Abstract
Smith-Lemli-Opitz syndrome (SLOS) is a severe autosomal recessive disorder resulting from defects in the cholesterol synthesising enzyme 7-dehydrocholesterol reductase (Δ7-sterol reductase, DHCR7, EC 1.3.1.21) leading to a build-up of the cholesterol precursor 7-dehydrocholesterol (7-DHC) in tissues and blood plasma. Although the underling enzyme deficiency associated with SLOS is clear there are likely to be multiple mechanisms responsible for SLOS pathology. In an effort to learn more of the aetiology of SLOS we have analysed plasma from SLOS patients to search for metabolites derived from 7-DHC which may be responsible for some of the pathology. We have identified a novel hydroxy-8-dehydrocholesterol, which is either 24- or 25-hydroxy-8-dehydrocholesterol and also the known metabolites 26-hydroxy-8-dehydrocholesterol, 4-hydroxy-7-dehydrocholesterol, 3β,5α-dihydroxycholest-7-en-6-one and 7α,8α-epoxycholesterol. None of these metabolites are detected in control plasma at quantifiable levels (0.5 ng/mL).
Keywords: Oxysterol, sterol, 7-dehydrocholesterol, 8-dehydrocholesterol, 7-dehydrocholesterol reductase, liquid chromatography – mass spectrometry
Graphical abstract
Introduction
Smith-Lemli-Opitz syndrome (SLOS, MIM no. 270400) was first described in 1964 [1]. It is an autosomal recessive disorder resulting from deficiency of the enzyme 7-dehydrocholesterol reductase (DHCR7, EC 1.3.1.21, 3β-hydroxysterol Δ7-reductase) [2]. DHCR7 reduces the Δ7-double bond in 7-dehydrodesmosterol (7-DHD, cholesta-5,7,24-trien-3β-ol) and in 7-dehydrocholesterol (7-DHC, cholesta-5,7-dien-3β-ol) leading to the formation of desmosterol (cholesta-5,24-dien-3β-ol) and cholesterol (cholest-5-en-3β-ol) via the Bloch and Kandutsch-Russel pathways, respectively (Figure 1A) [3]. SLOS patients show decreased levels of cholesterol and increased levels of 7-DHC and its isomer 8-dehydrocholesterol (8-DHC, cholesta-5,8(9)-dien-3β-ol) in serum and tissues [4]. SLOS was the first human syndrome discovered due to an inborn error of sterol synthesis [2]. The phenotypic spectrum of SLOS is extremely broad; while severe cases may die in utero, mild cases show only minor physical, learning and behavioural problems [5]. Limb abnormalities are common in SLOS, and patients often show a distinctive cognitive and behavioural phenotype, although normal intelligence is also possible [6].
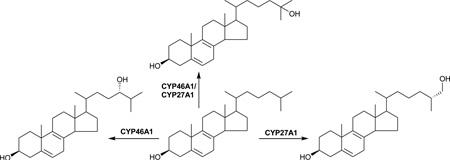
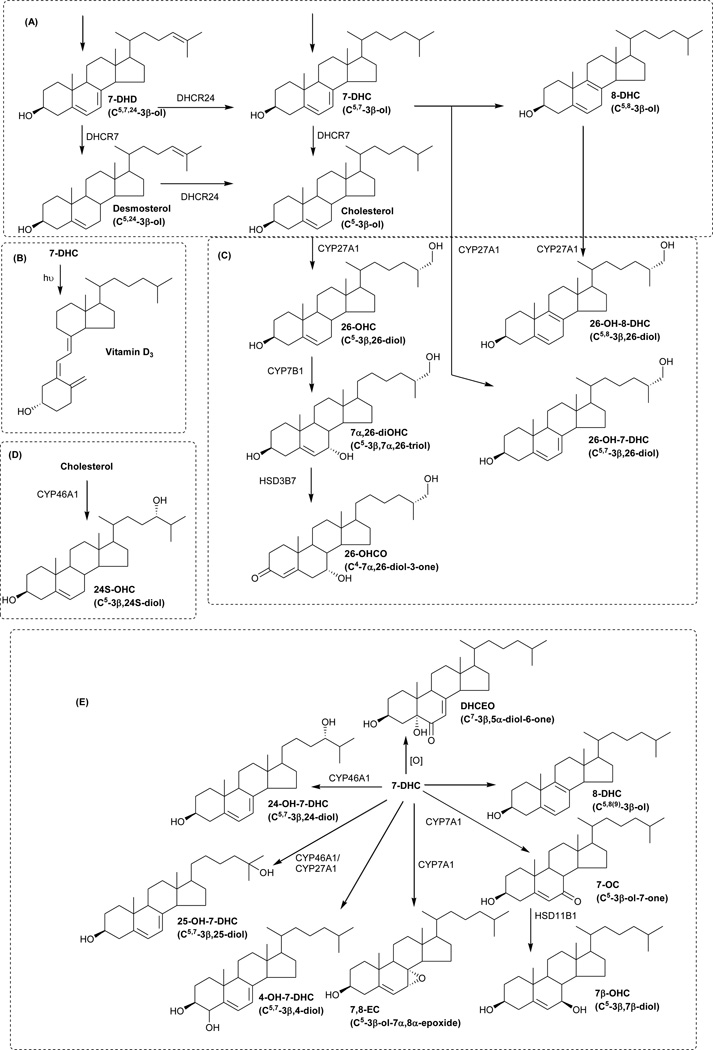
Figure 1.
Metabolism of cholesterol, 7-DHC and 8-DHC in SLOS patients. Where known, enzymes are shown.
The DHCR7 gene is encoded by nine exons, and over 100 mutations have been identified in SLOS patients [7]. Genotype-phenotype correlations are poor, although many missense mutations result in residual enzyme activity which is associated with a less severe phenotype [7]. SLOS has a high carrier frequency in Caucasians. In European populations the combined carrier frequency of two of the most common mutations c.964-1G>C (IVS8-1G>C) and p.W151X ranges from 1 – 2.3% [8]. Considering these numbers, the clinical incidence of SLOS (1:10,000 – 1:70,000 in Northern and Central European populations, 1:50,000 in the USA) is much lower than that predicted [5]. This is most likely due to several factors, including under-diagnosis of mild cases, and early prenatal pregnancy loss in severe cases. It is tempting to speculate that the high carrier frequency, particularly in populations from Northern and Central Europe, conveys a heterogeneous advantage [5]. 7-DHC is a precursor of vitamin D3 (Figure 1B), and increased vitamin D3 levels in the skin could protect against vitamin D deficiency.
SLOS can be diagnosed biochemically based on increased 7-DHC in serum and tissues [9]. 7-DHC levels are typically more than 50-fold elevated in SLOS cases, although there are equivocal cases of SLOS with serum 7-DHC levels just above normal levels [5]. Gene sequencing of DHCR7 is an alternative to biochemical analysis, but is limited by known pathogenic mutations.
Dietary supplementation with cholesterol to reduce de novo synthesis of 7-DHC and increase cholesterol levels is a standard treatment for SLOS. Dietary cholesterol supplementation is reported to improve behaviour [10], but as cholesterol does not pass the blood brain barrier (BBB), this improvement may be mediated by cholesterol metabolites, e.g. oxysterols, which can cross the BBB. Theoretically, statin therapy should also reduce 7-DHC biosynthesis and also tissue levels [11].
