Abstract
The leaves of Artemisia argyi Lev. et Vant. and A. princeps Pamp. are well known medicinal herbs used to treat patients in China, Japan, and Korea with skin problems such as eczema and itching, as well as abdominal pain and dysmenorrhoea. We investigated the anti-inflammatory effects of Artemisia leaf extract (ALE) using CD mice and Raw 264.7 cells. The effects of ALE on histopathological changes and cytokine production in ear tissues were assessed in mice with CD induced by 1-fluoro-2,4-dinitrobenzene (DNFB). Moreover, the anti-inflammatory effects on production levels of prostaglandin E2 (PGE2) and nitric oxide (NO) and expression levels of cyclooxygenase 2 (COX-2) and inducible nitric oxide synthase (iNOS) were investigated in Raw 264.7 cells. Topical application of ALE effectively prevented ear swelling induced by repeated DNFB application. ALE prevented epidermal hyperplasia and infiltration of immune cells and lowered the production of interferon- (IFN-) gamma (γ), tumour necrosis factor- (TNF-) alpha (α), and interleukin- (IL-) 6 in inflamed tissues. In addition, ALE inhibited expression of COX-2 and iNOS and production of NO and PGE2 in Raw 264.7 cells. These results indicate that Artemisia leaf can be used as a therapeutic agent for inflammatory skin diseases and that its anti-inflammatory effects are closely related to the inhibition of inflammatory mediator release from macrophages and inflammatory cytokine production in inflamed tissues.
1. Introduction
Contact dermatitis (CD) in the framework of occupational diseases remains prevalent among workers worldwide, impacting their quality of life and workability. The employees most affected by CD are hairdressers, healthcare workers, and metal workers [1] because they are continuously exposed to harmful environments when working. As a result, employees with CD tend to use anti-inflammatory and immunomodulatory agents such as corticosteroids repeatedly [2]. Corticosteroids are effective and powerful agents for CD, but their doses should be restricted because of their adverse side effects. Herbal medicines have recently emerged in the framework of complementary and alternative medicines (CAM) for corticosteroids because they have relatively lower cost and safety [3].
Herbs belonging to the Artemisia genus are widely used as medicine worldwide. The leaves of Artemisia argyi Lev. et Vant. and A. princeps Pamp. are frequently used as traditional or folk medicines for patients with abdominal pain, dysmenorrhoea, uterine haemorrhage, and inflammation in China, Japan, and Korea [4]. A. argyi, Chinese mugwort, is herbaceous perennial plant known in Japanese as gaiyou and in Chinese as aiye. A. princeps, Japanese mugwort, is a perennial and very vigorous plant known as yomogi in Japanese. Recently, the leaves of A. argyi and A. princeps and their components have been shown to have antitumour [5–8], antifungal [9], anticoagulant [10], antidiabetic [11], and anti-inflammatory [12, 13] activity.
Based on these findings, we examined the effects of Artemisia leaf extract (ALE) on inflamed tissues in mice with CD and anti-inflammatory activities in Raw 264.7 cells. Specifically, the effects of ALE on histopathological changes including ear swelling, epidermal hyperplasia, immune cell infiltration, and cytokine production such as interferon- (IFN-) gamma (γ), tumour necrosis factor- (TNF-) alpha (α), interleukin- (IL-) 6, and IL-10 in ear tissues were assessed in mice with CD induced by topical application of 1-fluoro-2,4-dinitrobenzene (DNFB). The anti-inflammatory effects on production levels of prostaglandin E2 (PGE2) and nitric oxide (NO) and the expression levels of cyclooxygenase 2 (COX-2) and inducible nitric oxide synthase (iNOS) were investigated in Raw 264.7 cells.
2. Materials and Methods
2.1. Preparation of ALE
Artemisia leaf was purchased from Gwangmyungdang (Ulsan, Korea). The Artemisia leaf consisted of a mixture of A. argyi and A. princeps and was authenticated by Professor Jung-Hoon Kim, one of the authors of this study. Twenty grams of Artemisia leaf was immersed in 500 mL of methyl alcohol and sonicated for 15 min, after which they were extracted for 24 h. Following extraction, the supernatant was transferred and the Artemisia leaf was again extracted with 500 mL of methanol for 24 h. The two extracts were then combined and filtered through Whatman no. 20 filter paper, after which they were condensed using a rotary evaporator (EYELA, Tokyo, Japan). The evaporated extract was subsequently dried using a lyophilizer (Labconco, Kansas City, MO, USA), which yielded 1.04 g of freeze-dried powder (yield, 5.21%). Specimens of crude material and Artemisia leaf methanol extract (ALE, Voucher no. MH2013-040) were deposited in the herbarium located in the School of Korean Medicine, Pusan National University.
