Abstract
Voltage imaging has the potential to unravel the contributions that rapid changes in membrane voltage make to cellular physiology, especially in the context of neuroscience. In particular, small molecule fluorophores are especially attractive because they can, in theory, provide fast and sensitive measurements of membrane potential dynamics. A number of classes of small molecule voltage indicators will be discussed, including dyes with improved two-photon voltage sensing, near infrared optical profiles for use in in vivo applications, and newly developed electron-transfer based indicators, or VoltageFluors, that can be tuned across a range of wavelengths to enable all-optical voltage manipulation and measurement. Limitations and a “wish-list” for voltage indicators will also be discussed.
Introduction
The cellular plasma membrane separates the internal cellular environment from external surroundings, enabling the accumulation of nutrients, ions, biomolecules, and genetic material that allows for the existence of life on earth. One particularly important facet of membrane biology is the ability to sequester ionic species in unequal concentrations, setting up electrochemical potential gradients, or membrane voltage (Vmem), that cells harness for a variety of physiological roles. Cells that rapidly change their Vmem are known as “excitable” and use Vmem changes, on the order of 1 to 100 ms in duration, to drive their unique physiology. For example, membrane depolarization drives neurotransmitter release in neurons and contraction in myocytes. Mounting evidence points to important roles for Vmem in a number of basic physiological processes such as cell division, migration, and differentiation, underlying the centrality of Vmem dynamics that stretches far beyond those typically associated with specialized, excitable cells [1].
Tools to assess Vmem
Despite the central importance of Vmem to cellular physiology, in both excitable and non-excitable cells, methods to monitor Vmem dynamics remain limited. Patch clamp electrophysiology remains the “gold standard” to probe Vmem. While patch-clamp methodologies deliver extremely sensitive measurements of Vmem, they are highly invasive, limited to cell bodies, and exceptionally low-throughput. Multi-electrode arrays enable parallel electrode recordings, but cannot provide cellular spatial resolution. Imaging approaches represent an attractive solution to interrogating Vmem, because they are relatively non-invasive, high throughput, and provide spatial information regarding Vmem dynamics that would not be achievable through more traditional methods [2, 3].
Voltage Indicator Challenges
Voltage imaging with fluorescent indicators has been a long-standing goal of the scientific community [2–4]; however, the broad implementation of voltage imaging remains elusive, due in part to the challenge of developing optical tools for sensing Vmem. First, because neuronal action potentials are fast, on the order of several ms, optical voltage sensors must be able to respond quickly (sub-millisecond). Furthermore, unlike Ca2+ indicators, whose wide-spread implementation represent a triumph of chemical biology, voltage indicators must localize to the plasma membrane in order to sense Vmem. This represents both a design challenge (localization) and a limit on the number of dye molecules that can generate useful signal, since the volume of the cytosol dwarfs that of the thin plasma membrane. The double jeopardy of fast biological events and small volumes requires bright, photostable, highly sensitive, and non-disruptive voltage-sensitive dyes. There have been a number of promising approaches put forward for optical voltage sensing, including, more recently, the use of fluorescent proteins[5, 6], opsins[7, 8], second-harmonic generation[9], and nanomaterials [10, 11]. This review will focus on recent[12–14] developments in small molecule fluorescent voltage indicators.
Small Molecule Approaches to Voltage Imaging
Voltage imaging with small molecules has a long history. In the early 1970s, Larry Cohen and coworkers screened thousands of available dyes searching for compounds that display voltage-sensitive optical properties. Merocyanine 540 (Fig. 1) displayed voltage-sensitive fluorescence, enabling tracking of action potentials in a giant squid axon [4]. A comprehensive history is available from Cohen himself and acknowledges the myriad contributions made in the early days of voltage imaging [15]. Since that time, a number of strategies for voltage imaging with small molecules have been explored in the search for more sensitive compounds. The dyes can generally be divided into two classes, “fast” and “slow” response dyes.
