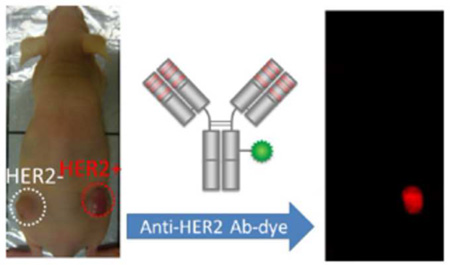
Abstract
The monoclonal antibody (mAb) has proven to be a good platform for designing specific molecular imaging probes due to its superior binding specificity. Several optical imaging probes have been developed for surgical navigation in patients and are in early phase clinical trials. However, an inherent limitation of using the mAb is its pharmacokinetics which result in a prolonged circulating half-life and slow clearance from the body. This results in undesirable target to background ratios during imaging. In this review, we first describe the mAb as a platform material for optical probe design and then discuss optimizing the design of monoclonal antibody-based optical molecular imaging probes by focusing on chemistry, biology and pharmacology.
Graphical Abstract
Introduction
Over 100 years ago, Erhlich proposed the idea that antibodies could be the “magic bullet” for detecting and treating disease. Now, there are more than 30 therapeutic antibodies, antibody-drug conjugates, antibody fragments, antibody-based imaging probes, and combined imaging and therapy antibody-based probes that have been approved by the FDA. Numerous others are in clinical trials or in earlier stages of development. Antibodies and their fragments can be engineered to specifically target a wide variety of cell surface proteins, either for diagnosis or to deliver localized therapy. [1] High specificity and target accumulation make monoclonal antibodies promising scaffolds for molecular imaging.
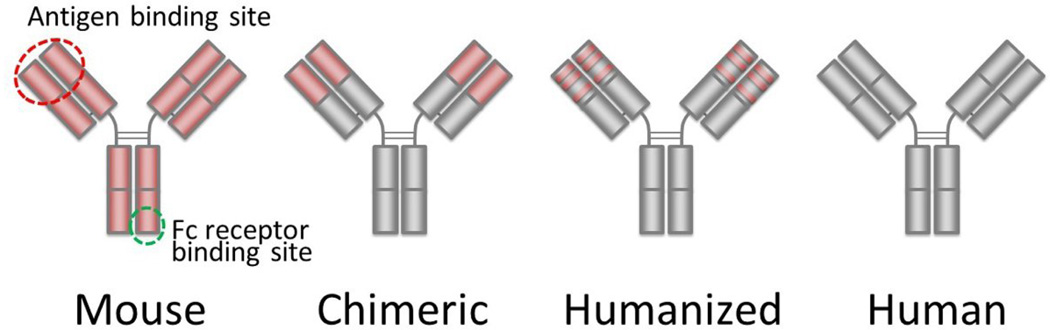
The binding sites of naturally produced monoclonal antibodies and their genetically engineered fragments can be modified to target a broad variety of cell surface epitopes. Originally, targeted monoclonal antibodies were produced by immunizing mice with target molecules. Then, antibody-producing murine B-cells were removed from the spleen and fused with myeloma cells to develop immortal hybridomas which were capable of producing monoclonal antibodies based on rearranged murine immunoglobulin genes. As a consequence, these monoclonal antibodies consist of mouse proteins. However, when used in humans, these monoclonal antibodies induce an immune response known as a human anti-mouse antibody (HAMA) which limits their repeated use in most patients. Interestingly, the HAMA response is less limiting in immunocompromised patients such as those with B-cell lymphoma or hematologic malignancies. This problem was largely eliminated by creating chimeric or humanized antibodies, which combine the variable region or the complimentary determining regions (CDRs), respectively, of murine antibodies with the constant regions of human antibodies, [2] Finally, large phage display libraries or humanized mice can be used to produce fully human antibodies, though the latter method permits only a limited variety of antibody production. (Figure 1).
Figure 1.
Protein structures of mouse, chimeric, humanized and entirely human antibodies. Parts derived from murine protein are shown in red.
Every antibody consists of two heavy chains (CH) and two light chains (CL), each of which contains a variable region (VH or VL), one to four constant regions (CH1, CH2, CH3, CH4, and CL), and sometimes a hinge region between the CH1 and CH2 regions. As the name suggests, the variable regions, which together compose the binding pocket, are extremely heterogeneous and therefore recognize a multitude of epitopes. The constant regions of the heavy chains interact with the host immune system and thus determine the antibody function (blocking or immunostimulatory), method of immunogenicity, and class: IgM, IgD, IgG, IgA, or IgE. Imaging probes are usually constructed with one of the four IgG subclasses (IgG1-4), which weigh ~150 kDa. [3] The vast majority of therapeutic monoclonal antibodies are of the IgG1 subclass, and these are particularly good for opsonizing targets by antibody-dependent cellular cytotoxicity (ADCC) or compliment-dependent cytotoxicity (CDC). [4] Therefore, when these therapeutic antibodies are used as imaging probes and not as therapeutic agents, the dose of the monoclonal antibody ranges from one fifth to one thirtieth compared to the therapeutic dose.
