Protocol 3
Reconstitution of ER InsP3R to BLM
General methods for making bilayers and for ion channel reconstitution to BLM has been extensively described in an excellent manual (Miller, 1986). Thus, here I focus on specfic details relevant for InsP3R recordings. The goal of the described procedures is to perform single channel recordings of native or recombinant InsP3Rs in BLM. Similar procedures are used to study native or recombinant RyanRs in BLM.
Materials
BLM recording setup as described in (Miller, 1986).
Tank with purified argon
Ice backet
Vortex
Lipid storage bottles
Glass rodes for painting bilayers. These are prepared by flaming 100 µl glass pipettes. Make the tip of the rod smooth to avoid making holes in the Teflon film
3.1 BLM chambers and Teflon film
Commercial cylindrical BLM chambers are available, such as BCH-13A chamber (1.2 ml solution volume) from Warner Instruments. In these chambers bilayer is formed on a cylindrical cup made of polystyrene, Delrin (acetyl resin) or polysulfone. The volume of solution in the chamber is 1.2 ml and the standard available aperture diameters are 150, 200 or 250 µm. The apertures machined by Warner. Custom aperture sizes from 50 µm to 1 mm can be custom ordered from Warner.
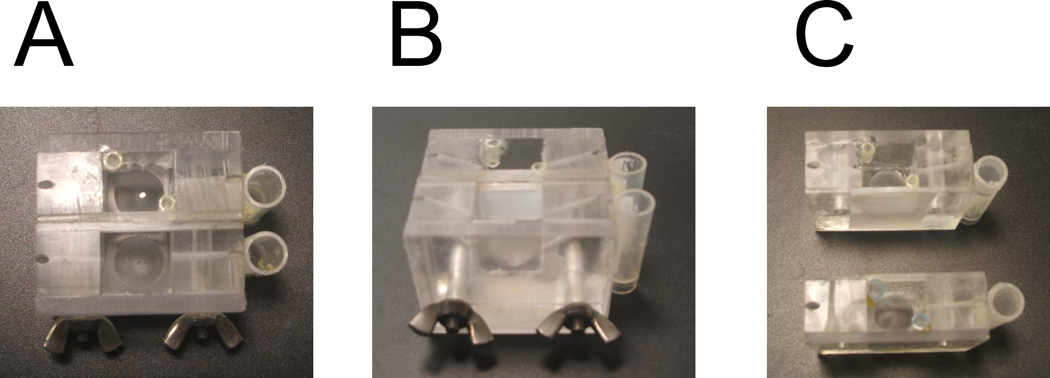
In our studies we use custom-made flat BLM chambers manufactured from Lucite (Fig 1). The reservoirs for electrodes are glued to the side of the chambers by hot glue. The piece of Teflon film of 0.005” thickness (from Small Parts Ins) is clamped between the chambers (Fig 1C). Vaseline is used to make sure that there is no solution leakage between Teflon film and the wall of Lucite chamber. The tight clamp is achieved by the screws holding the chambers together (Fig1 A, 1B). There is a indentation on the bottom of each chamber to place magnetic stir bar. The holes drilled in the side of the chamber enable agar bridge connections between the reservoirs for electrodes and solutions in the chamber. The chamber closest to experimentator is called cis and the opposite chamber is called trans.
Fig 1.
