Abstract
Quitting smoking is the single best change in behavior that smokers can make to improve their health and extend their lives. Although most smokers express a strong desire to stop using cigarettes, the vast majority of quit attempts end in relapse. Relapse is particularly likely when smokers encounter cigarette cues. A striking number of relapses occur very quickly, with many occurring within as little as 24 hrs. Characterizing what distinguishes successful quit attempts from unsuccessful ones, particularly just after cessation is initiated, is a research priority. We addressed this significant issue by examining the association between functional connectivity during cigarette cue exposure and smoking behavior during the first 24 hrs of a quit attempt. Functional MRI was used to measure brain activity during cue exposure in nicotine-deprived daily smokers during the first day of a quit attempt. Participants were then given the opportunity to smoke. Using data collected in two parent studies, we identified a subset of participants who chose to smoke and a matched subset who declined (n = 38). Smokers who were able to resist smoking displayed significant functional connectivity between the left anterior insula and the dorsolateral prefrontal cortex, whereas there was no such connectivity for those who chose to smoke. Notably, there were no differences in mean levels of activation in brain regions of interest, underscoring the importance of assessing interregional connectivity when investigating the links between cue-related neural responses and overt behavior. To our knowledge, this is the first study to link patterns of functional connectivity and actual cigarette use during the pivotal first hours of attempt to change smoking behavior.
Keywords: smoking, fMRI, cue reactivity, connectivity, insula, dorsolateral prefrontal cortex
1. Introduction
Recent estimates indicate that nearly one billion men and women in the world smoke cigarettes on a daily basis (Ng et al., 2014). With respect to health-related behavior change, quitting smoking is the single best step that these individuals can take to reduce their risk for a host of negative outcomes, including premature death (USDHHS; 2014). Fortunately, most smokers express a strong desire to quit using cigarettes (CDC, 2011). Yet for many, translating the motivation to stop using cigarettes into sustained behavior change is a major barrier to smoking cessation. As many as 95–97% of untreated smokers relapse (return to regular smoking) within 6–12 months of initiating a quit attempt (Hughes, Keely, & Naud, 2004). Even when receiving the best treatments currently available, roughly 70% of smokers relapse within one year (Piasecki, 2006). One of the most significant challenges currently facing tobacco researchers is fully characterizing what distinguishes successful quit attempts from unsuccessful ones, information that is crucial for devising ways to increase the likelihood of success for the large proportion of smokers who want to stop using cigarettes.
Relapse is particularly likely when smokers encounter cigarette-related stimuli (Ferguson & Shiffman, 2009). The use of functional brain imaging methods has become a particularly common approach to studying cigarette cue-reactivity (Engelmann et al., 2012). This work has demonstrated that cigarette cue exposure is associated with widely distributed increases in brain activation, with the precise pattern varying as a function of several variables (Jasinska, Stein, Kaiser, Naumer, & Yalachkov, 2014; Wilson & Sayette, 2015; Yalachkov, Kaiser, & Naumer, 2012).
Dual-systems models of brain functioning provide a useful framework for conceptualizing how such activation patterns may relate to smoking behavior. Broadly, dual-systems models posit that decision making is influenced by two distinct but interacting neural systems: an “automatic” system comprised of areas such as the ventral striatum that is driven by affective, reward-related, and visceral influences, and a “deliberative” system comprised of areas such as the dorsolateral prefrontal cortex (DLPFC) that supports working-memory and other “cold” cognitive functions required for planning and inhibitory control (Bechara, 2005; Bickel et al., 2007; McClure & Bickel, 2014; Metcalfe & Mischel, 1999). From a dual-systems perspective, smoking cues may contribute to relapse because they evoke an imbalance between these brain systems, such that the automatic system exerts greater influence over behavior than the deliberative system (Heatherton & Wagner, 2011).
Emerging research indicates that the insula may play a particularly important role in altering the balance between the automatic and deliberative systems in a way that increases the likelihood of relapse (Addicott, Sweitzer, Froeliger, Rose, & McClernon, 2015; Janes et al., 2010; Naqvi & Bechara, 2015; Naqvi, Gaznick, Tranel, & Bechara, 2014; Noel, Brevers, & Bechara, 2013a). Several lesion studies (Naqvi, Rudrauf, Damasio, & Bechara, 2007); Contreras, Ceric, & Torrealba, 2007; Gaznick, Tranel, McNutt, & Bechara, 2014; Suner-Soler et al., 2012) suggest that cigarette craving depends (at least partially) on the functional integrity of the insula. These studies are complemented by functional brain imaging research demonstrating that cigarette cues elicit increases in insular activation in smokers (Engelmann et al., 2012) which positively correlate with self-reported cigarette craving (Kuhn & Gallinat, 2011), and that greater functional connectivity between the insula and regions linked to executive (Janes et al., 2010) and motor (Addicott et al., 2015) control predicts improved smoking cessation outcomes.
Despite convincing evidence that the insula plays an important role in cue-elicited craving and relapse, there are important questions about the nature of the interactions between the insula and other areas in the context of smoking behavior. Whereas prior research provides some support for the idea that poorer smoking cessation outcomes are associated with weaker cognitive control over insular functions (Janes et al., 2010), it has also been proposed that the insula may drive drug use by simultaneously increasing craving and redirecting attention towards the goal of drug taking (i.e., “hijacking” deliberative functioning) (e.g., Naqvi & Bechara, 2009; Noel, Brevers, & Bechara, 2013b). Currently, it remains unclear whether the link between insula connectivity and smoking relapse reflects a weakening or redirection of processes that inhibit drug use, a strengthening of processes that promote drug use, or some alternative to these possibilities (e.g., lack of information flow or network coordination; Bressler & Tognoli, 2006)).
A second critical gap in the literature concerns the time frame over which connectivity between the insula and other brain regions predicts clinically meaningful behavior in quitting smokers. Although two recent studies have demonstrated smoking cessation outcomes are predicted by insula connectivity over a period of weeks (Addicott et al., 2015; Janes et al., 2010), it is not known whether insula connectivity also predicts more immediate relapse outcomes. This is an important question, as research has repeatedly shown that relapse rates are highest during the earliest phases of a quit attempt. Data indicate that 50–75% of untreated smokers relapse within one week (Hughes et al., 2004). A sizeable proportion are unable to maintain a quit attempt for as little as 24 hours (Allen, Bade, Hatsukami, & Center, 2008; Carpenter & Hughes, 2005), a time during which abstinence has been found to improve the likelihood of later success (Westman, Behm, Simel, & Rose, 1997). It is crucial, conceptually and clinically, to distinguish processes involved in cigarette abstinence or relapse to smoking during the critical early moments of a quit attempt when behavior change often falls apart.
