Abstract
Root morphological/physiological modifications are important for phosphorus (P) acquisition of plants under P deficiency, but strategies differ among plant species. Detailed studies on the response of maize roots to P deficiency are limited. Nitrogen (N) form influences root morphology/physiology, and thus may influence root responses to P deficiency. This work investigated adaptive mechanisms of maize roots to low P by comparison with white lupin and faba bean supplied with two N forms. Plants were grown for 7–16 days in hydroponics with sufficient (250 µmol L−1) and deficient P supply (1 µmol L−1) under supply of NH4NO3 or Ca(NO3)2. Plant growth and P uptake were measured, and release of protons and organic acid anions, and acid phosphatase activity in the root were monitored. The results showed that P deficiency significantly decreased shoot growth while increased root growth and total root length of maize and faba bean, but not white lupin. It enhanced the release of protons and organic acid anions, and acid phosphatase activity, from the roots of both legumes but not maize. Compared with Ca(NO3)2, NH4NO3 dramatically increased proton release by roots but did not alter root morphology or physiology of the three species in response to low P. It is concluded that the N form did not fundamentally change root morphological/physiological responses of the three species to P deficiency. Morphological variation in maize and morpho-physiological modifications in white lupin and faba bean were the main adaptive strategies to P deficiency.
Keywords: Low P availability, organic acids, rhizosphere, root exudation, root morphology, species variation
Introduction
Low phosphorus (P) availability in the soil is one of the most limiting factors for crop production (Schachtman et al. 1998; Lynch 2007). Plants have evolved different mechanisms in roots in order to increase P acquisition under P-limiting conditions. These mechanisms include morphological modifications, mycorrhizal symbioses, rhizosphere acidification, release of carboxylates and phosphatases, and up-regulation of P transporters (Neumann and Römheld 1999; Raghothama 1999; Hinsinger 2001; Lambers et al. 2006). Root morphological variations are beneficial for labile P acquisition since P is relatively immobile in soil, and enhanced root growth increases exploration of localized P sources (Barber 1995); while root physiological modifications can increase mobilization of non-labile P in soil (Neumann and Römheld 1999; Hinsinger 2001).
Although the adaptive mechanisms of plants to P deficiency are well understood, relatively few studies have addressed the differences in root responses to P deficiency among plant species. White lupin (Lupinus albus), a model plant to study root response to P deficiency, shows a superior ability to utilize sparingly soluble P and bound P from soil by developing proteoid roots that exude protons (Dinkelaker et al. 1989), citrate and acid phosphatases (APase) (Dinkelaker et al. 1989; Gerke et al. 1994). Although faba bean (Vicia faba) does not form proteoid roots under P deficiency, its extensive root system, a morphological advantage, enables exploration of a larger volume of soil to access inorganic P (Nuruzzaman et al. 2005a, b). It also releases large amounts of protons, phenolics and APase which enhance mobilization of soil non-labile P and hence P uptake (Nuruzzaman et al. 2005a; Li et al. 2007).
Maize is widely cultivated, for both staple food and industrial usage, in tropical and temperate soils worldwide (Calderón-Vázquez et al. 2011). Results of the adaptive responses in maize roots to P deficiency, however, are controversial. Maize roots always showed an extensive morphological variation when the roots had restricted growth (Yan et al. 2011) and grew in nitrate-rich patches (Yu et al. 2013) and under P deficiency (Anghinoni and Barber 1980; Zhang et al. 2012). Phosphorus deficiency has been shown to either increase (Gaume et al. 2001) or decrease (Liu et al. 2004; Corrales et al. 2007) the release of organic acid anions from the roots in solution culture. It enhanced (Kummerová 1986) or did not change (Corrales et al. 2007) the activity of APase on root surface. Furthermore, P deficiency did not change rhizosphere pH (Neumann and Römheld 1999; George et al. 2002), or resulted in a strong alkalization in the rhizosphere (Li et al. 2007).
Nitrogen form influences the balance of cation and anion uptake by plant roots, and thus alters the rhizosphere pH. The change of rhizosphere pH caused by differential uptake of cations and anions is more prominent than that caused by root exudates such as protons and organic acid anions (Jaillard et al. 2003; Tang and Rengel 2003; Marschner 2012). Ammonium nutrition leads to preferential cation uptake and thus net proton excretion by roots, causing rhizosphere acidification; whilst nitrate supply induces hydroxyl secretion and causes rhizosphere alkalization (Gahoonia and Nielsen 1992; Mengel et al. 2001; Tang et al. 2011). In legumes, on one hand, N2 fixation generally leads to rhizosphere acidification due to excess uptake of cations over anions (Tang et al. 1997; Hinsinger et al. 2003; Li et al. 2008). On the other hand, N source is one of the factors affecting P supply of plants. Ammonium supply often enhances plant uptake of P from soil via rhizosphere acidification (Miller 1974; Marschne 2012). A lower pH in the rhizosphere could increase the H2/ ratio and the solubility of Ca-phosphates. However, interactions of N form and P supply on rhizosphere pH are not fully understood. For example, P deficiency could decrease, increase or have no effect on rhizosphere pH in -fed plants (e.g. Dinkelaker et al. 1989; Neumann and Römheld 1999; Tang et al. 2009). These different responses to P supply and N form depend on plant species.
The aim of this study was to elucidate the adaptive strategies of maize roots in response to P deficiency, and to investigate the influence of N form on these responses, using white lupin and faba bean as reference species.
Methods
Plant materials and treatments
Two hydroponic experiments were conducted in the greenhouse under natural light with 28 °C/16 °C day/night temperatures, and 45–55 % relative humidity. Each experiment consisted of three factors which were P supply (LP, 1 µmol L−1 P and HP, 250 µmol L−1 P), N forms [NH4NO3 and Ca(NO3)2], and plant species [maize (Zea mays L. cv. Zhengdan 958), white lupin (Lupinus albus L. cv. Amiga), and faba bean (Vicia faba L. cv. Lincan No. 5)]. There were four replicates for each treatment.
Seeds of the three plant species were surface-sterilized in 10 % (v/v) H2O2 for 30 min, rinsed thoroughly in distilled water and then germinated between filter papers moistened with saturated CaSO4 solution in the dark. After a week, seven seedlings of each species were transferred into 7-L opaque pots (21 cm in diameter and 24 cm in height) filled with nutrient solution of the following composition (mol L−1): K2SO4 7.5 × 10−4, MgSO4 6.5 × 10−4, KCl 1 × 10−4, H3BO3 1.0 × 10−6, MnSO4 1.0 × 10−6, CuSO4 1.0 × 10−7, ZnSO4 1.0 × 10−6, (NH4)6Mo7O24 5.0 × 10−9 and Fe-EDTA 2.0 × 10−4 (Niu et al. 2007). Nitrogen was supplied as Ca(NO3)2 or NH4NO3 at the concentration of 2.0 × 10−3 mol L−1. Phosphorus was supplied as KH2PO4 at 1 or 250 µmol L−1, and KCl was added to the LP treatment to keep the same K concentration between the P treatments (Pang et al. 2010). The pH of nutrient solution was adjusted to 6.0 using 1 mol L−1 HCl or NaOH when the solution was renewed. The nutrient solution in each pot was continuously aerated and renewed every 3 days.
The first experiment was harvested 12 days after the commencement of treatments (DAT). Seven plants of each species in each pot were harvested as follows: (1) two plants were used to collect root exudates; (2) two plants were used to measure APase activity on root surface; (3) two plants were used to study rhizosphere acidification/alkalization using the agar technique; (4) one plant was used to measure rhizosphere pH using the scanning ion-selective electrode technique.
In the second experiment, nutrient solution pH was measured at 10:00 am daily during the experimental period using a pH meter (pH Testr30, USA). Plants were harvested 7, 12 and 16 days after the commencement of treatments. At each harvest, two roots of each species were kept at 4 °C for root length analysis. Five plants were separated into shoots and roots, and dried for dry weight and P measurements. In both experiments, no nodules were observed on roots of the two legumes, and no cluster roots were observed in white lupin at the last harvest (Wang et al. 2006).
