Abstract
This systematic review applied meta-analytic procedures to integrate primary research that examined blood pressure outcomes of medication adherence interventions. Random-effects model analysis calculated standardized mean difference effect sizes. Exploratory dichotomous and continuous moderator analyses using meta-analytic analogues of ANOVA and regression were performed. Codable data were extracted from 156 reports with 60,876 participants. The overall weighted mean difference systolic effect size was 0.235 across 161 treatment versus control comparisons. The diastolic effect size was 0.189 from 181 comparisons. Effect sizes were significantly heterogeneous. Common risks of bias included lack of allocation concealment, unmasked data collectors, and absent intention-to-treat analyses. Exploratory moderator analyses suggested that habit-based interventions may be most effective. The largest effect sizes were for interventions delivered by pharmacists. The modest magnitude effect sizes suggest future research should explore novel higher dose interventions that might address multiple levels of influence on adherence behavior.
Keywords: medication adherence, blood pressure, meta-analysis
Only about half of people with hypertension achieve adequate blood pressure control (CDC, 2015). Poor medication adherence (henceforth, adherence) is a prevalent cause of inadequate hypertension control (Burnier, 2014; Christensen et al., 2009; De Geest et al., 2014; Erdine & Arslan, 2013; Simpson et al., 2006; Wofford & Minor, 2009). About half of hypertensive patients cease taking medication by one year after prescription (Jung et al., 2013; Vrijens et al., 2008). Roughly half of patients with presumed resistant hypertension are medication non-adherent (De Geest et al., 2014; Dragomir et al., 2010). Hypertensive patients with poor adherence have more coronary disease, heart failure, cerebrovascular disease, and non-drug medical costs (Bramlage & Hasford, 2009; Dragomir et al., 2010). Nonadherence is a major barrier to reducing cardiovascular mortality (Gwadry-Sridhar et al., 2013).
Negative sequelae of poor adherence have prompted many trials testing interventions to improve hypertension medication-taking behaviors with the goal of improving blood pressure outcomes. The studies have reported mixed results. Prior syntheses of extant research have limitations: they have been restricted to specific interventionist disciplines (Clark et al., 2011; Jayasinghe, 2009; Morgado et al., 2011) and specific interventions such as telecare or home blood pressure monitoring (Cappuccio et al., 2004; Uhlig et al., 2013; Verberk et al., 2011), included interventions to increase adherence with other treatments (Glynn et al., 2010), reviewed few studies with blood pressure outcomes (Gwadry-Sridhar et al., 2013; Lewis, 2012; Lewis et al., 2012; Matthes & Albus, 2014; Morgado et al., 2011), did not meta-analyze blood pressure outcomes (Gwadry-Sridhar et al., 2013; Matthes & Albus, 2014; Morgado et al., 2011; Nieuwlaat et al., 2014; Schroeder et al., 2004), or focused on adherence outcomes instead of blood pressure outcomes (Chapman et al., 2010; Christensen et al., 2009; Gupta et al., 2010; Iskedjian et al., 2002; Sherrill et al., 2011; Takiya et al., 2004).
No comprehensive review has summarized and synthesized existing knowledge about blood pressure outcomes of adherence interventions. This systematic review and meta-analysis addressed the following questions: 1) What is the overall mean effect of adherence interventions on blood pressure outcomes among subjects with hypertension? 2) Do effects of interventions vary depending on sample and study characteristics? 3) Do the effects vary depending on intervention features? 4) What risks of bias are present in existing research, and are they associated with effect sizes?
Methods
Standard systematic review and meta-analytic methods following PRISMA guidelines were used to conduct and report this project (Cooper et al., 2009; Liberati et al., 2009).
Information Sources and Search Strategy
Multiple comprehensive searches strategies were employed to minimize the bias associated with limited searches (Dickersin et al., 1994; Rothstein & Hopewell, 2009; White, 2009). Searches in PubMED, MEDLINE, PsycINFO, Cochrane Database of Systematic Reviews, Cochrane Central Trials Register, EBSCO, CINAHL, PQDT, ERIC, IndMed, EBM Reviews - Database of Abstracts of Reviews of Effects, International Pharmaceutical Abstracts, and Communication and Mass Media were conducted by an expert health sciences librarian. The primary MeSH terms upon which searches were constructed were Patient Compliance and Medication Adherence. Patient Compliance was used to locate studies published prior to 2009. The MeSH term Medication Adherence, which came into MeSH usage in 2009, was used to locate studies published from that year and later. Other MeSH terms used in constructing search strategies were: prescription drugs, pharmaceutical preparations, dosage forms, drugs, or generic. Keywords used in searches were: prescription(s), prescribed, medication(s), regimen(s), drug(s), pill(s), tablet(s), agent(s), compliant, compliance, noncompliant, noncompliance, adherent, adherence, nonadherent, nonadherence, improve, promote, address, influence, enhance, increase, encourage, incentive, ensure, remind, foster, advocate, optimize, impact, prevent, decrease. Additional searching was conducted using the terms execution, persistence, and discontinuation to address international differences in adherence terminology.
Hand searches were conducted in 57 journals where multiple eligible studies were published (Langham et al., 1999). Abstracts from forty-eight conferences were retrieved and evaluated for eligible studies. Author searches were completed for authors of more than one eligible study. Ancestry searches were conducted on eligible studies and review papers. Nineteen research registers (e.g., Research Portfolio Online Reporting Tool) were searched (Easterbrook, 1992; Stern & Simes, 1997).
Eligibility Criteria
Studies with interventions designed to increase adherence among adults with hypertension were eligible. Studies with any subjects without hypertension were excluded. Only studies comparing treatment subjects to control subjects were included. Intervention trials with data adequate to calculate blood pressure effect sizes in the reported study or available from corresponding authors were included. Both published and unpublished studies were included because the single most consistent difference between published and unpublished studies is the statistical significance of findings (Rothstein & Hopewell, 2009; Sutton, 2009).