2.2. Animals
Six-week-old male Balb/c mice were obtained from Samtako (Incheon, Korea). All mice used in this experiment were housed in the cages under specific conditions, including a 12 h light/dark cycle and specific pathogen-free conditions. In addition, mice were provided with free access to standard rodent feed and water. We conducted all animal experiments according to institutional guidelines and all experimental procedures were approved by our animal care committee (PNU-2012-0140).
2.3. CD Induction and Experimental Schedule
CD was induced using our standard method as previously described [14]. Briefly, 0.1% DNFB (50 μL) in vehicle composed of acetone and olive oil (4 : 1, AOO) was applied onto the shaved back of mice for three successive days (sensitization). Mice were then treated by application of 0.2% DNFB (30 μL) in vehicle onto the backside of their ears every two days. For topical treatment with drugs, dexamethasone (DEX) and ALE were dissolved in ethanol, filtered using a syringe filter (0.45 μm), and finally diluted in vehicle (AOO : ethanol, 4 : 1). ALE (30 or 300 μg/ear) was topically applied onto the backside of ears for 7 days. A total of 36 mice were used in this study (NOR group and DEX group, 6 mice; CTL group and ALE group, 8 mice). The experimental procedures are summarized in Figure 1.
Figure 1.
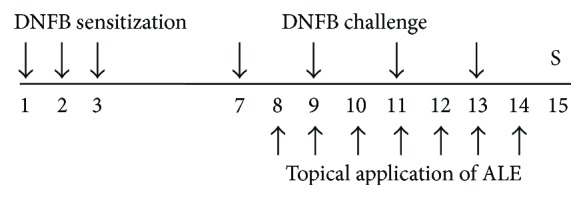
Experimental schedule. Mice in all experimental groups were sensitized by DNFB for three successive days and then challenged every other day (four times). The ALE group was topically treated with 30 or 300 μg/ear of ALE (n = 8). The DEX group was topically treated with 75 μg/ear of DEX for seven days from day 8 to day 14 (n = 6). All animals were sacrificed on day 15. S indicates sacrifice.
2.4. Effects on Ear Thickness and Weight
Mice were sacrificed with CO2, after which ear pieces (5 mm in diameter) obtained via dermal punch were weighed using a microbalance and the thicknesses of both ears were measured with digimatic calipers (Mitutoyo, Kanagawa, Japan) at the same time.
2.5. Tissue Preparation and Staining
Obtained tissues were fixed in 4% formalin for 24 h and then dehydrated using ethyl alcohol. Next, all tissues were soaked in xylene and finally embedded in paraffin. Ear tissues (4 μm) were subsequently resected, after which sections were stained with haematoxylin-eosin (H/E) and observed using a light microscope (50x).
2.6. Evaluation of Hyperplasia in the Epidermis and Infiltration of Immune Cells
To evaluate hyperplasia in the epidermis and infiltration of immune cells, five nonoverlapping fields per slide were randomly selected and captured with a light microscope. The height from the basal lamina to the top of the stratum granulosum was quantified to evaluate the epidermal thickness. Five lengths were used to calculate the mean epidermal thicknesses of each tissue slide. Total immune cell numbers were quantified by counting immune cells in the same size counting grid.
2.7. Measurement of Cytokine Production
Cytokine levels in ear tissues were evaluated using a mouse inflammation cytometric bead array (CBA) kit (BD Biosciences, San Jose, CA, USA). Briefly, to obtain tissue lysates, resected inflamed tissues were lysed using protein extraction solution (Intron Bio, Daejeon, Korea) and a homogenizer (Next Advance, NY, USA). Next, 50 μg of lysates was used to evaluate the levels of TNF-α, IFN-γ, IL-6, and IL-10.
2.8. Cell Culture
Raw 264.7 cells, the immortalized murine macrophage cell line, were cultured using DMEM (HyClone, Logan, UT, USA) containing foetal bovine serum (10%, FBS) and antibiotics (1%, penicillin-streptomycin). Cells were maintained at 37°C under 5% CO2.
2.9. Determination of Nitric Oxide (NO) Production
Cells were seeded in 96-well plates at a density of 1 × 104 cells/well and then incubated overnight. Next, cells were treated with the indicated concentrations of ALE for 4 h, after which they were stimulated with 1 μg/mL of lipopolysaccharide (LPS) for 20 h. Following stimulation, 100 μL of supernatants was mixed with 100 μL of Griess Reagent (2% sulphanilamide in 10% H3PO4 and 0.2% of N-(1-naphthyl)ethylenediamine in distilled water) and then incubated at room temperature for 10 min. The absorbance at 540 nm was subsequently measured using a spectrophotometer (TECAN, Männedorf, Switzerland). The production levels of NO were determined using a NaNO2 serial dilution standard curve.