Collection of root exudates and analysis of organic acids
Plant roots were carefully rinsed three times with deionized water and were then immersed in 100 mL collection solution in an opaque vessel and aerated for 2 h from 10:00 am to 12:00 noon for collection of root exudates. Collection of root exudates under non-sterile condition reflects the root exudates in nature (Shen 1998). The compositions of the collection solution were (μmol L−1): 200 MgCl2, 100 KCl, 600 CaCl2 and 5 H3BO3. These nutrients in the collection solution allowed for membrane integrity and minimizing osmotic stress and possible passive leakage. After collection, a subsample of 8 mL of collection solution was acidified by adding two drops of concentrated H3PO4. Microbe inhibitor (Micropur, Katadyn, Deutsschl and Gmbh, München, Germany) was added into the collected exudate solutions at 0.01 g L−1 to prevent microbial decomposition of root exudates (Shen et al. 2004). The 8 mL of collection solution was then filtered through 0.2-μm membrane and frozen at −20 °C until analysis.
Organic acids were analyzed by a reversed-phase high performance liquid chromatography (Wang et al. 2006). Separation was conducted on a 250 × 4.6 mm reversed phase column (Alltima C-18). The mobile phase was 25 mmol L−1 KH2PO4 (pH 2.5) with the flow rate of 1 mL min−1 at 28 °C and UV detection was set at 214 nm. Organic acids in the sample were identified by comparison with the retention times and absorption spectra of pure standards including tartaric, malic, citric, fumaric and T-aconitic acids, which are the reported major organic acids released by white lupin, faba bean and maize (Dinkelaker et al. 1989; Neumann and Römheld 1999; Gaume et al. 2001; Liu et al. 2004; Li et al. 2007).
Measurement of acid phosphatase activity
Plant roots were washed three times using deionized water, and blotted dry. The roots were placed in opaque vessels containing 0.5 g L−1 p-nitrophenyl phosphate (NPP) as a substrate in acetate buffer (0.1 mmol L−1, pH 5.6) for 1 h under nature illumination. Five mL of the reaction solution was mixed immediately with 2.5 mL NaOH (2 mol L−1) to terminate the reaction and to develop the colour (McLachlan 1980). The absorbance of the solution was determined at 405 nm using a spectrophotometer (UVmini 1240, Japan). The APase activity in the root was calculated on a basis of an oven-dried weight.
Rhizosphere acidification/alkalization
Rhizosphere acidification/alkalization was detected using an agar technique modified from Marschner and Römheld (1983). Intact roots of the plants cultured in treatment solutions for 12 days were washed in deionized water (pH 5.9), spread out in a flat tray (310 mm × 150 mm) and covered immediately with the agar solution (0.7 %, 38 °C) containing pH indicator 0.01 % bromocresol-purple at pH 5.9. After 30 min, images were recorded. A yellow colour indicates acidification whereas purple colour means alkalization.
Non-invasive measurement of H+ fluxes on root surface
Net H+ fluxes on root surface were measured using the scanning ion-selective electrode technique as described by Xu et al. (2006). Before measurement, microelectrodes were calibrated with pH 5.5 and pH 6.5 measuring solution, only those with a Nernst slope of 58 ± 6 mV were used. The H+ flux was recorded every 6 s for 10 min. Data were treated using Mageflux software (Version 1.0) developed by Xuyue Company (http://xuyue.net/mageflux; Sun et al. 2009). The results were presented as pmol cm−2 s−1.
After washing in deionized water, the roots were placed in a beaker containing measuring solution (0.1 mmol L−1 KCl, 0.1 mmol L−1 CaCl2, 0.1 mmol L−1 MgCl2, 0.5 mmol L−1 NaCl, 0.3 mmol L−1 MES, 0.2 mmol L−1 NaSO4, pH 6.0) (Sun et al. 2009). After 20 min, the roots were transferred into a Petri dish containing fresh measuring solution. Net H+ fluxes were continuously recorded for 10 min at 10 mm from the tip of lateral roots.
Dry weight and P content measurement
The shoots and roots harvested from the second experiment were dried at 105 °C for 30 min and then at 70 °C to a constant weight. After dry weights were recorded, the materials were ground into powder. Subsamples of ground materials were digested with a mixture of concentrated sulfuric acid and 30 % H2O2 (v/v), and total P content determined using the molybdivanadate method (Soon and Kalra 1995).
Root length analysis
The harvested roots were digitally scanned (Epson V700, Jakarta, Indonesia), and the resulting images were analyzed with the WinRHIZO version 5.0 (Regent Instruments, Quebec City, Canada). Axial root length was measured by a ruler before scanning, it stands for primary root length of legumes and the sum of axial root lengths of primary root, seminal roots and crown roots of maize. Lateral root density indicated the numbers of the first-order lateral roots per unit length of an axial root. Higher order lateral roots were not measured. Specific root length was the ratio of root length to root dry weight.
Calculations
Root P influx between the two harvests (7 and 16 DAT) in the second experiments was calculated according to Brewster and Tinker (1972)
| (1) |
where In refers to P influx (mg m−1 d−1), P refers to plant P content (mg plant−1), t refers to time (days) and L refers to root length (m plant−1). The indices 1 and 2 refer to 7 and 16 DAT, respectively.
Phosphorus-use efficiency (PUE) and P-absorption efficiency (PAE) were calculated according to Equations (2) and (3), respectively (Jakobsen et al. 1992; Corrales et al. 2007)
| (2) |
| (3) |
Root/shoot ratio was calculated according to Claassen and Jungk (1984)
| (4) |
where L refers to root length (m plant−1) and W refers to shoot dry weight (g plant−1).
Statistical analysis
The statistical means of different treatments were compared using the least significant difference (LSD) at a 0.05 level of probability using SAS 8.02. Differences between the P treatments were analyzed using the one-way PROC ANOVA. The effects of P supply and N forms on the variables and their interactions were tested using a two-way analysis of variance.
Results
Plant growth and P uptake
Low-P supply (1 µmol L−1) significantly decreased dry weights of maize at last two harvests, and of faba bean at the last harvest under both N forms, compared with HP (250 µmol L−1) supply (Table 1). These decreased plant dry weights were the consequence of the decreased shoot dry weights with the decrease being greater for maize than for faba bean [see Supporting Information—Figure S1A and C]. In contrast, LP increased root dry weights of the two species at three harvests under Ca(NO3)2, and of maize at the first two harvests under NH4NO3 [see Supporting Information—Figure S1B and D]. However, LP supply did not affect the dry weights of shoot or roots of white lupin at all three harvests irrespective of N form [Table 1; see Supporting Information—Figure S1].
Table 1.
Dry weights and P contents of the whole plant of white lupin, faba bean and maize grown with low (LP) (1 µmol L−1) and high P (HP) (250 µmol L−1) under two N forms [ca(NO3)2 and NH4NO3] for 7, 12 and 16 days.
| N forms | P supply | White lupin |
Faba bean |
Maize |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 7 d | 12 d | 16 d | 7 d | 12 d | 16 d | 7 d | 12 d | 16 d | ||
| Plant biomass (g dry weight per plant) | ||||||||||
| Ca(NO3)2-N | LP | 0.12 | 0.28 | 0.47 | 0.46 | 0.72 | 1.11 | 0.34 | 0.56 | 0.72 |
| HP | 0.14 | 0.30 | 0.53 | 0.42 | 0.80 | 1.38 | 0.27 | 0.84 | 1.57 | |
| NH4NO3-N | LP | 0.12 | 0.18 | 0.47 | 0.43 | 0.71 | 1.07 | 0.37 | 0.61 | 0.79 |
| HP | 0.12 | 0.21 | 0.50 | 0.43 | 0.77 | 1.37 | 0.34 | 1.01 | 1.99 | |
| LSD (P = 0.05) | 0.01 | 0.03 | 0.04 | 0.04 | 0.08 | 0.14 | 0.03 | 0.06 | 0.08 | |
| P level | n.s. | n.s. | n.s. | n.s. | n.s. | ** | ** | *** | *** | |
| N form | n.s. | *** | n.s. | n.s. | n.s. | n.s. | ** | ** | *** | |
| P × N | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | * | *** | |
| P content (mg P per plant) | ||||||||||
| Ca(NO3)2-N | LP | 1.04 | 1.62 | 1.67 | 3.39 | 4.06 | 4.33 | 0.89 | 0.73 | 0.75 |
| HP | 1.48 | 2.34 | 3.96 | 5.37 | 8.34 | 15.46 | 4.38 | 10.29 | 19.90 | |
| NH4NO3-N | LP | 1.05 | 1.18 | 1.67 | 3.53 | 4.60 | 5.01 | 0.97 | 0.87 | 0.96 |
| HP | 1.34 | 1.88 | 4.69 | 5.98 | 8.62 | 15.79 | 5.47 | 15.06 | 25.11 | |
| LSD (P = 0.05) | 0.12 | 0.26 | 0.38 | 0.60 | 1.52 | 2.03 | 0.62 | 1.28 | 2.17 | |
| P level | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| N form | n.s. | ** | n.s. | n.s. | n.s. | n.s. | * | ** | ** | |
| P × N | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | * | ** | ** | |
*P ≤0.05;
**P < 0.01;
***P < 0.001; n.s. not significant at P = 0.05.
Nitrogen forms did not affect whole plant dry weights of white lupin and faba bean at three harvests with the exception at 12 days for white lupin, with significant lower dry weights under NH4NO3 than under Ca(NO3)2. In comparison, the plant dry weight of maize was significant higher under NH4NO3 than under Ca(NO3)2. The interaction between P levels and N forms on plant dry weight was only significant in maize at the last two harvests (Table 1).