Study Selection
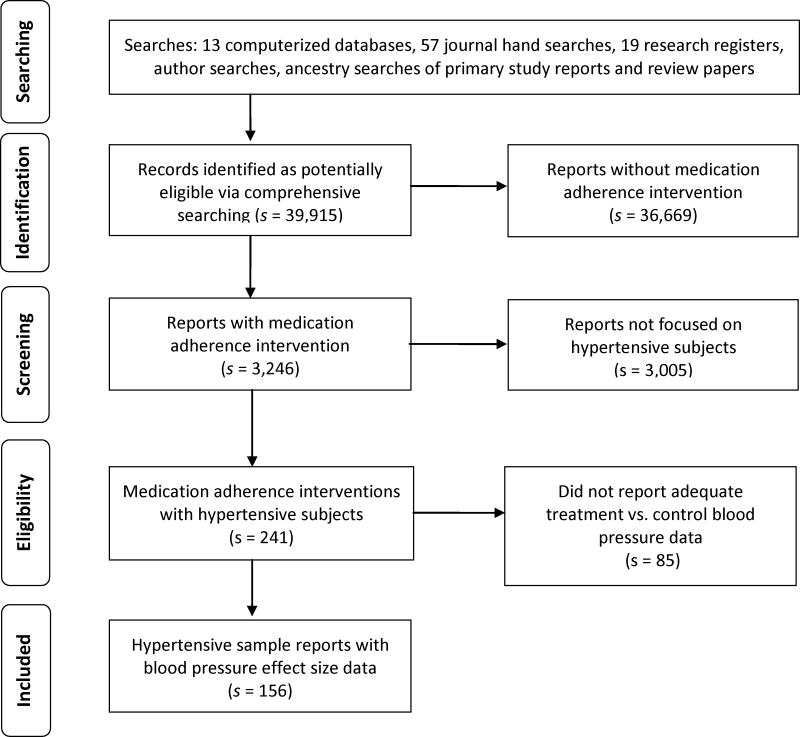
Figure 1 shows how potentially eligible primary studies flowed through the project (Liberati et al., 2009). Potential studies were imported into bibliographic software and tracked with customized fields and linked terms lists. Eligibility criteria were applied: presence of an intervention to increase adherence, hypertensive sample, and blood pressure outcome data. When necessary, additional publications about the study were sought or corresponding authors were contacted to confirm eligibility or provide outcome data. Author names for each study were compared against author names of other studies to ensure independence of data by avoiding sample overlap in coded studies (Wood, 2008).
Figure 1.
Flow diagram of potential studies through review process.
Note: s indicates the number of research reports
Data Items and Collection
Data items were developed by following suggestions from medication adherence and meta-analysis experts, examining adherence intervention review articles, and previewing primary studies (Devine, 1997; Orwin & Vevea, 2009). The coding frame was pilot tested with 20 studies prior to implementation. The coding frame included information about study source, study methods, participant characteristics, intervention features, and outcome data. Dissemination vehicle, presence and type of funding, and year of distribution were coded as source attributes. Assignment to groups, allocation concealment, type of control group (i.e., true control vs. attention control), data collector masking, attrition, and intention-to-treat analyses were coded as methodological features (Liberati et al., 2009). Primary study participants’ characteristics were coded (mean age, gender and racial/ethnic composition, education, socio-economic status, literacy, alcohol/substance abuse, mental health problems, homelessness, intentional non-adherence, unintentional non-adherence, poor adherence at enrollment). Clinical and setting characteristics were coded (interventions delivered to health care providers, interventions delivered to groups of subjects, interventions delivered to individual patients, interventions delivered in specific settings: ambulatory care, acute care, pharmacy).
Intervention features coded included theoretical foundation for intervention, delivery medium (e.g., face-to-face, mail), interventionist professional preparation (i.e., pharmacist, physician, advanced practice nurse, other nurse), and intervention dose (i.e., number and duration of sessions). Intervention content was coded and included in exploratory moderator analysis if reported by at least ten comparisons: strategies to manage barrier to adherence, feedback to patients about their adherence, researcher/health care provider feedback to patients about their blood pressure values, adherence goal setting, improved communication between patients and health care providers, increased integration of health care services, habit-based content such as linking medication consumption with well-established habits, motivational interviewing tactics, packaging medications such as in blister packs or pill boxes, patients self-monitoring adherence behavior, patients self-monitoring blood pressure, and providing written materials to patients. Diverse other adherence intervention content was coded but reported by fewer than 10 primary studies and so excluded from analyses.
Sample sizes, means, measures of variability, t statistics, and success rates were coded to calculate blood pressure effect sizes. Whenever multiple reports were available about the same subjects, all were used to code study information. Two extensively trained research specialists independently coded effect size data from each study to achieve 100% agreement.
Statistical Analysis: Summary Measure and Method of Analyses
A standardized mean difference effect size was calculated for each comparison (Borenstein et al., 2009; Lipsey & Wilson, 2001). Treatment vs. control post-intervention effect size refers to treatment group results compared to control group results after adherence interventions. A positive effect size indicates better results for treatment than for control subjects. To give more weight to studies with larger samples, each effect size was weighted by the inverse of variance (Hedges & Olkin, 1985; Lipsey & Wilson, 2001). Effect sizes were adjusted for bias. Effect sizes were converted to the original metric to facilitate interpretation (Lipsey & Wilson, 2001).
Effect sizes were calculated using the random-effects model, which assumes individual effect sizes vary due to variations at both study level and subject level (Hedges & Vevea, 1998; Raudenbush, 2009). The random effects model is consistent with this area of science, with common variations in samples, interventions, and methods. Q, a standard heterogeneity statistic, was used to assess effect size homogeneity (Shadish & Haddock, 2009). The I2 index was computed as a measure of heterogeneity beyond within-study sampling error (Borenstein et al., 2009). Heterogeneity was expected and handled in four ways (Conn et al., 2007). First, location parameters and measures of heterogeneity were reported. Second, random-effects models were used because the model assumes variations in studies beyond sampling error. Third, potential sources of heterogeneity were explored with moderator analyses. Finally, findings were interpreted in light of discovered heterogeneity.