The goal of the current study was to address these issues by examining the association between functional connectivity during cigarette cue exposure and smoking behavior during the first 24 hours of a quit attempt. Using dual systems theory as a guiding framework, we focused on connectivity among areas of the brain thought to be key constituents of the systems supporting automatic and deliberative processing. Importantly, our prior work has highlighted activation in and connectivity among these regions as significant in relation to cue-reactivity and craving (Wilson, Creswell, Sayette, & Fiez, 2013; Wilson, Sayette, & Fiez, 2004, 2012, 2013). We were particularly interested in functional connectivity of the insula. Based upon prior research (Addicott et al., 2015), we hypothesized that smokers who declined an opportunity to smoke immediately following cigarette cue exposure would exhibit stronger connectivity between the insula and other brain regions (particularly areas implicated in deliberative processing, such as the DLPFC) compared to those who chose to smoke.
2. Methods
2.1. Participants
Participants were drawn from two previous studies. Study 1 investigated the impact of quitting motivation and smoking opportunity on activation during smoking cue presentation (Wilson et al., 2012). Study 2 examined neural correlates of self-versus other-oriented strategies to cope with cue-elicited craving (Wilson, Sayette, et al., 2013). For each, participants were required to be right-handed native English speakers between ages 18–45, report smoking 15–40 cigarettes/day for the past year, and pass an MRI safety screening. Study 1 was composed of males and females, some of whom were motivated to quit smoking and some of whom were not; Study 2 included only males who were motivated to quit smoking. Participants from both studies who reported that they were motivated to quit smoking, who initiated a quit attempt 12-hrs before participating in an fMRI-based cigarette cue exposure protocol (described below), and who were given the opportunity to smoke immediately following cue exposure were considered for inclusion in the current study. We selected two subgroups from the pool of participants meeting these criteria: (1) those who declined the opportunity to smoke (Chose-No; n = 19); and (2) a matched subset who chose to smoke when given the opportunity (Chose-Yes; n = 19).1
2.2. Cue Exposure Task
Participants completed a cue exposure procedure adapted from prior research (Wilson, Sayette, Delgado, & Fiez, 2005). Each run began with a 48-sec period during which participants were instructed to remain still and relaxed. Participants then had an object placed in their left hand. The object was identified via intercom and instructions were given to hold and view the object (a live video feed projected on a screen allowed participants to view the object in real-time). Participants held the object for a period of 74-sec. Three runs of the task were completed, in which the objects were: a notepad (control), a roll of electrical tape (neutral), and a cigarette of the participant’s brand of choice (smoking cue). Upon presentation of the cigarette, a prerecorded message was delivered informing participants that they would be removed from the scanner in 40-sec and would be able to smoke immediately if they chose to do so. Participants verbally rated their smoking urge on a 0–100 scale prior to placement in the scanner (urge-baseline), after the conclusion of the neutral cue run (urge-neutral cue), and after the conclusion of the smoking cue run (urge-smoking cue). The first run served as a practice run and was excluded from analyses. Before the third run, participants in Study 2 (but not Study 1) were instructed to utilize the coping strategy that they had been trained. Because there is evidence that the presentation of smoking cues affects behavioral and neural responses to subsequently presented items (see Sayette, Griffin, & Sayers, 2010), the order in which objects were presented was fixed in the aforementioned sequence.
2.3. Procedure
Participants completed two sessions, described in detail elsewhere (Wilson et al., 2012; Wilson, Sayette, et al., 2013). Briefly, those deemed eligible based upon a telephone screening completed an initial baseline session during which they provided a baseline carbon monoxide (CO) sample and completed a battery of questionnaires and tasks. In addition, participants in Study 2 were trained to use either a self-focused or other-focused strategy for coping with smoking cue exposure (see Wilson, Sayette, et al., 2013). At the conclusion of the baseline session, participants were scheduled for the fMRI-based experimental visit (held within two weeks of baseline). For all participants included in the present analyses, the experimental session was scheduled to coincide with the first day of an attempt to quit smoking. Specifically, participants were instructed to initiate a cessation attempt 12-hrs before the onset of the experimental visit; they were told to abstain from smoking and using any other nicotine-containing products during this time. Participants were also instructed to refrain from consuming drugs or alcohol for the 24 hours preceding the experiment.
Upon arrival for the experimental session, participants reported the last time they smoked and CO was measured to check compliance with deprivation instructions. Participants had to have a CO level at least 50% lower than their baseline, a cutoff used in similar prior research (Sayette, Loewenstein, Griffin, & Black, 2008). Immediately before being placed in the scanner, participants were informed that they would get a break during the study, at which point they would have an opportunity to smoke a cigarette. After the collection of anatomical images, participants completed a working memory task (for details, see 29) and then the cue exposure procedure. Subsequently, participants were presented with the opportunity to smoke. Specifically, participants were asked to indicate whether or not they would like to smoke the cigarette that they were holding as they were being removed from the scanner. Due to the time required to exit the imaging facility (regulations prohibited smoking inside the building), participants who chose to do so were able to initiate smoking within approximately three minutes of being removed from the scanner. After smoking or taking a break, participants completed post-task questionnaires and were given an opportunity to participate in a follow-up study. Finally, participants were debriefed and paid.
2.4. fMRI Data Acquisition
Scanning was conducted using a 3-Tesla head-only Siemens Allegra magnet (Siemens Corporation, New York, NY) equipped with a standard transmit/receive head coil. Prior to functional scanning, a 40 slice oblique-axial anatomical series (3.125 × 3.125 × 3.0 mm voxels) was acquired parallel to the anterior commissure-posterior commissure plane using a standard T2-weighted pulse sequence. Additionally, a high-resolution (1 × 1 × 1 mm voxels) three-dimensional structural volume was collected using a magnetization-prepared rapid gradient-echo sequence. Next, functional images were acquired in the same plane as the 40-slice anatomical series with coverage limited to the 38 center slices using a one-shot echo-planar imaging pulse sequence [TR = 2000 ms, TE = 25 ms, FOV = 20 cm, flip angle = 79°].