Although the underlying enzymatic defect in SLOS is well established there are likely to be multiple mechanisms responsible for SLOS pathology. For instance, cholesterol has numerous biological functions and substitution of 7-DHC for cholesterol, and 7-DHD for desmosterol, may alter physiochemical properties and function of membranes. Also 7-DHC, its isomer 8-DHC, their metabolites and 7-DHD analogues may have a direct toxic effect on cells [12]. Cholesterol is the precursor of steroid hormones and bile acids and dehydrocholesterol analogues of pregnenolone, pregnanetriol, dehydroepiandrosterone and androstenediol have been reported [13]. 7-DHC derived bile acid precursors have been reported to be formed in liver mitochondrial incubations from a rat model of SLOS, including 26-hydroxy-7-dehydrocholesterol (26-OH-7-DHC, cholesta-5,7-diene-3β,26-diol) and 26-hydroxy-8-dehydrocholesterol (26-OH-8-DHC, cholesta-5,8(9)-dien-3β,26-diol) (Figure 1C) [14]. Note, we use here the systematic nomenclature where a hydroxy group introduced to the terminal carbon of the sterol side-chain is at carbon-26 [15]. Unless stated otherwise, this is assumed to introduce R stereochemistry at carbon-25. Further metabolism remains to be fully elucidated, although Natowicz and Evans reported unusual bile acids in the urine of SLOS patients [16]. These results have not been confirmed by others. 26-OH-7-DHC and 26-OH-8-DHC have been reported to be present in plasma from SLOS patients at levels of 0.04 – 0.51 µM (16 – 204 ng/mL), the Δ7 isomer being an inhibitor of sterol synthesis and a ligand to the liver X receptor α [17]. The mitochondrial enzyme, cytochrome P450 (CYP) 27A1, oxidises cholesterol to 26-hydroxycholesterol (26-OHC, cholest-5-en-3β,(25R)26-diol) and it is likely that this is the mitochondrial enzyme which also oxidises 7- and 8-DHC to Δ7 and Δ8 analogues of 26-OHC (Figure 1C) [18]. In a study of infants with SLOS, Björkhem et al found reduced plasma levels of 24S-hydroxycholesterol (24S-OHC, cholest-5-ene-3β,24S-diol), but increased levels of 26-OHC [19]. The reduced level of brain derived 24S-OHC was not surprising in light of the reduced abundance of its precursor, cholesterol, but the elevated level of 26-OHC was less easy to explain [19].
In a more recent study Liu et al have identified 4α- and 4β-hydroxy-7-dehydrocholesterol (4α- and 4β-OH-7-DHC, cholesta-5,7-diene-3β,4α/β-diol) in plasma of SLOS patients [20]. The 4β-hydroxy compound could be formed enzymatically via a CYP3A4 catalysed reaction analogous to that which forms 4β-hydroxycholesterol from cholesterol. Liu et al also found elevated levels of 7-oxocholesterol (7-OC, 3β-hydroxycholest-5-en-7-one) in SLOS plasma which correlated positively with SLOS severity scores [20]. Interestingly, 7-OC, is a product of the CYP7A1 oxidation of 7-DHC (Figure 1E) [21]. Goyal et al have also found 7-DHC to be a substrate for other CYP enzymes [22]. They found that CYP46A1 can oxidise 7-DHC to 24-hydroxy-7-dehydrocholesterol (24-OH-7-DHC, cholesta-5,7-dien-3β,24-diol) and to 25-hydroxy-7-dehydrocholesterol (25-OH-7-DHC, cholesta-5,7-dien-3β,25-diol, Figure 1E) [22]. Endo-Umeda et al have shown that 7-DHC can also be metabolised by CYP27A1 to 25-OH-7-DHC and that this oxysterol and 26-OH-7-DHC are present in SLOS plasma at levels of 4 ng/mL and 33 ng/mL, respectively [18]. 24-OH-7-DHC, 4α- and 4β-OH-7-DHC, 7-OC and also 3β,5α-dihydroxycholest-7-en-6-one (DHCEO) have been identified in tissues and fluids from a rat model of SLOS [23,24] and/or a Dhcr7-null mouse embryos [25,26]. DHCEO is formed non-enzymatically via free radical oxidation of 7-DHC (Figure 1E) [26]. This reaction occurs in vivo, at least in Dhcr7-deficient Neuro2a cells and SLOS fibroblasts [27], but the propensity of 7-DHC to undergo free radical oxidation reactions highlights the importance of sample handling procedures to avoid the ex vivo formation of 7-DHC oxidation products. In regard of the free radical oxidation of 7-DHC, Porter and colleagues found 7-DHC to be 200 times more reactive towards free radical chain oxidation than cholesterol and identified 15 oxysterols derived from 7-DHC through these reactions [28]. This makes sample handling of SLOS samples a key aspect of their analysis.
Historically, biochemical diagnosis of SLOS has been by gas chromatography (GC) – mass spectrometry (MS) based on 7-DHC levels in blood [9], although in recent years atmospheric pressure ionisation (API) - and liquid chromatography (LC)–MS methods have been adopted [20,29–31]. In addition to 7-DHC, its metabolites, formed either enzymatically or non-enzymatically, have a potential as biochemical markers [20]. An advantage of profiling for 7- and/or 8-DHC metabolites is that their identity may reveal more details of the aetiology of the SLOS phenotype. In the current study we have exploited an LC - electrospray ionisation (ESI) - MS with multistage fragmentation (MSn) approach for the profile-analysis of cholesterol, 7-DHC, 8-DHC and their oxysterol metabolites [32,33]. By using a charge-tagging approach analytical sensitivity is maximised (Supplementary Figure S1A).
Materials and Methods
SLOS Samples
Historical residual clinical plasma samples from SLOS patients were analysed along with samples from newly diagnosed patients and controls provided with written informed consent and institutional review board ethical approval and were collected according to the principles of the Declaration of Helsinki [29,32,34]. Data from two patient samples was previously reported in [34]. Additional control samples were those reported earlier in [35].
Analytical Methods
Sterols and oxysterols were analysed by LC-ESI-MSn using a charge-tagging approach (enzyme-assisted derivatisation for sterol analysis, EADSA) described fully in [32,33] and in supplementary information. In brief, non-polar sterols including cholesterol, 7-DHC and 8-DHC were separated from more-polar oxysterols by reversed-phase solid phase extraction (RP-SPE). The separated fractions were individually treated with cholesterol oxidase to convert 3β-hydroxy-5-ene and 3β-hydroxy-5,7(or 8)-diene to their 3-oxo-4-ene and 3-oxo-4,7(or 8)–diene equivalents, then derivatised with Girard P (GP) reagent (Supplementary Figure S1A) to add a charged quaternary nitrogen group to the analytes which greatly improve their LC-ESI-MS and MSn response. When fragmented by MS2 GP-tagged analytes give an intense [M-Py]+ ion, corresponding to the loss of the pyridine (Py) ring (Figure S1B), which can be fragmented further by MS3 to give a structurally informative pattern (Figure S1C–J). Some sterols and oxysterols naturally contain an oxo group and can be differentiated from those oxidised to contain one by omitting the cholesterol oxidase enzyme from the sample work-up procedure [32]. No hydrolysis step was carried out so free sterols and oxysterols were exclusively analysed.
Statistical Analysis
All values are shown as mean (± standard error of mean). The unpaired two grouped two tailed Student’s t-test was performed to asses significant differences.