2.10. Measurement of Prostaglandin E2 (PGE2) Production
The production of PGE2 was measured by enzyme-linked immunosorbent assay (ELISA) using PGE2 ELISA assay kit (Enzo Life Science, Farmingdale, NY, USA). Briefly, cells were treated as previously described. The culture supernatants were then collected, after which the optical densities were measured using a spectrophotometer (TECAN, Männedorf, Switzerland) at a wavelength of 405 nm.
2.11. Western Blotting
The changes in protein expression following treatment were evaluated by western blot analysis. Briefly, ALE and LPS treated Raw 264.7 cells were harvested, lysed, and homogenized as previously described. The protein concentration of each cell lysate was then calculated using a bicinchoninic acid (BCA) assay. Primary antibodies against iNOS (482728, Merck Millipore, Darmstadt, Germany, 1:1000), COX-2 (sc-19999, Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1:1000), β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and horseradish-conjugated secondary antibody (Enzo Life Sciences, Farmingdale, NY, USA, 1:3000) were used to detect specific protein levels. In addition, a West-Q Chemiluminescent Substrate Kit (GenDEPOT, Barker, TX, USA) and a LAS 4000 mini (GE Healthcare, Piscataway, NJ, USA) were used to visualize the antigen-antibody complex.
2.12. Statistical Analysis
The Mann-Whitney U test was used for data obtained from in vivo experiments; Student's t-test was used for data obtained from in vitro experiments, and Prism 5 for window version 5.01 (GraphPad Software Inc., CA, USA) was used for all analyses. All data are presented as the means ± standard deviation. A P < 0.05 was considered significant.
3. Results
3.1. ALE Prevented Ear Swelling Induced by Repeated DNFB Application
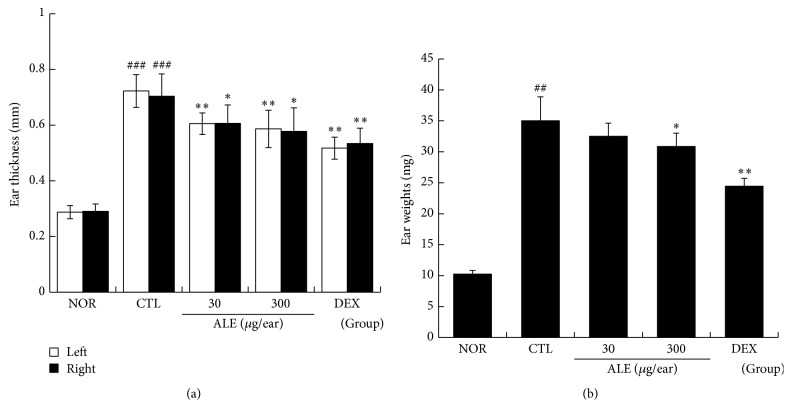
At the end of experiment, the thicknesses and weights of both ears were evaluated. In CTL group, repeated treatment with DNFB elevated the levels of ear thickness and weight more than three times relative to the normal group. 30 and 300 μg/ear of ALE treatment effectively inhibited enlargement of ear thickness (Figure 2(a)) and 300 μg/ear of ALE inhibited ear weight gain significantly (Figure 2(b)). DEX was more effective than ALE (Figure 2).
Figure 2.
Effect of ALE on ear swelling in CD mice. The ear thickness and weight were measured on day 15. NOR: nontreated normal; CTL: nontreated CD; 30 or 300, 30, or 300 μg/ear of ALE; DEX: 75 μg/ear of DEX. (a) Ear thickness. (b) Ear weight. Values are presented as the means ± SD. ## P < 0.01 and ### P < 0.001 compared to the NOR group and ∗ P < 0.05 and ∗∗ P < 0.01 compared to the CTL group.