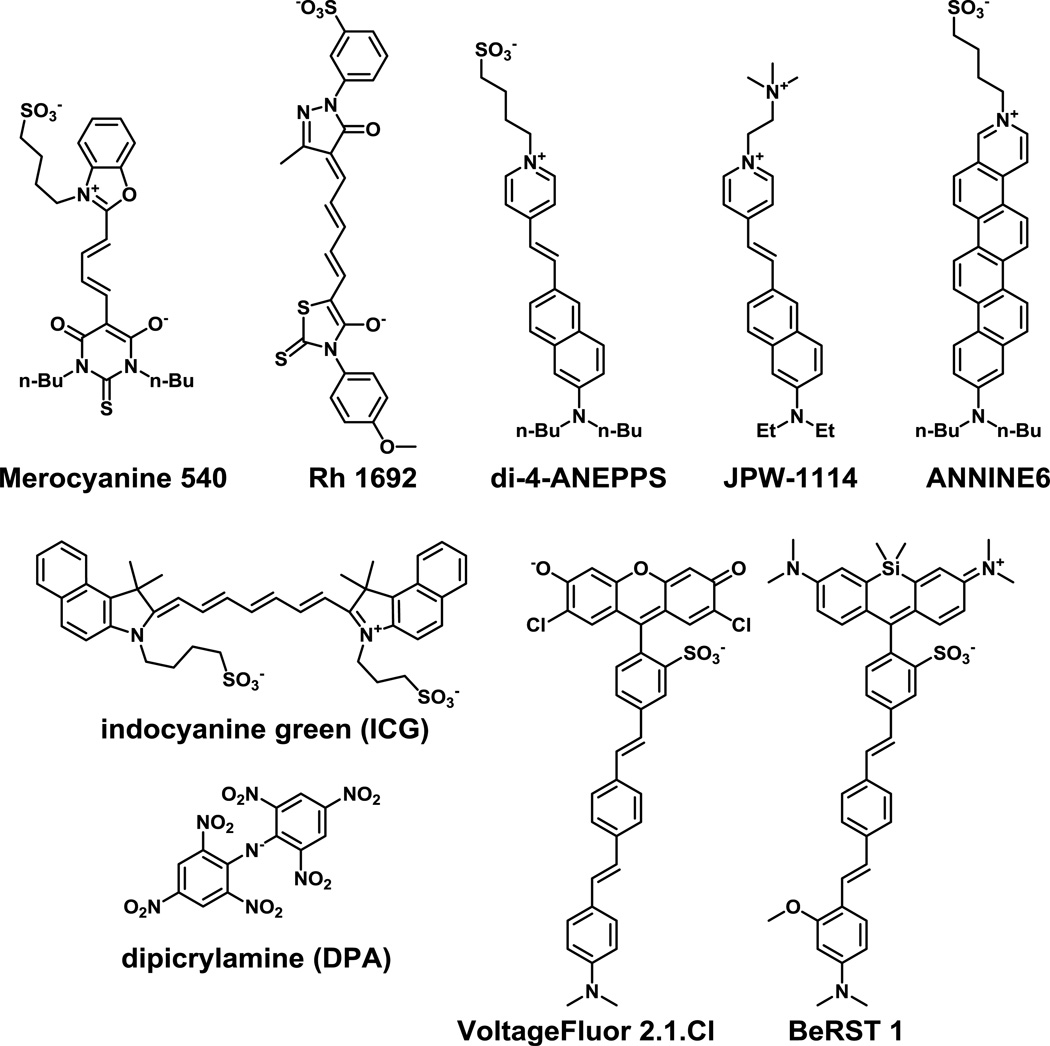
Figure 1.
Structures of representative small-molecule voltage-sensitive dyes. Merocyanine 540 was one of the first voltage-sensitive fluorescent dyes to find wide usage. RH1692 finds wide application in network-level voltage imaging. di-4-ANNEPS, JPW 1114, and ANNINE6 are all examples of charge-shift, or “fast” electrochromic voltage sensors. Indocyanine green is an FDA-approved near-infrared dye that exhibits voltage sensitivity. Dipicrylamine (DPA) is a lipophilic anion that partitions in the membrane in a potential-dependent manner. VoltageFluor 2.1.Cl and BeRST 1 are potentiometric dyes that make use of photoinduced electron transfer (PeT) to sense voltage.
Electrochromic dyes (Fig. 2b), often referred to as “fast” dyes, provide excellent response speed, making them ideally suited to monitoring fast neuronal voltage changes. Voltage sensitivity in electrochromic dyes arises from of a molecular Stark effect, in which the cellular electric field perturbs the energy levels of the chromophore dipole, resulting in fast (fs to ps) shifts in both the absorption and emission profiles. The color change can be used for ratiometric imaging, but the shifts themselves are actually quite small (<10 nm), meaning that maximum voltage sensitivity arises at the edge of the excitation and emission spectrum. Practically speaking, this means that instrumental parameters must be highly tuned to the dye of interest and that the majority of excitation and emission photons are not used, further diminishing an already-starved photon budget.