Results
Sterols
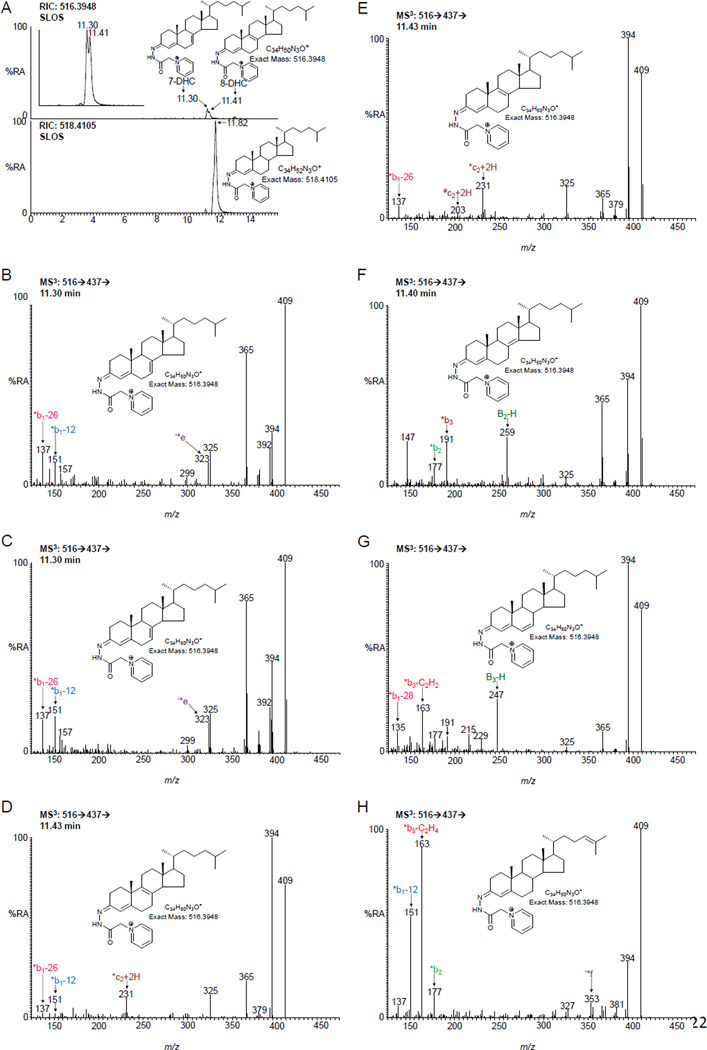
The non-polar sterol fraction from control adult samples is totally dominated by cholesterol. Desmosterol, 7-DHC and 8-DHC are almost invisible without overloading the chromatographic and MS systems with cholesterol. In control samples the ratio of 7-DHC plus 8-DHC to cholesterol is about 1:1000 (1 in units of ng/µg), and the desmosterol to cholesterol ratio is similar (Figure 2A). The situation is different with samples from most SLOS patients where 7-DHC and 8-DHC are readily quantifiable allowing diagnosis of the syndrome (Figures 2A & 3A). Note, one SLOS patient sample gave a similar DHC to cholesterol ratio to that measured in controls but showed an oxysterol pattern characteristic of SLOS (see below). As is evident from Figure 3A (inset), 7-DHC and 8-DHC can only just be chromatographically resolved, however, they give distinct MS3 spectra (Figures 3B–E and S1C–1D). There are in-fact two isomers of 8-DHC, cholesta-5,8(9)-diene-3β-ol and cholesta-5,8(14)-diene-3β-ol, which essentially co-elute. However, they give different MS3 spectra allowing their differentiation (Figure 3E–F and S1D–1E). Two other dehydrocholesterol isomers, i.e. cholesta-4,6-dien-3β-ol (6-DHC) (Figure 3G and S1F) and desmosterol (Figure 3H and Figure S1G) both give different MS3 spectra to the other isomers and are also chromatographically resolved from 7- and 8-DHC. A further non-polar sterol identified in SLOS samples but absent from controls corresponds to a cholestatrien-3β-ol. The MS3 spectrum (Figure S2) does not correspond to 7-DHD [36] and may correspond to cholesta-5,7,9(11)-trien-3β-ol an ex vivo photoxoidation product of 7-DHC [23].
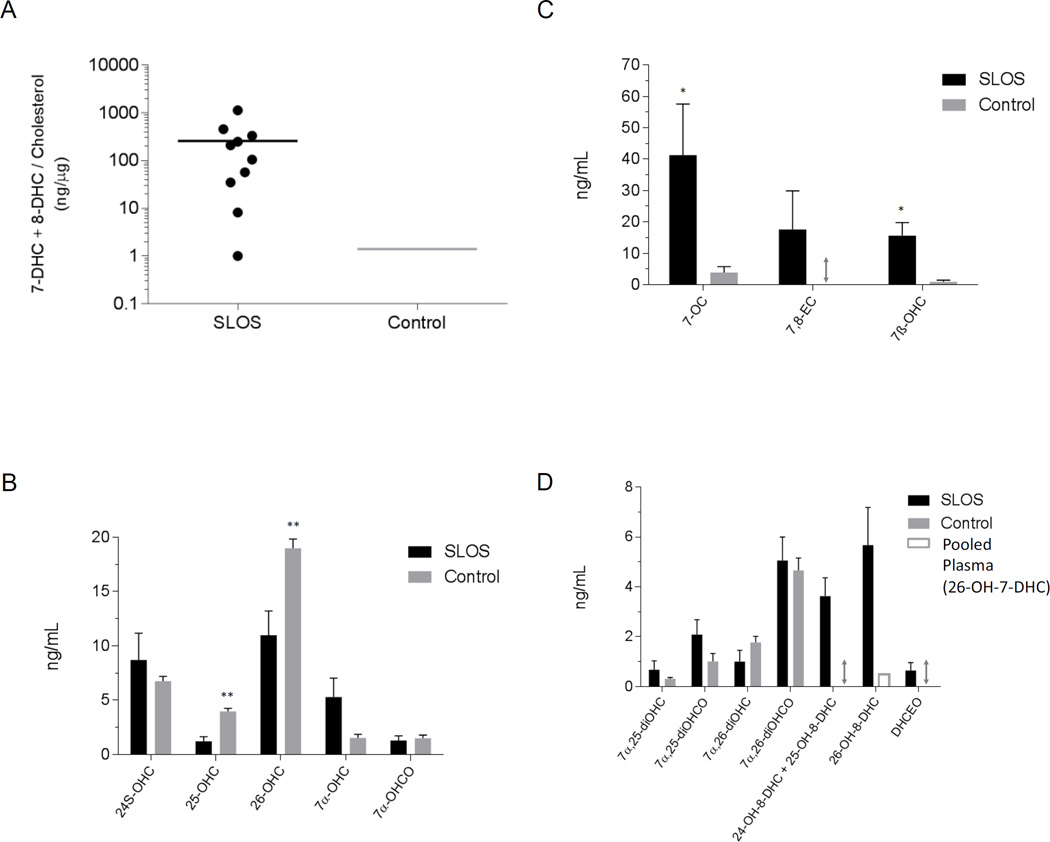
Figure 2.