Exploratory moderator analyses were conducted to identify characteristics of interventions associated with larger reductions in blood pressure outcomes. Dichotomous moderator analysis used a meta-analytic analogue of ANOVA and continuous moderator analysis used a meta-analytic version of regression (Borenstein et al., 2009).
Risk of Bias
Meta-analysis approaches to address variable methodological quality among primary studies use a priori inclusion criteria related to rigor, post hoc procedures for considering quality as an empirical question, and combination approaches. This meta-analysis used combination approaches. Effect sizes were reported only for two-group post-intervention comparisons to partially address design bias in single-group pre-post comparisons.
Comprehensive search strategies helped avoid the bias of including studies with larger effect sizes, which are often easier to locate (Sutton, 2009). Publication bias was assessed using funnel plots and Begg’s test using Kendall’s method (Borenstein et al., 2009).
Variations in primary study sample size were managed statistically. Small-sample studies were included because, although they may lack statistical power in primary research, they contribute to effect size estimates, and meta-analysis does not rely on p values from primary research. Effect size estimates were weighted so more precise estimates (e.g., due to larger sample sizes) would be given proportionally more influence on our findings.
Moderator analysis was used as a form of sensitivity analysis to examine potential links between effect sizes and common indicators of methodological strength (random assignment of subjects to groups, allocation concealment, data collector masking, and intention-to-treat analyses) (Liberati et al., 2009). Sensitivity analysis to compare effect sizes between studies reporting dichotomous and continuous blood pressure outcomes was used to explore this potential bias. Effect sizes were calculated separately for studies with three of fewer risks of bias (e.g., random assignment to groups, allocation concealment, use of attention control comparison groups, data collector masking, intention-to-treat analyses) to further address methodological rigor. Effect sizes were not weighted by overall quality scores because existing quality instruments lack validity and study quality is not a unitary construct (Conn & Rantz, 2003; de Vet et al., 1997; Higgins et al., 2011; Valentine, 2009).
Results
Comprehensive searching located 156 eligible primary study reports (list of primary studies is in electronic supplementary materials). Forty-four additional papers that reported on the same studies were used as companion papers to enhance coded data. The earliest study was published in 1974; 116 reports were published in 2000 or later. The project included four papers published in Spanish, thirteen dissertations, two conference presentations, and one unpublished report. While many studies were conducted or published in the United States, the sample of studies reflected international research. For example, 19 European journals or international journals with European editors published eligible reports.
Since behavioral intervention studies often contain multiple treatment groups, some primary study reports included multiple treatment vs. control comparisons. For systolic outcomes, there were 12 papers with two comparisons, 5 papers with three comparisons, and 1 paper with seven comparisons. For diastolic outcomes, there were 14 papers with two comparisons, 7 papers with three comparisons, and 1 paper with seven comparisons. Not all studies reported both systolic and diastolic outcomes; 165 systolic treatment vs. control comparisons and 188 diastolic comparisons were available. All results are reported for treatment versus control comparison studies (i.e., not for reports).
Primary Study Characteristics
The primary studies included a total of 60,876 subjects (32,595 treatment, 28,281 control). Table 1 provides descriptive characteristics of the included studies. The median sample size was 110 participants. The median attrition rate was 8%. The median of mean age was 59 years. Women were well-represented in samples. Of the 82 studies reporting minority participants, the median percentage of minority subjects was 75%.
Table 1.
Characteristics of Primary Studies Included in Blood Pressure Meta-analyses
| Characteristic | k | Min | Q1 | Mdn | Q3 | Max |
|---|---|---|---|---|---|---|
| Total post-test sample size per study | 193 | 4 | 51 | 110 | 253 | 10,110 |
| Percentage attrition | 156 | 0 | 0 | 8.05 | 21.74 | 68.25 |
| Mean age (years) | 149 | 39 | 53.7 | 59.1 | 64.2 | 77.4 |
| Percentage female | 160 | 0 | 48.9 | 57 | 65.5 | 100 |
| Percentage ethnic minority | 82 | 2 | 27 | 75 | 100 | 100 |
Note. Includes all studies that contributed to primary analyses at least one effect size for either systolic or diastolic comparisons. k=number of comparisons providing data on characteristic; Q1=first quartile, Q3=third quartile.
Co-morbidities were poorly reported in these studies. Only six studies reported the mean number of chronic diseases, and only seven reported the mean number of medications. The most common co-morbidities were diabetes (k = 68), cardiac disease (k = 36), hyperlipidemia (k = 21), stroke (k = 21), renal disease (k = 19), and osteoporosis (k = 15) (k is the number of comparisons).
Behavior Change Intervention Characteristics
Some 39 studies reported using a behavior change theory or model to guide the interventions, including social cognitive theory (13 studies) and the health belief model (9). Intervention characteristics included: written materials provided to patients to improve medication adherence (62 studies), blood pressure self-monitoring by participants (64), feedback about blood pressure values provided to subjects (19), medication adherence behavior goal setting (19), intervention content focused on managing barriers to adherence (17), asking participants to self-monitor medication administration (16), providing rewards for medication adherence behavior change (15), providing feedback to subjects about medication adherence behavior (15), special packaging such as blister packs and pill boxes (12), habit analysis and linking medication adherence with habits (12), and motivational interviewing to change health behavior (10).
Some interventions targeted health care delivery activities, including attempts to increase integration of care across health care providers as a strategy to increase medication adherence (38 studies), and efforts to improve communication between patients and health care providers (11).
Overall Effects of Intervention on Blood Pressure Outcomes
Overall blood pressure outcome effect sizes are presented in Table 2. The overall standardized mean difference systolic blood pressure effect size for treatment vs. control comparisons was 0.235. Analysis of residuals revealed four outliers, and those effect sizes were excluded from estimation of the overall effect size. The overall mean effect size with the outliers included was 0.292. The effect size represents the degree of difference between treatment and control groups. The 0.235 effect size is consistent with a difference of 3 mm Hg in systolic blood pressure.
Table 2.