Among the three species, total root length (TRL) was the greatest in maize and the smallest in white lupin. Compared with the HP treatment in maize, LP increased TRL, axial root length (ARL) and lateral root density (LRD) at all three harvests under Ca(NO3)2, and increased specific root length (SRL) at the last two harvests under Ca(NO3)2. Under NH4NO3 supply, however, LP increased TRL, ARL and LRD at first two harvests, while inhibited SRL of maize at the last harvest. For faba bean, LP increased TRL, due to the increased LRD at the last two harvests under Ca(NO3)2, while decreased TRL due to the decreased LRD at the last harvest under NH4NO3. In most cases, LP did not change the measured root length parameters of white lupin under both N forms compared with the HP treatment (Table 2).
Table 2.
Root length parameters of white lupin, faba bean and maize grown with low (LP) (1 µmol L−1) and high P (HP) (250 µmol L−1) under two N forms [ca(NO3)2 and NH4NO3] for 7, 12 and 16 days.
| Parameters | Ca(NO3)2 as N source |
NH4NO3 as N source |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White lupin |
Faba bean |
Maize |
White lupin |
Faba bean |
Maize |
|||||||
| LP | HP | LP | HP | LP | HP | LP | HP | LP | HP | LP | HP | |
| 7 days | ||||||||||||
| TRL (m) | 1.6 b | 2.0 a | 4.6 b | 6.1 a | 14.5 a | 12.4 b | 1.8 a | 1.7 a | 4.7 a | 4.8 a | 20.9 a | 14.9 b |
| ARL (cm) | 28.3 b | 31.5 a | 32.3 a | 36.0 a | 251.6 a | 219.5 b | 23.8 b | 26.9 a | 26.1 a | 29.7 a | 221.4 a | 189.9 b |
| LRD (no. cm−1) | 2.6 a | 2.4 a | 1.4 b | 1.7 a | 4.0 a | 3.4 b | 2.7 a | 2.1 b | 3.5 a | 2.5 b | 3.9 a | 3.4 b |
| SRL (m g−1) | 41.2 a | 41.4 a | 27.0 a | 33.5 a | 111.3 a | 113.6 a | 43.7 a | 38.0 b | 28.5 a | 28.0 a | 159.0 a | 147.9 a |
| 12 days | ||||||||||||
| TRL (m plant−1) | 3.9 a | 4.9 a | 11.0 a | 8.8 b | 33.6 a | 24.5 b | 3.9 a | 4.3 a | 9.0 a | 9.8 a | 47.4 a | 26.9 b |
| ARL (cm) | 36.0 a | 35.7 a | 38.7 a | 42.4 a | 497.4 a | 336.8 b | 34.9 a | 38.9 a | 39.0 a | 41.2 a | 420.5 a | 301.7 b |
| LRD (no. cm−1) | 3.9 a | 2.9 b | 2.0 a | 1.6 b | 3.7 a | 3.5 b | 3.2 a | 3.5 a | 2.8 a | 2.9 a | 4.8 a | 4.1 b |
| SRL (m g−1) | 86.0 a | 73.9 b | 36.7 a | 43.0 a | 176.2 a | 150.0 b | 82.4 a | 80.7 a | 42.8 a | 36.8 b | 226.0 a | 216.1 a |
| 16 days | ||||||||||||
| TRL (m) | 6.2 a | 6.3 a | 23.5 a | 13.1 b | 43.2 a | 36.2 b | 5.8 a | 6.9 a | 10.7 b | 18.2 a | 52.7 a | 50.5 a |
| ARL (cm) | 52.9 a | 48.6 a | 51.8 a | 1.6 b | 506.8 a | 454.3 b | 36.6 b | 46.8 a | 46.8 a | 46.2 a | 508.3 a | 450.1 a |
| LRD (no. cm−1) | 3.7 a | 3.5 a | 1.8 a | 1.6 b | 4.0 a | 3.5 b | 3.6 a | 3.5 a | 2.1 b | 2.8 a | 4.5 a | 4.4 a |
| SRL (m g−1) | 74.6 a | 68.1 a | 73.6 a | 63.8 b | 196.9 a | 189.1 b | 79.7 a | 82.5 a | 56.2 b | 72.7 a | 201.1 b | 269.7 a |
Values of each pair in rows of individual plant species followed by different letters represent significant differences between the P treatments (P < 0.05). Means ± SE, n = 4. Abbreviations: TRL: total root length (m plant−1); ARL: axial root length (cm); LRD: lateral root density (number of lateral roots per unit axial root, no./cm); SRL, specific root length (m g−1).
All three species had significantly lower P content of the whole plant supplied with LP than with HP under both N forms. Similar to the influence on plant dry weight, N forms influenced plant P content only in maize, with more P uptake under NH4NO3, but not in two legumes. In maize, increasing P supply increased the P content more under NH4NO3 than under Ca(NO3)2, leading to an interaction between P levels and N forms (Table 1). Low P supply significantly decreased whole plant N and K contents of maize and faba bean at the last two harvests, but not of white lupin at either harvest, compared with the HP supply [see Supporting Information—Table S1]. Nitrogen forms significantly influenced whole plant N and K contents of the three species with few exceptions at the first harvest. NH4NO3 facilitated N uptake of all three species while Ca(NO3)2 facilitated K uptake of legumes. Unlike the legumes, maize took up more K under NH4NO3 than under Ca(NO3)2 at the last harvest, under both P levels [see Supporting Information—Table S1].
Plant P concentration and P efficiency
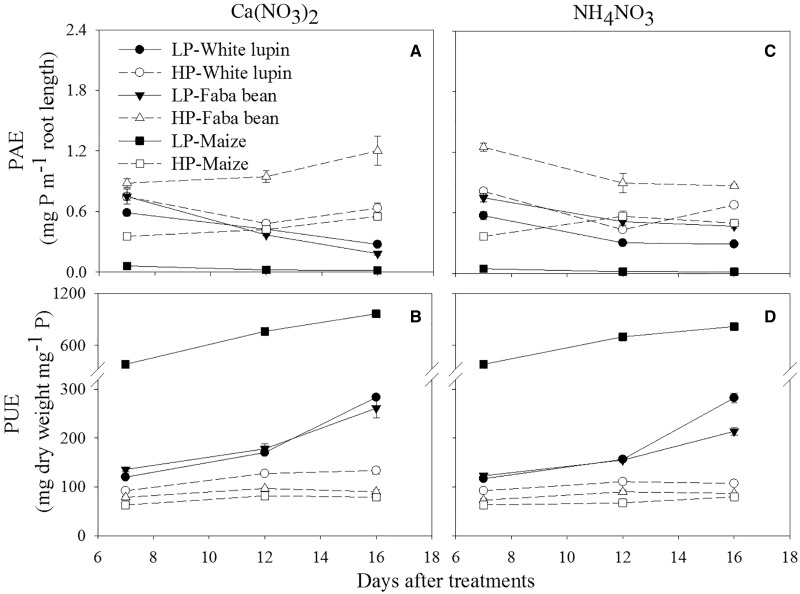
Compared with HP supply, LP significantly decreased plant P concentration (g P per 100 g DW) in all three species under both N forms. Maize showed the lowest P concentration under LP level while the highest value under HP level. Consistent with these results, maize had the lowest P-influx rate between the first and the third harvest (Table 3) and P-absorption efficiency (PAE) at three harvests under LP level and both N forms (Fig. 1A and C), when P influx and PAE were calculated per unit of root length. The P-influx rate was calculated by Equation (1), using the data in Tables 1 and 2. Because the P content of maize plants grown at LP did not increase between Days 7 and 16 (Table 1), the calculated P-influx rates of maize under LP and both N forms were zero (Table 3). However, irrespective of N form, maize had the highest P-use efficiency among the three plant species when LP was supplied (Fig. 1B and D). Nitrogen form did not affect P concentration in maize or faba bean at a given P level (Table 3).