7-DHC + 8-DHC and their metabolites are elevated in SLOS plasma. (A) Dot plot showing 7-DHC + 8-DHC concentrations in ng/µg of cholesterol for the ten SLOS samples analysed. The bar shows the mean value. For comparison the 7-DHC + 8-DHC level in the NIST standard reference material 1950 (Control) is shown [37]. This is a pooled plasma sample representative of the US population [38]. (B) Concentrations of monohydroxycholesterols (OHC) and of 7α-hydroxycholest-4-en-3-one (7α-OHCO) formed enzymatically from cholesterol. (C) Levels of oxysterols enzymatically derived from 7-DHC via oxidation of C-7. (D) Levels of dihydroxycholesterols (diOHC), dihydroxychelestenones (diOHCO) and hydroxy-8-dehydrocholesterols (OH-8-DHC). For (B) – (D) control data is from 50 samples reported in Theofilopoulos et al [35] or if not measured in that study from a pool of 8 adult plasma samples analysed in this work. The absence of a metabolite in the controls is indicated by a double-headed arrow. 26-OH-7-DHC was present in the pooled plasma but below the limit of quantification (0.5 ng/mL). All data is for free sterols as a hydrolysis step was not carried out. *, P < 0.05; **, P < 0.01
Figure 3.
7-DHC and 8-DHC are identified at elevated levels in plasma from a SLOS patient. (A) Upper panel, reconstructed ion chromatogram (RIC) of m/z 516.3948 ± 10 ppm displaying partially resolved 7-DHC and 8-DHC. The inset shows the two isomers partially separated in a chromatogram for the MS3 transition 516→437→ ([M]+→[M-Py]+→). Lower panel, RIC of m/z 518.4105 showing cholesterol. Both panels are plotted on the same scale. MS3 ([M]+→[M-Py]+→) spectrum (B) of the peak eluting at 11.30 min and corresponding to 7-DHC, (C) of authentic 7-DHC, (D) of the peak eluting at 11.43 min and corresponding to 8-DHC (8,9-isomer), (E) of authentic 8-DHC (8,9-isomer), (F) of authentic 8-DHC (8,14-isomer), (G) of authentic 6-DHC, and (H) of authentic desmosterol. Sterols were analysed as GP-derivatives. All data is for free sterols as a hydrolysis step was not carried out.
Enzymatically Derived Oxysterols
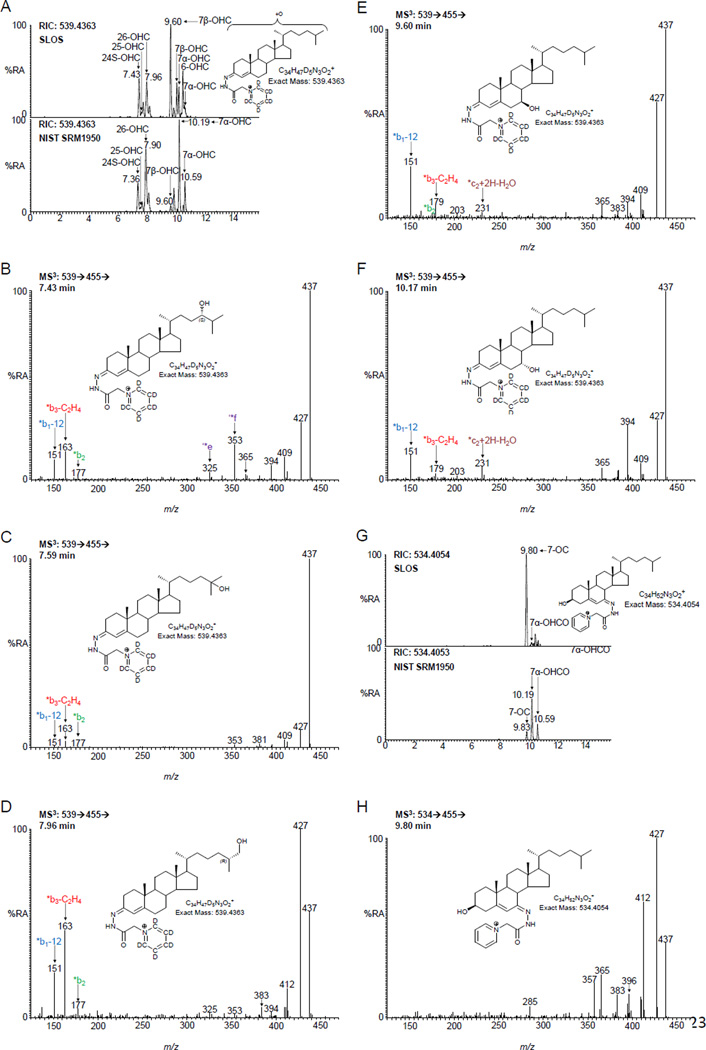
The pattern of enzymatically derived oxysterols in plasma from SLOS patients resembles that of controls, but there are differences (Figure 2B–D). Considering the monohydroxycholesterols first (Figure 4), the levels of 24S-OHC, 25-hydroxycholesterol (25-OHC, cholest-5-en-3β,25-diol) and 26-OHC show only small differences between SLOS patients and controls, and for the latter two compounds these are statistically significant. 7α-Hydroxycholesterol (7α-OHC, cholest-5-ene-3β,7α-diol) and 7α-hydroxycholest-4-en-3-one (7α-OHCO) are both formed enzymatically, and like 7β-hydroxycholesterol (7β-OHC, cholest-5-ene-3β,7β-diol) and 7-OC, 7α-OHC can also be formed non-enzymatically. 7-OC is often thought of as an ex vivo autoxidation product of cholesterol [37,38], however, Shinkyo et al showed that it can also be formed from 7-DHC in a CYP7A1 catalysed reaction (Figure 1E) [21], while others suggest it can be formed via free radical pathways in vivo [39]. In all SLOS samples studied the level of either 7-OC and/or of 7β-OHC was elevated (Figure 2C). 7β-OHC may be derived enzymatically from 7-OC (Figure 1E). 11β-Hydroxysteroid dehydrogenase 1 (HSD11B1) can act as an oxo-reductase inter-converting 7-OC and 7β-OHC in man [40,41]. Shinkyo et al also suggested that cholesterol-7,8-epoxide (3β-hydroxycholest-5-en-7α,8α-epoxide, 7,8-EC) was formed from 7-DHC by CYP7A1 in a side-reaction to the formation of 7-OC [21]. This was confirmed in a recent study by Björkhem et al who showed that plasma from some patients with cerebrotendinous xanthomatosis or SLOS had a marked increase in 7,8-EC [34]. We confirm this finding in the current study where patients with SLOS have elevated levels of 7,8-EC (Figures 2C & 5A) which is absent from control plasma.
Figure 4.
The oxysterols 7β-hydroxycholesterol and 7-oxocholesterol are identified at elevated levels in plasma from SLOS patients. (A) RIC for m/z 539.4363 ± 10 ppm corresponding to mono-hydroxycholesterols (OHC). Upper panel, an SLOS patient. For comparison, the lower panel shows the NIST standard reference material 1950 [37]. Both panels are plotted on the same scale. MS3 (539→455→) spectra of (B) 24S-OHC, (C) 25-OHC, (D) 26-OHC, (E) 7β-OHC, and (F) 7α-OHC from the SLOS patient. In (A) – (F) oxysterols were analysed as [2H5]GP-derivatives. (G) RIC for m/z 534.4054 ± 10 ppm corresponding to [2H0]GP-derivatives of 7-OC and 7α-OHCO. The upper panel is from the SLOS patient, the lower panel from the NIST sample, both panels are plotted on the same scale. (H) MS3 (534→455→) spectrum of 7-OC from the SLOS patient. All data is for free oxysterols as a hydrolysis step was not carried out. GP-derivatives give syn and anti conformers, some of which e.g. 7β-OHC are resolved, while others e.g. 7-OC give a single peak.