In this review, we will focus on the synthesis of imaging probes based on clinically available humanized or human monoclonal antibodies as the starting platform and review conjugation methods to optimize probe design.
Pharmacokinetic characteristics of monoclonal antibodies
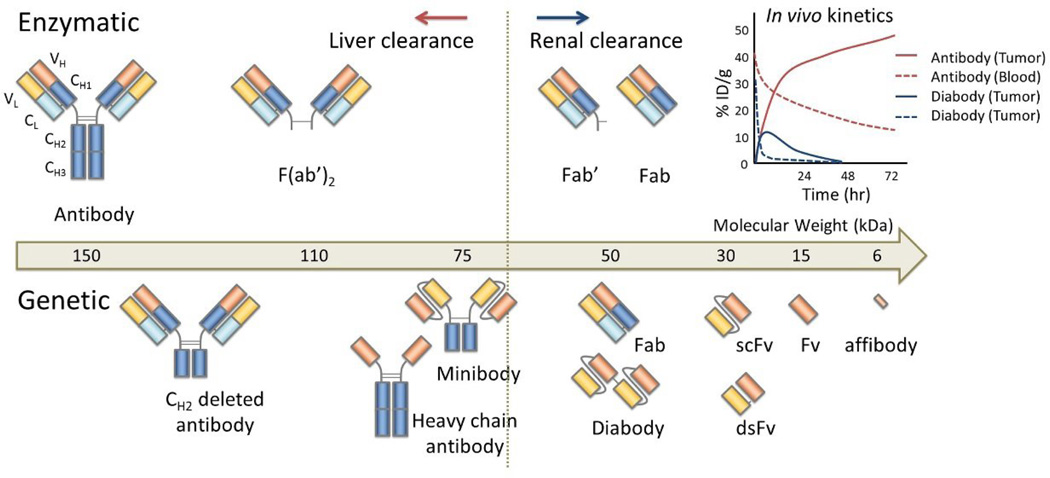
The biodistribution and pharmacokinetics of antibody-based molecules determine their safety and efficacy. The large size of intact antibodies (~150 kDa) results in slow clearance, high background signal, and nonspecific accumulation. Technologies based on enzymatic or genetic modifications of antibodies have been used to generate smaller antibody derivatives with improved pharmacokinetic properties (Figure 2). For instance, antibody fragments and other related constructs with sizes below the renal filtration threshold, clear rapidly, reducing background signal but also result in less tumor uptake. Unfortunately, these conflicting trends result in a trade-off between maximal target accumulation and minimal background signal. [5] Constructs based on antibody fragments can be engineered to optimize these parameters, although few antibody fragments have been FDA approved.
Figure 2.
The different types of enzymatically (upper) and genetically (lower) produced antibody fragments. Small fragments that are smaller than 60 kD are generally excreted through the kidneys, resulting in rapid blood clearance. The tumor uptake is generally higher in long circulating antibodies and their fragments due to superior input function.
Enzymatic digestion of intact antibodies was first used to produce antibody fragments which retained their targeting capabilities but also clear rapidly. Specifically, papain, pepsin or fucin were used to produce Fab’ and F(ab’)2 fragments, which include one or two antigen binding regions, respectively, each with a variable and constant domain (VH, VL, CH1, and CL), respectively. These Fab fragments exhibit rapid renal clearance and increased tumor penetration, particularly when compared to the whole antibody. Five Fab’ fragments have been approved by the FDA, three of which are for SPECT imaging. [6] Unfortunately, these fragments cannot be prepared from all subclasses of antibodies. Newer genetic engineering technology has enabled the isolation and easy production of the variable region as a unit. Single-chain variable fragments (scFv) contain only the antigen-binding variable regions (VH and VL) and a short peptide chain to link the C-terminus of one Fv region with the N-terminus of the other (Figure 2). [7] As a result, they are quite small, weighing only 26 kDa. Once designed, a single sequence codes the entire scFv. By including only variable regions, this construct also avoids secondary immune responses normally induced by antibody constant regions, particularly important for imaging applications where immunogenicity is a disadvantage. The two variable regions can instead be linked by a genetically engineered interchain disulfide bond, creating disulfide-stabilized Fv, or dsFv, or by both as in scdsFv fragments. [8] An even smaller construct type, affibodies (~6 kDa) are phage-selected from a library based on the Ig binding region of a staphylococcal protein with 13 randomized amino acids. [9] Rapid clearance and decreased avidity for the target (due to one binding site rather than two) of both scFv and affibodies, however, necessitate further alterations or strategies, such as genetic modification to enhance affinity, multimerization, the addition of other regions, or multistep targeting to achieve the necessary targeting.