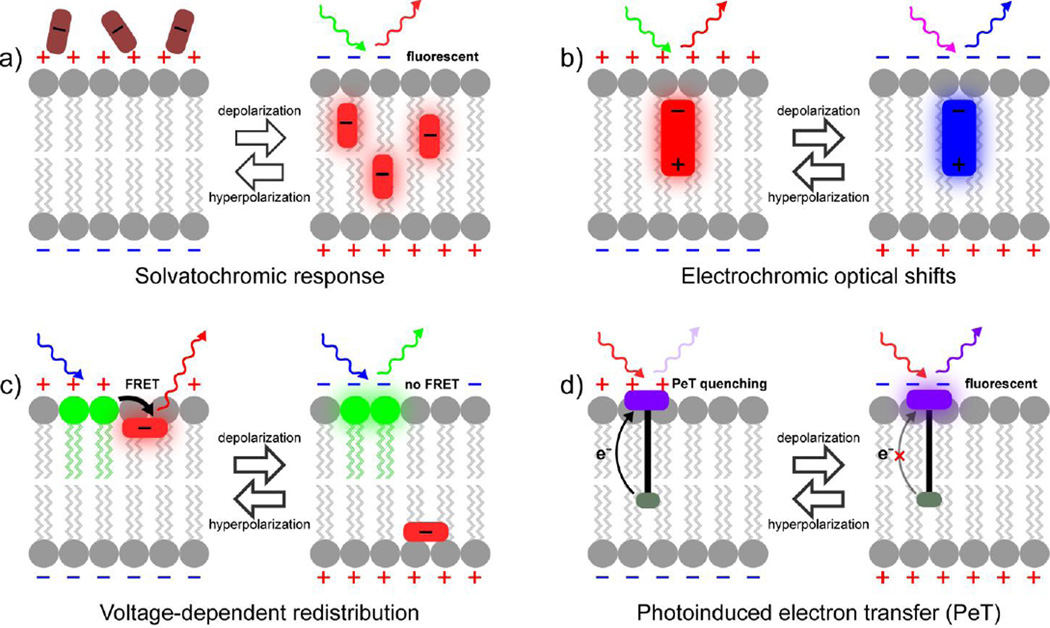
Figure 2.
Methods of voltage sensing with small molecules. a) Some voltage-sensitive dyes display voltage-dependent accumulation in cell membranes. Typically this is associated with a solvatochromic change, where the dye becomes more fluorescent upon association with the relatively non-polar lipid bilayer. An example of this is the slow response component of merocyanine 540. b) Electrochromic, or “fast” voltage-sensitive dyes, interact directly with the electric field across a cell membrane, causing a change in the molecular orbital energetics of the dye. This results in small wavelength changes depending on the relative orientation of the fluorophore dipole with the membrane-associated electric field. For the case of dyes such as di-4-ANEPPS, this results in a bathochromic shift upon hyperpolarization and a hypsochromic shift upon depolarization. c) Lipophilic anions traverse the membrane in a potential-dependent fashion. Several configurations are possible, however, all include a stationary fluorophore, pictured here on the outer leaflet of the membrane (green), and a mobile fluorophore or quenching group (red). Changes in membrane potential cause a redistribution of the mobile anion, which changes the efficiency of energy transfer or quenching. d) Voltage sensing via photoinduced electron transfer (PeT) through a molecular wire takes advantage of the sensitivity of eT to externally applied electric fields. At rest, the hyperpolarized, negative membrane potential enhances PeT from the electron-rich donor into the fluorophore (purple), quenching and diminishing fluorescence. Upon depolarization, the positive membrane potential slow the rate of PeT, resulting in a fluorescence brightening.
Among the most widely used fast dyes are the amino-naphthyl-ethylene-pyridinium-propylsulfonate, or ANEPPS dyes, first developed by Leslie Loew and co-workers [16, 17]. The 4- and 8-carbon substituted varieties, di-4- and d-8-ANEPPS (Fig. 1), respectively, provide fractional changes in fluorescence of approximately 10% per 100 mV. A more water-soluble version, JPW-1114 or di-2-ANEPEQ (Fig. 1), features a quaternary ammonium solubilizing group in place of the sulfonate and has found application for single-cell loading in brain slice via a patch pipet [18]. Another class of popular “fast” dyes have been developed Rina Hildesheim and Amiram Grinvald and are designated with the prefix “RH”. Oxonols RH 1691 and 1692 (Fig. 1) [19, 20] are often used as indicators of local field potential for intact brain imaging in primates and rodents [21].
Annulation of styryl dyes give the “ANellated hemicyaNINE,” or ANNINE, class (Fig. 1), which show improved voltage sensitivity on account of a rigidized π-system that enhances the dipole of the chromophore, increasing the molecular Stark effect on the dye [22, 23]. More recently, the Loew group introduced a palette of fluorinated styryl dyes that show improved photostability [24]. In addition to spanning wavelengths ranging from 440 to 670 nm, the dyes have varying degrees of voltage sensitivity, from 10% to 22% per 100 mV. Importantly, they showed improved efficacy under two-photon illumination and enabled voltage imaging in intact heart and brain slice.
In 2014 Bezanilla and co-workers reported the use of indocyanin green, or ICG, (Fig. 1) as a voltage-sensitive dye. ICG shows a modest 2% decrease in fluorescence intensity upon 100 mV depolarization, but has sufficient signal-to-noise to track action potentials in frog oocytes expressing sodium channels and in rat dorsal root ganglia neurons. In rat brain slices, ICG could monitor field evoked stimuli. Importantly, because ICG is FDA-approved for use in humans and has long-wavelength excitation and emission profiles, this opens up the possibility to use ICG in humans or in deep tissue context [25].