Figure 5.
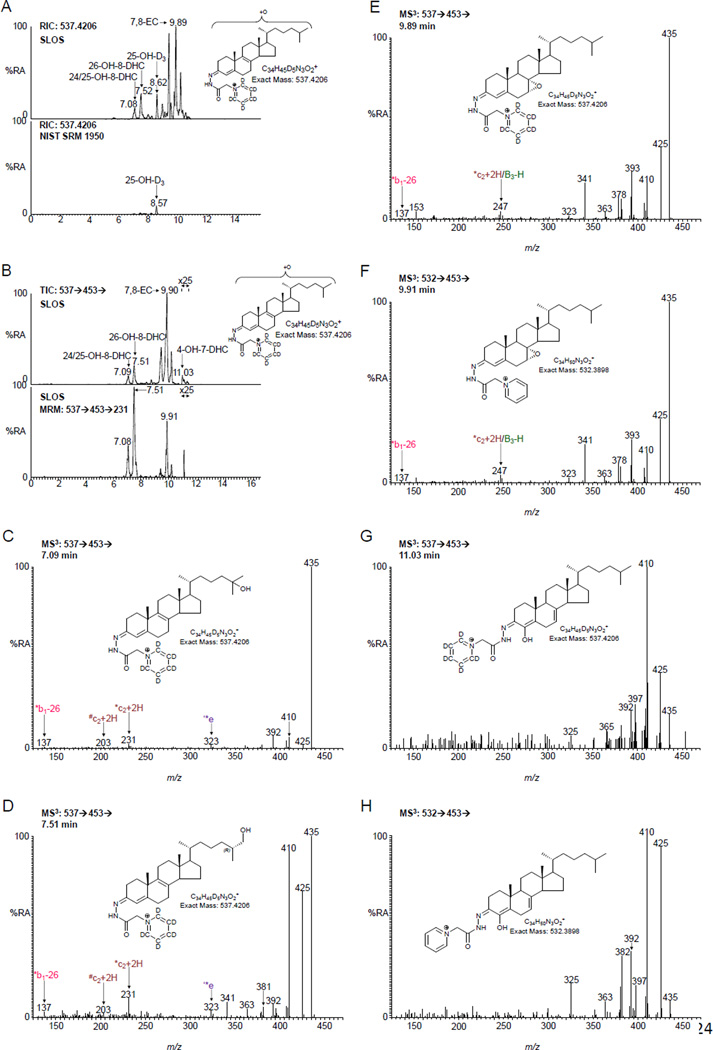
Oxysterols derived from 7- or 8-DHC are identified at elevated levels in SLOS plasma. (A) RIC for m/z 537.4206 ± 10 ppm corresponding to mono-hydroxydehydrocholesterols (OH-DHC). Upper panel, an SLOS patient. For comparison, the lower panel shows NIST standard reference material 1950 [37]. Both panels are plotted on the same scale. The peak at 8.62 min corresponds to 25-hydroxyvitamin D3 (25-OH-D3). (B) Upper panel, total ion chromatogram (TIC) for the 537→453→ ([M]+→ [M-Py]+→) transition. Lower panel, RIC for the transition 537→453→231 targeting on metabolites derived from 8-DHC (see figure S1D). Both panels are from the same SLOS sample. MS3 (537→453→) spectra of (C) 24- and/or 25-OH-8-DHC, (D) 26-OH-8-DHC, (E) 7,8-EC, (F) 7,8-EC authentic standard, (G) 4-OH-7-DHC and (H) 4β-OH-7-DHC authentic standard. Oxysterols in (A) – (E) and (G) were analysed as [2H5]GP-derivatives, those in (F) and (H) as [2H0]GP-derivatives. All data is for free oxysterols as a hydrolysis step was not carried out.
25- and 26-OHC are 7α-hydroxylated by CYP7B1 to 7α,25-dihydroxycholesterol (cholest-5-ene-3β,7α,25-triol, 7α,25-diOHC) and 7α,(25R)26-dihydroxycholesterol (cholest-5-ene-3β,7α,(25R)26-triol, 7α,26-diOHC), respectively, then oxidized by HSD3B7 to 7α,25-dihydroxycholest-4-en-3-one (7α,25-diOHCO) and 7α,(25R)26-dihydroxycholest-4-en-3-one (7α,26-diOHCO), respectively. All four metabolites are found in plasma. The levels in the SLOS patients are similar to those in controls (Figure 2D).
There is evidence in the literature for enzymatically formed 24-OH-7-DHC and 25-OH-7-DHC from 7-DHC by CYP46A1 [22], and Xu et al have identified the former compound in plasma from a rat model of SLOS [23,24]. Additionally, 26-OH-7-DHC and 26-OH-8-DHC, 4α-OH-7-DHC and 4β-OH-7-DHC have been identified plasma from SLOS patients [17,18,20]. We therefore searched for the presence of OH-7-DHCs and OH-8-DHCs in plasma samples from SLOS patients (Figure 5A–B). We identified two chromatographic peaks with retention times and giving MS3 spectra compatible with (i) either 24-OH-8-DHC (cholesta-5,8(9)-diene-3β,24-diol) and/or 25-OH-8-DHC (cholesta-5,8(9)-diene-3β,25-diol), and (ii) 26-OH-8-DHC (Figure 5C–D). These compounds are present in SLOS plasma but absent from control samples. The earlier eluting peak may well be a composite of 24- and 25-OH-8-DHC, while the latter peak is predominantly 26-OH-8-DHC, but could contain a small amount of unresolved 26-OH-7-DHC. Identification of these components in the absence of authentic standards is facilitated by the MS3 spectra recorded and by reference to the equivalent spectra of the DHC precursor molecules and to their hydroxycholesterol analogues. Chromatographic elution time provides another dimension for identification with the presence of an additional double bond reducing elution time by about 0.5 min. With our chromatographic system we cannot resolve 4α- from 4β-OH-7-DHC. However, we do find a compound eluting with the appropriate retention time (Figure 5B) and giving an MS3 spectrum identical to that of the authentic standard (Figure 5G–H). This compound was at a level below our limit of quantification (0.5 ng/mL).
Non-Enzymatically Derived Oxysterols
There is always considerable debate whether non-enzymatically formed oxysterols are generated in vivo or ex vivo during sample handling or storage [37,42]. The analytical protocol used in this work essentially eliminates ex vivo autoxidation during sample work-up by separating polar oxysterols from non-polar sterols like cholesterol, 7-DHC and 8-DHC. However, the possibility of autoxidation during sample collection and storage is difficult to eliminate when dealing with clinical samples from patients, especially when historical samples are analysed, as in the present study.