A variety of methods use scFv molecules as building blocks for multivalent particles, improving binding characteristics, lengthening serum half-lives, and increasing tumor retention. For example, reduction of the peptide linker to 3-12 amino acids can force dimerization leading to the formation of diabodies, while shorter chain lengths, differences in composition and a switch in the V-domain orientation can induce the formation of tribodies and tetrabodies. Other multimers can be generated by including an additional covalent linker between units (sc(Fv)n) or multimer-inducing protein domains. [10,11]
Thoroughly investigated for therapeutic uses, scFv and multibodies are actively under investigation for imaging applications. Additional genetic modifications can improve specificity or multimerization. Monomeric, multibody and minibody constructs based on scFv continue to be developed for targeting a variety of cancer epitopes including EGFR, HER2/neu, PSMA, CEA, MUC1, Lewis Y, cMet, mindin/RG-1, and prostate specific stem cell antigen (PSCA) etc. [11–13]
Other techniques can be used to alter the pharmacokinetic properties of antibodies and antibody fragments. Other regions can be added to the antibody, such as a CH3 region (called a “minibody”), or the FC region. [5,14] The constant regions can be manipulated or genetically engineered to alter the pharmacokinetic properties of the antibody. The neonatal Fc receptor (FcRn) naturally maintains IgG levels in the serum by promoting recycling rather than degradation. As a result, antibodies with murine or human Fc regions will have different clearance properties: Antibodies with murine Fc will clear slower in mice and faster in humans while antibodies with human Fc will clear faster in mice and slower in humans. [15] Murine antibodies can be used only in immunocompromised patients whose HAMA response is minimal, to enable faster clearance but this strategy clearly limits the utility of the antibody construct. Genetic modifications in the Fc region alter the binding properties between FcRn and the antibody constant regions, with implications for the serum half-life of the antibody. [16] This property was used to create a series of scFv-Fc antibody fragments with serum half-lives ranging from 8 to 83 hours, compared to the 12 day half-life of the wild-type fragment. In another method, human serum albumin was genetically added to an anti-CEA scFv, resulting in more rapid and higher total tumor uptake than the bare scFv. [17] A variety of other methods, such as post-translational modifications and conjugation to peptides, carbohydrates or polymers have been used to extend the circulation time of antibody fragments by increasing their hydrodynamic volume. [18]
Monoclonal antibody as a platform for conjugation chemistry
From the viewpoint of conjugation chemistry, each antibody varies not only in its variable region but also in the subclass of the constant region. However, currently most clinically used mAbs are genetically engineered. Specifically, most humanized antibodies use the IgG1 framework and then use grafted amino acid sequences, named CDRs, for producing specific binding to each epitope. Therefore, since CDRs represent only an additional 2 % in number of amino acids, genetically engineered mAbs have around 98% homology in amino acid sequence and identical conformational protein structures. [19] Therefore, when bifunctional molecules are conjugated with different humanized antibodies, an identical chemical reaction can be achieved resulting in obtaining uniform products with similar yield. Therefore, once an optimal reaction condition is determined, that method can be applied to most humanized mAbs.
The most important consideration regarding altering the chemical characteristics of a mAb-dye conjugate is the chemical characteristics of the dye. This can change the in vivo pharmacokinetics and tumor-to-background ratio. Small, hydrophilic dyes have the least effect on pharmacokinetics of mAb-dye conjugates. Such dyes usually emit visible light of short wavelength. [20] However, for in vivo imaging, near infrared (NIR) emission is preferred because of the low background autofluorescence and superior tissue penetration of NIR light. Cyanine derivatives, for instance, are frequently used as near infrared fluorescent dyes for in vivo fluorescence imaging. [21] There are several useful pointers in designing optimal near infrared fluorescent cyanine dyes; 1. Charged residues on both aromatic rings minimize non-covalent protein binding, [20] 2. Stable charges help the dye retain its hydrophilicity, 3. Zwitterionic charge increases renal excretion of catabolites of mAb-dye conjugates, [22] 4. C4-N-alkyl linkers minimize changes in pharmacokinetics after conjugation of multiple dyes to a single mAb, 5. The symmetric structure of bifunctional cyanine dyes lends itself to high yield synthesis. [23] Items 4 and 5 might become particularly important from the regulatory and commercial perspective, when cyanine dyes are used in clinical preparations. These 5 points only begin to cover the range of considerations in preparing mAb-NIR dye conjugates.
Another important factor for achieving superior results with antibody-dye conjugates is the choice of antibodies. Most IgGs generally show similar pharmacokinetics. However, some mAbs change their pharmacokinetics more readily upon modification than others.. For example, both cetuximab and panitumumab recognize the same epitope on EGFR, yet show different in vivo pharmacokinetics when conjugated with signaling moieties including chelates for holding a radiometal for radionuclide imaging or fluorescent cyanine dyes for fluorescence imaging. In this case, cetuximab is highly sensitive to the effects of conjugation and its pharmacokinetics are drastically altered compared with the same alterations using panitumumab. [24,25] Therefore, selection of a stable mAb that can withstand the effects of conjugation will permit more consistent mAb-dye conjugates.