In a complementary fashion, “slow” dyes, which are based on voltage-dependent accumulation in (Fig. 2a) or redistribution within (Fig. 2c) the membrane can display much larger fractional changes in fluorescence (up to 80% ΔF/F per 100 mV) [26], compared to “fast” dyes, but suffer from drawbacks in slow response time and prohibitive capacitive load on the membranes. Because the voltage sensing mechanism relies on the redistribution of a lipophilic dye within a lipid bilayer, this molecular diffusion can be much slower than the biological event of interest, making “slow” voltage dyes unable to track fast action potentials. Additionally, because charged dye molecules move through the membrane at a time scale similar to the underlying biological change, this adds an artificial gating charge or capacitive current that can severely disrupt normal membrane and cellular function [27, 28].
While these of “slow” dyes, which also include positively charged rhodamines, can be used in isolation, they are much more effective when paired with a stationary chromophore that enables ratiometric measurements of voltage via fluorescence-resonance energy transfer, or FRET (Fig. 2c). Tsien and Gonzalez established a small molecule FRET-based system mobile, containing a voltage-sensitive oxonol and stationary coumarin FRET pair [26]. This approach has since been elaborated by a number of groups, typically by replacing the stationary fluorophore with a genetically encoded fluorophore, such as green fluorescent protein, or GFP [29]. Optimized versions in which the position of the fluorescent protein relative the membrane are altered show improved sensitivity [28, 30, 31]. More recent incarnations of this approach couple a mobile, lipophilic anion like dipicrylamine (DPA) as a fluorescence quencher of a membrane-associated fluorophore such as DiO to achieve voltage sensing in a variety of systems [32, 33]. The DiO/DPA pair is compatible with two-photon imaging, making it useful for interrogating membrane potential in thick tissues.
Our lab has recently undertaken a program to develop new voltage sensing fluorophores that combine both speed and sensitivity with a tunable platform for readily optimizing sensitivity, color profile, and cellular localization. We couple a fluorescent reporter to an electron-rich donor that quenches fluorescence via photoinduced electron transfer, or PeT [34]. PeT directly competes with fluorescence as a pathway out of the excited state. Fluctuations in the transmembrane potential alter the rate of PeT, which in turn alter the brightness of the fluorophore. In the case of voltage sensors, the fluorophore/donor pair is positioned in the plasma membrane so that the resting, or hyperpolarized, potential of the plasma membrane accelerates PeT, and decreases fluorescence. Upon depolarization, the altered membrane potential decreases the rate of PeT resulting in fluorescence brightening (Fig. 2d). Because the rate of electron transfer decreases exponentially with distance, we fuse the fluorophore to the donor via a phenylenevinylene molecular wire, thereby decreasing the distance-dependence of eT [35].
Our first generation of PeT-based voltage sensors, VoltageFluors, or VF dyes (Fig. 1), feature a dichlorosulfofluorescein reporter, phenylenevinylene molecular wire, and a dimethylaniline donor [36, 37]. As predicted from the model proposed above, VF dyes show a fast fluorescence turn-on in response to depolarizations, with a sensitivity of 27% ΔF/F per 100 mV that is linear over the physiological range of ±100 mV. Additionally, VF dyes exhibit no measurable capacitive loading, and can track action potential spikes in single trials in mammalian neurons and in ex vivo leech preparations [36]. Consistent with a PeT-based mechanism, modulation of the relative electron affinities of the donor aniline and acceptor fluorophore alters the apparent voltage sensitivity, improving a 2nd generation of VFs to sensitivities of approximately 49% ΔF/F per 100 mV, while maintaining the speed and linearity of the first VF dye [37].
Although VF dyes are fast, sensitive, and non-disruptive, their pan-membrane staining limits spatial resolution in heterogeneous samples. Cell-type specific labeling with voltage-sensitive PeT probes would enable coupling of the speed and sensitivity of a small molecule approach with the specificity achieved through genetic means. To address this, we developed a small-molecule, photoactivatable optical sensor of transmembrane potential, or SPOT [38]. In this approach, the fluorescence of VF2.1.Cl is quenched by formation of a nitrobenzyl ether at the 3’ hydroxyl of VF2.1.Cl. This minimally fluorescent, caged VF dye intercalates into the plasma membrane and remains optically silent until photoactivation. Unmasking with spatially defined light enables single cells to be activated for fluorescence sensing. Specific cells can be identified with a red fluorescent protein, generating local contrast for improved voltage sensing. Other approaches to targeting voltage-sensitive dyes have made use of exogenously expressed enzymes, such as alkaline phosphatase. Cell-surface expression of alkaline phosphatase enables the selective accumulation of phosphate-modified electrochromic dyes [39, 40]. Removal of the phosphate groups from the lipophilic tails dramatically alters the hydrophilicity of the dye, causing accumulation of VSD in the cell membrane. We hope to apply strategies employing enzymatic activation to VF dyes.