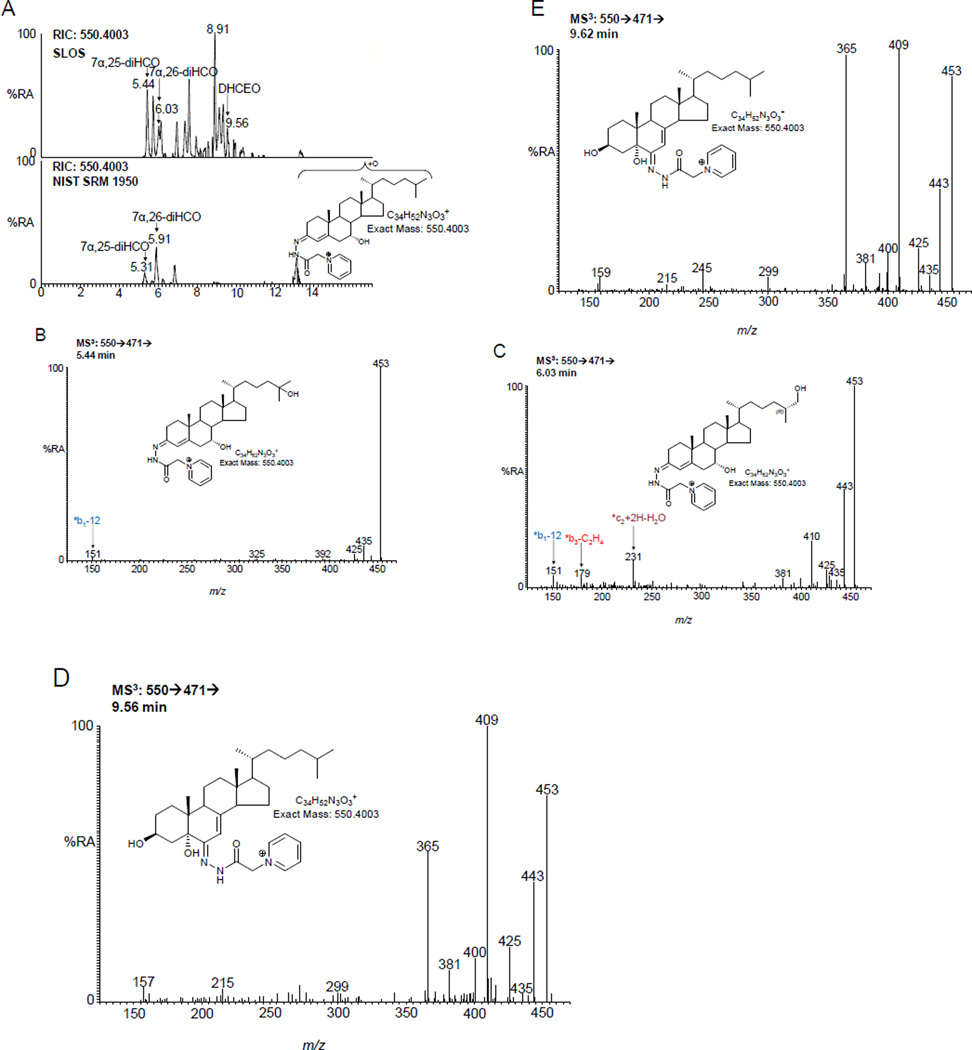
DHCEO is the major metabolic product of 7-DHC in SLOS fibroblasts generated through free radical oxidation (Figure 1E) [27] and it has been identified in brain from a Dhcr7 knock-out mouse model of SLOS [25,26]. We thus searched for the presence of DHCEO in plasma from SLOS patients. Shown in Figure 6 is the appropriate chromatogram for dihydroxycholestenones. DHCEO elutes late in the chromatogram and was not found in plasma from all SLOS patients. However, it was not detected in any control plasma (Figure 2D).
Figure 6.
DHCEO is elevated in SLOS plasma (A) RIC for m/z 550.4003 ± 10 ppm corresponding to dihydroxycholestenones. Upper panel, an SLOS patient. For comparison, the lower panel shows NIST standard reference material 1950 [37]. Both panels are plotted on the same scale. MS3 (550→471→) spectra of (B) 7α,25-dihydroxycholest-4-en-3-one, (C) 7α,26-dihydroxycholest-4-en-3-one, (D) DHCEO and (E) authentic DHCEO. Oxysterols were analysed as GP-derivatives. All data is for free oxysterols as a hydrolysis step was not carried out.
As is evident from Figures 5A and 6A there numerous other peaks present in the SLOS plasma which are absent from controls. Although their MS3 spectra were recorded, their identification was not obvious.
Discussion
In the current study we are able to identify 24- or 25-OH-8-DHC and 26-OH-8-DHC at elevated levels in plasma of SLOS patients (Figure 2D). This was found for each of the SLOS samples analysed. These molecules were not detected at quantifiable levels in control plasma (<0.5 ng/mL). Wassif et al have previously identified 26-OH-8-DHC in SLOS plasma in the range of 16 – 204 ng/mL [17], somewhat higher than the current range of 1.41 – 15.75 ng/mL, but neither 24- or 25-OH-8-DHC have previously been found in human plasma. We also confirmed the earlier findings of elevated levels of 7-OC and 7,8-EC in SLOS plasma [20,34]. 7,8-EC was not found in control plasma and was detected in seven of the ten SLOS patients studied. 7-OC can be converted to 7β-OHC by HSD11B1 (Figure 1E) [40,41] and every SLOS patient showed elevated 7-OC and/or 7β-HC. In the present study we do not have data on disease severity so we are not able to correlate metabolite levels with SLOS severity. We also identified 4-OH-7-DHC in some SLOS samples, but only at the limit of detection (0.1 ng/mL), below the limit of quantification (0.5 ng/mL). 4α-and 4β-OH-7-DHC have previously been identified in SLOS plasma [20].
In an earlier study Björkhem et al found reduced plasma levels of 24S-OHC in SLOS patients, this was readily explained by reduced cholesterol content of brain, the source of this metabolite [19]. 24S-OHC levels are known to vary with age, and in the current study we were not able to age match SLOS patients with controls so differences between SLOS and controls are likely lost in the case of 24S-OHC. In contrast to the previous study by Björkhem et al we found lower levels of 26-OHC in SLOS plasma than controls [19]. The difference is likely to be methodological as in the earlier study total 26-OHC was measured following alkaline hydrolysis of sterol esters while here only free sterols were measured.
7-DHC is known to readily undergo free radical oxidation [23], one of the products of which, DHCEO (Figure 1E), has been found in rodent models of SLOS. Here we were able to identify DHCEO in four of our ten SLOS samples. DHCEO is not detected in control plasma.
One of the SLOS patient samples investigated in this study showed an almost normal DHC to cholesterol ratio. Unfortunately, there was limited clinical information available relating to this patient. However, the pattern of plasma oxysterols from this patient clearly identifies SLOS. Of particular note were the high levels of 7β-OHC (24.46 ng/mL cf. 0.92±0.49 ng/mL) and 7-OC (130.71 ng/mL cf. 3.86±1.91 ng/mL) in plasma compared to controls. Further confirmation of SLOS was provided by the presence of elevated 26-OH-8-DHC (1.41 ng/mL cf. <0.5 ng/mL) in the patient plasma.
In summary, we have identified a number of metabolites derived from 7- or 8-DHC in SLOS plasma. Further studies will be directed at investigating how their values vary with disease severity and their merit as markers for disease stratification.
Supplementary Material
Highlights.