3.2. ALE Prevented Epidermal Hyperplasia and Infiltration of Immune Cells in Inflamed Tissues
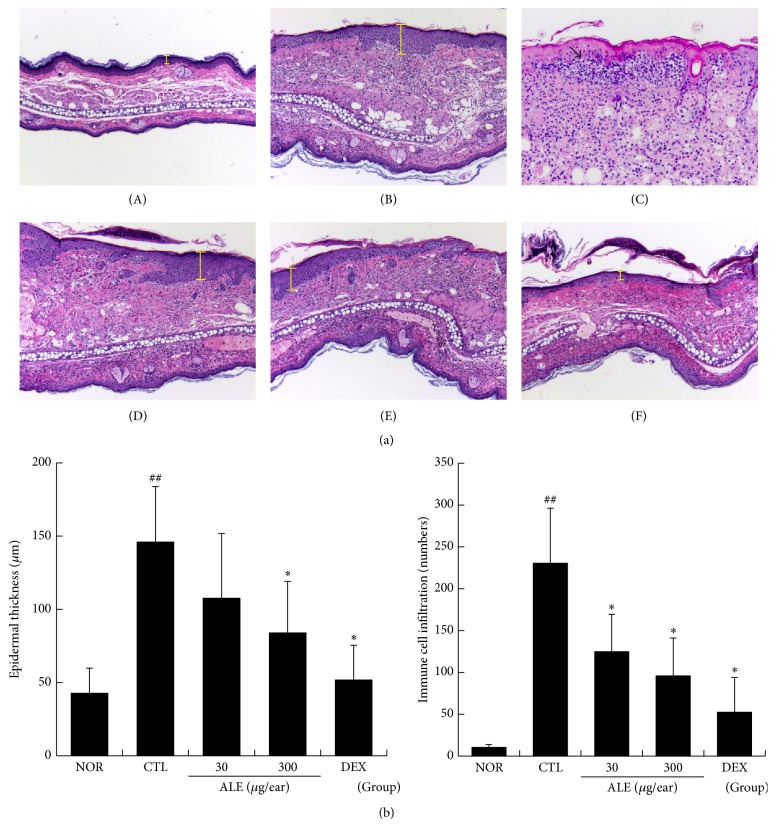
The effects of ALE on epidermal hyperplasia, one of the major features of CD, and immune cell infiltration were investigated. Marked increases in epidermal thickness (yellow bars) and infiltration of immune cells into inflamed tissues were observed in the nontreated CD mice. The main populations of infiltrated immune cells were neutrophil and macrophage. In addition, a large pustule (filled arrow) and vesicles were also observed (Figure 3(a)). Treatment with ALE effectively inhibited epidermal hyperplasia and reduced immune cell infiltration compared to the CTL mice (Figure 3(b)). DEX treatment was most effective among all experimental groups (Figure 3).
Figure 3.
Effect of ALE on epidermal hyperplasia and infiltration of immune cells in CD mice. Skin tissue was observed under a light microscope. (A) NOR; ((B) and (C)) CTL; (D) 30 μg/ear of ALE; (E) 300 μg/ear of ALE; (F) 75 μg/ear of DEX. Magnification, 50x. Yellow bars indicate the epidermis. Filled arrows indicate pustule areas (a). The epidermal thickness (A) and infiltration of immune cells (B) were evaluated using a quantitative method. Abbreviations are the same as in Figure 2. (b). Values are presented as the means ± SD. ## P < 0.01 compared to the NOR group and ∗ P < 0.05 compared to the CTL group.
3.3. ALE Lowered the Production Levels of TNF-α, IFN-γ, and IL-6 in Inflamed Tissues
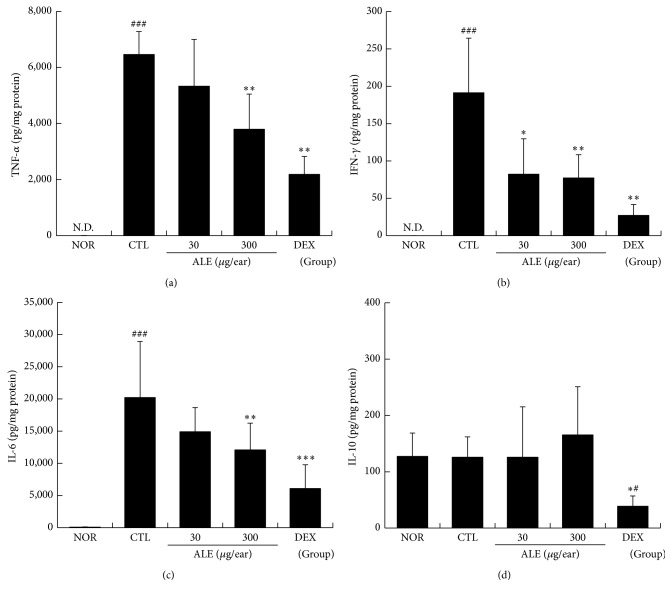
We also checked the effects of ALE on cytokine productions in inflamed tissues. In our results, elevated levels of TNF-α, IFN-γ, and IL-6 production were observed in CTL mice (Figure 4). These increases in inflammatory cytokines were significantly prevented by topical application of ALE. Treatment with 300 μg/ear of ALE significantly lowered the production levels of TNF-α, IFN-γ, and IL-6, respectively. The IL-10 production levels were not affected by induction of CD or treatment with ALE, and only the DEX treated group showed lower production of IL-10 than the normal and control groups (Figure 4).
Figure 4.
Effect of ALE on production levels of cytokines in inflamed tissues. The cytokine levels in inflamed tissues were evaluated. (a) TNF-α; (b) IFN-γ; (c) IL-6; (d) IL-10. N.D., not detected. Values are presented as the means ± SD. # P < 0.05 and ### P < 0.001 compared to the NOR group and ∗ P < 0.05, ∗∗ P < 0.01, and ∗∗∗ P < 0.001 compared to the CTL group.