Activatable monoclonal antibody-based optical imaging probes
The conventional contrast agents used in computed tomography (CT), magnetic resonance imaging (MRI), and angiography, continuously emit signals and hence are “always on”. Although radionuclide imaging is targeted to a specific target the radioactive signaling isotope is also “always on”. The fundamental disadvantage of “always on” probes is that they emit signal regardless of their proximity or interaction with the target tissues, and as a result, there is considerable background signal to contend with. In order to design superior molecular imaging probes, one seeks to either (1) maximize signal from the target, (2) minimize signal from the background, or (3) do both. Doing any of these leads to improved target-to-background ratio (TBR), which, in turn, improves the sensitivity and specificity for detecting a particular disease state with imaging. [26]
When using an “always on” imaging probe, the signal is dependent on the biodistribution of the probe. However, because mAb-conjugates have slow clearance they tend to lower TBR. Post-processing of imaging, including kinetic analysis, is a method to improve the detection of specific binding. Kinetic analysis requires that an accurate quantitative readout be obtained during radionuclide imaging. Using the time-activity curve in each image voxel, kinetic parameters can be calculated that can depict the specific binding fraction as a map. However, since optical imaging is not as quantitative as radionuclide imaging, parametric analysis of this type is unreliable.
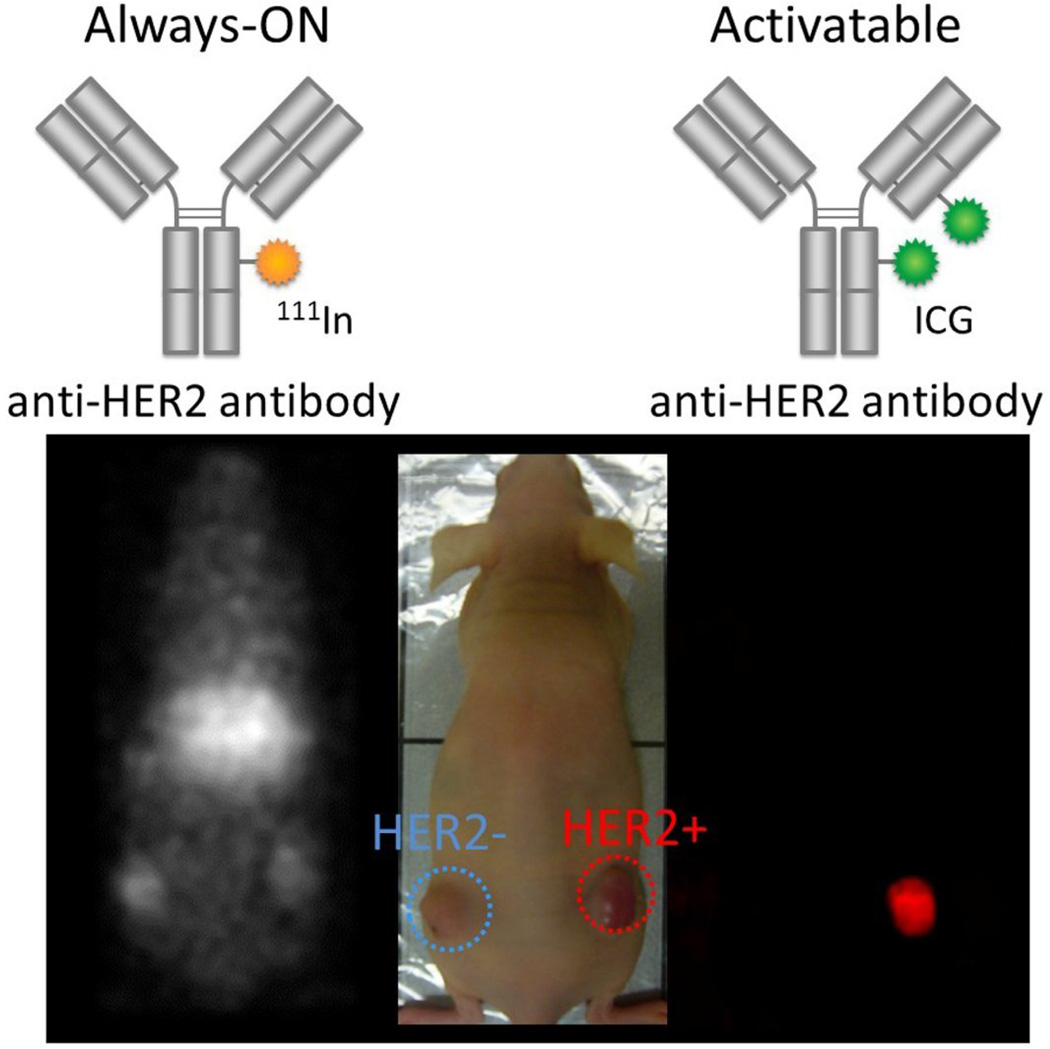
In contrast to the “always on” probe used in most kinds of imaging, the fluorescence signal is unique in that it can be suppressed or “turned on”. Optical imaging probes can be designed so that they do not emit light prior to binding to their target, but generate light only upon binding to their target molecules. The attribute of “turning on” only at the target has been termed “activatable” or “smart”. Therefore, activatable agents have low to no background signal and generate signal only after binding to the specific molecular target. These activatable fluorescence probes maximize the target signal while minimizing the background signal, thereby achieving the highest target-to-background ratios. When employing the antibody as a platform for activatable imaging probes, the whole IgG is usually the best platform molecule because it demonstrates the highest accumulation and binding to target cells due to its long lasting high input function. Therefore IgG-based activatable probes typically yield both the highest signal (due to high binding) as well as highest TBR (due to absent background signal). [26,27] (Figure 3)
Figure 3.