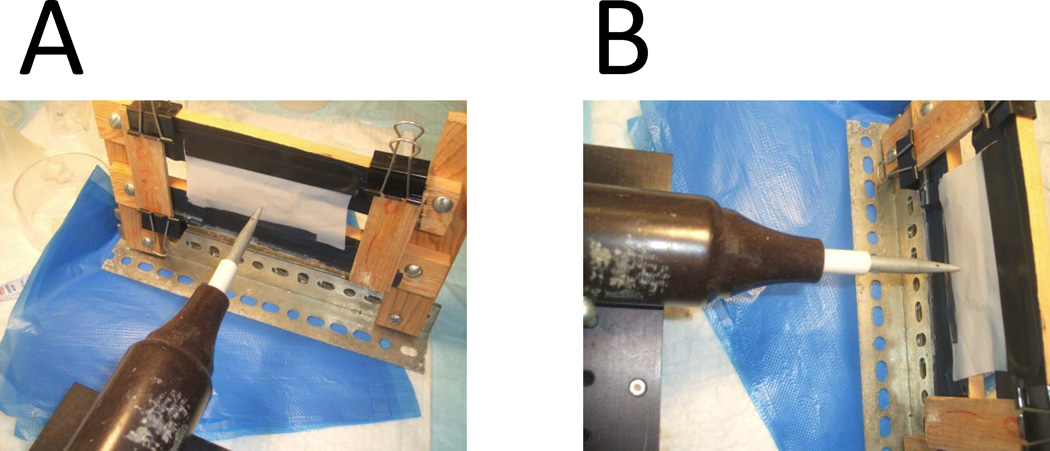
Small hole about 100–150 µm in diameter is made in Teflon film to support the BLM formation. Teflon film is obtained from Small Parts Ins. To make the hole in Teflon we use commercial High Frequency Generator Testers for Leak Detection to burn the hole in Teflon film. We use model BD-10A generator from Electro-Technic Products, Inc (4642 N. RAVENSWOOD, CHICAGO, ILLINOIS 60640-4510). Please see http://www.electrotechnicproduct.com/pinhole.html. The generator produces 10–50 kV discharge at frequency of approx. 0.5 megahertz at the tip of output electrode. To make a hole Teflon film is taped to the custom made frame. The electrode tip of BD-10A generator positioned at the distance of about 1 inch on one side of Teflon film (Fig 2). Thick cooper wire with sharp end is positioned on another side of the Teflon film at 0.5 inch distance (not shown). The cooper wire is connected with the ground in the electrical power socket. The BD-10A is powered on and slowly moved towards the Teflon film. At the distance of about 0.5 inch electrical disharge will occur between the tip of BD-10A electrode and the tip of the copper wire. This electrical discharge will burn a hole in Teflon film. Continious discharges will increase diamater of the hole until desired size is achieved.
Fig 2.
The initial dischange make a hole in Teflon in the random location, depends on a location of local defect in the structure of the film. After initial hole is made, the tip of the BD-10A generator and the tip of the cooper wire have to be repositioned to make sure that hole is located on the straight line connecting the tips. The shape and size of each hole need to be examined visually under the microscope. Most films will be discarded because holes are not round, too big, too small or multiple holes are made next to each other. Only films with a single round hole of approximately 100–150 µm in diameter need to be selected.
After film with the appropriate hole is selected, the Teflon is cut to size, covered with Vaseline and clamped between 2 parts of Lucite chamber (Fig 1C). Too much Vaseline results in Vaseline getting to the recording chambers, which is not acceptable. Too little Vaselien results in short-circuit between the chambers. Thus, amount of Vaseline used to cover the Teflon film need to be chosen carefully.
3.2 Lipids
The synthetic lipids for forming BLM are ordered from Avanti Polar Lipids (http://www.avantilipids.com/). The quality of lipids is extremely important. In our experiments we use Phosphatidylcholine (PC), Phosphatidylserine (PS), and Phosphatidylethanolamine (PE). Specifically, in our experiments we use
PC: 16:1 (Δ9-Cis) PC 1,2-dipalmitoleoyl-sn-glycero-3-phosphocholine, Avanti cat 850358
PS: 18:1 PS (DOPS) 1,2-dioleoyl-sn-glycero-3-phospho-L-serine (sodium salt), Avanti cat 840035
PE: 18:1 (Δ9-Cis) PE (DOPE) 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, Avanti cat 850725
Each of these lipids is ordered as 5 mg/ml fractions in chloroform aliquoted at 2 ml/vial and sealed vials shipped by Avanti Polar Lipids on dry ice. The lipids are stored at −80 C and new batch of lipids need to be ordered every 3–4 months.
A single vial with each lipid (PC, PE, PS) is warmed up to room temperature and the content of the vial transferred to dark glass bottle pre-filled with Argon gas. To minimize oxidation every effort needs to me made to avoid exposure of lipids to air or moister. These storage bottles are tightly closed and stored at −20. Every week new lipid vials from −80 freezer are opened and storage bottles from −20 freezer discarded.