3.4. ALE Inhibited iNOS Expression and NO Production in Raw 264.7 Cells
The inhibitory effects of ALE on iNOS expression induced by LPS were investigated. Expression of iNOS was elevated in the LPS treated CTL group compared to the normal group. Treatment with more than 25 μg/mL of ALE lowered iNOS expression in a concentration dependent manner (Figure 5(a)). In addition, treatment with LPS elevated NO production level more than nine times relative to neither LPS nor ALE treated group. This increase in No production was effectively inhibited by ALE in a dose dependent fashion (Figure 5(b)).
Figure 5.
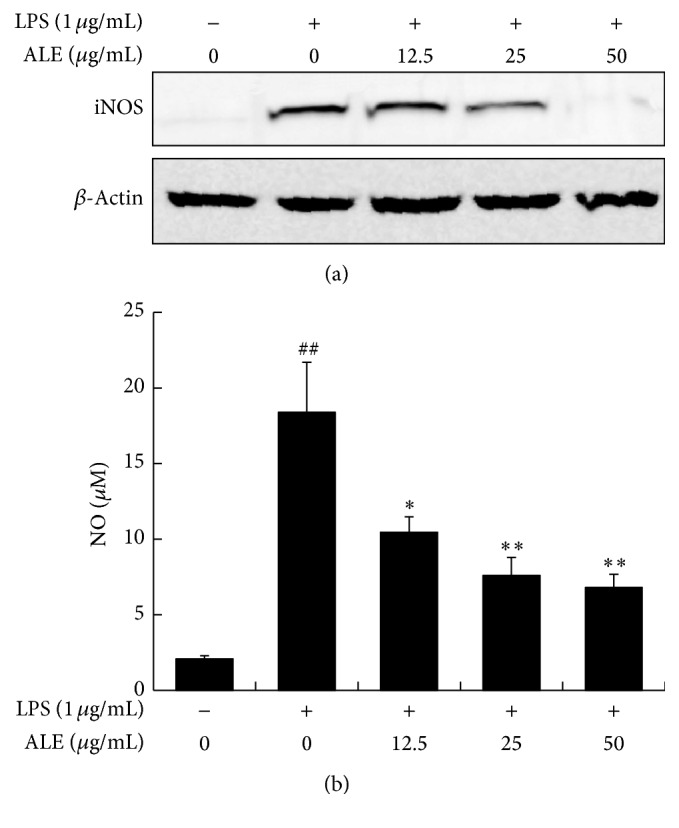
Effects of ALE on iNOS expression and NO production in Raw 264.7 cells. Cells were incubated with various concentrations of ALE for 4 h and then activated with 1 μg/mL of LPS for 20 h. (a) iNOS expression; (b) NO production. Values are presented as the means ± SD. ## P < 0.01 compared to the NOR group and ∗ P < 0.05 and ∗∗ P < 0.01 compared to the CTL group.
3.5. ALE Inhibited COX-2 Expression and PGE2 Production in Raw 264.7 Cells
The LPS stimulated Raw 264.7 cells showed marked increases in COX-2, representative COX enzyme, and treatment with ALE inhibited COX-2 expression (Figure 6(a)). In addition, the production of PGE2, one of the major final metabolites, was markedly induced by LPS stimulation. Treatment with 50 mg/mL of ALE inhibited PGE2 production significantly (Figure 6(b)).
Figure 6.
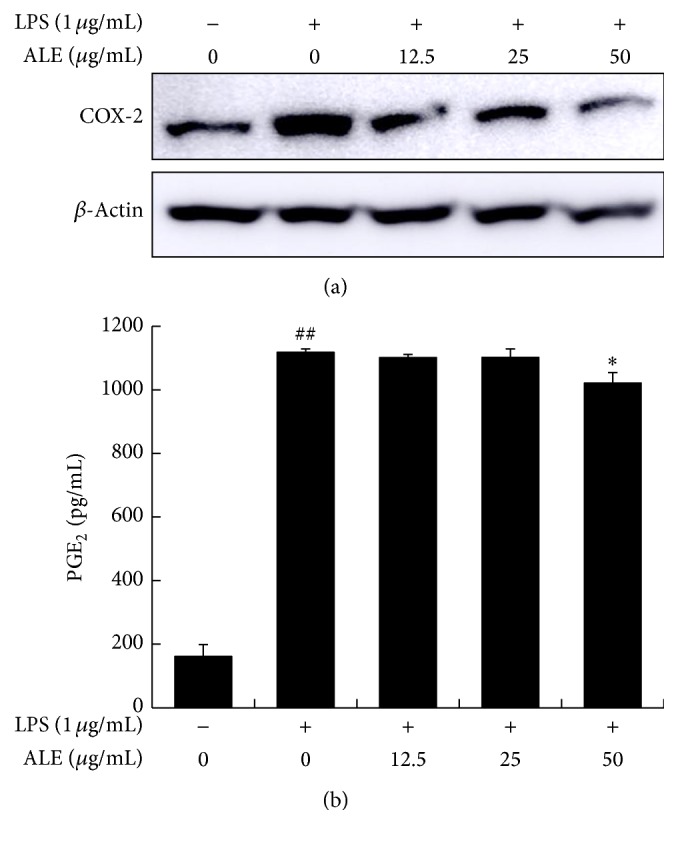
Effects of ALE on COX-2 expression and PGE2 production in Raw 264.7 cells. Cells were treated with various concentrations of ALE for 4 h and then activated with 1 μg/mL of LPS for 20 h. The COX-2 expression levels were then detected by western blot analysis (a), while PGE2 production was measured by ELISA. Values represented are the means ± SD. ## P < 0.01 compared to the NOR group and ∗ P < 0.05 compared to the CTL group (b).