Comparison of molecular (HER2) target images using always-ON and activatable probes. Radiolabeled trastuzumab that emits always-ON signal depicts both bound and unbound agents (left and right tumors) resulting in poor TBR. In contrast, the activatable fluorescent probe, ICG-labeled trastuzumab, depicts only a target-expressing tumor (right tumor) without background signal resulting in superior TBR.
Self-quenching with multiple fluorophores based on Förster resonance energy transfer (FRET) or dimer formation enables a fluorophore to become activatable. In both mechanisms, quenching of fluorescence signal relies on the distance between fluorophores. [26] By increasing the distance between a quenched pair of fluorophores, fluorescence signal can be turned back on. For instance, xanthene fluorophores, such as TAMRA, can be made activatable by designing molecules so that the dye molecules form H-dimers, thus quenching fluorescence. In this case, the fluorescence of mAb-TAMRA conjugate was quenched before binding the target cell but was turned on only after binding to and internalization within the target. As a result, target tumors were visualized with high TBR in fluorescent images after injection of activatable mAb-TAMRA. However, not all fluorophores are amenable to H-dimer formation. For instance, highly charged fluorophores, such as AlexaFluor488, cannot be induced to form H-dimers and therefore remain as unquenched “always on” probes even after the conjugation of multiple fluorophore molecules. [28]
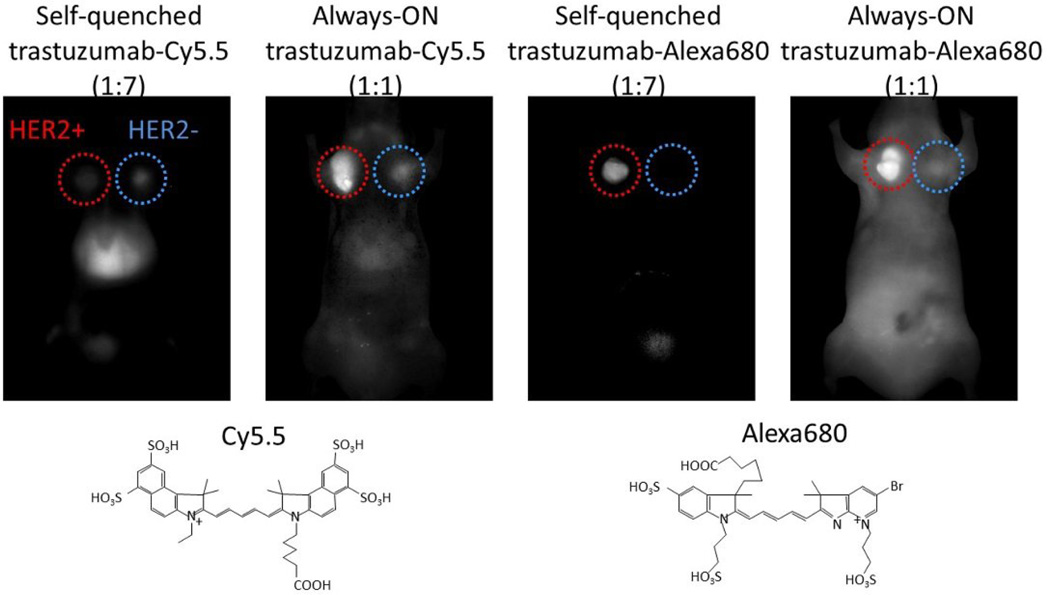
A drawback of activatable probes that utilize the self-quenching mechanism is that multiple fluorophores are needed to achieve quenching. Multiple fluorophore conjugation could interfere with the affinity of the antibody to its antigen, and furthermore, might alter the in vivo pharmacokinetics of the conjugate. For instance, conjugation of multiple molecules of Cy5.5, which is a widely used NIR fluorophore both in vitro and in vivo, altered the kinetics of the antibody and did not show specific accumulation in target tumors in vivo due to loss of specific binding affinity. In contrast, when a different NIR fluorophore with similar fluorescence profile with Cy5.5, AlexaFluor680, was conjugated with the antibody, the conjugates were activatable in vivo. In this case, the multiple fluorophore conjugation did not alter the conjugate’s behavior either in vitro or in vivo. [29] An explanation is that the strongly anionic Cy5.5 likely changes the hydrophobicity of the conjugate, thus altering the pharmacokinetics. (Figure 4)
Figure 4.
Chemical properties of fluorescent dyes affect the in vivo pharmacokinetics of mAb-dye conjugates. Target-specific tumor detection was successful only when self-quenched Alexa680-Ab (activatable probe) was employed.