Random-Effects Blood Pressure Outcome Estimates and Tests
| k | Effect size | p (ES) | 95% Confidence interval | Standard error | I2 | Q | p (Q) | |
|---|---|---|---|---|---|---|---|---|
| Systolic blood pressure outcomes, outliers excludeda | 161 | 0.235 | <.001 | 0.190, 0.281 | 0.023 | 74.245 | 621.230 | <.001 |
| Systolic blood pressure outcomes, all studies included | 165 | 0.292 | <.001 | 0.235, 0.348 | 0.029 | 84.722 | 1073.450 | <.001 |
| Diastolic blood pressure outcomes, outliers excludedb | 181 | 0.189 | <.001 | 0.146, 0.232 | 0.022 | 74.705 | 711.615 | <.001 |
| Diastolic blood pressure outcomes, all studies included | 188 | 0.260 | <.001 | 0.207, 0.312 | 0.027 | 84.550 | 1210.389 | <.001 |
Note. k denotes number of comparisons, Q is a conventional heterogeneity statistic, I2 is the percentage of total variation among studies’ observed effect sizes due to heterogeneity.
Four comparisons were excluded as outliers.
Seven comparisons excluded as outliers.
The diastolic blood pressure mean difference effect size for treatment-vs.-control comparisons was 0.189. Analysis revealed seven outliers that were excluded from estimation of the effect size. The effect size with the outliers included was 0.260. The 0.189 effect size is consistent with a 2 mm Hg difference between treatment and control subjects in diastolic blood pressure following interventions.
Tests of the heterogeneity statistic Q indicated significant between-studies variation for all systolic and diastolic effect size estimates. I2 values from 74 to 85 indicate the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error.
Post-hoc Exploratory Analyses of Studies with High and Low Blood Pressure Effect Sizes
Limited post-hoc analyses were conducted following the discovery of small overall blood pressure effect sizes. Studies with the largest and smallest blood pressure effect sizes were reexamined to determine if they reported medication adherence behavior outcome information. Many studies with blood pressure outcomes did not report adherence outcomes. For example, five of the ten studies with the highest blood pressure effect sizes did not report adherence behavior. The following results pertain to the ten highest and lowest blood pressure effect size studies that also reported adherence outcomes. For diastolic blood pressure, the ten studies with the largest blood pressure effect sizes that also reported adherence behavior data had an overall mean difference adherence effect size of 1.259 (CI: 0.688, 1.830). In contrast, the ten studies with the smallest blood pressure effect sizes had an adherence effect size of 0.087 (CI: −0.117, 0.288). For systolic blood pressure, the ten studies with the largest blood pressure effect sizes that also reported adherence behavior data had an overall mean difference adherence effect size of 0.881 (CI: 0.398, 1.364). In contrast, the ten studies with the smallest blood pressure effect sizes had an adherence effect size of 0.032 (CI: −0.083, 0.147).
Moderator Analyses of Study, Sample, and Intervention Characteristics
Study Features
Unfunded studies reported significantly larger effect sizes (systolic d = 0.304, diastolic d = 0.341) than studies that reported funding (systolic d = 0.205, diastolic d = 0.159). No difference in effect sizes for either systolic or diastolic effect sizes were reported between published and unpublished studies. Studies published more recently reported slightly larger blood pressure effect sizes than older studies for both systolic (p < .001) and diastolic (p < .001) outcomes.
Sample Attributes
For diastolic blood pressure, studies with younger subjects reported larger effect sizes than studies with older subjects (p < .001). Age was unrelated to effect size for systolic outcomes. The percentages of minority subjects were unrelated to either systolic or diastolic effect sizes. Studies with a smaller proportion of women reported slightly larger diastolic effect sizes than studies with more females (p = .003). No association between gender and effect sizes was noted for systolic outcomes. No link between socio-economic status and either systolic or diastolic blood pressure was found. Studies which limited their sample to participants who were literate did not report significantly different systolic or diastolic blood pressure when compared to studies which did not limit their sample by literacy. Several other study population characteristics were coded but could not be analyzed because they were infrequently reported: education, participants with cognitive impairment, homeless participants, participants with mental health problems, and participants with alcohol misuse or other substance abuse.
Although coded from the primary studies, too few studies reported information about whether subjects were recruited because they had intentional or nonintentional adherence difficulties for moderator analyses. Few studies recruited subjects specifically because the subjects had inadequate medication adherence. The effect size for systolic outcomes among the nine studies with non-adherent participants was not significantly different from studies which did not recruit subjects with adherence problems. For diastolic outcomes, the effect size among the 11 studies with non-adherent participants was not significantly different from studies which did not recruit subjects with adherence problems.
Intervention Characteristics
Intervention characteristics reported by at least 10 studies were analyzed to determine if they were linked with blood pressure effect sizes. The presence or absence of a behavior change theory (e.g., social cognitive theory, transtheoretical model) as a foundation for the adherence intervention was not associated with differences in systolic or diastolic effect sizes.
Habit-based interventions were effective (habit d = 0.477; no habit d = 0.181) in improving diastolic outcomes (p < .001). The difference in these interventions (habit d = 0.330, no habit d = 0.234) was not statistically significant for systolic blood pressure.
Several specific adherence behavior change strategies reported in adherence interventions were not linked with differences in blood pressure effect sizes: adherence barrier management, rewards for adherence, feedback to patients about their adherence, researcher/health care provider feedback about blood pressure values, adherence goal setting, improved communication between patients and health care providers, increased integration of health care services, motivational interviewing tactics, packaging medications, patients self-monitoring adherence behavior, patients self-monitoring blood pressure, and providing written materials to patients.
Effect sizes for different interventionist professional characteristics appear in Table 3. The largest effect sizes were for pharmacists (d = 0.235 – 0.317) and the smallest were for registered nurses who were not advanced practice nurses (d = 0.142 – 0.149). Interventions delivered face-to-face (d = 0.221) were significantly more effective in reducing diastolic blood pressure than those delivered in a mediated manner such as by mail (d = 0.060). A similar pattern for systolic outcomes (d = 0.256 vs d = 0.179) did not achieve statistical significance.
Table 3.