2.5. A Priori Regions of Interest (ROIs)
Our primary aim was to examine the association between smoking behavior during the first day of a quit attempt and functional connectivity among areas of the brain implicated in automatic versus deliberative processing, using contemporary dual systems theory as a guiding framework. In order to restrict analyses to brain areas that have been reliably linked to each of these broad domains in prior research, results from two quantitative meta-analyses were used to create 10 ROIs: five linked to automatic processing and five linked to deliberative processing (see Table 1). The set of automatic processing ROIs were taken from a meta-analysis of 206 published fMRI studies examining reward-related processing and subjective valuation (Bartra, McGuire, & Kable, 2013) and consisted of: left and right ventral striatum (VS), left and right anterior insula, and ventromedial prefrontal cortex (VMPFC). Among these, we were particularly interested in connectivity of the insula. The set of deliberative processing regions were taken from a meta-analysis of 113 published fMRI studies examining working memory (Rottschy et al., 2012), a core component of executive control (Courtney, 2004), and consisted of: left and right superior frontal gyrus (SFG), left and right DLPFC, and dorsal anterior cingulate cortex (dACC). A sphere 8mm in diameter was created around the center coordinate for each ROI.
Table 1.
A Priori Regions of Interest
| Region | MNI Coordinates | Associated With: | From: | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Left superior frontal gyrus | −28 | 0 | 56 | deliberative system | Rottschy et al. (2012) |
| Right superior frontal gyrus | 30 | 2 | 56 | deliberative system | Rottschy et al. (2012) |
| Left dorsolateral prefrontal cortex | −46 | 26 | 24 | deliberative system | Rottschy et al. (2012) |
| Right dorsolateral prefrontal cortex | 44 | 34 | 32 | deliberative system | Rottschy et al. (2012) |
| Dorsal anterior cingulate cortex | 2 | 18 | 48 | deliberative system | Rottschy et al. (2012) |
| Left anterior insula | −30 | 22 | −6 | automatic system | Bartra et al. (2013) |
| Right anterior insula | 32 | 20 | −6 | automatic system | Bartra et al. (2013) |
| Left ventral striatum | −12 | 12 | −6 | automatic system | Bartra et al. (2013) |
| Right ventral striatum | 12 | 10 | −6 | automatic system | Bartra et al. (2013) |
| Ventromedial prefrontal cortex | 2 | 46 | −8 | automatic system | Bartra et al. (2013) |
2.6. fMRI Data Analysis
Several standard preprocessing steps were conducted prior to fMRI data analysis, including motion correction and adjustment for drift (for details, see Wilson et al., 2012; Wilson, Sayette, et al., 2013). Participants’ anatomical images were co-registered with a reference anatomy using a six-parameter rigid-body automated registration algorithm, producing a transformation matrix that was applied to participants’ functional images. Functional images were then mean-normalized and smoothed using a 3D Gaussian filter (4-mm full width at half maximum).
Primary analyses were conducted using unified structural equation modeling (uSEM; J. Kim, Zhu, Chang, Bentler, & Ernst, 2007) for each individual. A unique strength of uSEMs is that they take into account both contemporaneous and lagged effects, both of which exist in fMRI data and must be accounted for to decrease bias in estimates (Gates, Molenaar, Hillary, Ram, & Rovine, 2010). The current study necessitates a data-driven approach for arriving at connectivity maps that account for heterogeneity in the sample to test the competing outcomes/connectivity models that may be predicted from theory. Two major concerns exist when conducting individual-level analysis on fMRI data: 1) results may be driven by noise and 2) traditional techniques are unable to correctly detect the directionality of relations among regions at the individual level (Smith et al., 2011). A recently developed model search technique, Group Iterative Multiple Model Estimation (GIMME;Gates & Molenaar, 2012), circumvents these issues to recover the true connectivity patterns and individual-level estimates at rates higher than many competing approaches (Mumford & Ramsey, 2014).
GIMME ultimately conducts uSEMs for each individual separately, allowing for unique paths among ROIs as needed. The data-driven model search begins by first selecting paths that would significantly improve the model fits for the majority of individuals in the sample. Following the expected ability to detect signal from noise in individual-level fMRI maps (Smith et al., 2011), 75% of individuals must have the path present for it to be considered a group-level path. Importantly, unlike traditional approaches that concatenate data across individuals, GIMME does not assume homogeneity across individuals or that one connectivity map can be used to describe the individuals comprising the group. Rather, it detects signal from noise by looking for consistency in connections across individuals’ brain processes.
GIMME then uses these “group-level” connectivity map pattern as a prior for iterative searches for individual-level connections among ROIs that exist in addition to the group-level paths. Starting with these known group-level paths prior to individual-level searches has been shown to vastly improve the recovery of the direction of effects and recovery of true connections when compared to traditional approaches for individual-level analysis (Gates & Molenaar, 2012; see also Smith et al., 2011 for comparison). In the end, all weights for the group and individual level paths are estimated separately for each individual. GIMME is freely available as an R package (gimme; Lane, Gates, & Molenaar, 2015).
Time series were extracted from Studies 1 and 2 corresponding to when participants held the neutral object (tape), the smoking cue object (cigarette), and the relaxation periods between runs. This resulted in 118 observations for each individual, which provides sufficient power to detect effects in time series analysis such as uSEM (Box & Jenkins, 1970) and is consistent with the length used in prior studies using GIMME (e.g., Nichols, Gates, Molenaar, & Wilson, 2014). Connectivity networks were obtained for each group of smokers using GIMME, which were then examined for group differences. Since the data-driven search generates group-level connectivity maps by only including paths that are significant for the majority of individuals, the GIMME group-level results directly indicate similarities and differences between the groups from a statistical standpoint.
Although our primary goal was to characterize outcome-related differences in directed functional connectivity, we also conducted analyses to determine whether the groups exhibited differences in mean activation in the preselected ROIs. Toward this end, a two-way mixed ANOVA was run for each ROI, with smoking choice as the between-subjects factor, cue (smoking or neutral) as the within-subjects factor, and mean activation level as the dependent measure. In order to maintain consistency with our prior work (Wilson et al., 2005; Wilson et al., 2012; Wilson, Sayette, et al., 2013), the mean calculated for each ROI excluded the initial 26 seconds of cue exposure (see 29 for details). (The entire time course for each exposure was included in the uSEM analysis described above.)