Some dyes are particularly sensitive to quenching after conjugation. When only one functionalized ICG dye was conjugated with a single antibody molecule the dye quenched. Hydrophobic interaction between ICG and the antibody might explain this highly sensitive fluorescence quenching. [30,31] Thus, Ab-ICG conjugates are capable of becoming activatable with conjugation ratios as low as 1:1 to 1.5:1, however, during purification processes, complete removal of non-covalently associated ICG-derivative to antibody was rather difficult. [32–34] An important caveat is that idiosyncratic biodistributions of mAb-conjugates can defeat attempts to create an activatable probe. For instance when a diabody (Db) was activatably labeled with ICG it was not a successful imaging agent. Radiolabeled Db is rapidly cleared from the circulation through the kidney resulting in high tumor-to-background ratio on radionuclide imaging. However, when Db-ICG was first tested it was surprising that it did not yield a high tumor-to-background ratio. In reality, the tumor-to-background ratio decreased because a large amount of injected Db-ICG probe rapidly accumulated in the kidney and was catabolized and reabsorbed in the renal tubules. Small unquenched ICG-derivatives were then released into the circulation. These fluorescing ICG-derivatives were excreted through the liver into the bile to the intestine. Therefore, ICG fluorescence signal remained high up to 2 days after injection of Db-ICG by which time most of the catabolites had cleared from the intestine. Since the percentage of injected dose of Db-ICG probe in the tumor was not high because of rapid clearance from the circulation the conjugate had a low input function and the tumor-to-background ratio of Db-ICG probe remained low at all time points. This low tumor-to-background ratio was not observed with radionuclide labeling because radionuclides were not reabsorbed in the kidney and were generally excreted into the urine. [32] Thus, when designing fluorescence imaging probe, it is important to consider the excretion route and timing of activated catabolites.
Practical clinical applications of monoclonal antibody-based fluorescent imaging probes
There are two scenarios in which mAb-based fluorescent probes could become clinically useful. One is in fluorescence guided navigation to aid surgeons in detecting tiny lesions and determining the margin between cancer and normal tissue. Another is in selecting patients whose cancer cells express a sufficient amount of target on the cellular membrane to enable molecularly targeted therapies such as antibody-drug conjugates (ADC) or antibody-photo-absorber conjugates (APC) to be successful.
A first-in-human clinical trial of fluorescence guided navigation to aid surgery in head and neck cancers is currently underway utilizing the cetuximab-IRDye800 conjugate that targets EGFR which is commonly overexpressed on head and neck cancers. [35,36] This agent depicted the extent of squamous cell carcinoma in patients undergoing surgery. It was noted that the conjugation of the IRDye800 significantly shortened the circulating half-life of cetuximab even though a low antibody-to-dye conjugation ratio (approximately 1) was chosen. [36] Rapid clearance of mAb-dye conjugates might help lower the background signal, but at the same time it can also compromise tumor accumulation due to decreasing input function. Taken together, these alterations could lower the performance of the agent.
When performing NIR-PIT, the photo-absorber, IRDye700DX, typically results in an “always on” type of fluorescence signal. [37] Therefore, fluorescence signal of IRDye700DX in the cancer tissue permits the estimation of the accumulation of mAb-IRDye700DX in the tumor. However, the signal cannot differentiate between bound and unbound mAb-IRDye700DX. In order to show sufficient expression of target molecules, activatable fluorescent probes would be useful for selecting eligible patients who could be efficiently treated with an ADC or APC such as in NIR-PIT.
Conclusions
In this review, we have focused on designing and synthesizing optimal mAb-based fluorescent imaging probes for clinical application especially from the conjugation chemistry point of view. Designing appropriate fluorescent dyes and conjugating them with stable humanized mAbs ideally will not alter in vivo pharmacokinetics of conjugate compared with the parental mAb. Having the conjugate behave similarly to the unconjugated antibody helps in achieving regulatory approval and, therefore, clinical translation. When it is possible to design them, activatable fluorescent imaging probes yield higher target-to-background ratios than “always on” probes, thereby improving sensitivity and specificity for detecting cancers.
Highlights.