4. Discussion
Artemisia species have traditionally been utilized for amelioration of diseases including malaria, hepatitis, parasites, and various cancers worldwide. The anti-inflammatory effects of A. princeps in antigen-stimulated T cells and regulatory T cells have been reported by Chang et al. [15]. Additionally, the major active components of Artemisia species are sesquiterpenoids and flavonoids such as eupatilin and jaceosidin, which are extracted from most herbs in Artemisia species, and these components have been reported to have anti-inflammatory effects in mice [16]. Based on the anti-inflammatory and immunomodulatory effects of Artemisia species, we investigated whether ALE can reduce inflammatory reactions in an animal model of CD to ameliorate its symptoms.
The skin of CD patients tends to thicken because of chronic and repeated inflammatory reactions, which are closely related to epidermal and dermal hyperplasia [17]. In our experiment, repeated application of DNFB induced ear swelling, which was effectively inhibited by topical application of ALE (Figure 2). Given that the degree of ear swelling is recognized as an index of inflammatory reaction, these results imply that ALE can exert anti-inflammatory action in animal models of CD.
In our animal model of CD, repeated application of DNFB induced hyperplasia in the epidermis and massive infiltration of immune cells to the epidermis and into the connective tissues. In addition, spongiotic changes, vesicles, and pustules were seen in the control group (Figure 3(a)(B)). Topical application of ALE effectively inhibited hyperplasia in the epidermis and infiltration of immune cells. In addition, the area of spongiotic changes and vesicles was diminished and pustules were rarely seen in the ALE and DEX group (Figure 3). These findings indicate that ALE can act as an anti-inflammatory agent in CD to reduce immune cell infiltration, resulting in inhibition of spongiotic changes, as well as pustule and vesicle formation.
TNF-α and IFN-γ, hallmarks of Th1 skewing reaction, can stimulate keratinocytes, which subsequently proliferate, resulting in epidermal hyperplasia [18]. These two cytokines are also closely related to infiltration of immune cells into the inflamed tissues. In the inflammatory cascade of CD, activated keratinocytes can release TNF-α and IL-6, which are growth promoting cytokines, resulting in accelerated immune cell infiltration and prolonged lifespan of immune cells [18]. IL-6 has also been reported to accelerate proliferation and migration of keratinocytes, leading to skin disorders, including psoriasis [19]. In this study, topical treatment with ALE effectively reduced production levels of TNF-α, IFN-γ, and IL-6 in ear tissues (Figure 4). When combined with previous results, these findings imply that ALE can inhibit hyperplasia in the epidermis and infiltration of immune cells via regulation of proinflammatory cytokines, finally leading to inhibition of enlargement of skin thickness.
Histopathological analyses revealed that many types of inflammatory cells, including macrophages, infiltrate into the dermis and epidermis in CD. Macrophages also play an important role by releasing inflammatory mediators such as NO, prostaglandins, and cytokines during initiation of CD. For these reasons, we also investigated anti-inflammatory effects of ALE in vitro using a macrophage cell line, Raw 264.7. Treatment with up to 100 μg/mL of ALE had no effect on the viability of Raw 264.7 cells (data not shown). ALE significantly inhibited iNOS expression and NO production in a dose dependent manner (Figure 5). In addition, ALE inhibited COX-2 expression and PGE2 production induced by LPS stimulation (Figure 6). These findings indicate that ALE inhibits inflammatory response in macrophages via regulation of NO and PGE2 production. As shown in Figures 5 and 6, ALE strongly inhibited iNOS expression and NO production, while it had little effect on PGE2 production or COX-2 expression. This difference in efficacy may indicate that ALE can affect other subtypes of prostaglandin.