Table 3.
Plant P concentration and root/shoot ratio at the final harvest (16 days after treatment) and P influx rate between 7 and 16 days of white lupin, faba bean and maize grown with low (LP) and high P supply (HP) under two N forms [ca(NO3)2 and NH4NO3].
| Parameters | Crops | Ca(NO3)2-N |
NH4NO3-N |
LSD | P level | N form | P × N | ||
|---|---|---|---|---|---|---|---|---|---|
| LP | HP | LP | HP | ||||||
| P concentration | White lupin | 0.35 | 0.76 | 0.36 | 0.94 | 0.07 | *** | * | * |
| (g P 100 g−1 DW) | Faba bean | 0.39 | 1.12 | 0.47 | 1.15 | 0.09 | *** | n.s. | n.s. |
| Maize | 0.10 | 1.27 | 0.12 | 1.26 | 0.05 | *** | n.s. | n.s. | |
| P influx | White lupin | 0.02 | 0.07 | 0.02 | 0.10 | 0.01 | *** | n.s. | * |
| (mg P m−1 root length d−1) | Faba bean | 0.01 | 0.12 | 0.02 | 0.11 | 0.02 | *** | n.s. | n.s. |
| Maize | 0.00 | 0.08 | 0.00 | 0.08 | 0.01 | *** | n.s. | n.s. | |
| root/shoot ratio | White lupin | 16.5 | 15.6 | 15.6 | 18.1 | 1.8 | n.s. | n.s. | n.s. |
| (m root length g−1 shoot DW) | Faba bean | 31.3 | 12.2 | 14.9 | 18.0 | 3.5 | *** | ** | *** |
| Maize | 105.3 | 28.0 | 112.6 | 30.5 | 8.0 | *** | n.s. | n.s. | |
*P ≤0.05;
**P < 0.01;
***P < 0.001; n.s., not significant at P = 0.05.
Figure 1.
P-absorption efficiency (PAE) and P-use efficiency (PUE) of white lupin, faba bean and maize grown with low (LP) and high P (HP) under two N forms for 7, 12 and 16 days. Nitrogen was supplied as Ca(NO3)2 on left panels and NH4NO3 on right panels. Values are means of four replicates and error bars represent the standard error of the mean.
The supply of LP significantly increased the root/shoot ratio (m root length g−1 shoot DW) of maize under both N forms and of faba bean under Ca(NO3)2, but did not affect the root/shoot ratio of white lupin under either N form, compared with HP supply (Table 3).
Proton release in roots of three plant species under two N forms
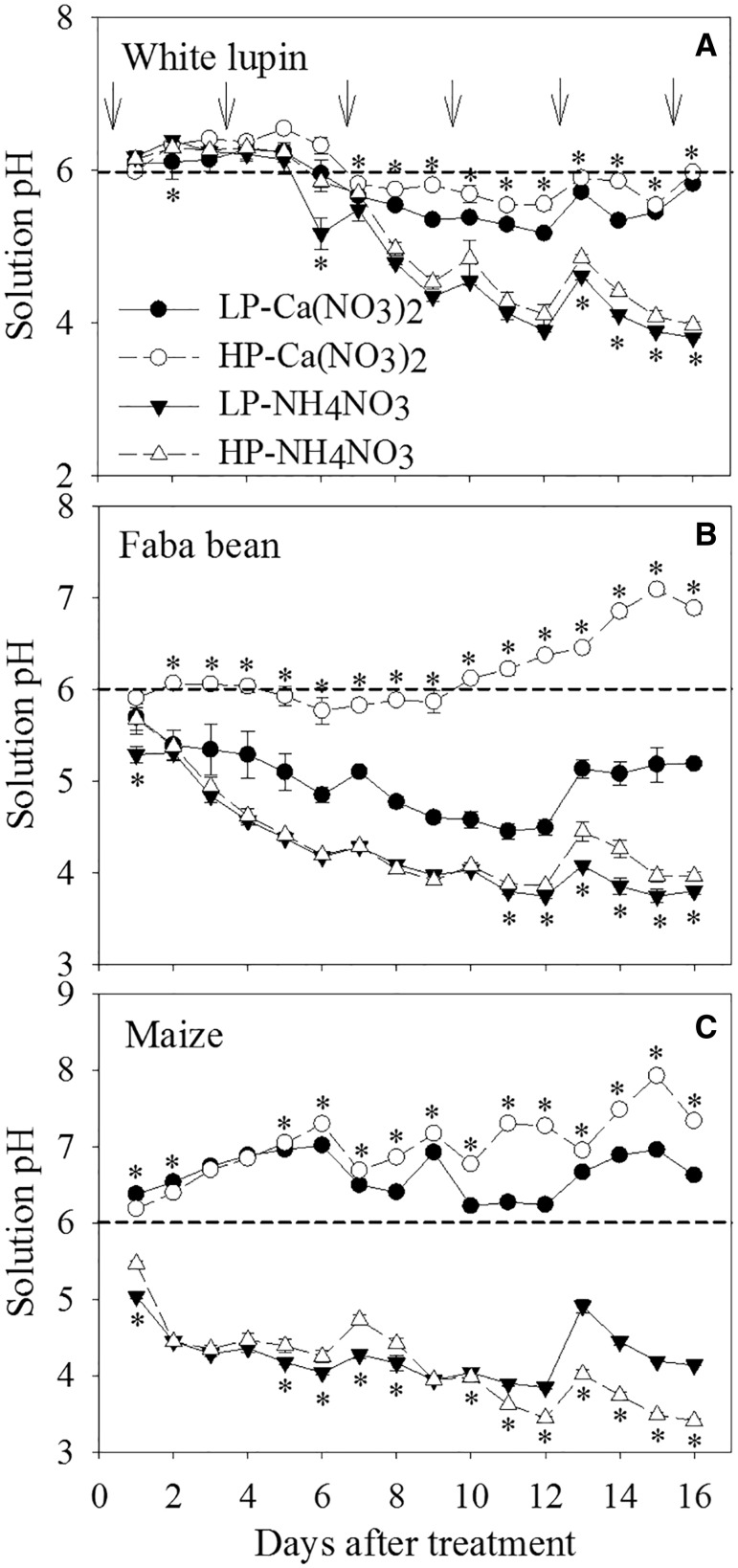
When Ca(NO3)2 was used as the N source, LP treatment increased proton release and decreased solution pH under both legumes, especially faba bean (Fig. 2A and B). In contrast, the solution pH under maize was higher than the initial pH value throughout the experimental period under both P levels, indicating an alkalization process (Fig. 2C). When NH4NO3 was supplied, the pH of solution grown with all three species declined dramatically over time with an exception of white lupin during the first 6 days (Fig. 2A). LP treatment further decreased solution pH under both legumes during the later cultural period, while increasing solution pH in maize during the same period. In comparison, the solution pH was lower with maize and faba bean than with white lupin under NH4NO3 supply (Fig. 2).
Figure 2.
Changes over time in pH of nutrient solutions grown with white lupin, faba bean and maize supplied with low (LP) and high P (HP) under supply of Ca(NO3)2 or NH4NO3 during the cultural period. The pH was measured at 10:00 am daily. Arrows indicate the times when nutrient solutions were replaced. Values are means of four replicates, and the bars represent the standard error of the mean. Asterisks represent significant differences between the P levels at each measurement (P < 0.05).