Monoclonal antibody is a good platform for designing optical molecular imaging probe
Conjugation reaction to humanized can be similarly performed
Optimal florescent dyes do not alter in vivo pharmacokinetics of conjugates
Charge and hydrophilicity of fluorescent dyes should be considered for the selection
Antibody-based fluorescent probes has started to be used for clinical trials
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- 2.Carter P, Merchant AM. Engineering antibodies for imaging and therapy. Current Opinion in Biotechnology. 1997;8:449–454. doi: 10.1016/s0958-1669(97)80067-5. [DOI] [PubMed] [Google Scholar]
- 3.Jain M, Kamal N, Batra SK. Engineering antibodies for clinical applications. Trends in Biotechnology. 2007;25:307–316. doi: 10.1016/j.tibtech.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 4. Weiner LM, Surana R, Wang SZ. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nature Reviews Immunology. 2010;10:317–327. doi: 10.1038/nri2744. (* an excellent review covering cancer immunotherapy using monoclonal antibody as a platform material)
- 5. Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23:1137–1146. doi: 10.1038/nbt1141. (** an excellent review overviewing armed monoclonal antibody and its fragments for cancer therapy)
- 6.Kenanova V, Wu AM. Tailoring antibodies for radionuclide delivery. Expert Opin Drug Deliv. 2006;3:53–70. doi: 10.1517/17425247.3.1.53. [DOI] [PubMed] [Google Scholar]
- 7.Huston JS, McCartney J, Tai MS, Mottola-Hartshorn C, Jin D, Warren F, Keck P, Oppermann H. Medical applications of single-chain antibodies. Int Rev Immunol. 1993;10:195–217. doi: 10.3109/08830189309061696. [DOI] [PubMed] [Google Scholar]
- 8.Reiter Y, Brinkmann U, Lee BK, Pastan I. Engineering antibody Fv fragments for cancer detection and therapy: Disulfide-stabilized Fv fragments. Nature Biotechnology. 1996;14:1239–1245. doi: 10.1038/nbt1096-1239. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson FY, Tolmachev V. Affibody (R) molecules: New protein domains for molecular imaging and targeted tumor therapy. Current Opinion in Drug Discovery & Development. 2007;10:167–175. [PubMed] [Google Scholar]
- 10.Cuesta AM, Sainz-Pastor N, Bonet J, Oliva B, Alvarez-Vallina L. Multivalent antibodies: when design surpasses evolution. Trends Biotechnol. 2010;28:355–362. doi: 10.1016/j.tibtech.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Xiong CY, Natarajan A, Shi XB, Denardo GL, Denardo SJ. Development of tumor targeting anti-MUC-1 multimer: effects of di-scFv unpaired cysteine location on PEGylation and tumor binding. Protein Engineering Design & Selection. 2006;19:359–367. doi: 10.1093/protein/gzl020. [DOI] [PubMed] [Google Scholar]
- 12.Kelly MP, Lee FT, Tahtis K, Power BE, Smyth FE, Brechbiel MW, Hudson PJ, Scott AM. Tumor targeting by a multivalent single-chain Fv (scFv) anti-Lewis Y antibody construct. Cancer Biotherapy and Radiopharmaceuticals. 2008;23:411–423. doi: 10.1089/cbr.2007.0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider DW, Heitner T, Alicke B, Light DR, McLean K, Satozawa N, Parry G, Yoo J, Lewis JS, Parry R. In Vivo Biodistribution, PET Imaging, and Tumor Accumulation of Y-86- and In-111-Antimindin/RG-1, Engineered Antibody Fragments in LNCaP Tumor-Bearing Nude Mice. Journal of Nuclear Medicine. 2009;50:435–443. doi: 10.2967/jnumed.108.055608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferl GZ, Kenanova V, Wu AM, DiStefano JJ. A two-tiered physiologically based model for dually labeled single-chain Fv-Fc antibody fragments. Molecular Cancer Therapeutics. 2006;5:1550–1558. doi: 10.1158/1535-7163.MCT-06-0072. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi H, Sakahara H, Saga T, Hosono M, Shirato M, Kanda H, Ishibashi K, Watanabe T, Endo K, Ishiwata I, et al. A Human Mouse Chimeric Monoclonal Antibody against CA-125 for Radioimmunoimaging of Ovarian Cancer. Cancer Immunology Immunotherapy. 1993;37:143–149. doi: 10.1007/BF01525427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghetie V, Popov S, Borvak J, Radu C, Matesoi D, Medesan C, Ober RJ, Ward ES. Increasing the serum persistence of an IgG fragment by random mutagenesis. Nature Biotechnology. 1997;15:637–640. doi: 10.1038/nbt0797-637. [DOI] [PubMed] [Google Scholar]
- 17.Kenanova V, Olafsen T, Crow DM, Sundaresan G, Subbarayan M, Carter NH, Ikle DN, Yazaki PJ, Chatziioannou AF, Gambhir SS, et al. Tailoring the pharmacokinetics and positron emission tomography imaging properties of anti-carcinoembryonic antigen single-chain Fv-Fc antibody fragments. Cancer Research. 2005;65:622–631. [PMC free article] [PubMed] [Google Scholar]
- 18.Kontermann RE. Strategies to extend plasma half-lives of recombinant antibodies. BioDrugs. 2009;23:93–109. doi: 10.2165/00063030-200923020-00003. [DOI] [PubMed] [Google Scholar]
- 19.Gonzales NR, De Pascalis R, Schlom J, Kashmiri SV. Minimizing the immunogenicity of antibodies for clinical application. Tumour Biol. 2005;26:31–43. doi: 10.1159/000084184. [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi H, Ogawa M, Alford R, Choyke PL, Urano Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem Rev. 2010;110:2620–2640. doi: 10.1021/cr900263j. (** an excellent review covering chemical designs of fluorescent imaging probes for medical applications)