The use of fluorescein-based VF dyes precludes usage alongside a variety of powerful optical tools with overlapping optical profiles, such as Ca2+ indicators like GCaMP or Oregon Green BAPTA or optical actuators like ChannelRhodopsin2 (ChR2). To address this point, we developed a sulfonated, far-red silicon-rhodamine (Berkeley Red) and incorporated it into the phenylenevinylene molecular wire scaffold to create a Berkeley Red Sensor of Transmembrane potential, or BeRST 1 (Fig. 1) [41]. BeRST 1 is 5 times more photostable than first generation VF dyes and shows good voltage sensitivity at 25% ΔF/F per 100 mV. BeRST 1 can be used with minimal optical interference alongside GFP-based sensors such as GCaMP6. In conjunction with ChR2, it enables “all-optical” electrophysiology in which cyan light causes ChR2-mediated neuronal depolarization which can be monitored with BeRST 1 using red excitation (Fig. 3). Optical tools such as BeRST 1 pave the way for a more sophisticated interrogation of cellular membrane potential dynamics in a number of different biological systems.
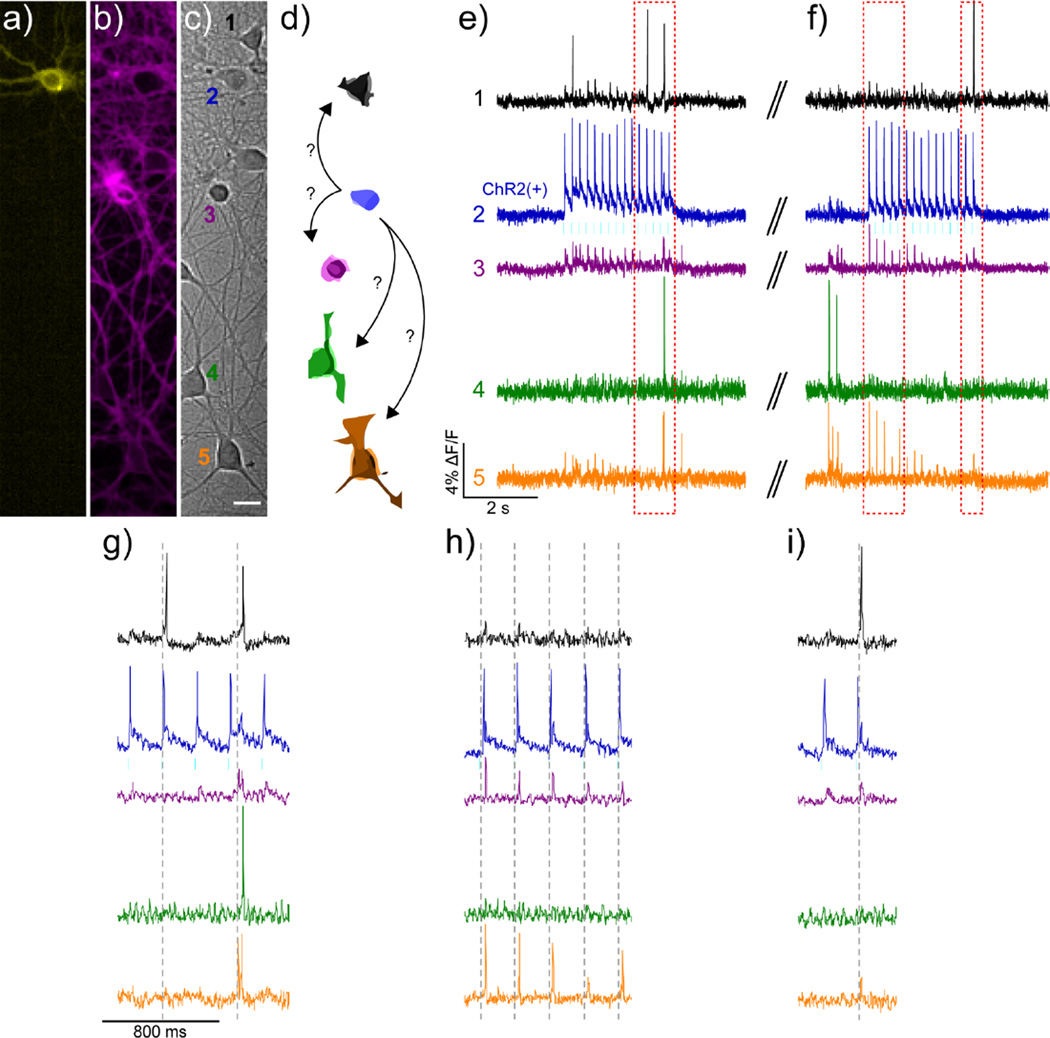
Figure 3.