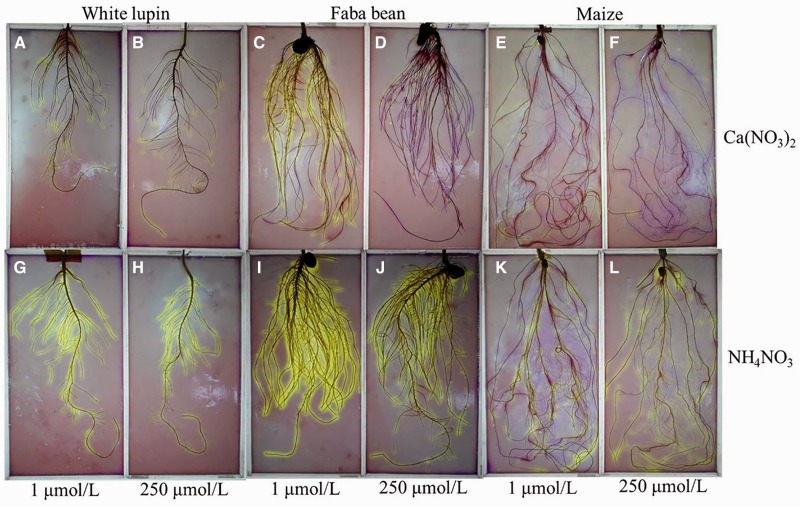
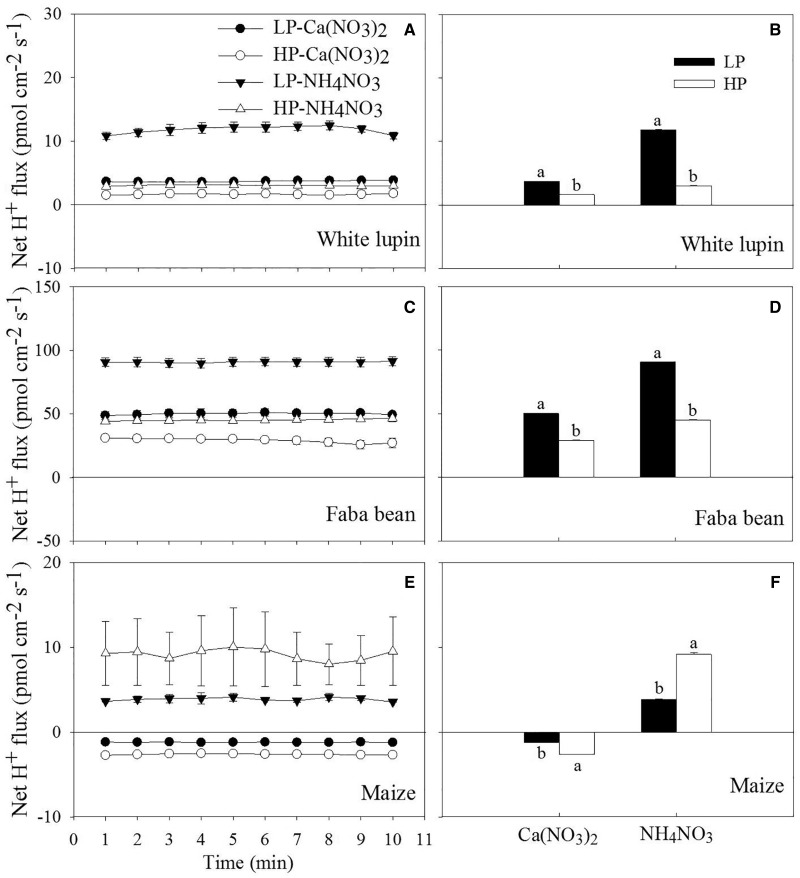
Rhizosphere acidification/alkalization and net H+ flux were compared among the three plant species on 12 days after treatments (Figs 3 and 4). When Ca(NO3)2 was supplied, yellow colour was observed surrounding the root tips of white lupin and LP-treated faba bean (Fig. 3A–C), indicating rhizosphere acidification, while purple colour along with the root surface of maize and HP-treated faba bean (Fig. 3D–F) revealed a rhizosphere alkalization. When NH4NO3 was supplied, yellow colour appeared on the root tips of maize, and on the surface of the entire root of the two legumes (Fig. 3G–L). The results were consistent with those of solution pH measurement (Fig. 2) and net H+ flux determined using the scanning ion-selective electrode technique (Fig. 4). Proton release from the root tips was greater under NH4NO3 than under Ca(NO3)2 (Fig. 4).
Figure 3.
Effect of P supply (1 and 250 µmol L−1) on the intensity of rhizosphere acidification/alkalization in white lupin, faba bean and maize grown with Ca(NO3)2 or NH4NO3. Rhizosphere pH changes were detected by embedding the roots of 12-d-old plants in agar with bromocresol-purple as a pH indicator (initial pH 5.9). The images were recorded 0.5 h after embedding. Yellow colour indicates pH <5.2 while purple colour indicates pH >6.8.
Figure 4.
Net (left) and mean (right) H+ fluxes over 10 min on root surface of white lupin (A and B), faba bean (C and D) and maize (E and F) that had grown for 12 days with low (LP) and high P (HP) supply under two N forms [Ca(NO3)2 and NH4NO3]. Values are means of four replicates, and the bars represent the standard error of the mean (A, C and E). Different letters (lower case) (B, D and F) represent significant differences between the P treatments within a plant species and N form (P <0.05). Positive values indicate net efflux while negative values indicate net influx.
Root release of organic acid anions and acid phosphatase activity
Release of organic acid anions and APase activity on root surface were measured 12 days after treatments. The total amounts of organic acid anions released by the legume roots under both N forms were greater in the LP than in the HP treatment, where the increases of malate, citrate and tartrate in white lupin and of tartrate in faba bean were significant. In contrast, the release of organic acid anions by maize roots was lower in the LP than in the HP treatment (Table 4).
Table 4.
Exudation of organic acid anions by roots of white lupin, faba bean and maize grown for 12 days with low (LP) and high P supply (HP) under two N forms [ca(NO3)2 and NH4NO3].
| Crops | Organic acids | Root exudation (µmol g−1 dry root h−1) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca(NO3)2-N |
NH4NO3-N |
LSD | P level | N form | P × N | ||||
| LP | HP | LP | HP | ||||||
| White lupin | Tartaric | 0.96 | 0.54 | 0.49 | 0.50 | 0.04 | *** | *** | *** |
| Malic | 1.67 | 0.92 | 1.66 | 1.24 | 0.27 | ** | n.s. | n.s. | |
| Citric | 0.59 | 0.47 | n.d. | n.d. | 0.04 | *** | * | * | |
| Fumaric | 0.02 | 0.03 | n.d. | n.d. | 0.00 | n.s. | n.s. | n.s. | |
| T-aconitic | 0.04 | n.d. | n.d. | 0.03 | 0.00 | n.s. | n.s. | *** | |
| Total organic acids | 3.29 | 1.95 | 2.15 | 1.82 | 0.29 | *** | ** | ** | |
| Faba bean | Tartaric | 1.35 | 0.83 | 2.87 | 2.25 | 0.20 | *** | *** | n.s. |
| Malic | 0.75 | 0.81 | 0.89 | 0.79 | 0.39 | n.s. | n.s. | n.s. | |
| Citric | 0.31 | 0.42 | 0.48 | 0.35 | 0.15 | n.s. | n.s. | n.s. | |
| Fumaric | 0.05 | n.d. | n.d. | n.d. | 0.02 | ** | ** | ** | |
| T-aconitic | n.d. | 0.01 | n.d. | 0.02 | 0.00 | *** | ** | ** | |
| Total organic acids | 2.47 | 2.08 | 4.24 | 3.81 | 0.35 | ** | *** | n.s. | |
| Maize | Tartaric | 0.41 | 3.62 | 0.49 | 2.19 | 1.08 | ** | n.s. | n.s. |
| Malic | n.d. | 1.20 | n.d. | 0.91 | 0.27 | *** | n.s. | n.s. | |
| Citric | n.d. | n.d. | n.d. | n.d. | 0.00 | n.s. | n.s. | n.s. | |
| Fumaric | 0.03 | 0.05 | 0.02 | 0.02 | 0.01 | n.s. | * | n.s. | |
| T-aconitic | 0.48 | 0.29 | n.d. | n.d. | 0.15 | n.s. | *** | n.s. | |
| Total organic acids | 0.92 | 5.16 | 0.51 | 3.12 | 1.42 | ** | n.s. | n.s. | |
*P ≤0.05;
**P < 0.01;
***P < 0.001; n.s., not significant at P = 0.05. n.d., not detected.
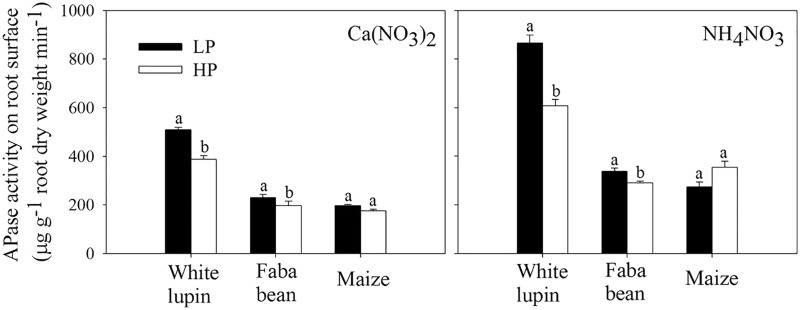
Low P supply significantly increased the APase activity on root surface of the legumes but not maize. The APase activity on root surface was higher under NH4NO3 than under Ca(NO3)2 for all three species, and was higher in white lupin than in faba bean and maize (Fig. 5).
