietin and spontaneous platelet aggregation in haemodialysis patients. Lancet. 1991 Nov 30;338(8779):1361–1362. doi: 10.1016/0140-6736(91)92239-x. [DOI] [PubMed] [Google Scholar]
- Teramura M., Kobayashi S., Hoshino S., Oshimi K., Mizoguchi H. Interleukin-11 enhances human megakaryocytopoiesis in vitro. Blood. 1992 Jan 15;79(2):327–331. [PubMed] [Google Scholar]
- Thompson C. B., Love D. G., Quinn P. G., Valeri C. R. Platelet size does not correlate with platelet age. Blood. 1983 Aug;62(2):487–494. [PubMed] [Google Scholar]
- Williams N., Eger R. R., Jackson H. M., Nelson D. J. Two-factor requirement for murine megakaryocyte colony formation. J Cell Physiol. 1982 Jan;110(1):101–104. doi: 10.1002/jcp.1041100116. [DOI] [PubMed] [Google Scholar]
- Williams N., Jackson H., Walker F., Oon S. H. Multiple levels of regulation of megakaryocytopoiesis. Blood Cells. 1989;15(1):123–133. [PubMed] [Google Scholar]