3. Results
3.1. Participant Characteristics and Urge
Participant characteristics are shown in Table 2. Smoking choice groups did not significantly differ in age, cigarettes per day, number of years smoking, nicotine dependence, baseline CO, or experiment CO (p values > .05). As described above, participants rated their urge to smoke prior to being placed in the MRI scanner and after the second and third runs of the cue exposure task. A repeated-measures ANOVA with a Greenhouse-Geisser correction for violation of sphericity revealed a main effect of time on urge, F(1.17, 42.19) = 6.043, p = .014. Post hoc tests using the Benjamini-Hochberg correction showed that reported urge increased over time: Although the difference between urge-neutral cue and urge-smoking cue did not reach statistical significance (p = .140), the difference between urge-baseline and urge-neutral cue (p = .036) and between urge-baseline and urge-smoking cue (p = .041) were each significant. The ANOVA also showed a significant difference in reported urge between the two smoking choice groups, such that urge ratings were higher for the Chose-Yes subgroup than the Chose-No subgroup, F(1,36) = 27.610, p < .001. The interaction between time and group was not significant, F(1.17, 42.19) = 1.532, p = .226.
Table 2.
Participant Characteristics and Urge Ratings
| Chose-Yes (n = 19) | Chose-No (n = 19) | |
|---|---|---|
| % Male | 89.47 | 89.47 |
| Mean Age (SD) | 33.89 (7.40) | 32.95 (7.34) |
| Mean Cigarettes per day (SD) | 18.53 (2.86) | 18.11 (2.47) |
| Mean Number of years smoking (SD) | 16.68 (6.86) | 14.63 (8.13) |
| Mean FTND (SD) | 5.11 (1.24) | 4.47 (1.54) |
| Mean Baseline CO (SD) | 33.53 (16.46) | 30.63 (10.13) |
| Mean Experiment CO (SD) | 13.47 (6.35) | 11.50 (4.82) |
| Mean Urge-Baseline (SD) | 61.42 (24.74) | 33.63 (24.86) |
| Mean Urge-Neutral Cue (SD) | 77.47 (19.01) | 38.37 (29.24) |
| Mean Urge-Smoking Cue (SD) | 82.11 (17.01) | 40.26 (31.95) |
Note: FTND = Fagerstrom Test for Nicotine Dependence
3.2. Functional Connectivity as a Function of Smoking Behavior
The uSEM analyses revealed a common model (i.e., connections that were estimated for all individuals because they were significant for the majority in that group) for participants who chose to smoke following cue exposure (Chose-Yes subgroup), as well as a common model for participants who declined the opportunity to smoke following cue exposure (Chose-No subgroup). It should be noted that individual-level paths were added as needed in addition to the common models via GIMME to improve upon the estimates of connection weights and final model fit but are not shown here as it is outside the scope of the present paper. The groups did not differ in terms of the number of final connections obtained for each individual, t(36) =.694, p = .492. However, they did differ in terms of how well the group-level model described the individuals. Those in the Chose-No group had a greater number of group-level paths (15) compared to the Chose-Yes group (11). Fitting the group-level maps to individuals did not reap an excellent fit on greater than one criteria for any individual in either group (i.e., individual-level paths had to be added for all individuals). Still, the Chose-No group was better described by the group model as evidenced by higher fit indices according to the four fit criteria (see Table 4). Overall, this suggests greater heterogeneity in the Chose-Yes group.
Table 4.
Mean Fit Indices for Individual-Level Effective Connectivity Maps Conducted Using only the Group-Level Model and the Final Models for Each Individual.
| Smoking Choice | ||
|---|---|---|
| No | Yes | |
| Group-level model | ||
| CFI* | .94 (.03) | .91 (.02) |
| NNFI* | .86 (.07) | .78 (.06) |
| SRMR* | .08 (.01) | .09 (.01) |
| RMSEA | .06 (.02) | .06 (.01) |
| Final models | ||
| CFI | .98 (.01) | .98 (.00) |
| NNFI | .95 (.02) | .95 (.01) |
| SRMR | .05 (.01) | .04 (.00) |
| RMSEA | .02 (.02) | .01 (.01) |
Note:
indicates difference between groups is significant at the p<.01 level.
CFI: confirmatory fit index; NNFI: non-normed fit index; SRMR: standardized root mean square residual (SRMR); RMSEA: root mean square error of approximation.
Final connectivity models had excellent fit to the individual-level data, as assessed by commonly used fit indices (see Table 4). The Chose-No group individuals had, on average, 10.21 additional individual-level paths added (SD = 4.22, range = 10–28), while the Chose-Yes group had 16.53 (SD = 5.06, range = 4–21) additional paths on average. The number of additional paths was significantly greater in the Chose-Yes group than the Chose-No group; t(36) = 4.18, p < .001. Both subgroup models are shown in Figure 1, where each arrow represents a connection present in the group model. One commonality between the groups is the absence of lagged effects between ROIs. This is consistent with previous work using uSEM (e.g., Nichols et al., 2013), and aligns with the underlying biology of relations among ROIs which occurs on a far faster scale than the temporal resolution provided by fMRI. Further supporting that contemporaneous relations best depict underlying brain functionality, findings from a large-scale simulation study of fMRI data found that effects occurring among ROIs were consistently captured by methods that identify contemporaneous or instantaneous effects as opposed to methods that only contained lagged effects (Smith et al., 2011). In both groups here the lagged effects are all autoregressive.
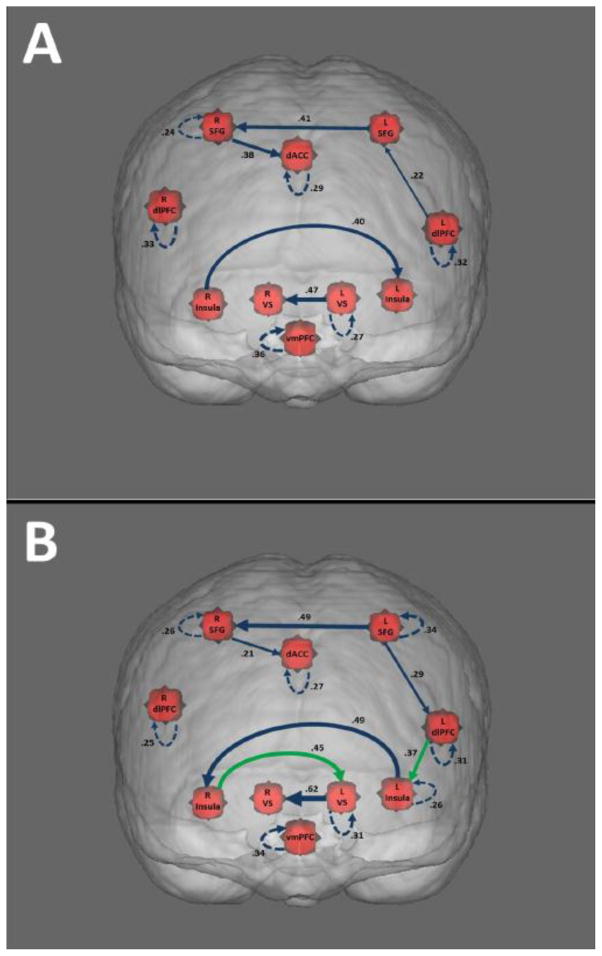
Figure 1. Effective Connectivity Models for Smoking Choice Groups.
