Abstract
Objective
The trauma caused during cochlear implant insertion can lead to cell death and a loss of residual hair cells in the cochlea. Various therapeutic approaches have been studied to prevent cochlear implant-induced residual hearing loss with limited success. In the present study, we show the efficacy of mild to moderate therapeutic hypothermia of 4 to 6°C applied to the cochlea in reducing residual hearing loss associated with the electrode insertion trauma.
Approach
Rats were randomly distributed in three groups: control contralateral cochleae, normothermic implanted cochleae and hypothermic implanted cochleae. Localized hypothermia was delivered to the middle turn of the cochlea for 20 minutes before and after implantation using a custom-designed probe perfused with cooled fluorocarbon. Auditory brainstem responses (ABRs) were recorded to assess the hearing function prior to and post-cochlear implantation at various time points up to 30 days. At the conclusion of the trials, inner ears were harvested for histology and cell count. The approach was extended to cadaver temporal bones to study the potential surgical approach and efficacy of our device. In this case, the hypothermia probe was placed next to the round window niche via the facial recess or a myringotomy.
Main Results
A significant loss of residual hearing was observed in the normothermic implant group. Comparatively, the residual hearing in the cochleae receiving therapeutic hypothermia was significantly conserved. Histology confirmed a significant loss of outer hair cells in normothermic cochleae receiving the surgical trauma when compared to the hypothermia treated group. In human temporal bones, a controlled and effective cooling of the cochlea was achieved using our approach.
Significance
Collectively, these results suggest that therapeutic hypothermia during cochlear implantation may reduce traumatic effects of electrode insertion and improve conservation of residual hearing.
Keywords: Therapeutic hypothermia, cochlear implant, trauma, electrode insertion, residual hearing, hair cell loss
1. Introduction
Conservation of residual hearing by reducing cochlear trauma has always been an important goal during inner ear surgeries, especially during cochlear implantation (CI). Patients with residual hearing are now being implanted to take advantage of bimodal electroacoustic stimulation (EAS) (Irving et al., 2014). The Food and Drug Administration (FDA)-approved indications for CI now allow bilateral implants in young children (Carlson et al., 2015; Ching et al., 2014), in whom residual hearing levels may be difficult to ascertain (Tharpe et al., 2008). Furthermore, in recent years, novel CI electrode designs have improved efficiency and performance by locating stimulation sites closer to spiral ganglion neurons and deeper into the scala tympani. As a result, in the future, the number of CI/EAS recipients with some degree of usable hearing is likely to increase. Depending upon the insertion depth and the intracochlear electrode position, however, CI can result in inflammation and oxidative stress leading to neural degeneration, hair cell loss and loss of residual hearing (Eshraghi et al., 2005; Wardrop et al., 2005). The nature of cochlear electrode insertion trauma has been characterized both in animal models and in temporal bone studies. Previous research has demonstrated the relationship between device-specific CI electrodes and surgical techniques with cochlear trauma (Adunka et al., 2005; Ahmad et al., 2012; Briggs et al., 2001). Auditory brainstem response (ABR) studies in animal models have observed significant increase in hearing thresholds within 7 days of implantation trauma (Balkany et al., 2005) and can be a result of both the intrinsic and extrinsic cell death signaling pathways (Bas et al., 2015). The loss of residual hearing due to electrode-induced trauma (EIT) can negatively impact the learning ability and speech recognition in younger patients. As such the importance of protecting residual hearing during and after implantation has grown proportionately. To reduce trauma-associated cellular response, refinements in surgical techniques and neuroprotective drug-eluting electrodes are being investigated (Dinh et al., 2008; Friedland et al., 2009; James et al., 2008; Jolly et al., 2010; Van De Water et al., 2010; Vivero et al., 2008). While surgical techniques and electrode designs have been advanced, residual hearing is still lost in many cochlear implant patients (Balkany et al., 2006; Kiefer et al., 2004).
The present study tested the hypothesis that localized therapeutic hypothermia applied to the cochlea prior to implantation can protect hair cells (HC) and neurons and hence may preserve residual hearing. Mild to moderate hypothermia is a promising neuroprotective intervention when induced during or after a central nervous system injury (Cappuccino et al., 2010; Dietrich et al., 2009; Kawai et al., 2000; Levi et al., 2010; Matsui et al., 2006). When localized and administered prior to trauma, these effects may be enhanced with little adverse effects on other biological phenomena or immune response (Purdy et al., 2013; Tzen et al., 2013). Mild therapeutic hypothermia, defined as a temperature decrease of 4 to 6 °C below body temperature, has the potential to reduce or prevent oxidative stress (Balkany et al., 2005; Henry et al., 1984; Shintani et al., 2010). Prior literature, both from animal studies and clinical trials, has shown protective effects of therapeutic hypothermia on ischemic and traumatic injuries to neurons (Levi et al., 2010; Ohta et al., 2007; Shintani et al., 2010; Yanamoto et al., 1999; Yokobori et al., 2011) in cases of mild traumatic brain injury (mTBI), seizures (Atkins et al., 2010), ischemia and inflammatory response (Cappuccino et al., 2010; Kawai et al., 2000; Levi et al., 2010; Matsui et al., 2006) after cardiac arrest, and related to spinal cord injuries (Cappuccino et al., 2010; Dietrich et al., 2009). Unfortunately, systemic hypothermia has multiple side effects on functioning of organs and organ systems including impairment to immune response, higher infection, altered effects of other drugs and compromising vascular function. These adverse effects restrict its use in clinical setting, and make it challenging to utilize hypothermia for preserving sensory functions of the inner ear.
That temperature is an important parameter and influences cochlear responses has been long known (Brown et al., 1983; Liberman et al., 1984; Ohlemiller et al., 1992; Ohlemiller et al., 1994) with demonstrated benefit of applied hypothermia (Henry et al., 1984; Watanabe et al., 2001). To test the beneficial effects of localized hypothermia in otoprotection, we designed a custom device to cool the cochlea that does not require any modifications to the current CI surgical approach. Direct comparisons were made between hearing thresholds post-surgery in normothermic and hypothermic-treated cochleae in normal hearing rats subjected to EIT. We also carried out a detailed histological study at the end of the long-term implantation and observed significant otoprotection with hypothermia.