It is well known that intracellular signaling pathways such as Erk, JNK, and p38 and transcription factors such as CREB and NF-κB play a central role in activation and inflammatory mediator production in macrophages [20]. We checked the effects of ALE on expression levels of signaling pathways and the NF-κB pathway. Treatment with ALE prevented phosphorylation of p38 but had no effect on the NF-κB pathway (data not shown). Considering these results, the anti-inflammatory effects of ALE may be closely related to the p38 signaling pathway, and the NF-κB pathway may not participate in the anti-inflammatory mechanisms of ALE.
Kim et al. reported immune-stimulatory effects of hot water extracts of the leaves of A. princeps [21]. Specifically, they found that extracts of A. princeps elevated production levels of NO and TNF-α, which is inconsistent with our results. These differences may be attributed to different extraction methods. Many previous studies of A. princeps have shown anti-inflammatory or antiallergic effects, as well as antioxidative effects. In addition, components isolated from A. princeps such as eupatilin and jaceosidin have been reported to have inhibitory effects against IgE-induced hypersensitivity and carrageenan-induced inflammation, respectively [16, 22]. However, Kim et al. did not describe why their findings were inconsistent with those of previous results. In addition, water extracts of plant materials should also be checked for microbial contamination before use because they are more easily contaminated than methanol or ethanol extracts.
Overall, the results of this study suggest that the anti-inflammatory effects of ALE in CD mice are closely related to regulation of the activation of immune cells, especially macrophages and cytokine production in inflamed tissues.
5. Conclusion
In this study, ALE inhibited the release of NO and PGE2 in macrophages. In addition, ALE effectively lowered the production levels of TNF-α, IFN-γ, and IL-6 in inflamed tissues. These anti-inflammatory actions prevented epidermal hyperplasia and immune cell infiltration. Finally, ALE effectively inhibited ear swelling induced by DNFB. These results imply that ALE can be used for the treatment of patients with CD as a complement and alternative medicine (CAM) to corticosteroids.
Acknowledgments
This research was supported by the National Research Foundation of Korea Grant funded by the Korean government (MSIP; Grant nos. 2015R1A2A2A04005619 and 2014R1A5A2009936).
Competing Interests
The authors declare that there is no conflict of interests regarding the publication of this article.
Authors' Contributions
Conception and study design were done by Hyungwoo Kim and Su-In Cho; funding was obtained by Hyungwoo Kim; data acquisition was done by Chanyong Yun, Youngchul Jung, Wonjoo Chun, Beodeul Yang, and Junghyun Ryu; data analysis and interpretation were done by Jung-Hoon Kim, Hyungwoo Kim, Chiyeon Lim, and Su-In Cho; statistical expertise was provided by Hyungwoo Kim, Chiyeon Lim, and Su-In Cho; Hyungwoo Kim and Su-In Cho provided administrative technical/logistic support and conducted data collection and assembly; critical revision of the article for important intellectual content was done by Hyungwoo Kim and Su-In Cho; all authors contributed to article drafting and approved final version. Chanyong Yun and Youngchul Jung equally contributed to this work.
References
- 1.Diepgen T. L., Kuss O., Blesius C. R., Schmidt A., Diepgen T. L. Occupational skin diseases in Northern Bavaria between 1990 and 1999: a population-based study. British Journal of Dermatology. 2001;145(3):453–462. doi: 10.1046/j.1365-2133.2001.04377.x. [DOI] [PubMed] [Google Scholar]
- 2.Cohen D. E., Heidary N. Treatment of irritant and allergic contact dermatitis. Dermatologic Therapy. 2004;17(4):334–340. doi: 10.1111/j.1396-0296.2004.04031.x. [DOI] [PubMed] [Google Scholar]
- 3.Wen M.-C., Wei C.-H., Hu Z.-Q., et al. Efficacy and tolerability of antiasthma herbal medicine intervention in adult patients with moderate-severe allergic asthma. The Journal of Allergy and Clinical Immunology. 2005;116(3):517–524. doi: 10.1016/j.jaci.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 4.Kim C. M., Shin M. G., An D. G., Lee K. S. The Encyclopedia of Oriental Herbal Medicine. Seoul, Republic of Korea: Jeongdam; 1997. [Google Scholar]
