Abstract
The high prevalence of noise-induced and age-related hearing loss in the general population has warranted the use of animal models to study the etiology of these pathologies. Quick and accurate auditory threshold determination is a prerequisite for experimental manipulations targeting hearing loss in animal models. The standard auditory brainstem response (ABR) measurement is fairly quick and translational across species, but is limited by the need for anesthesia and a lack of perceptual assessment. The goal of this study was to develop a new method of hearing assessment utilizing prepulse inhibition (PPI) of the acoustic startle reflex, a commonly used tool that measures detection thresholds in awake animals, and can be performed on multiple animals simultaneously. We found that in control mice PPI audiometric functions are similar to both ABR and traditional operant conditioning audiograms. The hearing thresholds assessed with PPI audiometry in sound exposed mice were also similar to those detected by ABR thresholds one day after exposure. However, three months after exposure PPI threshold shifts were still evident at and near the frequency of exposure whereas ABR thresholds recovered to the pre-exposed level. In contrast, PPI audiometry and ABR wave one amplitudes detected similar losses. PPI audiometry provides a high throughput automated behavioral screening tool of hearing in awake animals. Overall, PPI audiometry and ABR assessments of the auditory system are robust techniques with distinct advantages and limitations, which when combined, can provide ample information about the functionality of the auditory system.
Keywords: mouse, sound exposure, audiometric functions, hearing loss, temporary threshold shift, permanent threshold shift
1. Introduction
Quick and accurate assessment of auditory thresholds is a prerequisite for experimental manipulations in the field of auditory research. To date, a variety of protocols have been used to determine audiometric thresholds. Auditory brainstem responses (review by Stapells & Oates, 1997), behavioral audiograms (Heffner & Masterson, 1980; Radizwon et al., 2009), and startle reflex audiometry (Young & Fetchter, 1983; Walter et al., 2012) have each been used to assess hearing, yet each has limitations which should be taken into account when interpreting data related to threshold shifts and overall cochlear damage following sound or chemical lesions.
Perhaps the most ubiquitous test used to assess hearing performance is the auditory brainstem response. Rapid assessment of auditory brainstem circuitry makes ABR a good candidate for detecting gross changes in the auditory system. It is known that following an auditory insult, ABRs reliably identify elevated thresholds. This temporary threshold shift is thought to result from swelling of cochlear nerve terminals which is present for days after exposure. When measured with ABRs, thresholds return to baseline soon after noise exposure (Robertson, 1983). However, recent work has clearly demonstrated that this measure does not account for the trauma-induced damage to ribbon synapses (Kujawa & Liberman, 2009). ABR wave one amplitudes have been shown to more accurately represent suprathreshold hearing loss, as they correlate strongly with ribbon synapse denervation following sound exposure (Liberman & Liberman, 2015). However, the ABR methodology is not without some caveats. Immediately following sound exposure, ABR thresholds are often elevated past the point of detection where they cannot be accurately measured which also precludes wave one amplitudes from being assesed. Furthermore, ABR’s are typically collected under anesthesia and are challenging to measure in animals with larger body mass (Chambers et al., 2012; Cederholm et al., 2012). Lastly, some have contended that while ABRs thresholds are useful in detecting noise-induced damage to the auditory brainstem, they do not provide any perceptual indications of hearing loss (Davis, 1984). Alternatively, others have stated that ABRs can closely approximate behavioral thresholds in humans, yet ABRs are known to be less precise (Stapells 2011). For these reasons, it is useful to explore alternatives for hearing assessment.
Many years ago it was found that prepulse modulation of the acoustic startle reflex (ASR) could be employed to assess behavioral response thresholds (Fechter et al., 1988). Prepulse inhibition, a decrease of ASR magnitude when a preceding weaker sound (prepulse) is presented before the startle, has been used for over half a century to objectively measure complex neurological systems (Hoffman & Searle, 1965; Hoffman & Wible, 1970; Graham, 1975; Gerrard & Ison, 1990). Much like behavioral audiograms collected by operant conditioning methods (Heffner & Masterson, 1980; Radizwon et al., 2009), the prepulse was varied in intensity and frequency to differentially modulate the startle response. This method has successfully identified behavioral correlates of cochlear damage due to ototoxic drugs (Young & Fetchter, 1983) and temporary threshold shifts due to pure tone acoustic exposure (Walter et al., 2012). Advantages of this approach for assessing hearing thresholds are numerous. First, the measure is based on a reflex and does not require experience in animal behavior and months of animal training as in other commonly used behavioral paradigms. Second, in contrast to the ABR approach, PPI audiometric functions can be collected in awake animals, avoiding confounds of anesthesia. Finally, a key advantage is that many animals can be tested at once with a short preparation, allowing for high data throughput, and timely data collections at various experimental conditions.