2. Methods
2.1. Animal preparation and surgical approach
The use of Brown Norway rats with normal hearing in this study was approved by the University of Miami Animal Care and Use Committee and was in compliance with USDA and NIH Guidelines for the Care and Use of Laboratory Animals. The animals were anesthetized with an initial intraperitoneal injection of Ketamine and Xylazine (22–60 and 5–10 mg/kg respectively) and maintained with supplemental doses. Lidocaine HCl 1% was used for local analgesia prior to surgical incision. Withdrawal reflex was measured by extension of one leg and pinching the web of skin between the toes or pinching the ear. A positive reflex was indicated by flexion of the limb with toe, paw pinch or movement of the head or whiskers with ear pinch. Once anesthetized, a bland, sterile ophthalmic base ointment was applied to corneas to prevent eye dryness. The skin over the skull and behind the ears was shaved, and prepped with Betadine scrub. All surgical procedures were performed under aseptic conditions. For the approach to the inner ear organs, a post-auricular incision was made extending from the dorsal skin defect down behind the ear to terminate approximately over the posterior edge of the mandible. The soft tissues were dissected to expose the temporal bone over the bulla. A small defect in the bone of the bulla was created using the tip of a scalpel blade or a hand-held micro-drill to expose both the round window membrane niche and the lateral bony wall of the cochlea adjacent to the niche. Electrode trauma to the cochlea was accomplished by the insertion of an electrode analog into the scala tympani via the round window to a depth of 5 mm. The electrode analog was 0.28mm monofilament as described previously (Bas et al., 2012; Bas et al., 2015) to allow for insertion in the smaller scala tympani of the rat. The site around the electrode was secured by packing with a graft of fascia obtained locally from the site of the surgical approach to the bulla. Once the placement and stability of the electrode analog were established, the defect in the ventro-lateral wall of the temporal bone bulla was covered with carboxylate cement, with care taken not to allow the cement to enter into the bulla. A subcuticular closure was made using vicryl absorbable sterile sutures and the skin was closed with vicryl absorbable sutures. A topical antibiotic silver sulfadiazine 1% or Bacitracin Zinc was applied to the wound sites. To prevent post-op dehydration the animals received IP or SC injection of sterile Ringer’s solution (up to 1 mL). To prevent post-operative pain, buprenorphine (Buprenex, 0.05 mg/kg) was given once at the time of surgery and sequential dosages were provided for 48 hours to aid recovery. To prevent hypothermia post-op, the animal holding/recovery cages where placed on water-circulating heating pads. There were three experimental groups: in the first group, the left ear was implanted under normothermic condition and in the second group, therapeutic hypothermia (cooling by 3–6 °C measured at the round window) was provided using the newly developed hypothermia device. The right contralateral ear always served as the intra-animal control.
2.2. Delivery of therapeutic hypothermia
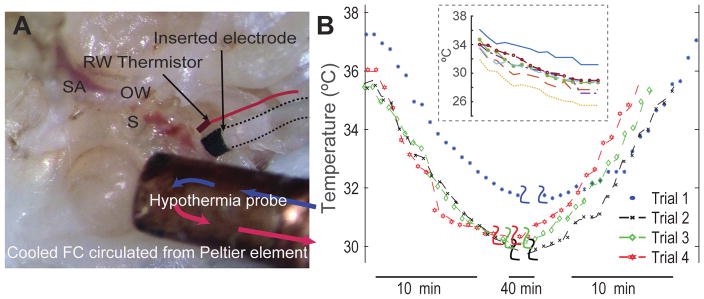
A novel copper hypothermia probe attached to a custom thermoelectric Peltier device was placed in the middle ear adjacent to the cochlea under direct visualization. Fluorocarbon cooled by the Peltier system was used as the refrigerant, and circulated through the metal probe. In acute experiments (n=7), the temperatures at the apex and the basal turn of the cochlea were measured using (QTI Sensing Solutions’ T320/E320) microthermistors over time. The temperature of the cochlea was reduced by a 5–6°C with our device (Figure 1) and cooling was maintained within ±0.3°C over the duration of the experiment. Using the acute experiments as guide, we tested the following protocol for chronic experiments: localized hypothermia was applied for 20 minutes before, and for 20 minutes after cochlear implantation for a total hypothermia duration of 40 minutes.
Figure 1.
(A) shows placement of the hypothermia probe in contact with an excised rat left cochlea. The inserted electrode analog and a thermistor placed at the round window for temperature measurement are highlighted. Fluorocarbon cooled by an external peltier element is circulated through the copper tip of the probe. RW-round window, SA-stapedial artery, OW-oval window, S-stapes. (B) With the custom-designed thermoelectric cooler and probe positioned anteroinferior to the RW, the cochleae were cooled between 4–6°C within 10 minutes. The temperature was maintained for 20 minutes prior to insertion of the electrode analog. Hypothermia was maintained for further 20 minutes prior to a gradual rewarming of the cochleae over 10 minutes. Inset shows the level of cooling achieved at the round window measured prior to electrode insertion in animals undergoing chronic insertion.
2.3. Measurement of hearing thresholds: auditory brainstem responses (ABR)
All animals were naïve with normal hearing prior to surgery. ABRs were measured for acoustic tones between 0.5 and 32 kHz before surgery to determine the pre-surgical hearing thresholds (Figure 2). On average the thresholds were ~40 dB SPL at 0.5 kHz and 1 kHz, 30 dB at 2 kHz, 15 dB at 4 kHz, 8 kHz, and 16 kHz, and 30 dB at 32 kHz. ABRs were also measured at various time points post-surgery up to 30 days in the contralateral control, normothermic implanted and hypothermia-treatment implanted ears and compared to respective pre-surgical thresholds. The changes in residual hearing with electrode insertion trauma were tested on the frequencies of 0.5, 1, 2, 4, 8, 16, 24, and 32 kHz using 1000 sweeps of tone bursts of a rate of stimulation of 21.1 Hz amplified using an Opti-Amp bioamplifier from Intelligent Hearing Systems (IHS, Miami, FL) connected to the Smart EP system. Recording negative electrodes were attached to the each ipsilateral superior post auricular area and with a positive reference electrode to the vertex. A ground electrode was inserted subcutaneously on the left leg. The ipsilateral ABR information was assessed bilaterally by averaging 1000 samples at each interval starting from a level of 80 dB SPL tone bursts and decreasing by 10 dB steps until no identifiable ABR response was present at each frequency. Threshold was defined as the minimum stimulating level where an ABR response was both identifiable and repeatable. The hearing function was compared between the three groups: cochleae that did not receive hypothermia during surgery (normothermic trauma-only group, n=7), cochleae that received localized mild hypothermia (hypothermia treated group, n=7) and the contralateral cochleae of each animal (control group, n=14). Two animals did not survive past 14 days and 4 animals were not sampled at day 14. ABR information was additionally reviewed by two blinded investigators for accuracy with no knowledge of the animal group classification.