All-optical electrophysiology using BeRST 1 and ChannelRhodopsin 2 in cultured rat hippocampal neurons. Cultured rat hippocampal c) neurons transfected with a) YFP-ChR2 and stained with b) BeRST 1 were stimulated with 475 nm light at a frequency of 5 Hz (cyan bars) during two separate 3 second periods to evoke activity in the YFP-ChR2-expressing cell. Scale bar is 20 μm. d) Schematic representation of neurons from DIC image in panel c), color-coded to match the corresponding traces in e–i). The blue ChR2(+) cell is depicted making possible connections to other neurons in the field of view. Optical records of BeRST 1 responses were acquired at 500 Hz with an sCMOS camera during e) an optical recording session and f) subsequent trial, separated by approximately 30 seconds (double hash). Numbers and colors of traces refer to specific neurons in panels a–d. Red boxes indicate areas of the trace that have been magnified for clarity in panels g–h). Dotted grey lines are provided in panel g–h) to help visually estimate the spike timing of BeRST 1-stained neurons, which can be resolved down to 2 ms.
Outlook
The ability to optically probe Vmem dynamics with spatial and temporal resolution promises to uncover basic insights into not only neurobiology, but fundamental cellular physiology. Small molecule voltage indicators offer a promising route to addressing a wish-list for future generations of voltage sensors, including tunable wavelengths, ratiometric detection, cellular and sub-cellular targeting in conjunction with genetically-encoded components, enhanced multiphoton brightness for imaging in thick tissue, and improved photostability for long-term imaging. We have made some progress in addressing targeting using a photoactivation strategy, and we have recently tuned the wavelength of voltage sensors in our lab to reach far-red/near-infrared optical profiles. Although this review focused on small molecule approaches to voltage sensing, indicators that use genetically encoded proteins or nanomaterials offer complementary routes to exploring the contributions membrane voltage makes to human health and disease.
Highlights.
Optical voltage sensors promise high-speed tracking of membrane potential in neurons
Several classes of small molecule voltage indicators exist
Voltage indicators using electron transfer as a trigger provide speed and sensitivity
Electron transfer voltage indicators can be tuned across a range of colors
Photoactivatable voltage indicators improve labeling in heterogeneous systems
Acknowledgments
Research in the Miller lab is and has been generously supported by the University of California, Berkeley, the NIH (R00NS078561), the Brain Research Foundation, and the Hellman Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levin M. Molecular bioelectricity: how endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol Biol Cell. 2014;25(24):3835–3850. doi: 10.1091/mbc.E13-12-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scanziani M, Hausser M. Electrophysiology in the age of light. Nature. 2009;461(7266):930–939. doi: 10.1038/nature08540. [DOI] [PubMed] [Google Scholar]
- 3.Peterka DS, Takahashi H, Yuste R. Imaging voltage in neurons. Neuron. 2011;69(1):9–21. doi: 10.1016/j.neuron.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davila HV, Salzberg BM, Cohen LB, Waggoner AS. A large change in axon fluorescence that provides a promising method for measuring membrane potential. Nat New Biol. 1973;241(109):159–160. doi: 10.1038/newbio241159a0. [DOI] [PubMed] [Google Scholar]
- 5.St-Pierre F, Marshall JD, Yang Y, Gong YY, Schnitzer MJ, Lin MZ. High-fidelity optical reporting of neuronal electrical activity with an ultrafast fluorescent voltage sensor. Nat Neurosci. 2014;17(6):884–889. doi: 10.1038/nn.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin L, Han Z, Platisa J, Wooltorton JRA, Cohen LB, Pieribone VA. Single action potentials and subthreshold electrical events imaged in neurons with a fluorescent protein voltage probe. Neuron. 2012;75(5):779–785. doi: 10.1016/j.neuron.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gong YY, Huang C, Li JZ, Grewe BF, Zhang YP, Eismann S, Schnitzer MJ. High-speed recording of neural spikes in awake mice and flies with a fluorescent voltage sensor. Science. 2015;350(6266):1361–1366. doi: 10.1126/science.aab0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hochbaum DR, Zhao Y, Farhi SL, Klapoetke N, Werley CA, Kapoor V, Zou P, Kralj JM, Maclaurin D, Smedemark-Margulies N, Saulnier JL, et al. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat Methods. 