3.3. BLM formation
Take PC, PE, PS working stocks from −20 C, warm up to room temperature (at least 20 min).
-
Open the vials, aliquote with glass micropipets to glass tubes pre-filled with argon.
Prepaint solution: 150 µl PC + 50 µl PS
Paint solution: 150 µl PE + 50 µl PS
Vortex 1 min, evaporate chloroform under slow Argon stream until dry film is formed (15 min), add 50 ul of n-decane, close with parafilm
Vortex extensively (3 min or more)
Top with Aron (10 sec), close with parafilm. Now Prepaint and Paint solutions are ready.
Prepaint the hole in Teflon on both sides with prepaint solution (large drops on both sides)
Wait until decan completely evaporates (at least 30 min) to allow formation of dry lipid film covering the hole in Teflon.
Fill cis and trans chambers with 3 ml of Cis and Trans solutions, put stirring bars and stir for 30 sec
Put 3 M KCl and insert agar bridges to electrode side chambers
-
Insert Ag/Cl electrodes connected to the BLM amplifier to the electrode side.
Important: Cis chamber is connected to a ground electrode and Trans chamber is connected to a signal electrode.
Under visual control (microscope) with glass rod remove excess prepaint lipids from the hole until it opens and the circuit is shorted.
Adjust junction potential on the amplifier.
With visual control start to paint over the hole with Paint solution using glass rod
Monitor formation of the BLM visually and by capacitance measurements on the BLM amplifier.
Capacitance of the BLM should be in the range 150–200 pF. If it is smaller than 100 pF it means that BLM was not formed and the hole is occluded by drop of lipids in decane. This should be mechanically removed and the painting procedure started over. If capacitance is more than 200 pF it means hole is too large and Teflon film needs to be replaced.
The BLM formed with this procedure much be stable for at least 1 hour. Should be no current activity and resistance of the BLM should be at least 10 GΩ. The capacitance of BLM should stay in the range above 100 pF. If BLM is unstable, breaks quickly, has low resistance or “channel-like” activity that will indicate problems with lipid stocks preparation. New lipids stocks need to be prepared (section 3.2) taking care to avoid exposure to air or moister.
3.4. InsP3R reconstitution
Take an aliquote of ER microsomes (from rat cerebellum – Protocol 1 or from Sf9 cells – protocol 2) from −80 C storage, put on ice.
Add to cis compartment 3 X 200 µl of 3 M KCl stock with constant stirring (~ 600 mM KCl final concentration). After each addition remove 200 µl from the cis compartment so volume stays fixed at 3 ml
Add to cis compartment 150 ul of 100 mM CaCl2 with stirring (~ 5 mM CaCl2 final concentration). Remove 150 µl from cis compartment so volume stays fixed at 3 ml
-
Add 2 µl microsomes in storage buffer directly to the BLM - no stirring.
Note: The density of microsomal storage buffer containing 10% sucrose should be exactly the same as the density of the buffer in cis chamber at this point. This can be confirmed by visual observation. “Milky cloud” of ER microsomes should stay close to the BLM. If “milky cloud” of ER microsomes sinks, it means density of storage buffer is too high. If “milky cloud” of ER microsomes floats up it means density of storage buffer is too low.
Wait 1 min to give ER microsomes chance to pre-fuse to the BLM.
Start stirring for 1 min
Add 200 ul 3M KCl to cis compartment with stirring (increasing final concentration to ~800 mM KCl). Remove 200 µl from the cis compartment so volume stays fixed at 3 ml. Increase in osmolarity on the cis side causes “prefused” microsomes to fuse with the BLM due to water flux.
Wait for fusion (2–5 min) - monitor current at 0 mV holding potential. The fusion should register as appearance of large potassium or chloride currents across the BLM. These currents are very large and can be easily detected by looking at current reading on the BLM amplifier.
If no fusion or insufficient fusion, break and reform BLM. Add new portion of microsomes directly to the newly formed BLM.
Repetitevly break and reform BLM with glass rod.