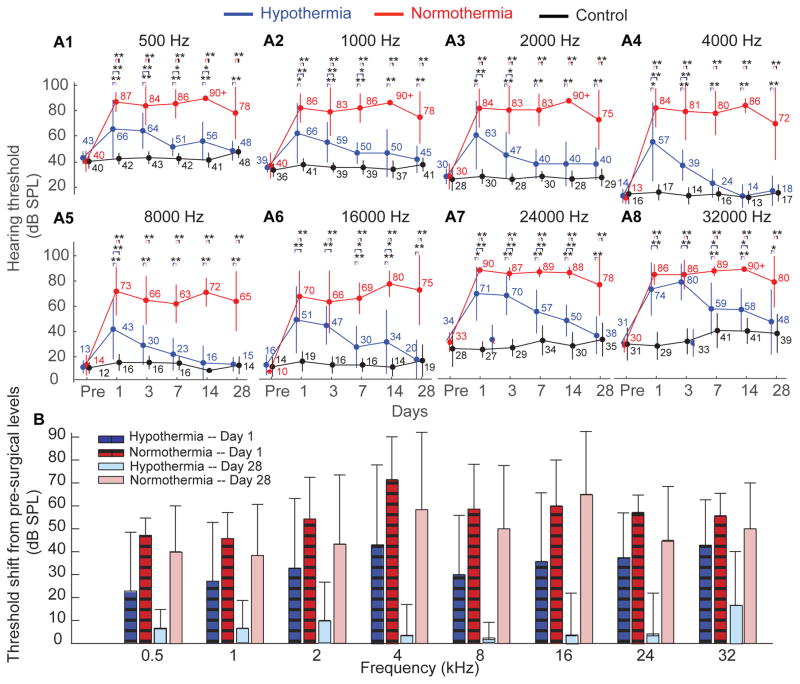
Figure 2.
shows the mean±S.D. of the auditory brainstem responses at .5 (3A1) −32 (3A8) kHz tones were recorded up to 30 days following cochlear implant surgery (at day 0) in rats. The responses were compared for three groups: normothermic cochleae receiving EIT (red, n=7 except for days 14, where n=5 and 28, where n=6), hypothermic cochleae receiving EIT (blue, n=7 except for days 14, where n=5 and 28, where n=6) and non-operated, contralateral control cochleae (black, n=14 except for days 14, where n=10 and 28, where n=12). The electrode was chronically implanted and remained in place throughout the duration. 3B) shows the results represented as mean±S.D. of the threshold shift on days 1, 3, 7, 14 and 28 following electrode insertion from intranimal pre-surgical thresholds.
2.4. Histology
At the conclusion of the trials, inner ears were harvested and fixed with 4% paraformaldehyde for histologic evaluation of the organ of Corti (OC). The fixed explants were washed three times in PBS the OC was dissected from the bony capsule and subsequently incubated in 5% normal goat serum (Sigma Aldrich, MO) and 1% Triton X-100 (Sigma Aldrich) in PBS for 30 min at 25°C. OC specimens were then incubated with FITC-labeled phalloidin (Sigma-Aldrich) for 45 min at 25°C and washed three times. The OC were placed in DAPI solution to label the nuclei for 5 min and washed three times. After washing, these OC explants were mounted on a glass slide and viewed under a confocal microscope Zeiss LSM 700. Stereocilia bundles of HCs stained with phalloidin-FITC were recognized. A HC was counted if it possessed an intact cuticular plate with an intact stereociliary bundle. Total HCs were counted for the apex, middle and basal images taken from regions of the OC explants and expressed as percentage of HCs lost on each. Each image count was additionally verified by two blinded investigators individually selecting from a non-descript total set. Counts were then segregated into their respective groups. The HC count was compared between the three groups: normothermic, hypothermic and control contralateral cochleae.
2.5. Surgical approach in human temporal bone
The hypothermia device and probe were tested on human temporal bones (n=3) to test the feasibility of achieving mild to moderate therapeutic hypothermia during surgery. The bone was placed on a heat pad to maintain it near room temperature. The facial recess was approached by canal wall up mastoidectomy. The mucosa and bone of the round window niche were taken down using 1mm coarse diamond burr and instrument. The round window membrane was visualized and pierced using Rosen needle. The hypothermia probe was placed anteroinferior to the round window via a myringotomy. The bones were perfused via the round window with egress via a cochleostomy on the apical surface with water heated at 45 °C that translated into approxim ate 36 °C measured near or at the round window and at a hole drilled on the inside of the bone to expose the cochlea apex. The custom device with probe placed near the promontory was used to lower the temperature by 4–6 °C at the meas uring locations. A microthermistor was placed on the surface of the mastoid to measure temperature over time. Two other microthermistors were placed on the cochleae: one at on the bone covering apical surface (via middle fossa approach) and another near the round window membrane.
2.6. Statistical Analysis
The results were assessed by using two-way ANOVA implemented in MATLAB and presented as mean±S.D. For each frequency, group was the between subject factor, whereas decibels was the within-subject factor. Not significant values are represented with ns, values of p < 0.05 are represented by *, values of p < 0.01 are represented by ** and values of p < 0.001 are represented by ***.
3. Results
With the current surgical approach and placement of the hypothermia probe in close proximity to the cochlear wall, we could successfully achieve 4–6°C of cooling required for therapeutic hypothermia in rat cochleae within 5–8 minutes (Figure 1). The design of our device and its efficacy were tested in two acute experiments (Figure 1B). The temperature was maintained within ±0.3°C over the duration of the experiments. In the present set of experiments, hypothermia was maintained for 20 minutes prior to and post electrode insertion for a total of 40 minutes. Contralateral cochleae were used as an intra-animal reference.