2014;11(8):825–833. doi: 10.1038/nmeth.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reeve JE, Corbett AD, Boczarow I, Kaluza W, Barford W, Bayley H, Wilson T, Anderson HL. Porphyrins for probing electrical potential across lipid bilayer membranes by second harmonic generation. Angew Chem Int Ed Engl. 2013;52(34):9044–9048. doi: 10.1002/anie.201304515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall JD, Schnitzer MJ. Optical strategies for sensing neuronal voltage using quantum dots and other semiconductor nanocrystals. ACS Nano. 2013;7(5):4601–4609. doi: 10.1021/nn401410k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park K, Deutsch Z, Li JJ, Oron D, Weiss S. Single molecule quantum-confined stark effect measurements of semiconductor nanoparticles at room temperature. ACS Nano. 2012;6(11):10013–10023. doi: 10.1021/nn303719m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minta A, Kao JPY, Tsien RY. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. Journal of Biological Chemistry. 1989;264(14):8171–8178. [PubMed] [Google Scholar]
- 13.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260(6):3440–3450. [PubMed] [Google Scholar]
- 14.Paredes RM, Etzler JC, Watts LT, Zheng W, Lechleiter JD. Chemical calcium indicators. Methods. 2008;46(3):143–151. doi: 10.1016/j.ymeth.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braubach O, Cohen LB, Choi Y. Historical overview and general methods of membrane potential imaging. Adv Exp Med Biol. 2015;859:3–26. doi: 10.1007/978-3-319-17641-3_1. [DOI] [PubMed] [Google Scholar]
- 16.Fluhler E, Burnham VG, Loew LM. Spectra, membrane binding, and potentiometric responses of new charge shift probes. Biochemistry. 1985;24(21):5749–5755. doi: 10.1021/bi00342a010. [DOI] [PubMed] [Google Scholar]
- 17.Loew LM, Bonneville GW, Surow J. Charge shift optical probes of membrane potential. Theory. Biochemistry. 1978;17(19):4065–4071. doi: 10.1021/bi00612a030. [DOI] [PubMed] [Google Scholar]
- 18.Zhou WL, Yan P, Wuskell JP, Loew LM, Antic SD. Intracellular long-wavelength voltage-sensitive dyes dynamics of action potentials in axons and thin for studying the dendrites. J Neurosci Meth. 2007;164(2):225–239. doi: 10.1016/j.jneumeth.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoham D, Glaser DE, Arieli A, Kenet T, Wijnbergen C, Toledo Y, Hildesheim R, Grinvald A. Imaging cortical dynamics at high spatial and temporal resolution with novel blue voltage-sensitive dyes. Neuron. 1999;24(4):791–802. doi: 10.1016/s0896-6273(00)81027-2. [DOI] [PubMed] [Google Scholar]
- 20.Lebeuf R, Ferezou I, Rossier J, Arseniyadis S, Cossy J. Straightforward synthesis of the near-infrared fluorescent voltage-sensitive dye RH1691 and analogues thereof. Org Lett. 2009;11(21):4822–4825. doi: 10.1021/ol901846g. [DOI] [PubMed] [Google Scholar]
- 21.Mohajerani MH, Chan AW, Mohsenvand M, LeDue J, Liu R, McVea DA, Boyd JD, Wang YT, Reimers M, Murphy TH. Spontaneous cortical activity alternates between motifs defined by regional axonal projections. Nat Neurosci. 2013;16(10) doi: 10.1038/nn.3499. 1426-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhn B, Fromherz P. Anellated hemicyanine dyes in a neuron membrane: Molecular Stark effect and optical voltage recording. J Phys Chem B. 2003;107(31):7903–7913. [Google Scholar]
- 23.Fromherz P, Hubener G, Kuhn B, Hinner MJ. ANNINE-6plus, a voltage-sensitive dye with good solubility, strong membrane binding and high sensitivity. Eur Biophys J Biophy. 2008;37(4):509–514. doi: 10.1007/s00249-007-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yan P, Acker CD, Zhou WL, Lee P, Bollensdorff C, Negrean A, Lotti J, Sacconi L, Antic SD, Kohl P, Mansvelder HD, et al. Palette of fluorinated voltage-sensitive hemicyanine dyes. Proc Natl Acad Sci U S A. 2012;109(50):20443–20448. doi: 10.1073/pnas.1214850109. • Several new fluorinated styryl dyes are described. The best of the dyes shows improved photostability and voltage sensitivity under two-photon illumination and can be used in acute cerebellar slices and intact guinea pig heart.
- 25. Treger JS, Priest MF, Iezzi R, Bezanilla F. Real-time imaging of electrical signals with an infrared fda-approved dye. Biophysical Journal. 2014;107(6):L9–L12. doi: 10.1016/j.bpj.2014.07.054. •• The authors use indocyanine green (ICG), an FDA-approved dye to conduct voltage imaging in frog oocytes, HEK cells, cultured dorsal root ganglia neurons, and cardiomyocyte syncytia. The infrared (780 nm) excitation and emission (>815 nm) profiles provide an opportunity to conduct thick-tissue and in vivo imaging.