Models are depicted for the Chose-Yes (A) and Chose-No (B) groups. Line thickness represents the average beta weight of the connection, with thicker lines indicating higher beta weights. Solid lines represent contemporaneous relationships, whereas dotted lines represent lagged relationships. Arrow directionality is based on BOLD activity in one ROI predicting BOLD activity in another ROI (or in the same ROI at a later time point). Green lines (seen only in Model B) represent connections between regions that were present in one smoking choice group but not the other.
Figure 1A depicts the group model for Chose-Yes subgroup. This model shows connectivity between several regions associated with deliberative processing (left DLPFC, left SFG, right SFG, and dACC), as well as connectivity between several regions associated with automatic processing (left and right VS, left and right insula). Notably, the model did not contain any paths connecting these broadly defined systems. As shown in Figure 1B, participants who chose not to smoke demonstrated many of the same connectivity patterns as those who chose to smoke, but there were noteworthy differences. Namely, the insula appears to play a more important role in the Chose-No model, showing increased connectivity with other areas. For instance, the model contains a path connecting the right insula and the left ventral striatum, which was not seen in the Chose-Yes model. Of high importance is the connection between the left DLPFC (an area associated with deliberative processing/cognitive control) and the left insula, which was found in the Chose-No model (but not the Chose-Yes). Since the selection of group-level connections via GIMME is subject to a threshold (i.e., at least 75% of individuals have the path), it is possible that individuals in the Chose-Yes group could have a strong connection between these two regions if it were estimated. To statistically test this, we conducted a post hoc analysis whereby we added this specific path to all of the Chose-Yes individuals’ models. The comparison of the estimated weights on this connection between individuals revealed that the connection between DLPFC and the left insula was significantly higher for the Chose-No group than for the Chose-Yes group, t(36) = 3.33, p =.002. This provides further evidence for important differences in connectivity between these regions as a function of smoking choice behavior. Tests for correlation showed that strength of connections (as measured by path beta weights) in both models were not related to urge or nicotine dependence measures.
3.3. Effects on Activation within Individual Regions
A series of two-way mixed ANOVAs were run to examine the effects of group and cue on activity in the preselected ROIs. The Benjamini–Hochberg method (Benjamini & Hochberg, 1995) was used to control for possible inflation of Type I error rate as a result of the number of comparisons performed. Consistent with prediction and with prior findings indicating greater fMRI responses among smokers to smoking cues as opposed to neutral cues in several brain regions (see Engelmann et al., 2012) including those selected for the current study, results showed a significant main effect of cue, such that activation was significantly higher in the cigarette as opposed to tape condition for all but one ROI (see Table 3). The remaining ROI (vmPFC) also showed more activation in the cigarette condition as opposed to the tape condition; however this difference did not reach statistical significance. The results failed to show a main effect of group: there were no significant differences when comparing independent ROI activation between participants who chose to smoke and participants who declined smoking (p > .05 for each ROI). Likewise, the interaction between cue and group was nonsignificant across all ROIs (p > .05 for each ROI).
Table 3.
ANOVA results: Smoking Cue > Neutral Cue
| ROI | F (df = 1,36) | p |
|---|---|---|
| L SFG* | 6.30 | .021 |
| R SFG* | 6.80 | .022 |
| L dlPFC* | 8.23 | .018 |
| R dlPFC** | 16.01 | .000 |
| dACC* | 9.35 | .020 |
| L Insula* | 9.60 | .013 |
| R Insula* | 5.99 | .021 |
| L VS* | 7.35 | .020 |
| R VS* | 6.52 | .021 |
| vmPFC | 2.19 | .148 |
p < .05
p < .01
ROI KEY: L SFG = Left superior frontal gyrus, R SFG = Right superior frontal gyrus, L dlPFC = Left dorsolateral prefrontal cortex, R dlPFC = Right dorsolateral prefrontal cortex, dACC = Dorsal anterior cingulate cortex, L Insula = Left insula, R Insula = Right insula, L VS = Left ventral striatum, R VS = Right ventral striatum, vmPFC = Ventromedial prefrontal cortex
4. Discussion
Quitting-motivated smokers who were able to resist smoking when given the opportunity displayed significant functional connectivity between the left DLPFC and the left anterior insula during cue exposure, whereas there was no such connectivity between these regions for those who chose to smoke. This finding is consistent with recent work demonstrating that smokers who lapsed during an eight-week smoking cessation intervention exhibited weaker prequit functional connectivity between a network containing the insula and specific control-related brain regions (e.g., DLPFC and dACC) than those who did not lapse (Janes et al., 2010). Results from the current study extend previous research by confirming that cue-related connectivity of the insula predicts smoking behavior during the critical first hours of a quit attempt, mere moments before the actual decision to smoke is made.
Our findings add to mounting evidence that the insula plays a central role in addictive behavior (Cisler et al., 2013; Goldstein et al., 2009; McHugh et al., 2013; Naqvi et al., 2014; Noel et al., 2013a; Sutherland, McHugh, Pariyadath, & Stein, 2012; Viswanath et al., 2015; Volkow & Baler, 2015; Wisner, Patzelt, Lim, & MacDonald, 2013; Zhang et al., 2015), including cigarette addiction (Addicott et al., 2015; Clewett et al., 2014; Dinur-Klein et al., 2014; Forget, Pushparaj, & Le Foll, 2010; Gaznick, Tranel, McNutt, & Bechara, 2014; Janes et al., 2010; Naqvi et al., 2007; Suner-Soler et al., 2012; Zanchi et al., 2015). The link between the insula and addiction has largely been conceptualized as one that relates to the interoceptive functions commonly attributed to the region. It has become increasingly clear, however, that the insula may best be thought of as an area that serves as a key hub for interactions among large-scale brain networks, rather than one narrowly dedicated to interoception in isolation (Chang, Yarkoni, Khaw, & Sanfey, 2013; Menon & Uddin, 2010; Sridharan, Levitin, & Menon, 2008). Most relevant to the current study, the anterior insula – particularly the left anterior insula – has been implicated as a junction between networks associated with the processing of information from internal (e.g., bodily sensations) and external (e.g., biologically relevant environmental cues) information (62, 63). While it has been suggested that addictive behavior may result from a “hijacking” of the cognitive control system by the insula (64), the current study suggests that vulnerability to relapse may stem instead (at least in part) from deficient connectivity between the insula and other regions/networks; i.e. the anterior insula may not be functioning appropriately as a junction between brain networks. Indeed, a similar systems-level view of insula functioning has recently been integrated into neurobiological models of addiction (Janes, Farmer, Peechatka, Frederick, & Lukas, 2015; Lerman et al., 2014; Naqvi et al., 2014; Noel et al., 2013a; Sutherland et al., 2012; Volkow & Baler, 2015).