3.1. Hypothermia prevented a significant elevation in hearing thresholds following cochlear implant trauma
In order to investigate the efficacy of therapeutic hypothermia, we studied the changes in hearing threshold evoked post cochlear implantation in normothermic and hypothermic-treated implanted cochleae (Figure 2). The results show the comparison of ABRs between the three groups at different frequency locations (mean±S.D). For example, at frequency location of 16 kHz a significant increase in hearing thresholds was observed at day 1 post-surgery (two-way ANOVA, p<0.01, Figure 2A6). In the normothermic implanted cochleae, the significant loss of residual hearing post-surgical trauma persisted until 28 days. The hearing threshold increased by 60±20 dB (n=7) on the day after surgical implantation and it remained elevated for up to 28-days (an elevation of 65±27 dB over pre-surgical levels). In comparison, thresholds in the hypothermic-implanted cochleae increased by 35±26 dB on the day after surgical implantation. Starting day 3 post-implantation, a recovery was observed with the hearing thresholds returning to pre-surgical levels by day 28 (elevation of 4±15 dB). The hearing thresholds of the contralateral cochleae did not change significantly over the duration of the experiments (19±7 dB at day 28 compared to 14±6 dB measured at the day before surgery). All ABR measurements were carried out in euthermic conditions (37°C body temperature).
The effects of EIT on residual hearing and the efficacy of therapeutic hypothermia were consistent across multiple frequency locations. ABRs were measured between 500 Hz (Figure 2A1) and 32 kHz (Figure 2A8) for changes in hearing threshold evoked post cochlear implantation in normothermic and hypothermic-treated implanted cochleae. These hearing threshold were repeated on day 1, 3, 7, 14 and 28. The results similarly display increased hearing threshold (two-way ANOVA, p<0.01) for normothermic cochleae after surgical implantation with an average increase of 56±12 dB. The hearing threshold remained elevated with an average change of 48±24 dB at day 28 (as compared to pre-surgical levels). On the contrary, the hypothermic treated cochleae had post-surgical increase in hearing thresholds ~35±24 dB and displayed a significant recovery at 28 days (a non significant change of 7±13 dB compared to pre-surgical levels). The contralateral control cochleae did not show significant changes or progressive loss of residual hearing at any frequency (5±9 dB at day 28 compared to 2±7 dB measured pre-surgical). Figure 2B shows the same results as a threshold shift in residual hearing over pre-surgical levels measured at post-implant days 1, 3, 7, 14 and 28.
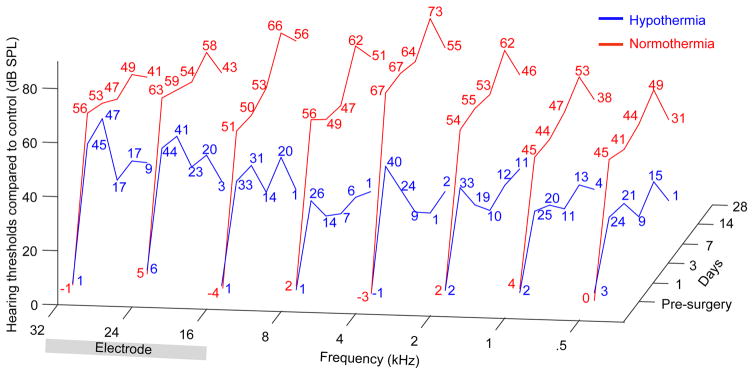
Hearing threshold measured in the normothermic and hypothermia-treated groups were normalized with respect to intra-animal control, contralateral cochleae that were not subjected to any surgery or treatment. Figure 3 shows the averaged change in the two groups measured at various frequencies and over the duration of the experiment. In normothermic implanted cochleae, a significant loss of residual hearing is observed across all frequencies including at the lower frequencies corresponding to apical sites within the cochlea. Comparatively, the hypothermia-treated cochlea recovered significantly starting day 3 and had hearing thresholds near normal by day 28. In present experiments, the electrode analog was inserted at the round window and approximately to a depth of 5 mm. The placement of the electrode and the depth of insertion were estimated by using frequency representation in the rat cochlea derived in a previous study (Muller, 1991). The approximate electrode position is visualized in Figure 3 to highlight the primary site of traumatic injury.
Figure 3.
shows hearing threshold in hypothermia-treated (blue) and normothermic (red) EIT cochleae normalized with control, contralateral cochleae. The hearing thresholds at each tested frequency (0.5, 1, 2, 4, 8, 16, 24 and 32 kHz) and the days at which residual hearing was assessed (pre-surgery and post-surgical day 1, 3, 7, 14 and 28) are represented. The approximate site of insertion and length of the inserted electrode are highlighted.
3.2. Hypothermia prevented outer hair cell loss following EIT
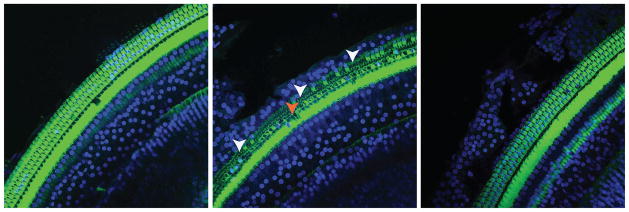
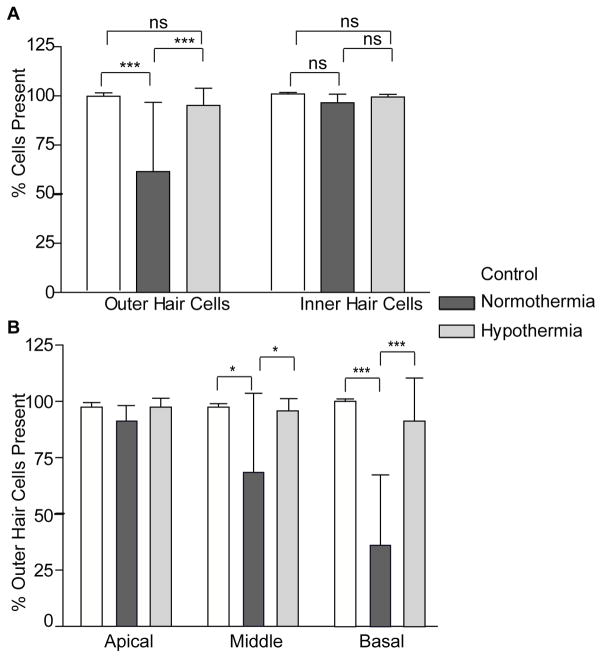
Cochleae were harvested at day 30 for histologic evaluation of the OC. Typical photomicrographs from the middle segment of the cochlea showed stereocilia bundles of HCs stained with phalloidin-FITC and nuclei stained with DAPI. Contralateral, control cochlea showed little or no evidence of hair cell loss and normal OHC and IHC morphology (Figure 4A). As shown in Figure 4b, a significant loss of the outer hair cells was observed in the normothermic cochlea when compared with hypothermia-treated cochlea. Hypothermia preserved the outer hair cells as seen in the photomicrographs (Figure 4c). The inner hair cells were significantly less affected in all the samples, with a small but not significant loss in normothermic-implanted cochleae.