Figure 5.
Activity of acid phosphatase (APase) on the root surface of white lupin, faba bean and maize grown for 12 days with low (LP) and high P (HP) supply under two N forms [Ca(NO3)2 and NH4NO3]. Values are means of four replicates and the error bars represent the standard error of the mean. Different letters (lower case) represent significant differences between the P treatments within a plant species and N form (P < 0.05).
Discussion
Morphological modification was the main adaptive response of maize roots to P deficiency
This study demonstrated that the growth of maize was more sensitive to P deficiency than that of the two legumes. This is consistent with the findings that maize has a high soil-fertility requirement to attain maximal grain yield (Paponov and Engels 2003). Under P deficiency, more carbon was distributed to roots and was used for the formation of new roots, but not for increases in the release of protons, organic acid anions and APase activity although the two N forms had contrasting effects on net H+ release (Figs 2–5 and Table 4). These responses were also true for different maize genotypes (Anghinoni and Barber 1980; Corrales et al. 2007; Li et al. 2012; Fernández and Rubio 2015) under a broad range of growing conditions, including different P levels and growth media like hydroponics (Anghinoni and Barber 1980; Mollier and Pellerin 1999; Gaume et al. 2001; Li et al. 2012; Fernández and Rubio 2015), sand culture (Corrales et al. 2007) and field conditions (Fernández et al. 2009; Zhang et al. 2012). Additionally, the density and the length of maize root hairs were also found to increase under P deficiency (Zhu et al. 2005). While distributing more carbon to roots increases root length and surface area for exploration and acquisition of P from the soil (Tang et al. 2009), P-deficiency decreased photosynthesis and reproductive growth of the plant (Lynch and Ho 2005). In fact, low P supply dramatically decreased maize shoot growth [Table 1; see Supporting Information—Figure S1]. In other studies, continuous P deficiency decreased the growth of maize shoot and roots, and subsequently grain yields (Plénet et al. 2000; Zhang et al. 2012; Krey et al. 2013; Deng et al. 2014). It is not clear why maize has the priority to invest more carbon for root morphological over physiological modifications under P deficiency.
Maize responded to P deficiency similarly to wheat and does not increase proton release or exudation of organic acid anions in roots (Neumann and Römheld 1999; Li et al. 2008). In contrast, maize and wheat roots exhibit a remarkable increase in total root length and root/shoot ratio in response to P deficiency (Table 3; Vlek et al. 1996; Bhadoria et al. 2002). A larger root system is obviously beneficial for a better access of inorganic P because of the low mobility of P in the soil (Hinsinger 2001). Phosphorus uptake by plants depends on the root length and surface area, and on lateral roots to explore a large soil volume (Richardson et al. 2009; Balemi and Negisho 2012; Lambers et al. 2015). According to the results in the literature and the present study, it is concluded that the main adaptive strategy of maize roots to P deficiency is morphological variation.
White lupin and faba bean exhibited morpho-physiological strategy in roots in response to P deficiency
Unlike maize, low P did not influence shoot and root growth of white lupin under both N forms in the present study [see Supporting Information—Figure S1], although the shoot and root P content and concentration decreased (Tables 1 and 3). In comparison, low P increased the root dry weight, total root length and root/shoot ratio of faba bean under Ca(NO3)2 supply [Tables 2 and 3; see Supporting Information—Figure S1], indicating that faba bean has the morphological advantage of an extensive root system favouring to access P by exploring a larger volume of soil. The P concentration of white lupin and faba bean was higher than that of maize under low P supply (Table 3), indicating that both legumes have a higher internal P requirement than maize (Föhse et al. 1988).
It is well known that P-deficient white lupin adapts P deficiency by morphological and physiological variations, i.e. formation of cluster roots and increased release of organic acid anions and protons (Dinkelaker et al. 1989; Gerke et al. 1994). In the present study, white lupin supplied with low P had not yet formed cluster roots because the duration of low-P treatment was not long enough (Dinkelaker et al. 1989; Gerke et al. 1994; Neumann and Martinoia 2002; Wang et al. 2006). Furthermore, malate was the main organic acid anion released by the roots (Table 4), yet the major organic acid anion released by root clusters is citrate while that released by root tips, including non-proteoid roots and proteoid roots, is malate (Gardner et al. 1982, 1983; Neumann et al. 1999). Apparently, white lupin roots adapted P deficiency by morphological/physiological modifications, although the carbon costs for root physiological variation are greater than that for morphological modification (Gardner et al. 1983; Dinkelaker et al. 1989; Lynch and Ho 2005). Since white lupin grows in P-impoverished habitats, the best strategy of the P-deficient white lupin is to mine soil P by releasing protons and organic compounds, rather than modifying root growth (Neumann and Martinoia 2002; Lambers et al. 2008, 2011).
Although reflecting different aspects of proton release by roots, the results obtained using solution pH measurement, agar technique and non-invasive measurement of net H+ fluxes were consistent and showed that low P stimulated proton release in the two legumes, in agreement with the previous findings (Dinkelaker et al. 1989; Nuruzzaman et al. 2005a). The proton release induced by P deficiency was greater in faba bean than in white lupin, as presented by the intensive rhizosphere acidification within 30 min (Fig. 3) and more H+ efflux on root tips of faba bean (Fig. 4). On one hand, the increased proton release by the roots of P-deficient faba bean would contribute greatly to mobilization of non-labile P in soil, especially in high-pH soils (Li et al. 2004). On the other hand, the increased release of protons under P deficiency may compensate for both excess uptake of cations (Dinkelaker et al. 1989) and a concomitant release of organic acid anions (Neumann and Römheld 1999).
Nitrogen form did not affect the root morphological/physiological response to low P
The present study investigated whether N forms influence root responses to P deficiency. NH4NO3 facilitated N uptake of all three species while Ca(NO3)2 facilitated K uptake of legumes [see Supporting Information—Table S1]. In comparison with Ca(NO3)2, NH4NO3 supply resulted in an increase in proton release from the roots of all three species, especially maize under the high-P condition. Low-P supply increased proton release in white lupin and faba bean at the supply of both N forms, and in maize at the supply of NH4NO3 (Figs 2–4). The marked increase in proton release of the NH4NO3-fed maize under both P levels could be explained by the preferential uptake of over under mixed supply of ammonium and nitrate, which causes more proton release (Clark 1982). There is a competition for transport across the tonoplast between chloride and nitrate, which affects their accumulation and uptake (White and Veneklaas 2012). In the present study, KCl was added to the low-P treatment to keep the K supply constant between the P treatments, which increased Cl concentration (250 µmol) in the low-P solution (see Methods section). In comparison with the high-P treatment, however, low P did not decrease N uptake in white lupin, but decreased it in faba bean at the final harvest and maize at the last two harvests under both N forms [see Supporting Information—Table S1]. The increased uptake of N could be explained by the larger plant biomass and thus high demand of the faba bean and maize supplied with high P (Table 1).