- 21.Kobayashi H, Longmire MR, Ogawa M, Choyke PL. Rational chemical design of the next generation of molecular imaging probes based on physics and biology: mixing modalities, colors and signals. Chem Soc Rev. 2011;40:4626–4648. doi: 10.1039/c1cs15077d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi HS, Gibbs SL, Lee JH, Kim SH, Ashitate Y, Liu F, Hyun H, Park G, Xie Y, Bae S, et al. Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nat Biotechnol. 2013;31:148–153. doi: 10.1038/nbt.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato K, Nagaya T, Nakamura Y, Harada T, Nani RR, Shaum JB, Gorka AP, Kim I, Paik CH, Choyke PL, et al. Impact of C4'-O-Alkyl Linker on in Vivo Pharmacokinetics of Near-Infrared Cyanine/Monoclonal Antibody Conjugates. Mol Pharm. 2015;12:3303–3311. doi: 10.1021/acs.molpharmaceut.5b00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrett T, Koyama Y, Hama Y, Ravizzini G, Shin IS, Jang BS, Paik CH, Urano Y, Choyke PL, Kobayashi H. In vivo diagnosis of epidermal growth factor receptor expression using molecular imaging with a cocktail of optically labeled monoclonal antibodies. Clin Cancer Res. 2007;13:6639–6648. doi: 10.1158/1078-0432.CCR-07-1119. [DOI] [PubMed] [Google Scholar]
- 25.Sato K, Watanabe R, Hanaoka H, Harada T, Nakajima T, Kim I, Paik CH, Choyke PL, Kobayashi H. Photoimmunotherapy: comparative effectiveness of two monoclonal antibodies targeting the epidermal growth factor receptor. Mol Oncol. 2014;8:620–632. doi: 10.1016/j.molonc.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kobayashi H, Choyke PL. Target-cancer-cell-specific activatable fluorescence imaging probes: rational design and in vivo applications. Acc Chem Res. 2011;44:83–90. doi: 10.1021/ar1000633. (* an excellent review for describing chemical designs of activatable fluorescent imaging probes for medical diagnosis)
- 27.Ogawa M, Regino CA, Seidel J, Green MV, Xi W, Williams M, Kosaka N, Choyke PL, Kobayashi H. Dual-modality molecular imaging using antibodies labeled with activatable fluorescence and a radionuclide for specific and quantitative targeted cancer detection. Bioconjug Chem. 2009;20:2177–2184. doi: 10.1021/bc900362k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogawa M, Kosaka N, Choyke PL, Kobayashi H. H-type dimer formation of fluorophores: a mechanism for activatable, in vivo optical molecular imaging. ACS Chem Biol. 2009;4:535–546. doi: 10.1021/cb900089j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogawa M, Regino CA, Choyke PL, Kobayashi H. In vivo target-specific activatable near-infrared optical labeling of humanized monoclonal antibodies. Mol Cancer Ther. 2009;8:232–239. doi: 10.1158/1535-7163.MCT-08-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakajima T, Mitsunaga M, Bander NH, Heston WD, Choyke PL, Kobayashi H. Targeted, activatable, in vivo fluorescence imaging of prostate-specific membrane antigen (PSMA) positive tumors using the quenched humanized J591 antibody-indocyanine green (ICG) conjugate. Bioconjug Chem. 2011;22:1700–1705. doi: 10.1021/bc2002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ogawa M, Kosaka N, Choyke PL, Kobayashi H. In vivo molecular imaging of cancer with a quenching near-infrared fluorescent probe using conjugates of monoclonal antibodies and indocyanine green. Cancer Res. 2009;69:1268–1272. doi: 10.1158/0008-5472.CAN-08-3116. (* an original work for designing practical actovatable fluorescent antibody-based imaging probes with functionalized indocyanine green)
- 32.Sano K, Nakajima T, Ali T, Bartlett DW, Wu AM, Kim I, Paik CH, Choyke PL, Kobayashi H. Activatable fluorescent cys-diabody conjugated with indocyanine green derivative: consideration of fluorescent catabolite kinetics on molecular imaging. J Biomed Opt. 2013;18:101304. doi: 10.1117/1.JBO.18.10.101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sano K, Nakajima T, Miyazaki K, Ohuchi Y, Ikegami T, Choyke PL, Kobayashi H. Short PEG-Linkers Improve the Performance of Targeted, Activatable Monoclonal Antibody-Indocyanine Green Optical Imaging Probes. Bioconjug Chem. 2013;24:811–816. doi: 10.1021/bc400050k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe R, Sato K, Hanaoka H, Harada T, Nakajima T, Kim I, Paik CH, Wu AM, Choyke PL, Kobayashi H. Minibody-indocyanine green based activatable optical imaging probes: the role of short polyethylene glycol linkers. ACS Med Chem Lett. 2014;5:411–415. doi: 10.1021/ml400533y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Boer E, Warram JM, Tucker MD, Hartman YE, Moore LS, de Jong JS, Chung TK, Korb ML, Zinn KR, van Dam GM, et al. In Vivo Fluorescence Immunohistochemistry: Localization of Fluorescently Labeled Cetuximab in Squamous Cell Carcinomas. Sci Rep. 2015;5:10169. doi: 10.1038/srep10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosenthal EL, Warram JM, de Boer E, Chung TK, Korb ML, Brandwein-Gensler M, Strong TV, Schmalbach CE, Morlandt AB, Agarwal G, et al. Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin Cancer Res. 2015;21:3658–3666. doi: 10.1158/1078-0432.CCR-14-3284. (* an original first-in-human work of fluorescent surgery mavigation using an antibody-based near infrared fluorescent imaging probe)
- 37.Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2011;17:1685–1691. doi: 10.1038/nm.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]