Figure 4.
shows the organ of Corti of chronically implanted rats harvested at Day 28. Stereocilia bundles of HCs were stained with phalloidin-FITC (green) and the nuclei with DAPI (blue). A) shows the organ of Corti of a hypothermia-treated cochlea undergoing EIT. B) shows an EIT normothermic cochlea that had significant outer HC loss (OHC) near the electrode insertion site indicated by the arrows. C) shows a control, non-operated contralateral cochlea.
Quantification analyses of the microphotographs show an average outer hair cell loss of 39.5% of normothermic samples compared to 4.8% loss in hypothermia treated cochleae (Figure 5A). Furthermore based on distance, we divided the cochlear samples into three regions: the basal region encompassing high frequencies up to 24 kHz, the middle region encompassing the 16 to 8 kHz band, and the apical region encompassing the low frequencies between 4 to 0.5 kHz. The outer hair cell counts in the three regions were confirmed by two blinded subjects and has been represented as percentage of total OHCs present in Figure 5B. A significant loss of OHCs was observed in the middle (*P≤0.05) and in the base (***P≤0.005) of the cochlea. A profound loss occurred in the normothermic implanted cochleae with an average loss of 64% of all outer hair cells in the basal area, 32.7% in the middle turn and ~10% in the apex. On the other hand, the hypothermia-treated cochleae did not show a significant loss of OHCs when compared to controls.
Figure 5.
A) shows the hair cell count comparison between outer and inner normothermia treated, hypothermia treated and control cochleae harvested 28 days post implantation. There was no significant inner hair cell loss, but we observed a pronounced outer hair cell loss in normothermic cochleae. B) Quantification of the total number of outer hair cells (OHCs) present at the end of chronic experiment in basal, middle and apical turn regions of the cochleae in control, normothermic and hypothermia-treated implanted cochleae. Note the significant loss of OHCs in the middle (*P≤0.05) and in the base (***P≤0.005) of the normothermic cochlea. Error bars are S.D.
3.3. Developing a surgical approach for human application
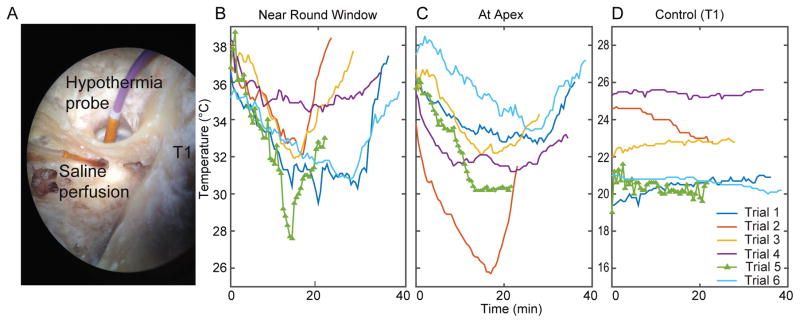
To study the translational potential of this device, we carried out temperature measurements in cadaver temporal bones using the current hypothermia probe designed for the rats (Figure 6, n=3). The microthermistor location is shown in Figure 6A (control, T1). As can be seen in Figure 6B, C, the cochlear temperature was reduced gradually by ~3–6°C within 10 minutes while the mastoid cooled by less than 1°C to equilibrate with room temperature. The cochlear temperature was maintained near within ±0.5°C, following which a slow rewarming protocol was carried out to return the temperature to baseline levels.
Figure 6.
shows temperature measurements taken from a human temporal bone during applied hypothermia. (A) shows the placement of the hypothermia probe anteroinferior to the round window via a myringotomy. The hypothermia probe was placed anteroinferior to the round window via a myringotomy. The temperature was reduced by 4–6°C in on the bone cov ering apical surface (via middle fossa approach) (B) and at the round window membrane (C). The mastoid temperature varied less than 1°C during the experiment (D).
4. Discussion
Previous reports have documented that EIT results in a significant loss of residual hair cells either by direct trauma to sensitive cochlear structures such as basilar membrane, osseous spiral lamina, and modiolus or by cascading molecular effects (Bas et al., 2012; Eshraghi et al., 2013). While it is likely that a combination of mechanisms is responsible for the loss of residual function, recent studies have begun to unravel the molecular mechanisms that underlie the loss of residual hearing. It has been shown that increased levels of inflammation and oxidative stress following trauma could initiate apoptosis of hair cells. In particular, the mRNA levels of pro-inflammatory cytokines such as TNFα and IL-1β, and pro-inflammatory enzymes, iNOS and COX-2 increased significantly following trauma (Bas et al., 2012). Cytokines, such as interleukin-1 (IL-1) (Touzani et al., 1999) and tumor necrosis factor alpha (TNF-α) (Dinh et al., 2008; Keithley et al., 2008), are known to contribute to brain damage similarly to free radicals and may cause auditory hair cell and spiral ganglion loss. The apoptotic pathways include the extrinsic or death receptor pathway and the intrinsic or mitochondrial pathway, with both involving the activation of caspase-3, which induces chromatin condensation and DNA fragmentation (Elmore, 2007).