Interventionist Blood Pressure Outcome Estimates and Tests
| Systolic blood pressure | Diastolic blood pressure | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| k | Effect size | p (ES) | 95% Confidence interval | k | Effect size | p (ES) | 95% Confidence interval | |
| Pharmacist interventionist | 39 | 0.317 | <.001 | 0.206, 0.427 | 43 | 0.235 | <.001 | 0.145, 0.324 |
| Physician interventionist | 25 | 0.218 | <.001 | 0.124, 0.313 | 27 | 0.199 | <.001 | 0.092, 0.307 |
| Advanced practice registered nurse | 8 | 0.298 | .001 | 0.127, 0.469 | 12 | 0.224 | .030 | 0.021, 0.427 |
| Registered nurse (not advanced practice) | 12 | 0.142 | .089 | −0.022, 0.305 | 15 | 0.149 | .101 | −0.029, 0.327 |
Note. k denotes number of comparisons.
Intervention dose (minutes per session and number of sessions) was poorly reported. Only 40 studies with systolic outcome and 44 studies with diastolic outcomes reported this information. Studies with a larger intervention dose were more effective in improving both systolic (p = .021) and diastolic (p = .027) outcomes.
Clinical/Setting Characteristics
Several clinical/setting characteristics were examined as potential moderators of systolic and diastolic blood pressure effect sizes. The analyses revealed no differences in systolic or diastolic effect sizes based on whether the interventions targeted patients and families as compared to those which delivered interventions directly to health care providers expecting the health care provider to improve patient adherence. Effect sizes did not differ between interventions delivered in ambulatory care settings as compared to those delivered in other locations such as participants’ home or community centers. Too few studies reported interventions delivered in acute-care in-patient clinical settings for moderator analyses.
Interventions delivered in pharmacies were more effective than those delivered in other locations. For systolic blood pressure, the effect size was 0.360 (k = 9, CI: 0.247, 0.472) for pharmacy delivered interventions and the effect size for other locations was 0.226 (k = 152, CI: 0.179, 0.272, Qb = 4.637, p = .031). The diastolic effect size for pharmacy delivered interventions was 0.356 (k = 10, CI: 0.231, 0.501) which was significantly larger than interventions not delivered in pharmacies (effect size = 0.177, k = 171, CI: 0.133, 0.226, Qb = 6.783, p = .009). Interventions delivered to groups of patients were more effective than interventions delivered to individual patients and/or patient families. For systolic blood pressure, the effect size was 0.399 (k = 10, CI: 0.254, 0.545) for group interventions and 0.228 for individual/family delivered interventions (k = 151, CI: 0.181, 0.274, Qb = 4.848, p = .029). The diastolic effect size for group delivered interventions was 0.376 (k = 12, CI: 0.219, 0.534) which was significantly larger than intervention delivered to individual or families (effect size = 0.179, k = 169, CI: 0.135, 0.223, Qb = 5.606, p = .018).
Risks of Bias
Table 4 depicts dichotomous moderator analyses for risks of bias. Neither allocation method (individual randomization vs. other method of allocation) nor allocation concealment was related to systolic or diastolic effect sizes. The use of attention control groups was not analyzed as a moderator because only one study used such a group. The presence or absence of data collector masking was unrelated to systolic or diastolic outcomes.
Table 4.
Risk of Bias Sensitivity Analyses
| Systolic blood pressure | Diastolic blood pressure | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Moderator | k | Effect size | Qbetween | p (Qbetween) | k | Effect size | Qbetween | p (Qbetween) |
| Allocation to treatment groups | 0.005 | .943 | 0.087 | .768 | ||||
| Randomization of individual subjects | 112 | 0.238 | 128 | 0.184 | ||||
| Subjects not individually randomized | 49 | 0.234 | 53 | 0.198 | ||||
| Allocation concealment | 0.782 | .377 | 2.408 | .121 | ||||
| Allocation concealed | 36 | 0.202 | 36 | 0.124 | ||||
| Did not report allocation concealed | 125 | 0.248 | 145 | 0.211 | ||||
| Data collector masking | 0.008 | .928 | 1.101 | .294 | ||||
| Data collectors masked to group assignment | 36 | 0.234 | 37 | 0.141 | ||||
| Did not report data collectors masked to group assignment | 125 | 0.228 | 144 | 0.201 | ||||
| Intention-to-treat approach | 0.581 | .446 | 6.630 | .010 | ||||
| Reported intention-to-treat approach | 37 | 0.208 | 35 | 0.102 | ||||
| Did not report intention-to-treat approach | 124 | 0.247 | 146 | 0.220 | ||||
| Reported continuous vs. dichotomous blood pressure outcomes | 0.743 | .389 | 1.194 | .275 | ||||
| Continuous data | 132 | 0.224 | 131 | 0.170 | ||||
| Dichotomous data | 29 | 0.297 | 50 | 0.235 | ||||
| Publication status | 0.081 | .776 | 0.242 | .623 | ||||
| Published articles | 145 | 0.240 | 166 | 0.192 | ||||
| Dissertations, presentations, unpublished reports | 16 | 0.218 | 15 | 0.142 | ||||
Note. k denotes number of comparisons, effect size is standardized mean difference, Q is a conventional heterogeneity statistic.
For diastolic outcomes, studies using intention-to-treat analysis (d = 0.102) reported significantly smaller effect sizes than studies without intention-to-treat analyses (d = 0.220). The analysis for systolic outcomes did not reveal a significant difference based on intention-to-treat analyses. Studies with lower attrition reported significantly larger diastolic blood pressure effect sizes (p < .001) than studies with higher attrition. The negative association between attrition and systolic effect sizes did not achieve statistical significance (p = .05).
Additional analyses were conducted on studies with three or fewer risks of bias to further examine methodological rigor. Risks of bias were not related to effect size for systolic outcomes. The effect size for diastolic outcomes among studies with fewer risks of bias was 0.119 (k = 73, CI = 0.063, 0.175) which was significantly lower than among studies with more risks of bias (effect size = .247, k = 108, CI = .187, .308, Qb = 9.4, p = .002).