Although PPI audiometry has several advantages over other currently used hearing assessment methodologies, several important questions need to be explored before it can be widely applied. First, it is still unknown whether it is sensitive enough to assess hearing in individual animals, which would be much more beneficial than group averages. Second, the extent of PPI threshold reliability from day to day is also unknown. Third, it is important to know whether PPI audiometry can be used to detect permanent threshold shifts caused by the most common hearing insult, noise-induced hearing loss. The goal of this study was to address these questions and compare PPI audiometry with the traditional ABR approach.
2. Methods
2.1. Animals
A total of 16 male CBA/CaJ mice obtained from Jackson Laboratories were used. To avoid startle variability which is known to result from hormone fluctuations of the estrous cycle, female mice were not used in this study (Plappert et al. 2005; Ison & Allen, 2007). This phenomenon has also been shown in human subjects (Kumari et al., 2004). Mice were 12 weeks old at the beginning of the experimental procedures. They were housed in pairs within a colony room with a 12-h light–dark cycle at 25°C.
Ten mice were sound exposed as described below while six unexposed mice were used as controls. The exposed mice (depicted throughout all figures in color: Blue: unexposed, Purple: one day after exposure, Orange: three months after exposure) were tested during a 3 month period to detect permanent threshold shifts. The 6 control mice were used to test for the consistency of PPI measurements across time. Procedures used in this study were approved by the Institutional Animal Care and Use Committee at the Northeast Ohio Medical University.
2.2. Acoustic trauma
Mice were anesthetized with an intramuscular injection of a ketamine/xylazine mixture (100/10 mg/kg). An additional injection (50% of the initial dose) was given 30 min after the initial injection. Mice were unilaterally exposed to a one octave narrow-band noise centered at 12.5 kHz (~8–17 kHz). This noise was generated using a waveform generator (Tektronix AFG 3021B), amplified (QSC RMX 2450) to 116 dB SPL, and played through a speaker (Fostex T925A Horn Tweeter). The output of the loudspeaker was calibrated with a 0.25-in. microphone (Brüel and Kjaer 4135) attached to a measuring amplifier (Brüel and Kjaer 2525) and found to be ±4 dB between 4 and 60 kHz. During exposure the speaker was located ~5 cm from the animal’s right ear. The left external ear canal was obstructed with a cotton plug and a Kwik-Sil silicone elastomer plug (World Precision Instruments), a manipulation which typically reduces sound levels by 30 to 50 dB SPL (Turner et al., 2006; Ropp et al., 2014).
2.3. Auditory Brainstem Response Testing
Mice were anesthetized with ketamine/xylazine as during the acoustic trauma. Sterile, stainless-steel recording electrodes (connected to a Tucker Davis Technologies (TDT) RA4LI Low Impedance Headstage) were placed subdermally, one behind each pinna with the reference electrode along the vertex. Tone bursts at 4, 12.5, 16, 20, 25, and 31.5 kHz were presented at increasing sound intensities ranging from 10 to 80 dB SPL in 10 dB steps. Tones were 5 ms duration, 0.5 ms rise/fall time and delivered at the rate of 50/s. ABRs were averaged over 300 repetitions. These waveforms were amplified (TDT RA4PA Medusa Preamplifier), digitized (TDT RZ6 Multi-I/O Processor), and analyzed offline using a customized program within OpenEx Software (TDT). Thresholds, the smallest sound amplitude that evoked a visible ABR, were determined by visually examining the ABR waveforms in response to every sound frequency presented at different sound levels. ABR wave one amplitudes (μV peak to peak) were measured at each intensity/frequency combination for all exposed mice at all time points tested (prior to exposure (control), one day after exposure, and three months after exposure).