The feasibility and safety of mild to moderate hypothermia is a useful therapeutic method for improving recovery from brain injuries, strokes, and neural function. Its efficacy has been evaluated in experimental settings and clinical trials. Previous studies have assessed the cortical infarct volume and shown that it was significantly decreased by hypothermic treatment, while others have identified an improvement in recovery from spinal cord injury after intravascular cooling. (Cappuccino et al., 2010; Dietrich et al., 2009; Kawai et al., 2000; Levi et al., 2010). The neuroprotective mechanisms of hypothermia include suppression of excitotoxicity, free radical production, neuroinflammation, blood brain barrier (BBB) disruption and regulation of early gene expression. (Darwazeh et al., 2013; Dietrich et al., 2011). Studies have shown that hypothermia related protective effects are also correlated with a significant increase in expression of Bcl-2, an important anti-apoptotic gene, in a rat model of transient global ischemia (Zhang et al., 2010)
Applications of hypothermia in a clinical setting have varied from systemic hypothermia to localized cooling of surgically exposed tissue. It has been shown that cochleae undergoing surgical implantation in animals receiving mild hypothermia had less functional loss compared to normothermic implanted cochlea up to 7 days (Balkany et al., 2005). While the results were positive, the application of systemic hypothermia for inner ear surgeries is not clinically feasible. In the present study, we evaluated the potential of localized hypothermia delivered to the inner ear during cochlear implant surgery to improve protection of critical structures and preservation of residual function. The results of the present study indicate that cooling the inner ear between 4–6°C protected residual hearing in rats. The hypothermia-treated cochleae showed a similar but significantly smaller shift in the ABR thresholds as the normothermic-implanted cochleae immediately following the CI. The ABR thresholds in normothermic-implanted group remained elevated over the next 4 weeks, while those of the hypothermic-implanted group recovered starting week 1. Histological results compared well with the functional data -- hypothermic-cochleae showed significant preservation of outer hair cells and minimal cell death compared to normothermic-cochleae. The approach utilized a novel copper hypothermia probe attached to a thermoelectric Peltier device placed under direct visualization in the surgical cavity adjacent to the site of implant. With continuously circulated refrigerant through the probe tip the temperature of the cochlea could be reduced by 4–6°C within 5 to 10 minutes. Furthermore, the temperature could be maintained within ±0.3°C over the duration of the experiments. Our approach will be feasible for human clinical application as shown here and has significant advantages over systemic or external application of hypothermia. Although the surgical approach and design of our device will need to be optimized in the future to deliver therapeutic hypothermia to the human cochleae, these results clearly demonstrated that effective cooling of the cochlea and inner ear structures is possible.
While the current investigation presents the utility of custom-designed probe to deliver localized hypothermia in vivo and the efficacy of hypothermia delivered pre-trauma, several limitations should be noted. First, the present study focused on normal hearing animals. The normal functioning cochlea is likely to be different from a cochlea in a hearing-impaired or deafened animal with respect to expression of inflammatory mediators and may present different mechanisms of injury not mitigated by therapeutic hypothermia. Second, the auditory function was studied over a relatively short post-implantation period (28 days) and we did not study potential damage caused by electrical activation of cochlear implants. It is possible that the protective effects of hypothermia may have simply delayed the progressive component of the hearing loss observed following EIT. Additional studies focusing on chronic effects of EIT, electrical activation and potential benefits of therapeutic hypothermia are therefore required prior to translating the application to clinical trials. Finally, prior literature has shown that duration of cooling and speed of rewarming can impact the beneficial effects of hypothermia (Polderman et al., 2009). In the present study, hypothermia was delivered for ~60 min intraoperatively, with 10 minutes of cooling and rewarming period and 40 minutes of maintained hypothermia. The relatively long duration may restrict widespread adoption of the technique. Future studies will need to focus on determining the critical duration for therapeutic hypothermia applied to the cochlea for CI.
5. Conclusion
In conclusion, the present results are promising and indicate that therapeutic hypothermia applied pre-trauma protects residual function in the cochleae following cochlear implantation. In the rat model, localized hypothermia can reduce the post-implant trauma and protect the cochleae against the progressive loss of hearing observed in normothermic implanted cochleae. Additionally, based on the temporal bone studies described, the present approach appears to be applicable to patients receiving cochlear implantation with localized hypothermia achieved during surgery.
Highlights.
Developed and tested safety and efficacy of a novel system to deliver therapeutic hypothermia locally to the inner ear in a rat model.
Localized therapeutic hypothermia conserves residual hearing following cochlear implant surgical trauma.
Significant protection of outer hair cells was obseved in hypothermia-treated cochleae when compared to the normothermic implanted group.
Present approach can deliver controlled and efficacious hypothermia treatment and shows promise of clinical translation.
Acknowledgments
Funding: This work was supported by R21DC014324 (SMR) and Pilot Award (SMR) from Grant Number 1UL1TR000460, Miami Clinical and Translational Science Institute.
Footnotes
Conflict of interest
The authors declare no competing interests.