Egger’s test (p < .0001) confirmed publication bias for systolic outcomes. Duval and Tweedie’s trim and fill method suggested the corrected effect size would be 0.157. No publication bias was detected for diastolic outcomes (Egger’s test p = .467).
Discussion
This is the first comprehensive systematic review and meta-analysis of the effect of medication adherence behavior change interventions on blood pressure outcomes. The significant overall mean difference documents that interventions are effective in decreasing systolic and diastolic blood pressure. The modest effect may reflect the difficulty in changing adherence behavior which decreases blood pressure. Our post-hoc exploratory analyses confirmed that interventions that improved adherence behavior successfully reduced blood pressure. A recent meta-analysis of medication adherence behavior outcomes of interventions to increase adherence reported an overall standardized mean difference effect size of only 0.300. This was consistent with treatment subjects taking 4% more of their prescribed daily doses than control subjects at outcome (Conn et al., in press). These findings suggest blood pressure interventions are not lowering blood pressure because they are not increasing medication adherence. Future research should report both blood pressure and medication adherence behavior outcomes.
The modest effect may also reflect the multiple factors influencing blood pressure, beyond medication adherence. This project was unable to quantify other influences on blood pressure, such as diet and exercise, because primary studies rarely reported them. Future intervention research should either control for other influences on blood pressure, or if the intervention is designed to address multiple health behaviors, a factorial approach would be useful to determine the differential impact of medication adherence, diet, and exercise interventions on blood pressure control.
The findings of 3 and 2 mm Hg reductions in systolic and diastolic blood pressure following adherence interventions are similar to the reductions of 4 for systolic and 3 for diastolic for exercise (Cornelissen & Smart, 2013); 3 and 4 for systolic and 2 for diastolic blood pressure for home blood pressure monitoring interventions (Cappuccio et al., 2004; Glynn et al. 2010; Uhlig et al., 2013); and 4 for both systolic and diastolic for weight reduction interventions (Neter et al., 2003). Intervention research has had limited effectiveness in producing the magnitude of changes in health behavior required to improve health, despite the well-documented importance of health behaviors in determining or strongly influencing many health outcomes.
Several explanations are possible for the limited magnitude of blood pressure improvements. The predominant focus of adherence interventions may account for limited effects. Most interventions focus on changing individual patients, especially their beliefs and knowledge. The focus on knowledge and beliefs may not be adequate to improve blood pressure outcomes. The exploratory moderator analyses suggested that habit-focused interventions more effectively reduce blood pressure. Habit-based interventions examine participants’ daily routines and link medication administration to existing habitual behaviors or routine events. A previous meta-analysis reported that habit-based interventions were more effective than other interventions at improving adherence among studies targeting chronically ill patients with poor medication adherence (Conn et al., 2015). Habit-based interventions may also be easily tailored to enhancing other healthy habits, such as diet and exercise, that may influence blood pressure outcomes. Such a multi-pronged approach may be useful to improve blood pressure across patient populations.
The focus on individuals may be another reason interventions have limited effects on blood pressure. Few interventions deal with the context of adherence behavior, such as family. Complex multi-level interventions that target individuals, families and social context, health care providers, and systems of health care may be necessary to achieve clinically significant reductions in blood pressure.
Treating all subjects identically in adherence behavior intervention research may be another potential explanation for limited effects. For example, none of the primary studies in this review addressed whether participants’ lack of adherence was intentional. Very different interventions may be needed for intentional and nonintentional adherence (Gwadry-Sridhar et al., 2013). Future research may benefit from assessing reasons for patients’ nonadherence and targeting interventions to address the reasons for poor medication adherence.
The moderator analyses revealed some interesting patterns related to intervention delivery which could be examined in future research and implemented in practice. For example, interventions delivered to groups were more effective than interventions to individual patients. Group interventions are likely less costly while being more effective. Future research could explore mechanisms, such as social support and group composition, by which group interventions are superior. Interventions delivered by pharmacists/in pharmacies were most effective. Patients may recognize pharmacists as experts in pharmacology therapy and be especially receptive to their suggestions. Additional research could examine differences in intervention delivery and content pharmacists provide to patients compared to other health care professionals.
Further exploration of sample characteristics as moderators of effect size may be possible if future research provides more details about samples. Racial/ethnic composition of samples was poorly reported. Hypertension is prevalent in some minority groups; however, only about 50% of the studies included in this meta-analysis reported sample racial/ethnic composition, the median of 75% minority participants was surprising. This could reflect a tendency for studies with strong minority participation to report this information while studies with limited minority participants may be less likely to report sample race/ethnicity distribution. It is also possible researchers are targeting studies to minority participants because of persistent health disparities related to blood pressure control. The high minority representation in some studies could reflect clinic demographics where researchers recruited subjects. Future intervention studies should strive to include data on sample racial/ethnic composition to strengthen generalizability to more diverse populations. Interventions designed specifically for some underrepresented groups may need to be developed.
This synthesis had both project-specific limitations and those inherent in meta-analysis research. Despite comprehensive searching, some relevant studies may have been missed. Moderator analyses should be considered exploratory and observational. Moderator analyses of some potentially interesting variables, such as the numbers of medications and co-morbid conditions, were not conducted because the variables were poorly reported in extant literature. The multiple factors that influence blood pressure other than medication adherence were not addressed in this review because they are inadequately described in primary research testing medication adherence interventions.
Future research should consider interventions that understand medication adherence behavior in the context of individual life patterns, such as habit-based interventions, and in the context of family environments. New research should provide detailed information about subjects’ medication regimens and co-morbidities. Risks of bias were common among primary research. Effect sizes of studies were smaller among more rigorous studies than among studies with more potential risks of bias. Future research should be more vigilant in limiting common sources of bias such as the absence of random assignment, allocation concealment, attention control groups, intention-to-treat analyses, and masking of data collectors.
Adherence behavior interventions are important, given the poor adherence among many patients with resistant hypertension (Burnier, 2014). This comprehensive review of adherence interventions documented significant but modest magnitude reductions in blood pressure.