Repeat steps 9–10 until chloride current > 100 pA at 0 mV holding potential is recorded by the BLM amplifier. Note. Smaller chloride current or potassium current indicates insufficient fusion. From our experience large chloride currents are the best predictor for successful InsP3R recording.
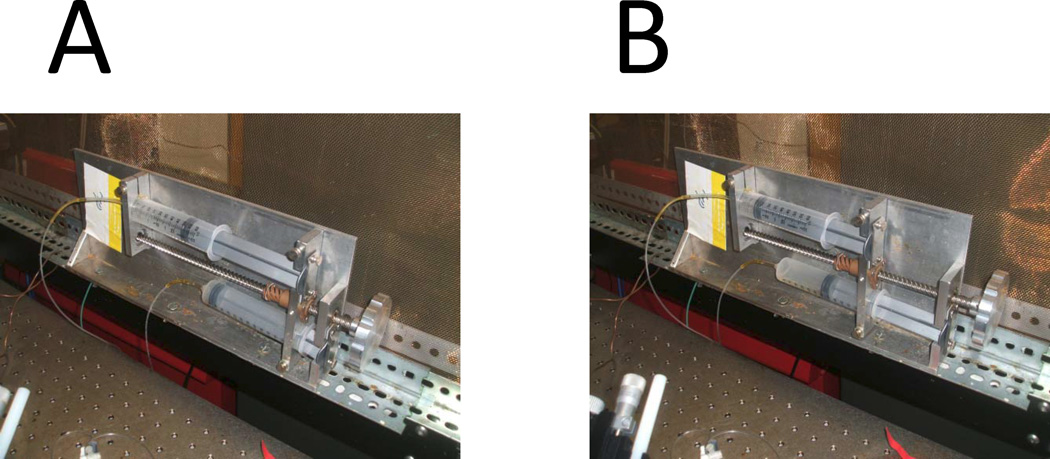
Perfuse very slowly cis chamber with 2 volumes of cis buffer (6 ml) without stirring using push-pull 2 syringe system (Fig 3). Lower density cis buffer is layered on the top, higher density cis+0.8 M KCl buffer is removed from the bottom. Note: Slow and careful perfusion is crucial. Perfusion that is too fast causes buffer mixing and insufficient KCl removal. Fluctuations of solution levels in the cis chamber during perfusion causes BLM to break. This step needs a lot of patience and practice.
Perfuse slowly cis chamber with 5 volumes of cis buffer (15 ml) with stirring using push-pull 2 syrnge system to completely remove remaining KCl.
Should see no current across the BLM at this point in experiments with Sf9 microsomes. In experiments with cerebellar microsomes may see RyanR activity. If baseline is noisy that indicates too much KCl left in the cis compartment and need to perfuse more.
Add 136 µl of Ca-EGTA solution to the cis compartment with stirring and remove 136 ul volume. This will result in final concentrations of 1 mM EGTA and 0.707 mM CaCl2, yielding pCa equal 6.7
If RyanR were active, they should close at this point.
Add 15 µl of 100 mM ATP to cis compartment with stirring, yielding final concentration 0.5 mM ATP
RyanR may get activated in BLM again at this point. Block RyanR activity by adding 6 µl of 1 mM ruthenium red stock (final concentration 2 µM) with stirring. Shold cause immediate and complete block of RyanR activity.
Add 6 µl of 1 mM InsP3 stock with stirring to cis compartment, resulting in final concentration of 2 µM InsP3.