In addition to different patterns of connectivity between the insula and the DLPFC, quitting-motivated smokers who refrained from cigarette use early during a quit attempt displayed significant functional connectivity between the right insula and the left ventral striatum, whereas these paths were absent for those who decided to smoke. It is tempting to interpret this pattern in terms of sequential regulatory processing (e.g., the DLPFC in firstly engaging in top-down control of urge based on interoceptive signals represented within the insula, followed by a modulation of reward/value signals represented in the ventral striatum). We believe that it is more justifiable, however, to view this as additional support for the broader idea that the insula serves as an important hub between networks in the service of goal-directed behavior (such as resisting smoking).
Results from the current study highlight the importance of assessing smoking behavior when studying cue-elicited brain activation, echoing a key point recently raised by Perkins (Perkins, 2009). More generally, results from the current study underscore the importance of examining patterns of functional connectivity, rather than focusing solely on mean levels of activation within brain areas in isolation, in the study of addictive behavior (Sutherland, Liang, Yang, & Stein, 2015). That is, while there were clear differences in functional connectivity between quitting smokers who did and who did not smoke when given the opportunity, there were no such group differences in mean levels of activation for any of the a priori regions of interest. Instead, activation of each of these regions was greater during cigarette cue exposure than during neutral cue exposure, regardless of smoking choice. Thus, functional connectivity – especially connectivity of the insula – revealed neurobiological effects linked to clinically meaningful behavior that were not detected using more traditional analytic strategies. Studies that use similar methods to further characterize functional connectivity as it relates to relapse would be valuable.
At a broad level, the current findings lend additional support to the idea that functional neuroimaging methods can provide unique insight into processes associated with behavior change (e.g., see Feldstein Ewing & Chung, 2013; Feldstein Ewing, Filbey, Sabbineni, Chandler, & Hutchison, 2011; Morgenstern, Naqvi, Debellis, & Breiter, 2013). With respect to the neural mechanisms associated with changing smoking behavior, specifically, our findings indicate that the ability to remain abstinent in the face of temptation is related to the functioning of the insula in relation to areas of the brain supporting cognitive control and reward-related processing. Accordingly, the findings suggest that facilitating connectivity between the insula and regions involved in relevant networks may be a potential point of intervention that could help facilitate smoking abstinence. For instance, advances in neurofeedback techniques, which have made it possible to train individuals to modulate connectivity between specific brain regions (e.g., see Kadosh et al., 2015; D. Y. Kim, Yoo, Tegethoff, Meinlschmidt, & Lee, 2015; Megumi, Yamashita, Kawato, & Imamizu, 2015), could be used to develop interventions that increase the functional coupling between the insula and the DLPFC in smokers. More broadly, research may benefit from focusing on the development and implementation of training geared explicitly at the interaction between deliberative and automatic processing, as opposed to targeting “top down” or “bottom up” processes in isolation (e.g., using cognitive control training to enhance deliberative processing).
4.1. Limitations
Although this study offers a novel methodology, meaningful findings, and promising directions for future research, some limitations should be considered. First, the sample size was constrained by the proportion of participants who elected to resist smoking and was relatively small. The payoff was that we were able to successfully match participants who did and did not choose to smoke along a host of relevant variables. Second, the number of regions of interest included for analysis was kept relatively constrained for analytical and interpretive purposes. Although ROIs were carefully selected on conceptual and empirical bases, future work exploring other areas might prove fruitful. A third potential limitation concerns differences between Study 1 and 2 (i.e., whether or not coping was used). Although participants were matched by study, as well as by which coping strategy they used (if relevant), instructing participants to resist smoking via a particular strategy could limit the variability of results; it is possible that some participants would otherwise utilize different strategies to attempt to resist smoking. Finally, while numerous studies have demonstrated that smoking cues robustly increase the urge to smoke (Carter & Tiffany, 1999), the design of the current experiment (i.e., the fixed order of cues) leaves open the possibility that observed increases in self-reported craving were attributable in part to the passage of time. We decided against counterbalancing the order of cues because of the concern that nicotine-deprived smokers exposed to smoking cues first would still be experiencing elevated urges during subsequent exposure to control cues (Sayette et al., 2010).
4.2. Conclusions
Consistent with prior work (Janes et al., 2010), it was found that smokers who were able to remain abstinent in the presence of smoking cues early during a quit attempt displayed increased connectivity between areas related to deliberative processing and the insula, namely the DLPFC and left anterior insula. The present study expands upon prior work by highlighting for the first time how early the links between functional connectivity and clinically meaningful differences in behavior emerge. Specifically, results from the current study demonstrate that cue-related connectivity of the insula predicts smoking behavior within the first hours of a quit attempt, a critical period during which the vulnerability to relapse is often highest. The observed links between functional connectivity and smoking behavior provide a compelling groundwork for future research with important clinical implications. Notably, there were no clear differences between those who were able to resist smoking and those who were not in mean activation of individual a priori regions of interest, further emphasizing the importance of examining connectivity in relation to smoking behavior. Future research using similar methods to characterize the association between functional connectivity of the insula and clinically relevant outcomes early during attempted smoking cessation would provide important data regarding the factors that shape smoking cessation and other forms of health-related behavior change.
Acknowledgments
We thank Deidra Rendinell, Alex Ciuca, and Maryam Khatami for their assistance with data collection and Corrine Durisko, Kate Fissel, Scott Kurdilla, and Deborah Viszlay for technical assistance. Financial support for this research was provided by National Institute on Drug Abuse Grant R01 DA02463 (Principal Investigator: JF) and the National Institute for Biomedical Imaging and Bioinformatics Grant R21 EB015573-01A1 (Principal Investigator: KMG). Portions of these data were presented at the Society for Research on Nicotine and Tobacco (Philadelphia, PA, February 2015).