Supplementary Material
Acknowledgments
Funding: The project was funded by the American Heart Association (13GRNT16550001) and the National Institutes of Health (R01NR011990). The content is solely the responsibility of the authors and does not necessarily represent the official views of the American Heart Association or the National Institutes of Health.
Footnotes
Conflicts of Interest: The authors declare that they have no conflict of interest.
Ethical approval: This project did not include human subjects.
References
- Borenstein M, Hedges L, Higgins JPT, Rothstein H. Introduction to Meta-Analysis. West Sussex, England: John Wiley & Sons, Ltd; 2009. [Google Scholar]
- Bramlage P, Hasford J. Blood pressure reduction, persistence and costs in the evaluation of antihypertensive drug treatment--a review. Cardiovascular Diabetology. 2009;8:18. doi: 10.1186/1475-2840-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnier M. Managing ‘resistance’: is adherence a target for treatment? Current Opinion in Nephrology and Hypertension. 2014;23:439–443. doi: 10.1097/MNH.0000000000000045. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Kerry SM, Forbes L, Donald A. Blood pressure control by home monitoring: meta-analysis of randomised trials. BMJ. 2004;329(7458):145. doi: 10.1136/bmj.38121.684410.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. High blood pressure fact sheet. Centers for Disease Control and Prevention; 2015. Retrieved from http://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_bloodpressure.htm. [Google Scholar]
- Chapman RH, Ferrufino CP, Kowal SL, Classi P, Roberts CS. The cost and effectiveness of adherence-improving interventions for antihypertensive and lipid-lowering drugs. International Journal of Clinical Practice. 2010;64:169–181. doi: 10.1111/j.1742-1241.2009.02196.x. [DOI] [PubMed] [Google Scholar]
- Christensen A, Osterberg LG, Hansen EH. Electronic monitoring of patient adherence to oral antihypertensive medical treatment: a systematic review. Journal of Hypertension. 2009;27:1540–1551. doi: 10.1097/HJH.0b013e32832d50ef. [DOI] [PubMed] [Google Scholar]
- Clark CE, Smith LF, Taylor RS, Campbell JL. Nurse-led interventions used to improve control of high blood pressure in people with diabetes: a systematic review and meta-analysis. Diabetic Medicine. 2011;28:250–261. doi: 10.1111/j.1464-5491.2010.03204.x. [DOI] [PubMed] [Google Scholar]
- Conn V, Ruppar T, Chase J, Enriquez M, Cooper P. Interventions to improve hypertension medication adherence behavior: systematic review and meta-analysis. Current Hypertension Reports. doi: 10.1007/s11906-015-0606-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn V, Ruppar T, Enriquez M, Cooper PS. Medication adherence interventions that target subjects with adherence problems: systematic review and meta-analysis. Research in Social and Administrative Pharmacy. 2015 doi: 10.1016/j.sapharm.2015.06.001. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn VS, Hafdahl AR, Mehr DR, LeMaster JW, Brown SA, Nielsen PJ. Metabolic effects of interventions to increase exercise in adults with type 2 diabetes. Diabetologia. 2007;50:913–921. doi: 10.1007/s00125-007-0625-0. [DOI] [PubMed] [Google Scholar]
- Conn VS, Rantz MJ. Research methods: managing primary study quality in meta-analyses. Research in Nursing and Health. 2003;26:322–333. doi: 10.1002/nur.10092. [DOI] [PubMed] [Google Scholar]
- Cooper H, Hedges LV, Valentine JC, editors. The Handbook of Research Synthesis and Meta-Analysis. 2. New York: Russell Sage Foundation; 2009. [Google Scholar]
- Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. Journal of the American Heart Association. 2013;2(1):e004473. doi: 10.1161/JAHA.112.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Geest S, Ruppar T, Berben L, Schonfeld S, Hill MN. Medication non-adherence as a critical factor in the management of presumed resistant hypertension: a narrative review. Euro Intervention. 2014;9:1102–1109. doi: 10.4244/EIJV9I9A185. [DOI] [PubMed] [Google Scholar]
- de Vet HC, de Bie RA, van der Heijden GJ, Verhagen AP, Sijpkes P, Kipschild P. Systematic review on the basis of methodological criteria. Physiotherapy. 1997;83:284–289. [Google Scholar]
- Devine E. Issues and challenges in coding interventions for meta-analysis of prevention research. Bukoski W, editor. Meta-analysis of Drug Abuse Prevention Programs. 1997:130–146. (NIDA research monograph; 170).). Retrieved from http://archives.drugabuse.gov/pdf/monographs/monograph170/monograph170.pdf. [PubMed]
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ. 1994;309:1286–1291. doi: 10.1136/bmj.309.6964.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragomir A, Cote R, Roy L, Blais L, Lalonde L, Berard A, Perreault S. Impact of adherence to antihypertensive agents on clinical outcomes and hospitalization costs. Medical Care. 2010;48:418–425. doi: 10.1097/MLR.0b013e3181d567bd. [DOI] [PubMed] [Google Scholar]
- Easterbrook PJ. Directory of registries of clinical trials. Statistics in Medicine. 1992;11:363–423. [PubMed] [Google Scholar]
- Erdine S, Arslan E. Monitoring treatment adherence in hypertension. Current Hypertension Reports. 2013;15:269–272. doi: 10.1007/s11906-013-0369-9. [DOI] [PubMed] [Google Scholar]
- Glynn LG, Murphy AW, Smith SM, Schroeder K, Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database of Systematic Reviews. 2010;(3):Art. No.: CD005182. doi: 10.1002/14651858.CD005182.pub4. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension. 2010;55:399–407. doi: 10.1161/HYPERTENSIONAHA.109.139816. [DOI] [PubMed] [Google Scholar]
- Gwadry-Sridhar FH, Manias E, Lal L, Salas M, Hughes DA, Ratzki-Leewing A, Grubisic M. Impact of interventions on medication adherence and blood pressure control in patients with essential hypertension: a systematic review by the ISPOR medication adherence and persistence special interest group. Value in Health. 2013;16:863–871. doi: 10.1016/j.jval.2013.03.1631. [DOI] [PubMed] [Google Scholar]
- Hedges L, Olkin I. Statistical Methods for Meta-Analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- Hedges L, Vevea J. Fixed- and random-effects models in meta-analysis. Psychological Methods. 1998;3:486–504. [Google Scholar]
- Higgins J, Altman D, Sterne J. Assessing risk of bias in included studies. Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. 2011 Retrieved from http://handbook.cochrane.org/chapter_8/8_assessing_risk_of_bias_in_included_studies.htm.