Financial conflict of interest
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adunka O, Kiefer J, Unkelbach MH, Radeloff A, Gstoettner W. Evaluating cochlear implant trauma to the scala vestibuli. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2005;30:121–7. doi: 10.1111/j.1365-2273.2004.00935.x. [DOI] [PubMed] [Google Scholar]
- Ahmad FI, Choudhury B, De Mason CE, Adunka OF, Finley CC, Fitzpatrick DC. Detection of intracochlear damage during cochlear implant electrode insertion using extracochlear measurements in the gerbil. The Laryngoscope. 2012;122:636–44. doi: 10.1002/lary.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CM, Truettner JS, Lotocki G, Sanchez-Molano J, Kang Y, Alonso OF, Sick TJ, Dietrich WD, Bramlett HM. Post-traumatic seizure susceptibility is attenuated by hypothermia therapy. The European journal of neuroscience. 2010;32:1912–20. doi: 10.1111/j.1460-9568.2010.07467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkany TJ, Eshraghi AA, Jiao H, Polak M, Mou C, Dietrich DW, Van De Water TR. Mild hypothermia protects auditory function during cochlear implant surgery. The Laryngoscope. 2005;115:1543–7. doi: 10.1097/01.mlg.0000173169.45262.ae. [DOI] [PubMed] [Google Scholar]
- Balkany TJ, Connell SS, Hodges AV, Payne SL, Telischi FF, Eshraghi AA, Angeli SI, Germani R, Messiah S, Arheart KL. Conservation of residual acoustic hearing after cochlear implantation. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2006;27:1083–8. doi: 10.1097/01.mao.0000244355.34577.85. [DOI] [PubMed] [Google Scholar]
- Bas E, Gupta C, Van De Water TR. A novel organ of corti explant model for the study of cochlear implantation trauma. Anatomical record. 2012;295:1944–56. doi: 10.1002/ar.22585. [DOI] [PubMed] [Google Scholar]
- Bas E, Goncalves S, Adams M, Dinh CT, Bas JM, Van De Water TR, Eshraghi AA. Spiral ganglion cells and macrophages initiate neuro-inflammation and scarring following cochlear implantation. Front Cell Neurosci. 2015;9:303. doi: 10.3389/fncel.2015.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs RJ, Tykocinski M, Saunders E, Hellier W, Dahm M, Pyman B, Clark GM. Surgical implications of perimodiolar cochlear implant electrode design: avoiding intracochlear damage and scala vestibuli insertion. Cochlear implants international. 2001;2:135–49. doi: 10.1179/cim.2001.2.2.135. [DOI] [PubMed] [Google Scholar]
- Brown MC, Smith DI, Nuttall AL. The temperature dependency of neural and hair cell responses evoked by high frequencies. The Journal of the Acoustical Society of America. 1983;73:1662–70. doi: 10.1121/1.389387. [DOI] [PubMed] [Google Scholar]
- Cappuccino A, Bisson LJ, Carpenter B, Marzo J, Dietrich WD, 3rd, Cappuccino H. The use of systemic hypothermia for the treatment of an acute cervical spinal cord injury in a professional football player. Spine. 2010;35:E57–62. doi: 10.1097/BRS.0b013e3181b9dc28. [DOI] [PubMed] [Google Scholar]
- Carlson ML, Sladen DP, Haynes DS, Driscoll CL, DeJong MD, Erickson HC, Sunderhaus LW, Hedley-Williams A, Rosenzweig EA, Davis TJ, Gifford RH. Evidence for the expansion of pediatric cochlear implant candidacy. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2015;36:43–50. doi: 10.1097/MAO.0000000000000607. [DOI] [PubMed] [Google Scholar]
- Ching TY, Day J, Van Buynder P, Hou S, Zhang V, Seeto M, Burns L, Flynn C. Language and speech perception of young children with bimodal fitting or bilateral cochlear implants. Cochlear implants international. 2014;15(Suppl 1):S43–6. doi: 10.1179/1467010014Z.000000000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwazeh R, Yan Y. Mild hypothermia as a treatment for central nervous system injuries: Positive or negative effects. Neural Regen Res. 2013;8:2677–86. doi: 10.3969/j.issn.1673-5374.2013.28.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich WD, Atkins CM, Bramlett HM. Protection in animal models of brain and spinal cord injury with mild to moderate hypothermia. Journal of neurotrauma. 2009;26:301–12. doi: 10.1089/neu.2008.0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich WD, Levi AD, Wang M, Green BA. Hypothermic treatment for acute spinal cord injury. Neurotherapeutics. 2011;8:229–39. doi: 10.1007/s13311-011-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh C, Hoang K, Haake S, Chen S, Angeli S, Nong E, Eshraghi AA, Balkany TJ, Van De Water TR. Biopolymer-released dexamethasone prevents tumor necrosis factor alpha-induced loss of auditory hair cells in vitro: implications toward the development of a drug-eluting cochlear implant electrode array. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2008;29:1012–9. doi: 10.1097/MAO.0b013e3181859a1f. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshraghi AA, Polak M, He J, Telischi FF, Balkany TJ, Van De Water TR. Pattern of hearing loss in a rat model of cochlear implantation trauma. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2005;26:442–7. doi: 10.1097/01.mao.0000169791.53201.e1. discussion 447. [DOI] [PubMed] [Google Scholar]
- Eshraghi AA, Gupta C, Van De Water TR, Bohorquez JE, Garnham C, Bas E, Talamo VM. Molecular mechanisms involved in cochlear implantation trauma and the protection of hearing and auditory sensory cells by inhibition of c-Jun-N-terminal kinase signaling. The Laryngoscope. 2013;123(Suppl 1):S1–14. doi: 10.1002/lary.23902. [DOI] [PubMed] [Google Scholar]
- Friedland DR, Runge-Samuelson C. Soft cochlear implantation: rationale for the surgical approach. Trends in amplification. 2009;13:124–38. doi: 10.1177/1084713809336422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KR, Chole RA. Hypothermia protects the cochlea from noise damage. Hearing research. 1984;16:225–30. doi: 10.1016/0378-5955(84)90111-4. [DOI] [PubMed] [Google Scholar]
- Irving S, Gillespie L, Richardson R, Rowe D, Fallon JB, Wise AK. Electroacoustic stimulation: now and into the future. BioMed research international. 2014;2014:350504. doi: 10.1155/2014/350504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James DP, Eastwood H, Richardson RT, O’Leary SJ. Effects of round window dexamethasone on residual hearing in a Guinea pig model of cochlear implantation. Audiology & neuro-otology. 2008;13:86–96. doi: 10.1159/000111780. [DOI] [PubMed] [Google Scholar]