- 5.Bao X., Yuan H., Wang C., Liu J., Lan M. Antitumor and immunomodulatory activities of a polysaccharide from Artemisia argyi . Carbohydrate Polymers. 2013;98(1):1236–1243. doi: 10.1016/j.carbpol.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Choi E.-J., Kim G.-H. Antioxidant and anticancer activity of Artemisia princeps var. orientalis extract in HepG2 and Hep3B hepatocellular carcinoma cells. Chinese Journal of Cancer Research. 2013;25(5):536–543. doi: 10.3978/j.issn.1000-9604.2013.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan M., Yu B., Rasul A., et al. Jaceosidin induces apoptosis in U87 glioblastoma cells through G2/M phase arrest. Evidence-Based Complementary and Alternative Medicine. 2012;2012:12. doi: 10.1155/2012/703034.703034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J.-H., Jung S.-H., Yang Y.-I., et al. Artemisia leaf extract induces apoptosis in human endometriotic cells through regulation of the p38 and NFκB pathways. Journal of Ethnopharmacology. 2013;145(3):767–775. doi: 10.1016/j.jep.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Wenqiang G., Shufen L., Ruixiang Y., Yanfeng H. Comparison of composition and antifungal activity of Artemisia argyi Lévl. et Vant inflorescence essential oil extracted by hydrodistillation and supercritical carbon dioxide. Natural Product Research. 2006;20(11):992–998. doi: 10.1080/14786410600921599. [DOI] [PubMed] [Google Scholar]
- 10.Ryu R., Jung U. J., Kim H.-J., et al. Anticoagulant and antiplatelet activities of artemisia princeps Pampanini and its bioactive components. Preventive Nutrition and Food Science. 2013;18(3):181–187. doi: 10.3746/pnf.2013.18.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto N., Kanemoto Y., Ueda M., Kawasaki K., Fukuda I., Ashida H. Anti-obesity and anti-diabetic effects of ethanol extract of Artemisia princeps in C57BL/6 mice fed a high-fat diet. Food and Function. 2011;2(1):45–52. doi: 10.1039/c0fo00129e. [DOI] [PubMed] [Google Scholar]
- 12.Wang S., Li J., Sun J., et al. NO inhibitory guaianolide-derived terpenoids from Artemisia argyi . Fitoterapia. 2013;85(1):169–175. doi: 10.1016/j.fitote.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Chung K.-S., Choi H.-E., Shin J.-S., et al. Chemopreventive effects of standardized ethanol extract from the aerial parts of Artemisia princeps Pampanini cv. Sajabal via NF-κB inactivation on colitis-associated colon tumorigenesis in mice. Food and Chemical Toxicology. 2015;75:14–23. doi: 10.1016/j.fct.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Han H.-Y., Ryu M. H., Lee G., et al. Effects of Dictamnus dasycarpus Turcz., root bark on ICAM-1 expression and chemokine productions in vivo and vitro study. Journal of Ethnopharmacology. 2015;159:245–252. doi: 10.1016/j.jep.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Chang S. H., Jung E. J., Park Y. H., et al. Anti-inflammatory effects of Artemisia princeps in antigen-stimulated T cells and regulatory T cells. The Journal of Pharmacy and Pharmacology. 2009;61(8):1043–1050. doi: 10.1211/jpp/61.08.0008. [DOI] [PubMed] [Google Scholar]
- 16.Min S.-W., Kim N.-J., Baek N.-I., Kim D.-H. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps on carrageenan-induced inflammation in mice. Journal of Ethnopharmacology. 2009;125(3):497–500. doi: 10.1016/j.jep.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Serup J. Characterization of contact dermatitis and atopy using bioengineering techniques. A survey. Acta Dermato-Venereologica. 1992;177:14–25. [PubMed] [Google Scholar]
- 18.Corsini E., Galli C. L. Epidermal cytokines in experimental contact dermatitis. Toxicology. 2000;142(3):203–211. doi: 10.1016/S0300-483X(99)00145-6. [DOI] [PubMed] [Google Scholar]
- 19.Kawakami M., Kaneko N., Anada H., Terai C., Okada Y. Measurement of interleukin-6, interleukin-10, and tumor necrosis factor- alpha levels in tissues and plasma after thermal injury in mice. Surgery. 1997;121(4):440–448. doi: 10.1016/S0039-6060(97)90315-9. [DOI] [PubMed] [Google Scholar]
- 20.Bode J. G., Ehlting C., Häussinger D. The macrophage response towards LPS and its control through the p38 MAPK-STAT3 axis. Cellular Signalling. 2012;24(6):1185–1194. doi: 10.1016/j.cellsig.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Kim T.-H., Lee S.-J., Rim H.-K., et al. In vitro and in vivo immunostimulatory effects of hot water extracts from the leaves of Artemisia princeps Pampanini cv. Sajabal. Journal of Ethnopharmacology. 2013;149(1):254–262. doi: 10.1016/j.jep.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 22.Lee S. H., Bae E.-A., Park E.-K., et al. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps in IgE-induced hypersensitivity. International Immunopharmacology. 2007;7(13):1678–1684. doi: 10.1016/j.intimp.2007.08.028. [DOI] [PubMed] [Google Scholar]