26-Hydroxy- and 24(or 25)-hydroxy-8-dehydrocholesterol identified in SLOS plasma
Enhanced concentrations of 7β-hydroxy- and 7-oxo-cholesterol in SLOS plasma
Free radical generated 3β,5α-dihydroxycholest-7-en-6-one found in SLOS plasma
Acknowledgments
This work was supported by the UK Biotechnology and Biological Sciences Research Council (BBSRC, grant numbers BB/I001735/1 to WJG, BB/L001942/1 to YW), National Institutes of Health USA (NICHD R01 HD064727 to NAP, NIH K99HD073270 to LX) and the Swedish Science Council (to IB). Members of the European Network for Oxysterol Research (ENOR, http://oxysterols.com/) are thanked for informative discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Smith D, Lemli, Opitz J. A newly recognized syndrome of multiple congenital anomalies. J Pediatr. 1964;64:210–217. doi: 10.1016/s0022-3476(64)80264-x. [DOI] [PubMed] [Google Scholar]
- 2.Irons M, Elias ER, Salen G, Tint GS, Batta AK. Defective cholesterol biosynthesis in Smith-Lemli-Opitz syndrome. Lancet. 1993;341:1414. doi: 10.1016/0140-6736(93)90983-n. [DOI] [PubMed] [Google Scholar]
- 3.Mazein A, Watterson S, Hsieh WY, Griffiths WJ, Ghazal P. A comprehensive machine-readable view of the mammalian cholesterol biosynthesis pathway. Biochem. Pharmacol. 2013;86:56–66. doi: 10.1016/j.bcp.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batta AK, Tint GS, Shefer S, Abuelo D, Salen G. Identification of 8-dehydrocholesterol (cholesta-5,8-dien-3 beta-ol) in patients with Smith-Lemli-Opitz syndrome. J Lipid Res. 1995;36:705–713. [PubMed] [Google Scholar]
- 5.Porter FD, Herman GE. Malformation syndromes caused by disorders of cholesterol synthesis. J Lipid Res. 2011;52:6–34. doi: 10.1194/jlr.R009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tierney E, Nwokoro NA, Kelley RI. Behavioral phenotype of RSH/Smith-Lemli-Opitz syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2000;6:131–134. doi: 10.1002/1098-2779(2000)6:2<131::AID-MRDD7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 7.Correa-Cerro LS, Porter FD. 3beta-hydroxysterol Delta7-reductase and the Smith-Lemli-Opitz syndrome. Mol. Genet. Metab. 2005;84:112–126. doi: 10.1016/j.ymgme.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Witsch-Baumgartner M, Schwentner I, Gruber M, Benlian P, Bertranpetit J, Bieth E, Chevy F, Clusellas N, Estivill X, Gasparini G, Giros M, Kelley RI, Krajewska-Walasek M, Menzel J, Miettinen T, Ogorelkova M, Rossi M, Scala I, Schinzel A, Schmidt K, Schonitzer D, Seemanova E, Sperling K, Syrrou M, Talmud PJ, Wollnik B, Krawczak M, Labuda D, Utermann G. Age and origin of major Smith-Lemli-Opitz syndrome (SLOS) mutations in European populations. J Med. Genet. 2008;45:200–209. doi: 10.1136/jmg.2007.053520. [DOI] [PubMed] [Google Scholar]
- 9.Kelley RI. Diagnosis of Smith-Lemli-Opitz syndrome by gas chromatography/mass spectrometry of 7-dehydrocholesterol in plasma, amniotic fluid and cultured skin fibroblasts. Clin. Chim. Acta. 1995;236:45–58. doi: 10.1016/0009-8981(95)06038-4. [DOI] [PubMed] [Google Scholar]
- 10.Kelley RI, Hennekam RC. The Smith-Lemli-Opitz syndrome. J Med. Genet. 2000;37:321–335. doi: 10.1136/jmg.37.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jira PE, Wevers RA, De JJ, Rubio-Gozalbo E, Janssen-Zijlstra FS, van Heyst AF, Sengers RC, Smeitink JA. Simvastatin. A new therapeutic approach for Smith-Lemli-Opitz syndrome. J Lipid Res. 2000;41:1339–1346. [PubMed] [Google Scholar]
- 12.Korade Z, Xu L, Shelton R, Porter NA. Biological activities of 7-dehydrocholesterol-derived oxysterols: implications for Smith-Lemli-Opitz syndrome. J Lipid Res. 2010;51:3259–3269. doi: 10.1194/jlr.M009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shackleton C, Roitman E, Guo LW, Wilson WK, Porter FD. Identification of 7(8) and 8(9) unsaturated adrenal steroid metabolites produced by patients with 7-dehydrosterol-delta7-reductase deficiency (Smith-Lemli-Opitz syndrome) J Steroid Biochem. Mol. Biol. 2002;82:225–232. doi: 10.1016/s0960-0760(02)00155-3. [DOI] [PubMed] [Google Scholar]
- 14.Honda A, Salen G, Shefer S, Batta AK, Honda M, Xu G, Tint GS, Matsuzaki Y, Shoda J, Tanaka N. Bile acid synthesis in the Smith-Lemli-Opitz syndrome: effects of dehydrocholesterols on cholesterol 7alpha-hydroxylase and 27-hydroxylase activities in rat liver. J Lipid Res. 1999;40:1520–1528. [PubMed] [Google Scholar]
- 15.Fakheri RJ, Javitt NB. 27-Hydroxycholesterol, does it exist? On the nomenclature and stereochemistry of 26-hydroxylated sterols. Steroids. 2012;77:575–577. doi: 10.1016/j.steroids.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Natowicz MR, Evans JE. Abnormal bile acids in the Smith-Lemli-Opitz syndrome. Am. J Med. Genet. 1994;50:364–367. doi: 10.1002/ajmg.1320500413. [DOI] [PubMed] [Google Scholar]
- 17.Wassif CA, Yu J, Cui J, Porter FD, Javitt NB. 27-Hydroxylation of 7-and 8-dehydrocholesterol in Smith-Lemli-Opitz syndrome: a novel metabolic pathway. Steroids. 2003;68:497–502. doi: 10.1016/s0039-128x(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 18.Endo-Umeda K, Yasuda K, Sugita K, Honda A, Ohta M, Ishikawa M, Hashimoto Y, Sakaki T, Makishima M. 7-Dehydrocholesterol metabolites produced by sterol 27-hydroxylase (CYP27A1) modulate liver X receptor activity. J Steroid Biochem. Mol. Biol. 2014;140:7–16. doi: 10.1016/j.jsbmb.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Björkhem I, Starck L, Andersson U, Lütjohann D, von BS, Pikuleva I, Babiker A, Diczfalusy U. Oxysterols in the circulation of patients with the Smith-Lemli-Opitz syndrome: abnormal levels of 24S- and 27-hydroxycholesterol. J Lipid Res. 2001;42:366–371. [PubMed] [Google Scholar]