Footnotes
Subgroups were matched according to age, ethnicity, smoking rate, years smoking, sex, coping strategy (for Study 2 – i.e. self vs. other strategy), and quitting motivation (motivated-to-quit vs. unmotivated-to-quit). Each subgroup included 17 males and 2 females. Twelve participants (6 Abstainers and 6 Lapsers) were selected from Study 1 and 26 participants (13 Abstainers and 13 Lapsers) were selected from Study 2.
Financial Disclosures
All authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addicott MA, Sweitzer MM, Froeliger B, Rose JE, McClernon FJ. Increased Functional Connectivity in an Insula-Based Network is Associated with Improved Smoking Cessation Outcomes. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SS, Bade T, Hatsukami D, Center B. Craving, withdrawal, and smoking urges on days immediately prior to smoking relapse. Nicotine and Tobacco Research. 2008;10(1):35–45. doi: 10.1080/14622200701705076. [DOI] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience. 2005;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995:289–300. [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug and Alcohol Dependence. 2007;90(Suppl 1):S85–91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box GEP, Jenkins GM. Time series analysis: Forecasting and control. San Francisco: Holden-Day; 1970. [Google Scholar]
- Bressler SL, Tognoli E. Operational principles of neurocognitive networks. International Journal of Psychophysiology. 2006;60(2):139–148. doi: 10.1016/j.ijpsycho.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Hughes JR. Defining quit attempts: what difference does a day make? Addiction. 2005;100(2):257–258. doi: 10.1111/j.1360-0443.2004.00952.x. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94(3):327–340. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention [CDC] Quitting smoking among adults--United States, 2001–2010. MMWR Morbidity and Mortality Weekly Report. 2011;60(44):1513–1519. [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cerebral Cortex. 2013;23(3):739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Elton A, Kennedy AP, Young J, Smitherman S, Andrew James G, Kilts CD. Altered functional connectivity of the insular cortex across prefrontal networks in cocaine addiction. Psychiatry Research. 2013;213(1):39–46. doi: 10.1016/j.pscychresns.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewett D, Luo S, Hsu E, Ainslie G, Mather M, Monterosso J. Increased functional coupling between the left fronto-parietal network and anterior insula predicts steeper delay discounting in smokers. Human Brain Mapping. 2014;35(8):3774–3787. doi: 10.1002/hbm.22436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney SM. Attention and cognitive control as emergent properties of information representation in working memory. Cognitive, Affective and Behavioral Neuroscience. 2004;4(4):501–516. doi: 10.3758/cabn.4.4.501. [DOI] [PubMed] [Google Scholar]
- Dinur-Klein L, Dannon P, Hadar A, Rosenberg O, Roth Y, Kotler M, Zangen A. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biological Psychiatry. 2014;76(9):742–749. doi: 10.1016/j.biopsych.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, … Cinciripini PM. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60(1):252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Chung T. Neuroimaging mechanisms of change in psychotherapy for addictive behaviors: emerging translational approaches that bridge biology and behavior. Psychology of Addictive Behaviors. 2013;27(2):329–335. doi: 10.1037/a0031491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Filbey FM, Sabbineni A, Chandler LD, Hutchison KE. How psychosocial alcohol interventions work: a preliminary look at what FMRI can tell us. Alcoholism, Clinical and Experimental Research. 2011;35(4):643–651. doi: 10.1111/j.1530-0277.2010.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. Journal of Substance Abuse Treatment. 2009;36(3):235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Forget B, Pushparaj A, Le Foll B. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biological Psychiatry. 2010;68(3):265–271. doi: 10.1016/j.biopsych.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Gates KM, Molenaar PC. Group search algorithm recovers effective connectivity maps for individuals in homogeneous and heterogeneous samples. Neuroimage. 2012;63(1):310–319. doi: 10.1016/j.neuroimage.2012.06.026. [DOI] [PubMed] [Google Scholar]
- Gates KM, Molenaar PC, Hillary FG, Ram N, Rovine MJ. Automatic search for fMRI connectivity mapping: an alternative to Granger causality testing using formal equivalences among SEM path modeling, VAR, and unified SEM. Neuroimage. 2010;50(3):1118–1125. doi: 10.1016/j.neuroimage.2009.12.117. [DOI] [PubMed] [Google Scholar]
- Gaznick N, Tranel D, McNutt A, Bechara A. Basal ganglia plus insula damage yields stronger disruption of smoking addiction than basal ganglia damage alone. Nicotine and Tobacco Research. 2014;16(4):445–453. doi: 10.1093/ntr/ntt172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13(9):372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends Cogn Sci. 2011;15(3):132–139. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Janes AC, Farmer S, Peechatka AL, Frederick BdB, Lukas SE. Insula-dorsal anterior cingulate cortex coupling is associated with enhanced brain reactivity to smoking cues. Neuropsychopharmacology. 2015;40:1561–1568. doi: 10.1038/npp.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, Frederick BdB, Chuzi S, Pachas G, … Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biological Psychiatry. 2010;67(8):722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadosh KC, Luo Q, de Burca C, Sokunbi MO, Feng J, Linden DE, Lau JY. Using real-time fMRI to influence differential effective connectivity in the developing emotion regulation network. Neuroimage. 2015 doi: 10.1016/j.neuroimage.2015.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Yoo SS, Tegethoff M, Meinlschmidt G, Lee JH. The inclusion of functional connectivity information into fMRI-based neurofeedback improves its efficacy in the reduction of cigarette cravings. Journal of Cognitive Neuroscience. 2015;27(8):1552–1572. doi: 10.1162/jocn_a_00802. [DOI] [PubMed] [Google Scholar]
- Kim J, Zhu W, Chang L, Bentler PM, Ernst T. Unified structural equation modeling approach for the analysis of multisubject, multivariate functional MRI data. Human Brain Mapping. 2007;28(2):85–93. doi: 10.1002/hbm.20259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. European Journal of Neuroscience. 2011;33(7):1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Lane ST, Gates KM, Molenaar PC. gimme. 2015 [Computer Software] https://cran.r-project.org/web/packages/gimme/index.html.
- Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry. 2014;71(5):523–530. doi: 10.1001/jamapsychiatry.2013.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Bickel WK. A dual-systems perspective on addiction: contributions from neuroimaging and cognitive training. Annals of the New York Academy of Sciences. 2014;1327:62–78. doi: 10.1111/nyas.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh MJ, Demers CH, Braud J, Briggs R, Adinoff B, Stein EA. Striatal-insula circuits in cocaine addiction: implications for impulsivity and relapse risk. American Journal of Drug and Alcohol Abuse. 2013;39(6):424–432. doi: 10.3109/00952990.2013.847446. [DOI] [PubMed] [Google Scholar]
- Megumi F, Yamashita A, Kawato M, Imamizu H. Functional MRI neurofeedback training on connectivity between two regions induces long-lasting changes in intrinsic functional network. Front Hum Neurosci. 2015;9:160. doi: 10.3389/fnhum.2015.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychological Review. 1999;106(1):3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- Morgenstern J, Naqvi NH, Debellis R, Breiter HC. The contributions of cognitive neuroscience and neuroimaging to understanding mechanisms of behavior change in addiction. Psychology of Addictive Behaviors. 2013;27(2):336–350. doi: 10.1037/a0032435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford JA, Ramsey JD. Bayesian networks for fMRI: a primer. Neuroimage. 2014;86:573–582. doi: 10.1016/j.neuroimage.2013.10.020. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends in Neurosciences. 2009;32(1):56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula. In: Wilson SJ, editor. The Wiley Handbook on the Cognitive Neuroscience of Addiction. Chichester, UK: John Wiley & Sons, Ltd; 2015. [Google Scholar]
- Naqvi NH, Gaznick N, Tranel D, Bechara A. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Annals of the New York Academy of Sciences. 2014;1316:53–70. doi: 10.1111/nyas.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, … Gakidou E. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA. 2014;311(2):183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- Nichols TT, Gates KM, Molenaar PC, Wilson SJ. Greater BOLD activity but more efficient connectivity is associated with better cognitive performance within a sample of nicotine-deprived smokers. Addiction Biology. 2014;19(5):931–940. doi: 10.1111/adb.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel X, Brevers D, Bechara A. A neurocognitive approach to understanding the neurobiology of addiction. Current Opinion in Neurobiology. 2013a;23(4):632–638. doi: 10.1016/j.conb.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel X, Brevers D, Bechara A. A triadic neurocognitive approach to addiction for clinical interventions. Front Psychiatry. 2013b;4:179. doi: 10.3389/fpsyt.2013.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA. Does smoking cue-induced craving tell us anything important about nicotine dependence? Addiction. 2009;104(10):1610–1616. doi: 10.1111/j.1360-0443.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- Piasecki TM. Relapse to smoking. Clinical Psychology Review. 2006;26(2):196–215. doi: 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, … Eickhoff SB. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage. 2012;60(1):830–846. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Griffin KM, Sayers WM. Counterbalancing in smoking cue research: a critical analysis. Nicotine and Tobacco Research. 2010;12(11):1068–1079. doi: 10.1093/ntr/ntq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Loewenstein G, Griffin KM, Black JJ. Exploring the cold-to-hot empathy gap in smokers. Psychological Science. 2008;19(9):926–932. doi: 10.1111/j.1467-9280.2008.02178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Miller KL, Salimi-Khorshidi G, Webster M, Beckmann CF, Nichols TE, … Woolrich MW. Network modelling methods for FMRI. Neuroimage. 2011;54(2):875–891. doi: 10.1016/j.neuroimage.2010.08.063. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suner-Soler R, Grau A, Gras ME, Font-Mayolas S, Silva Y, Davalos A, … Serena J. Smoking cessation 1 year poststroke and damage to the insular cortex. Stroke. 2012;43(1):131–136. doi: 10.1161/strokeaha.111.630004. [DOI] [PubMed] [Google Scholar]
- Sutherland MT, Liang X, Yang Y, Stein EA. Beyond functional localization: Advancing the understanding of addiction-related processes by examining brain connectivity. In: Wilson SJ, editor. The Wiley Handbook on the Cognitive Neuroscience of Addiction. Oxford, UK: John Wiley & Sons; 2015. [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage. 2012;62(4):2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services [USDHHS] The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- Viswanath H, Velasquez KM, Savjani R, Molfese DL, Curtis K, Molfese PJ, … Salas R. Interhemispheric insular and inferior frontal connectivity are associated with substance abuse in a psychiatric population. Neuropharmacology. 2015;92:63–68. doi: 10.1016/j.neuropharm.2014.12.030. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD. NOW vs LATER brain circuits: implications for obesity and addiction. Trends in Neurosciences. 2015 doi: 10.1016/j.tins.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Westman EC, Behm FM, Simel DL, Rose JE. Smoking behavior on the first day of a quit attempt predicts long-term abstinence. Archives of Internal Medicine. 1997;157(3):335–340. [PubMed] [Google Scholar]
- Wilson SJ, Creswell KG, Sayette MA, Fiez JA. Ambivalence about smoking and cue-elicited neural activity in quitting-motivated smokers faced with an opportunity to smoke. Addictive Behaviors. 2013;38:1541–1549. doi: 10.1016/j.addbeh.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA. Neuroimaging craving: urge intensity matters. Addiction. 2015;110(2):195–203. doi: 10.1111/add.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Instructed smoking expectancy modulates cue-elicited neural activity: a preliminary study. Nicotine and Tobacco Research. 2005;7(4):637–645. doi: 10.1080/14622200500185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: A neurocognitive analysis. Nature Neuroscience. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Quitting-unmotivated and quitting-motivated cigarette smokers exhibit different patterns of cue-elicited brain activation when anticipating an opportunity to smoke. Journal of Abnormal Psychology. 2012;121(1):198–211. doi: 10.1037/a0025112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Neural correlates of self-focused and other-focused strategies for coping with cigarette cue exposure. Psychology of Addictive Behaviors. 2013;27(2):466–476. doi: 10.1037/a0027055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KM, Patzelt EH, Lim KO, MacDonald AW., 3rd An intrinsic connectivity network approach to insula-derived dysfunctions among cocaine users. American Journal of Drug and Alcohol Abuse. 2013;39(6):403–413. doi: 10.3109/00952990.2013.848211. [DOI] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Naumer MJ. Functional neuroimaging studies in addiction: multisensory drug stimuli and neural cue reactivity. Neuroscience and Biobehavioral Reviews. 2012;36(2):825–835. doi: 10.1016/j.neubiorev.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Zanchi D, Brody AL, Montandon L, Kopel R, Emmert K, Preti MG, … Haller S. Cigarette smoking leads to persistent and dose-dependent alterations of brain activity and connectivity in anterior insula and anterior cingulate. Addiction Biology. 2015 doi: 10.1111/adb.12292. [DOI] [PubMed] [Google Scholar]
- Zhang JT, Yao YW, Li CS, Zang YF, Shen ZJ, Liu L, Fang XY. Altered resting-state functional connectivity of the insula in young adults with Internet gaming disorder. Addiction Biology. 2015 doi: 10.1111/adb.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]