- Jolly C, Garnham C, Mirzadeh H, Truy E, Martini A, Kiefer J, Braun S. Electrode features for hearing preservation and drug delivery strategies. Advances in oto-rhino-laryngology. 2010;67:28–42. doi: 10.1159/000262594. [DOI] [PubMed] [Google Scholar]
- Kawai N, Okauchi M, Morisaki K, Nagao S. Effects of delayed intraischemic and postischemic hypothermia on a focal model of transient cerebral ischemia in rats. Stroke; a journal of cerebral circulation. 2000;31:1982–9. doi: 10.1161/01.str.31.8.1982. discussion 1989. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Wang X, Barkdull GC. Tumor necrosis factor alpha can induce recruitment of inflammatory cells to the cochlea. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2008;29:854–9. doi: 10.1097/MAO.0b013e31818256a9. [DOI] [PubMed] [Google Scholar]
- Kiefer J, Gstoettner W, Baumgartner W, Pok SM, Tillein J, Ye Q, von Ilberg C. Conservation of low-frequency hearing in cochlear implantation. Acta oto-laryngologica. 2004;124:272–80. doi: 10.1080/00016480310000755a. [DOI] [PubMed] [Google Scholar]
- Levi AD, Casella G, Green BA, Dietrich WD, Vanni S, Jagid J, Wang MY. Clinical outcomes using modest intravascular hypothermia after acute cervical spinal cord injury. Neurosurgery. 2010;66:670–7. doi: 10.1227/01.NEU.0000367557.77973.5F. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW. Single-neuron labeling and chronic cochlear pathology. II. Stereocilia damage and alterations of spontaneous discharge rates. Hearing research. 1984;16:43–53. doi: 10.1016/0378-5955(84)90024-8. [DOI] [PubMed] [Google Scholar]
- Matsui T, Ishikawa T, Takeuchi H, Okabayashi K, Maekawa T. Mild hypothermia promotes pro-inflammatory cytokine production in monocytes. Journal of neurosurgical anesthesiology. 2006;18:189–93. doi: 10.1097/01.ana.0000188639.39844.f6. [DOI] [PubMed] [Google Scholar]
- Muller M. Frequency representation in the rat cochlea. Hearing research. 1991;51:247–254. doi: 10.1016/0378-5955(91)90041-7. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Siegel JH. The effects of moderate cooling on gross cochlear potentials in the gerbil: basal and apical differences. Hearing research. 1992;63:79–89. doi: 10.1016/0378-5955(92)90076-y. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Siegel JH. Cochlear basal and apical differences reflected in the effects of cooling on responses of single auditory nerve fibers. Hearing research. 1994;80:174–90. doi: 10.1016/0378-5955(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Ohta H, Terao Y, Shintani Y, Kiyota Y. Therapeutic time window of post-ischemic mild hypothermia and the gene expression associated with the neuroprotection in rat focal cerebral ischemia. Neuroscience research. 2007;57:424–33. doi: 10.1016/j.neures.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Critical care medicine. 2009;37:1101–20. doi: 10.1097/CCM.0b013e3181962ad5. [DOI] [PubMed] [Google Scholar]
- Purdy PD, Novakovic RL, Giles BP, Miller SL, Riegel MS. Spinal cord hypothermia without systemic hypothermia. AJNR American journal of neuroradiology. 2013;34:252–6. doi: 10.3174/ajnr.A3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani Y, Terao Y, Ohta H. Molecular mechanisms underlying hypothermia-induced neuroprotection. Stroke research and treatment. 2010;2011:809874. doi: 10.4061/2011/809874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharpe AM, Sladen DP. Causation of permanent unilateral and mild bilateral hearing loss in children. Trends in amplification. 2008;12:17–25. doi: 10.1177/1084713807313085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzani O, Boutin H, Chuquet J, Rothwell N. Potential mechanisms of interleukin-1 involvement in cerebral ischaemia. Journal of neuroimmunology. 1999;100:203–15. doi: 10.1016/s0165-5728(99)00202-7. [DOI] [PubMed] [Google Scholar]
- Tzen YT, Brienza DM, Karg PE, Loughlin PJ. Effectiveness of local cooling for enhancing tissue ischemia tolerance in people with spinal cord injury. The journal of spinal cord medicine. 2013;36:357–64. doi: 10.1179/2045772312Y.0000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Water TR, Abi Hachem RN, Dinh CT, Bas E, Haake SM, Hoosien G, Vivero R, Chan S, He J, Eshraghi AA, Angeli SI, Telischi FF, Balkany TJ. Conservation of hearing and protection of auditory hair cells against trauma-induced losses by local dexamethasone therapy: molecular and genetic mechanisms. Cochlear implants international. 2010;11(Suppl 1):42–55. doi: 10.1179/146701010X12671178390834. [DOI] [PubMed] [Google Scholar]
- Vivero RJ, Joseph DE, Angeli S, He J, Chen S, Eshraghi AA, Balkany TJ, Van de Water TR. Dexamethasone base conserves hearing from electrode trauma-induced hearing loss. The Laryngoscope. 2008;118:2028–35. doi: 10.1097/MLG.0b013e31818173ec. [DOI] [PubMed] [Google Scholar]
- Wardrop P, Whinney D, Rebscher SJ, Roland JT, Jr, Luxford W, Leake PA. A temporal bone study of insertion trauma and intracochlear position of cochlear implant electrodes. I: Comparison of Nucleus banded and Nucleus Contour electrodes. Hearing research. 2005;203:54–67. doi: 10.1016/j.heares.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Watanabe F, Koga K, Hakuba N, Gyo K. Hypothermia prevents hearing loss and progressive hair cell loss after transient cochlear ischemia in gerbils. Neuroscience. 2001;102:639–45. doi: 10.1016/s0306-4522(00)00510-8. [DOI] [PubMed] [Google Scholar]
- Yanamoto H, Nagata I, Nakahara I, Tohnai N, Zhang Z, Kikuchi H. Combination of intraischemic and postischemic hypothermia provides potent and persistent neuroprotection against temporary focal ischemia in rats. Stroke; a journal of cerebral circulation. 1999;30:2720–6. doi: 10.1161/01.str.30.12.2720. discussion 2726. [DOI] [PubMed] [Google Scholar]
- Yokobori S, Frantzen J, Bullock R, Gajavelli S, Burks S, Bramlett H, Dietrich WD. The Use of Hypothermia Therapy in Traumatic Ischemic / Reperfusional Brain Injury: Review of the Literatures. Therapeutic hypothermia and temperature management. 2011;1:185–192. doi: 10.1089/ther.2011.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Xu G, Zhang J, Murong S, Mei Y, Tong E. Mild hypothermia reduces ischemic neuron death via altering the expression of p53 and bcl-2. Neurological research. 2010;32:384–9. doi: 10.1179/016164110X12670144526228. [DOI] [PubMed] [Google Scholar]