- 20.Liu W, Xu L, Lamberson CR, Merkens LS, Steiner RD, Elias ER, Haas D, Porter NA. Assays of plasma dehydrocholesteryl esters and oxysterols from Smith-Lemli-Opitz syndrome patients. J Lipid Res. 2013;54:244–253. doi: 10.1194/jlr.M031732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shinkyo R, Xu L, Tallman KA, Cheng Q, Porter NA, Guengerich FP. Conversion of 7-dehydrocholesterol to 7-ketocholesterol is catalyzed by human cytochrome P450 7A1 and occurs by direct oxidation without an epoxide intermediate. J Biol. Chem. 2011;286:33021–33028. doi: 10.1074/jbc.M111.282434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goyal S, Xiao Y, Porter NA, Xu L, Guengerich FP. Oxidation of 7-dehydrocholesterol and desmosterol by human cytochrome P450 46A1. J Lipid Res. 2014;55:1933–1943. doi: 10.1194/jlr.M051508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu L, Liu W, Sheflin LG, Fliesler SJ, Porter NA. Novel oxysterols observed in tissues and fluids of AY9944-treated rats: a model for Smith-Lemli-Opitz syndrome. J Lipid Res. 2011;52:1810–1820. doi: 10.1194/jlr.M018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu L, Sheflin LG, Porter NA, Fliesler SJ. 7-Dehydrocholesterol-derived oxysterols and retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome. Biochim. Biophys. Acta. 2012;1821:877–883. doi: 10.1016/j.bbalip.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korade Z, Xu L, Mirnics K, Porter NA. Lipid biomarkers of oxidative stress in a genetic mouse model of Smith-Lemli-Opitz syndrome. J Inherit. Metab Dis. 2013;36:113–122. doi: 10.1007/s10545-012-9504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu L, Korade Z, Rosado DA, Jr, Liu W, Lamberson CR, Porter NA. An oxysterol biomarker for 7-dehydrocholesterol oxidation in cell/mouse models for Smith-Lemli-Opitz syndrome. J Lipid Res. 2011;52:1222–1233. doi: 10.1194/jlr.M014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu L, Korade Z, Rosado DA, Jr, Mirnics K, Porter NA. Metabolism of oxysterols derived from nonenzymatic oxidation of 7-dehydrocholesterol in cells. J Lipid Res. 2013;54:1135–1143. doi: 10.1194/jlr.M035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu L, Korade Z, Porter NA. Oxysterols from free radical chain oxidation of 7-dehydrocholesterol: product and mechanistic studies. J Am. Chem. Soc. 2010;132:2222–2232. doi: 10.1021/ja9080265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffiths WJ, Wang Y, Karu K, Samuel E, McDonnell S, Hornshaw M, Shackleton C. Potential of sterol analysis by liquid chromatography-tandem mass spectrometry for the prenatal diagnosis of Smith-Lemli-Opitz syndrome. Clin. Chem. 2008;54:1317–1324. doi: 10.1373/clinchem.2007.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson DW, ten Brink HJ, Jakobs C. A rapid screening procedure for cholesterol and dehydrocholesterol by electrospray ionization tandem mass spectrometry. J Lipid Res. 2001;42:1699–1705. [PubMed] [Google Scholar]
- 31.Paglia G, D'Apolito O, Gelzo M, Dello RA, Corso G. Direct analysis of sterols from dried plasma/blood spots by an atmospheric pressure thermal desorption chemical ionization mass spectrometry (APTDCI-MS) method for a rapid screening of Smith-Lemli-Opitz syndrome. Analyst. 2010;135:789–796. doi: 10.1039/b919622f. [DOI] [PubMed] [Google Scholar]
- 32.Crick PJ, William BT, Abdel-Khalik J, Matthews I, Clayton PT, Morris AA, Bigger BW, Zerbinati C, Tritapepe L, Iuliano L, Wang Y, Griffiths WJ. Quantitative charge-tags for sterol and oxysterol analysis. Clin. Chem. 2015;61:400–411. doi: 10.1373/clinchem.2014.231332. [DOI] [PubMed] [Google Scholar]
- 33.Griffiths WJ, Crick PJ, Wang Y, Ogundare M, Tuschl K, Morris AA, Bigger BW, Clayton PT, Wang Y. Analytical strategies for characterization of oxysterol lipidomes: liver X receptor ligands in plasma. Free Radic. Biol. Med. 2013;59:69–84. doi: 10.1016/j.freeradbiomed.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 34.Björkhem I, Diczfalusy U, Lövgren-Sandblom A, Starck L, Jonsson M, Tallman K, Schirmer H, Ousager LB, Crick PJ, Wang Y, Griffiths WJ, Guengerich FP. On the formation of 7-ketocholesterol from 7-dehydrocholesterol in patients with CTX and SLO. J Lipid Res. 2014;55:1165–1172. doi: 10.1194/jlr.P048603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Theofilopoulos S, Griffiths WJ, Crick PJ, Yang S, Meljon A, Ogundare M, Kitambi SS, Lockhart A, Tuschl K, Clayton PT, Morris AA, Martinez A, Reddy MA, Martinuzzi A, Bassi MT, Honda A, Mizuochi T, Kimura A, Nittono H, De MG, Carbone R, Criscuolo C, Yau JL, Seckl JR, Schule R, Schols L, Sailer AW, Kuhle J, Fraidakis MJ, Gustafsson JÅ, Steffensen KR, Björkhem I, Ernfors P, Sjövall J, Arenas E, Wang Y. Cholestenoic acids regulate motor neuron survival via liver X receptors. J Clin. Invest. 2014;124:4829–4842. doi: 10.1172/JCI68506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meljon A, Watson GL, Wang Y, Shackleton CH, Griffiths WJ. Analysis by liquid chromatography-mass spectrometry of sterols and oxysterols in brain of the newborn Dhcr7(Delta3–5/T93M) mouse: a model of Smith-Lemli-Opitz syndrome. Biochem. Pharmacol. 2013;86:43–55. doi: 10.1016/j.bcp.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffiths WJ, Crick PJ, Wang Y. Methods for oxysterol analysis: past, present and future. Biochem. Pharmacol. 2013;86:3–14. doi: 10.1016/j.bcp.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 38.Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, Bandyopadhyay S, Jones KN, Kelly S, Shaner RL, Sullards CM, Wang E, Murphy RC, Barkley RM, Leiker TJ, Raetz CR, Guan Z, Laird GM, Six DA, Russell DW, McDonald JG, Subramaniam S, Fahy E, Dennis EA. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010 doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang X, Sidhu R, Porter FD, Yanjanin NM, Speak AO, te Vruchte DT, Platt FM, Fujiwara H, Scherrer DE, Zhang J, Dietzen DJ, Schaffer JE, Ory DS. A sensitive and specific LC-MS/MS method for rapid diagnosis of Niemann-Pick C1 disease from human plasma. J Lipid Res. 2011;52:1435–1445. doi: 10.1194/jlr.D015735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hult M, Elleby B, Shafqat N, Svensson S, Rane A, Jörnvall H, Abrahmsen L, Oppermann U. Human and rodent type 1 11beta-hydroxysteroid dehydrogenases are 7beta-hydroxycholesterol dehydrogenases involved in oxysterol metabolism. Cell Mol. Life Sci. 2004;61:992–999. doi: 10.1007/s00018-003-3476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larsson H, Böttiger Y, Iuliano L, Diczfalusy U. In vivo interconversion of 7beta-hydroxycholesterol and 7-ketocholesterol, potential surrogate markers for oxidative stress. Free Radic. Biol. Med. 2007;43:695–701. doi: 10.1016/j.freeradbiomed.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 42.Schroepfer GJ., Jr Oxysterols: modulators of cholesterol metabolism and other processes. Physiol Rev. 2000;80:361–554. doi: 10.1152/physrev.2000.80.1.361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.