2.4.1 Acoustic Startle Hardware/Software
The equipment used to collect all acoustic startle data has been described in detail previously (Longenecker & Galazyuk, 2012). Commercial hardware/software equipment from Kinder Scientific, Inc. was used in behavioral experiments. Each behavioral testing station was lined with anechoic foam to prevent sound reflection and wave cancelling sound echoes (Sonex foam from Pinta Acoustics). A small customization of the hardware’s startle stimulus system was made by adding SLA-4 (ART) power amplifiers to adjust sound levels to correct for variations in speaker loudness between testing station. Mice restrainers were open walled to allow for maximum sound penetration (Fig. 3 in Longenecker & Galazyuk, 2012). Background sound levels within each testing chamber were calibrated with a 0.25-in. microphone (Brüel and Kjaer 4135) attached to a measuring amplifier (Brüel and Kjaer 2525) and found to be less than 40 dB SPL between 4 and 60 kHz. Startle waveforms were recorded using load cell platforms which measure actual force changes during an animal’s jump. Each load cell was calibrated with a 100g weight which corresponds to 1 newton of force. Offline waveform analysis converted these forces into center of mass displacement (in mm) (Grimsley et al., 2015).
2.4.2. Reflex modification audiometry: Prepulse detection testing
Testing sessions contained two types of stimuli. First, a startle stimulus (wide-band noise, 100dB SPL, 20ms in duration, 1ms rise/fall) was presented alone; this is referred to in the text as startle only (SO) (Fig 1A). The second stimulus type consisted of a congruent startle stimulus preceded by a prepulse (Fig 1A). Prepulse stimuli were 20 ms pure tones with a 1 ms rise/fall time presented at six different frequencies (4, 12.5, 16, 20, 25, and 31.5 kHz) 100 ms before the startle stimulus. These prepulses were played in a pseudo-randomized range of intensities (10–80dB SPL, 10 dB step) for a given sound frequency. Each frequency/intensity combination was presented 39 times. SOs were pseudo-randomly mixed throughout each testing session. In preliminary experiments, inter-trial intervals (ITIs) were randomized between 15 and 25 seconds. With this ITI, a complete testing period (1872 prepulse trials, and 468 startle alone trials) required 15 hours with a 1 hour break for the animal to rest between hour 7 and 8. In order to shorten the testing period to reduce stress on the animals and to allow similar time constraints as ABR testing, we tested animals at short ITIs of 4–6 s. In this condition, a complete testing session, in which 8 mice could be tested simultaneously, lasted roughly 1.5 hours. Interestingly, when the same mice were tested in both ITI conditions, response thresholds were lower when the shorter ITIs were used (Fig. 2). Following this experiment, all remaining PPI testing was completed at 4–6s ITIs. Startle-only responses were closely monitored throughout testing sessions to ensure that startle magnitudes were not significantly affected by habituation as described in detail below. Additionally, it has been shown that unlike rats (review by Koch, 1999), many strains of mice show little to no reduction in their acoustic startle reflex magnitude throughout testing sessions (Bullock et al., 1997; Ison, 2001). This is an important factor when assessing prepulse inhibition of the acoustic startle reflex across sessions, and experimental conditions.
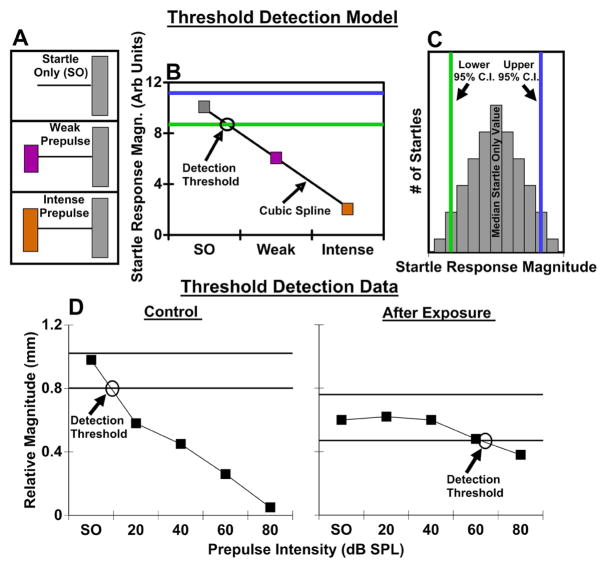
Figure 1.
Determining detection thresholds for PPI audiometry. A. Schematic of stimuli presented, as described in Methods. B. Detection function. For each frequency, a cubic spline was fitted to medians of the square-root-transformed response amplitudes for SO and each intensity prepulse. Detection threshold was defined as the sound level where this function intersected the 95% lower confidence interval of a bootstrapped Startle Only response distribution (C). C. Calculations of Startle Only 95% confidence intervals. A Startle Only response amplitude distribution was created by bootstrapping square-root-transformed response amplitudes. The lower 95% confidence interval was used for detection threshold identification (in B). D. Threshold detection data exemplar. Representative audiometric functions (tested at 12.5 kHz) from an individual mouse before and after exposure. In the control condition the PPI function is both steep and monotonic with the detection threshold located at 9.4 dB SPL. In the exposed condition PPI functions tend to become shallower and non-monotonic, leading to a higher detection threshold at 64.3 dB SPL.