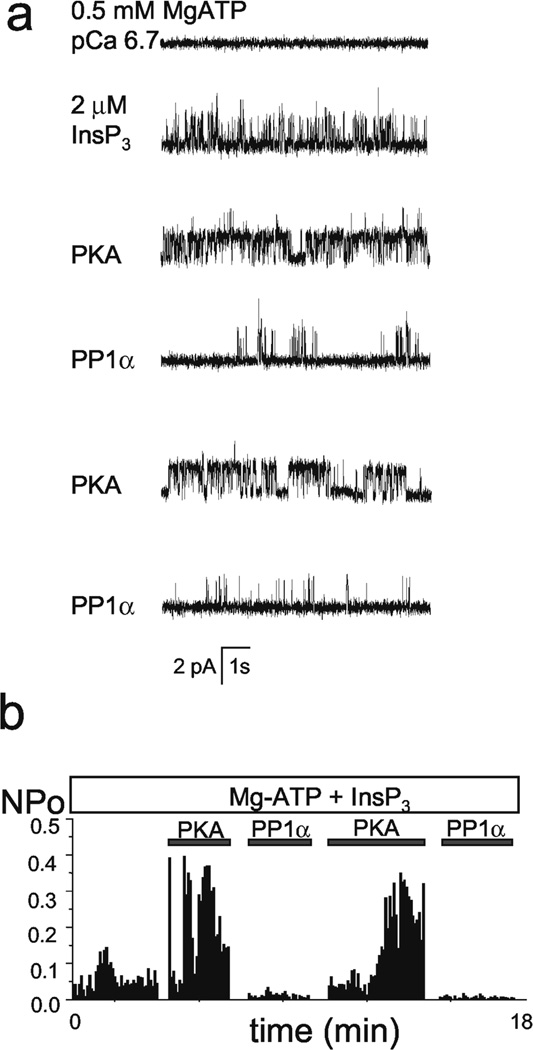
Observe activity of InsP3R in BLM. Record, analyse and study InsP3R properties. Example of BLM experiment used to study modulation of recombinant InsP3R1 by PKA and PP1α (Tang et al., 2003b) is shown on Fig 4.
To verify that recorded channels are indeed correspond to InsP3R, addition of 1 µM heparin solution to the cis chamber can be performed at the end of experiment. This should result in immediate and complete inhibition of InsP3R activity in BLM. Note: if RyanR were present in the BLM, addition of heparin may activate these channels, even in the presence of ruthenium red.
If no activity is observed in response to InsP3 addition then stop experiment, wash and rinse BLM chambers with water, dry with air and start over. Try not to get flustrated.
-
After several days of experiments the BLM chamber needs to be taken apart and Teflon film needs to be soaked in the mixture of chloroform and methanol (2:1) to remove all lipids and other organic material. After wash Teflon film can be dried out with the stream of Argon and reused again. Eventually Teflon fill will become deformed or develop cracks. At that point it needs to be replaced with a new film.
Note: quality of hole is very important. Treasure good films!
Fig 3.
Fig 4.
Buffer Recepies for BLM experiments
Trans Recording Solution (250 ml recepie). 53 mM Ba2+ and 250 mM HEPES, pH 7.35
Weight 4.19 g of Ba(OH)2 dry powder, yielding final concentration 53 mM Ba2+
Note: Ba(OH)2 is very hydroscopic, keep from excess exposure to air and replace with new lot periodically.
Add 14 g of HEPES dry powder.
Add water to yield 250 ml final volume
Stir until powder dissolves and measure pH.
Keep adding dry HEPES with stirring to yield pH 7.35
Record final amount of HEPES added. Final amount of HEPES should be in the range 14–16 g, yielding final HEPES concentration 25–260 mM
Filter solution to remove precipitate.
Cis Recording Solution (2 liters recepie) 250 mM HEPES and 110 mM Tris, pH 7.35
Take amount of HEPES used to make 250 ml Trans recording colution and multiple by 8.
Weight out exactly that amount of dry HEPES powder.
Take 30 g of highest purity Tris base and mix with dry HEPES powder
Add water to 2 liter, stir
Keep adding dry Tris with stirring to yield pH 7.35
Ca-EGTA solution
10.6 ml of 20 mM CaCl2 stock
3.0 ml of 100 mM EGTA stock, pH 7.35
Mix, aliquote and store frozen.
Protocol References
- Miller C, editor. Ion Channel Reconstitution. New York: Plenum Press; 1986. [Google Scholar]
- Tang TS, Tu H, Wang Z, Bezprozvanny I. Modulation of type 1 inositol (1,4,5)-trisphosphate receptor function by protein kinase A and protein phosphatase 1alpha. J Neurosci. 2003b;23:403–415. doi: 10.1523/JNEUROSCI.23-02-00403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]