- Iskedjian M, Einarson TR, MacKeigan LD, Shear N, Addis A, Mittmann N, Ilersich AL. Relationship between daily dose frequency and adherence to antihypertensive pharmacotherapy: evidence from a meta-analysis. Clinical Therapeutics. 2002;24:302–316. doi: 10.1016/s0149-2918(02)85026-3. [DOI] [PubMed] [Google Scholar]
- Jayasinghe J. Non-adherence in the hypertensive patient: can nursing play a role in assessing and improving compliance? Canadian Journal of Cardiovascular Nursing. 2009;19:7–12. [PubMed] [Google Scholar]
- Jung O, Gechter JL, Wunder C, Paulke A, Bartel C, Geiger H, Toennes SW. Resistant hypertension? Assessment of adherence by toxicological urine analysis. Journal of Hypertension. 2013;31:766–774. doi: 10.1097/HJH.0b013e32835e2286. [DOI] [PubMed] [Google Scholar]
- Langham J, Thompson E, Rowen K. Identification of randomized controlled trials from the emergency medicine literature: comparison of hand searching versus MEDLINE searching. Annals of Emergency Medicine. 1999;34:25–34. doi: 10.1016/s0196-0644(99)70268-4. [DOI] [PubMed] [Google Scholar]
- Lewis LM. Factors associated with medication adherence in hypertensive blacks: a review of the literature. Journal of Cardiovascular Nursing. 2012;27:208–219. doi: 10.1097/JCN.0b013e318215bb8f. [DOI] [PubMed] [Google Scholar]
- Lewis LM, Ogedegbe C, Ogedegbe G. Enhancing adherence of antihypertensive regimens in hypertensive African-Americans: current and future prospects. Expert Review of Cardiovascular Therapy. 2012;10:1375–1380. doi: 10.1586/erc.12.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Lipsey M, Wilson D. Practical Meta-analysis. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- Matthes J, Albus C. Improving adherence with medication: a selective literature review based on the example of hypertension treatment. Deutsches Arzteblatt International. 2014;111(4):41–47. doi: 10.3238/arztebl.2014.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgado MP, Morgado SR, Mendes LC, Pereira LJ, Castelo-Branco M. Pharmacist interventions to enhance blood pressure control and adherence to antihypertensive therapy: Review and meta-analysis. American Journal of Health-System Pharmacy. 2011;68:241–253. doi: 10.2146/ajhp090656. [DOI] [PubMed] [Google Scholar]
- Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42:878–884. doi: 10.1161/01.HYP.0000094221.86888.AE. [DOI] [PubMed] [Google Scholar]
- Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, … Haynes BR. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews. 2014;11 doi: 10.1002/14651858.CD000011.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwin R, Vevea J. Evaluating coding decisions. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2. New York, NY: Russell Sage Foundation; 2009. pp. 177–203. [Google Scholar]
- Raudenbush S. Random effects models. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2. New York: Russell Sage Foundation; 2009. pp. 295–315. [Google Scholar]
- Rothstein HR, Hopewell S. Grey literature. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2. New York: Russell Sage Foundation; 2009. pp. 103–125. [Google Scholar]
- Schroeder K, Fahey T, Ebrahim S. Interventions for improving adherence to treatment in patients with high blood pressure in ambulatory settings. Cochrane Database of Systematic Reviews. 2004:Art.No.:CD004804. doi: 10.1002/14651858.CD004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadish W, Haddock C. Combining estimates of effect size. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2. New York, NY: Russell Sage Foundation; 2009. pp. 257–277. [Google Scholar]
- Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. Journal of Clinical Hypertension. 2011;13:898–909. doi: 10.1111/j.1751-7176.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, Johnson JA. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333:15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JM, Simes RJ. Publication bias: evidence of delayed publication in a cohort study of clinical research projects. BMJ. 1997;315:640–645. doi: 10.1136/bmj.315.7109.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton AJ. Publicaton bias. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2. New York: Russell Sage Foundation; 2009. pp. 435–452. [Google Scholar]
- Takiya LN, Peterson AM, Finley RS. Meta-analysis of interventions for medication adherence to antihypertensives. Annals of Pharmacotherapy. 2004;38:1617–1624. doi: 10.1345/aph.1D268. [DOI] [PubMed] [Google Scholar]
- Uhlig K, Patel K, Ip S, Kitsios GD, Balk EM. Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysis. Annals of Internal Medicine. 2013;159:185–194. doi: 10.7326/0003-4819-159-3-201308060-00008. [DOI] [PubMed] [Google Scholar]
- Valentine J. Judging the quality of primary research. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2. New York: Russell Sage Foundation; 2009. pp. 129–146. [Google Scholar]
- Verberk WJ, Kessels AG, Thien T. Telecare is a valuable tool for hypertension management, a systematic review and meta-analysis. Blood Pressure Monitoring. 2011;16:149–155. doi: 10.1097/MBP.0b013e328346e092. [DOI] [PubMed] [Google Scholar]
- Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008;336:1114–1117. doi: 10.1136/bmj.39553.670231.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. Scientific communication and literature retrieval. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2. New York: Russell Sage Foundation; 2009. pp. 51–71. [Google Scholar]
- Wofford MR, Minor DS. Hypertension: issues in control and resistance. Current Hypertension Reports. 2009;11:323–328. doi: 10.1007/s11906-009-0055-0. [DOI] [PubMed] [Google Scholar]
- Wood JA. Methodology for dealing with duplicate study effects in a meta-analysis. Organizational Research Methods. 2008;11:79–95. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.