- 26.Gonzalez JE, Tsien RY. Improved indicators of cell membrane potential that use fluorescence resonance energy transfer. Chem Biol. 1997;4(4):269–277. doi: 10.1016/s1074-5521(97)90070-3. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez JM, Taylor RE, Bezanilla F. Induced capacitance in the squid giant axon. Lipophilic ion displacement currents. J Gen Physiol. 1983;82(3):331–346. doi: 10.1085/jgp.82.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang D, Zhang Z, Chanda B, Jackson MB. Improved probes for hybrid voltage sensor imaging. Biophys J. 2010;99(7):2355–2365. doi: 10.1016/j.bpj.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chanda B, Blunck R, Faria LC, Schweizer FE, Mody I, Bezanilla F. A hybrid approach to measuring electrical activity in genetically specified neurons. Nat Neurosci. 2005;8(11):1619–1626. doi: 10.1038/nn1558. [DOI] [PubMed] [Google Scholar]
- 30.Sjulson L, Miesenbock G. Rational optimization and imaging in vivo of a genetically encoded optical voltage reporter. J Neurosci. 2008;28(21):5582–5593. doi: 10.1523/JNEUROSCI.0055-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghitani N, Bayguinov PO, Ma Y, Jackson MB. Single-trial imaging of spikes and synaptic potentials in single neurons in brain slices with genetically encoded hybrid voltage sensor. J Neurophysiol. 2015;113(4):1249–1259. doi: 10.1152/jn.00691.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradley J, Luo R, Otis TS, DiGregorio DA. Submillisecond optical reporting of membrane potential in situ using a neuronal tracer dye. J Neurosci. 2009;29(29):9197–9209. doi: 10.1523/JNEUROSCI.1240-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fink AE, Bender KJ, Trussell LO, Otis TS, DiGregorio DA. Two-photon compatibility and single-voxel, single-trial detection of subthreshold neuronal activity by a two-component optical voltage sensor. PLoS One. 2012;7(8):e41434. doi: 10.1371/journal.pone.0041434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li LS. Fluorescence probes for membrane potentials based on mesoscopic electron transfer. Nano Lett. 2007;7(10):2981–2986. doi: 10.1021/nl071163p. [DOI] [PubMed] [Google Scholar]
- 35.Davis WB, Svec WA, Ratner MA, Wasielewski MR. Molecular-wire behaviour in p-phenylenevinylene oligomers. Nature. 1998;396(6706):60–63. [Google Scholar]
- 36.Miller EW, Lin JY, Frady EP, Steinbach PA, Kristan WB, Jr, Tsien RY. Optically monitoring voltage in neurons by photo-induced electron transfer through molecular wires. Proc Natl Acad Sci U S A. 2012;109(6):2114–2119. doi: 10.1073/pnas.1120694109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Woodford CR, Frady EP, Smith RS, Morey B, Canzi G, Palida SF, Araneda RC, Kristan WB, Jr, Kubiak CP, Miller EW, Tsien RY. Improved PeT molecules for optically sensing voltage in neurons. J Am Chem Soc. 2015;137(5):1817–1824. doi: 10.1021/ja510602z. • The relationship between donor and acceptor electron affiinty in photoinduced electron transfer (PeT)-based VoltageFluors is explored with the synthesis of 10 new voltage indicators. An improved probe, VF2.1(OMe).H shows enhanced voltage sensitivity, at a nominal sensitivity of 48% ΔF/F per 100 mV).
- 38. Grenier V, Walker AS, Miller EW. A small-molecule photoactivatable optical sensor of transmembrane potential. J Am Chem Soc. 2015;137(34):10894–10897. doi: 10.1021/jacs.5b05538. • A photoactivation strategy for generating local contrast in voltage imaging is disclosed. A nitrobenzyl modification of a PeT-based VoltageFluor dims fluorescence until activated with UV light. This enables enhanced contrast for improved voltage imaging in heterogenous samples.
- 39.Hinner MJ, Hbener G, Fromherz P. Enzyme-induced staining of biomembranes with voltage-sensitive fluorescent dyes. J Phys Chem B. 2004;108(7):2445–2453. doi: 10.1021/jp036811h. [DOI] [PubMed] [Google Scholar]
- 40.Ng DN, Fromherz P. Genetic targeting of a voltage-sensitive dye by enzymatic activation of phosphonooxymethyl-ammonium derivative. ACS Chem Biol. 2011;6(5):444–451. doi: 10.1021/cb100312d. [DOI] [PubMed] [Google Scholar]
- 41. Huang YL, Walker AS, Miller EW. A photostable silicon rhodamine platform for optical voltage sensing. J Am Chem Soc. 2015;137(33):10767–10776. doi: 10.1021/jacs.5b06644. •• A sulfonated silicon rhodamine (Berkeley Red) has been synthesized and displays pH-insensitive abosrbance and emission. Combination with a phenylenevinylene molecular wire provides BeRST 1, (Berkeley Red Sensor of Transmembrane potential) that shows good voltage sensitivity (approximately 25% ΔF/F per 100 mv) and excellent spectral separation from optical tools like ChannelRhodopsin-2.