Conclusions
The study demonstrated that root morphological variation was the main adaptive strategy in maize in response to P deficiency under the present condition. A large root system is, therefore, important for maize to access available nutrients in soil, especially immobile P. Breeding crops such as maize with large root systems and better architecture will also contribute to increasing the use efficiency of P and other nutrients. NH4NO3 enhanced the magnitude of proton release in white lupin and faba bean under P deficiency although N form did not fundamentally alter the responses of root morphology and physiology of the three species to P deficiency. It is important in practices to use appropriate N forms to maximize root/rhizosphere processes and to enhance P-use efficiency. The use of ammonium-based fertilizers may facilitate P acquisition of legumes in non-acidic P-deficient soils but further work is needed to elucidate the effects of N form on P acquisition strategies of N2-fixing legumes.
Sources of Funding
This research was supported by the State Key Basic Research and Development Plan of China (No. 2013CB127402).
Contributions by the Authors
C.L. conceived and designed research; H.L. performed the experiments and analyzed data; C.L. coordinated the research, provided all the technical support, and participated in data interpretation; H.L., C.T. and C.L. wrote the article; all authors participated in stimulating discussion and approved the final article.
Conflicts of Interest Statement
None declared.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Wei Ma from the Chinese Academy of Agricultural Science for providing experimental equipment for non-invasive measurement of H+ fluxes on root surface; Chao Wang and Yaping Zhou for their help in the experiments.
Supporting Information
The following additional information is available in the online version of this article —
Figure S1. Dry weights of shoot and root of white lupin, faba bean and maize grown with low (LP, 1 µmol L−1) and high P (HP, 250 µmol L−1) under two N forms for 7, 12 and 16 days. Nitrogen was supplied as Ca(NO3)2 on left panels and NH4NO3 on right panels. Values are means of four replicates, and error bars represent ± the standard error of the mean.
Table S1. Nitrogen and potassium contents of intact white lupin, faba bean and maize grown with LP (1 µmol L−1) and HP (250 µmol L−1) under two N forms [ca(NO3)2 and NH4NO3] for 7, 12 and 16 days.
Literature cited
- Anghinoni I, Barber S. 1980. Phosphorus influx and growth characteristics of corn roots as influenced by phosphorus supply. Agronomy Journal 72:685–688. [Google Scholar]
- Balemi T, Negisho K. 2012. Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: a review. Journal of Soil Science and Plant Nutrition 12:547–562. [Google Scholar]
- Barber SA. 1995. Soil nutrient bioavailability: a mechanistic approach. New York: John Wiley. [Google Scholar]
- Bhadoria PS, Steingrobe B, Claassen N, Liebersbach H. 2002. Phosphorus efficiency of wheat and sugar beet seedlings grown in soils with mainly calcium, or iron and aluminium phosphate. Plant and Soil 246:41–52. [Google Scholar]
- Brewster J, Tinker P. 1972. Nutrient flow rates into roots. Soils and Fertilizers 35:355–359. [Google Scholar]
- Calderón-Vázquez C, Sawers RJ, Herrera-Estrella L. 2011. Phosphate deprivation in maize: genetics and genomics. Plant Physiology 156:1067–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen N, Jungk A. 1984. Bedeutung der Kaliumaufnahmerate, Wurzelwachstum und Wurzelhaare für das Kaliumane ignungsvermögen verschiedener Pflanzenarten. Zeitschrift Fuer Pflanzenernährung Und Bodenkungde 147:276–289. [Google Scholar]
- Clark RB. 1982. Nutrient solution growth of sorghum and corn in mineral nutrition studies. Journal of Plant Nutrition 5:1039–1057. [Google Scholar]
- Corrales I, Amenós M, Poschenrieder C, Barceló J. 2007. Phosphorus efficiency and root exudates in two contrasting tropical maize varieties. Journal of Plant Nutrition 30:887–900. [Google Scholar]
- Deng Y, Chen K, Teng W, Zhan A, Tong Y, Feng G, Cui Z, Zhang F, Chen X. 2014. Is the inherent potential of maize roots efficient for soil phosphorus acquisition? PloS One 9:e90287.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkelaker B, Römheld V, Marschner H. 1989. Citric acid excretion and precipitation of calcium citrate in the rhizosphere of white lupin (Lupinus albus L.). Plant, Cell and Environment 12:285–292. [Google Scholar]
- Fernández MC, Belinque H, Boem FHG, Rubio G. 2009. Compared phosphorus efficiency in soybean, sunflower and maize. Journal of Plant Nutrition 32:2027–2043. [Google Scholar]
- Fernández MC, Rubio G. 2015. Root morphological traits related to phosphorus-uptake efficiency of soybean, sunflower, and maize. Journal of Plant Nutrition and Soil Science 178:807–815. [Google Scholar]
- Föhse D, Claassen N, Jungk A. 1988. Phosphorus efficiency of plants: I. External and internal P requirement and P uptake efficiency of different plant species. Plant and Soil 110:101–109. [Google Scholar]
- Gahoonia TS, Nielsen NE. 1992. The effects of root-induced pH changes on the depletion of inorganic and organic phosphorus in the rhizosphere. Plant and Soil 143:185–191. [Google Scholar]
- Gardner W, Barber D, Parbery D. 1983. The acquisition of phosphorus by Lupinus albus L. Plant and Soil 70:107–124. [Google Scholar]
- Gardner W, Parbery D, Barber D. 1982. The acquisition of phosphorus by Lupinus albus L. Plant and Soil 68:19–32. [Google Scholar]
- Gaume A, Mächler F, De León C, Narro L, Frossard E. 2001. Low-P tolerance by maize (Zea mays L.) genotypes: significance of root growth, and organic acids and acid phosphatase root exudation. Plant and Soil 228:253–264. [Google Scholar]
- George T, Gregory P, Robinson J, Buresh R. 2002. Changes in phosphorus concentrations and pH in the rhizosphere of some agroforestry and crop species. Plant and Soil 246:65–73. [Google Scholar]
- Gerke J, Römer W, Jungk A. 1994. The excretion of citric and malic acid by proteoid roots of Lupinus albus L.; effects on soil solution concentrations of phosphate, iron, and aluminum in the proteoid rhizosphere in samples of an oxisol and a luvisol. Zeitschrift Für Pflanzenernährung Und Bodenkunde 157:289–294. [Google Scholar]
- Hinsinger P. 2001. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant and Soil 237:25–41. [Google Scholar]
- Hinsinger P, Plassard C, Tang C, Jaillard B. 2003. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant and Soil 248:43–59. [Google Scholar]
- Jaillard B, Plassard C, Hinsinger P. 2003. Measurements of H+ fluxes and concentrations in the rhizosphere In: Rengel Z, eds. Handbook of soil acidity. New York: Dekker, 231–266. [Google Scholar]
- Jakobsen I, Abbott L, Robson A. 1992. External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. New Phytologist 120:371–380. [Google Scholar]
- Krey T, Vassilev N, Baum C, Eichler-Löbermann B. 2013. Effects of long-term phosphorus application and plant-growth promoting rhizobacteria on maize phosphorus nutrition under field conditions. European Journal of Soil Biology 55:124–130. [Google Scholar]
- Kummerová M. 1986. Localization of acid phosphatase activity in maize root under phosphorus deficiency. Biologia Plantarum 28:270–274. [Google Scholar]
- Lambers H, Clode PL, Hawkins HJ, Laliberte E, Oliveira RS, Reddell P, Shane MW, Stitt M, Weston P. 2015. Metabolic adaptations of the non-mycotrophic proteaceae to soils with low phosphorus. Annual Plant Reviews, Phosphorus Metabolism in Plants 48:289. [Google Scholar]
- Lambers H, Finnegan PM, Laliberte E, Pearse SJ, Ryan MH, Shane MW, Veneklaas EJ. 2011. Update on phosphorus nutrition in Proteaceae. Phosphorus nutrition of proteaceae in severely phosphorus-impoverished soils: are there lessons to be learned for future crops? Plant Physiology 156:1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Raven JA, Shaver GR, Smith SE. 2008. Plant nutrient-acquisition strategies change with soil age. Trends in Ecology and Evolution 23:95–103. [DOI] [PubMed] [Google Scholar]
- Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ. 2006. Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Annals of Botany 98:693–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Shen J, Zhang F, Clairotte M, Drevon J, Le Cadre E, Hinsinger P. 2008. Dynamics of phosphorus fractions in the rhizosphere of common bean (Phaseolus vulgaris L.) and durum wheat (Triticum turgidum durum L.) grown in monocropping and intercropping systems. Plant and Soil 312:139–150. [Google Scholar]
- Li L, Li SM, Sun JH, Zhou LL, Bao XG, Zhang HG, Zhang FS. 2007. Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. Proceedings of the National Academy of Sciences 104:11192–11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SM, Li L, Zhang FS, Tang C. 2004. Acid phosphatase role in chickpea/maize intercropping. Annals of Botany 94:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Xu C, Li K, Yan S, Qu X, Zhang J. 2012. Phosphate starvation of maize inhibits lateral root formation and alters gene expression in the lateral root primordium zone. BMC Plant Biology 12:89.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Mi G, Chen F, Zhang J, Zhang F. 2004. Rhizosphere effect and root growth of two maize (Zea mays L.) genotypes with contrasting P efficiency at low P availability. Plant Science 167:217–223. [Google Scholar]
- Lynch JP, Ho MD. 2005. Rhizoeconomics: carbon costs of phosphorus acquisition. Plant and Soil 269:45–56. [Google Scholar]
- Lynch JP. 2007. Turner review no. 14. Roots of the second green revolution. Australian Journal of Botany 55:493–512. [Google Scholar]
- Marschner H, Römheld V. 1983. In vivo measurement of root-induced pH changes at the soil–root interface: effect of plant species and nitrogen source. Zeitschrift Für Pflanzenphysiologie 111:241–251. [Google Scholar]
- Marschner P. 2012. Mineral nutrition of higher plants, 3rd edn London: Academic Press. [Google Scholar]
- McLachlan K. 1980. Acid phosphatase activity of intact roots and phosphorus nutrition in plants. 2. Variations among wheat roots. Crop and Pasture Science 31:441–448. [Google Scholar]
- Mengel K, Kosegarten H, Kirkby EA, Appel T. 2001. Principles of plant nutrition. New York: Springer Science and Business Media. [Google Scholar]
- Miller MH. 1974. Effects of nitrogen on phosphorus absorption by plants In: Carson E, eds. The plant root and its environment. Charlottesville: University Press of Virginia, 643–668. [Google Scholar]
- Mollier A, Pellerin S. 1999. Maize root system growth and development as influenced by phosphorus deficiency. Journal of Experimental Botany 50:487–497. [Google Scholar]
- Neumann G, Martinoia E. 2002. Cluster roots–an underground adaptation for survival in extreme environments. Trends in Plant Science 7:162–167. [DOI] [PubMed] [Google Scholar]
- Neumann G, Massonneau A, Martinoia E, Römheld V. 1999. Physiological adaptations to phosphorus deficiency during proteoid root development in white lupin. Planta 208:373–382. [Google Scholar]
- Neumann G, Römheld V. 1999. Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant and Soil 211:121–130. [Google Scholar]
- Niu J, Chen F, Mi G, Li C, Zhang F. 2007. Transpiration, and nitrogen uptake and flow in two maize (Zea mays L.) inbred lines as affected by nitrogen supply. Annals of Botany 99:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuruzzaman M, Lambers H, Bolland MD, Veneklaas EJ. 2005a. Phosphorus benefits of different legume crops to subsequent wheat grown in different soils of Western Australia. Plant and Soil 271:175–187. [Google Scholar]
- Nuruzzaman M, Lambers H, Bolland MD, Veneklaas EJ. 2005b. Phosphorus uptake by grain legumes and subsequently grown wheat at different levels of residual phosphorus fertiliser. Crop and Pasture Science 56:1041–1047. [Google Scholar]
- Pang J, Ryan MH, Tibbett M, Cawthray GR, Siddique KH, Bolland MD, Denton MD, Lambers H. 2010. Variation in morphological and physiological parameters in herbaceous perennial legumes in response to phosphorus supply. Plant and Soil 331:241–255. [Google Scholar]
- Paponov IA, Engels C. 2003. Effect of nitrogen supply on leaf traits related to photosynthesis during grain filling in two maize genotypes with different N efficiency. Journal of Plant Nutrition and Soil Science 166:756–763. [Google Scholar]
- Plénet D, Mollier A, Pellerin S. 2000. Growth analysis of maize field crops under phosphorus deficiency. II. Radiation-use efficiency, biomass accumulation and yield components. Plant and Soil 224:259–272. [Google Scholar]
- Raghothama K. 1999. Phosphate acquisition. Annual Review of Plant Biology 50:665–693. [DOI] [PubMed] [Google Scholar]
- Richardson AE, Hocking PJ, Simpson RJ, George TS. 2009. Plant mechanisms to optimise access to soil phosphorus. Crop and Pasture Science 60:124–143. [Google Scholar]
- Schachtman DP, Reid RJ, Ayling SM. 1998. Phosphorus uptake by plants: from soil to cell. Plant Physiology 116:447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J. 1998. Separation and determination of low-molecular-weight organic acids and Phenolic acids in root exudates. PhD Thesis, China Agricultural University, China.
- Shen J, Tang C, Rengel Z, Zhang F. 2004. Root-induced acidification and excess cation uptake by N2-fixing Lupinus albus grown in phosphorus-deficient soil. Plant and Soil 260:69–77. [Google Scholar]
- Soon Y, Kalra Y. 1995. Short communication: a comparison of plant tissue digestion methods for nitrogen and phosphorus analyses. Canadian Journal of Soil Science 75:243–245. [Google Scholar]
- Sun J, Dai S, Wang R, Chen S, Li N, Zhou X, Lu C, Shen X, Zheng X, Hu Z, Zhang Z, Song J, Xu Y. 2009. Calcium mediates root K+/Na+ homeostasis in poplar species differing in salt tolerance. Tree Physiology 29:1175–1186. [DOI] [PubMed] [Google Scholar]
- Tang C, Barton L, McLay C. 1997. A comparison of proton excretion of twelve pasture legumes grown in nutrient solution. Animal Production Science 37:563–570. [Google Scholar]
- Tang C, Conyers MK, Nuruzzaman M, Poile G, Li Liu D. 2011. Biological amelioration of subsoil acidity through managing nitrate uptake by wheat crops. Plant and Soil 338:383–397. [Google Scholar]
- Tang C, Han XZ, Qiao YF, Zheng SJ. 2009. Phosphorus deficiency does not enhance proton release by roots of soybean [Glycine max (L.) Murr.]. Environmental and Experimental Botany 67:228–234. [Google Scholar]
- Tang C, Rengel Z. 2003. Role of plant cation/anion uptake ratio in soil acidification In: Rengel Z, eds. Handbook of soil acidity. New York: Dekker, 57–81. [Google Scholar]
- Vlek P, Lüttger A, Manske G. 1996. The potential contribution of arbuscular mycorrhiza to the development of nutrient and water efficient wheat In: Tanner DG, Payne TS, Abdalla OS, eds. The ninth regional wheat workshop for eastern, central and Southern Africa . Addis Ababa, Ethiopia: CIMMYT, 28–46. [Google Scholar]
- Wang Z, Shen J, Zhang F. 2006. Cluster-root formation, carboxylate exudation and proton release of Lupinus pilosus Murr. as affected by medium pH and P deficiency. Plant and Soil 287:247–256. [Google Scholar]
- White PJ, Veneklaas EJ. 2012. Nature and nurture: the importance of seed phosphorus content. Plant and Soil 357:1–8. [Google Scholar]
- Xu Y, Sun T, Yin LP. 2006. Application of non-invasive microsensing system to simultaneously measure both H+ and O2 fluxes around the pollen tube. Journal of Integrative Plant Biology 48:823–831. [Google Scholar]
- Yan H, Li K, Ding H, Liao C, Li X, Yuan L, Li C. 2011. Root morphological and proteomic responses to growth restriction in maize plants supplied with sufficient N. Journal of Plant Physiology 168:1067–1075. [DOI] [PubMed] [Google Scholar]
- Yu P, Li X, Yuan L, Li C. 2013. A novel morphological response of maize (Zea mays) adult roots to heterogeneous nitrate supply revealed by a split-root experiment. Physiologia Plantarum 150:133–144. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yu P, Peng Y, Li X, Chen F, Li C. 2012. Fine root patterning and balanced inorganic phosphorus distribution in the soil indicate distinctive adaptation of maize plants to phosphorus deficiency. Pedosphere 22:870–877. [Google Scholar]
- Zhu J, Kaeppler SM, Lynch JP. 2005. Mapping of QTL controlling root hair length in maize (Zea mays L.) under phosphorus deficiency. Plant and Soil 270:299–310. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.