Figure 2.
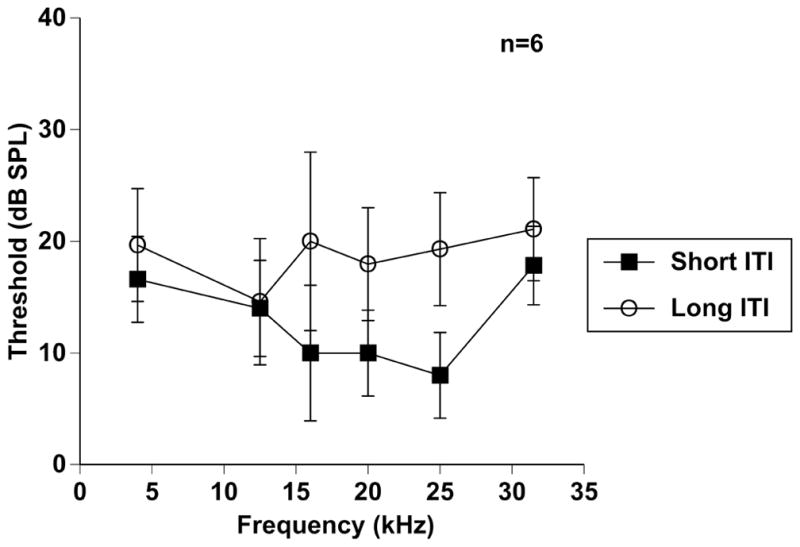
Short inter-trial interval (ITIs) are effective in PPI sessions. PPI functions across frequencies (4–31.5 kHz) in six mice tested with short (4–6 s) and long (15–25 s) ITIs. Error bars show SEMs.
2.4.3. Reflex modification audiometry: Startle waveform identification and measure
All waveforms collected during testing sessions were analyzed offline using a recently developed automatic method of startle waveform identification via a template matching paradigm (Grimsley et al., 2015). In this recent study we used high-speed video recordings (1,000 frames/s) to visualize animal startles in order to identify stereotyped waveforms associated with a startle. This allowed us to develop custom software which automatically separates data into either startles or non-startle-related movements. Based on this separation, we have only included trials that resulted in successful startle responses in our data analysis. We also used a mathematical approach to normalize startle response magnitudes of individual animals to their body mass (Grimsley et al., 2015). This mathematical conversion normalizes for mass, allowing legitimate comparisons between animals of different mass.
2.4.4. Reflex modification audiometry: Prepulse detection threshold identification
In each session, the magnitudes of the SOs were compared to the magnitudes of startles preceded by prepulses with various frequency and intensity (Fig 1A). A significant reduction in the magnitude of the startle response by the prepulse compared to the SO was defined as the prepulse detection threshold (Fig 1B). Identifying this threshold involved several steps. First, we examined the distribution of the raw startle magnitudes. Startle magnitude is known to be quite variable in mice, and indeed the startle magnitude typically was strongly positively skewed. This positive skew could allow aberrantly high values to obscure reliable changes in startle magnitude across stimulus parameters. Furthermore, a normal distribution of magnitudes is an assumption underlying the method for threshold determination that we use here. Thus we transformed the data (Tukey, 1977). A square root transform was found to be the best at generating a normal distribution of startle responses within each trial type, as assessed using the Anderson-Darling test. This standard statistical transform reduces skew by enhancing the lesser and reducing the larger startle magnitudes. Second, for each animal, the transformed SO data was bootstrapped to determine 95% confidence intervals for the SO response magnitudes (Fig 1C). Then we calculated medians of transformed magnitudes for each frequency and intensity, as the median value is a better measure of central tendency than the mean for skewed distributions. For each frequency, a detection function was calculated by fitting a cubic spline from the median transformed SO magnitude through the median transformed magnitudes for prepulses presented at various intensities. Detection threshold was defined as the sound level at which the fitted detection function crossed the lower 95% confidence interval (Fig 1B). The detection threshold increases after sound exposure because the slope of the detection curve becomes “flatter,” likely due to hearing loss (Fig 1D).
2.5. Statistical analyses
Both ABR threshold data and PPI thresholds were evaluated with a repeated measures design because the same animals were used in each condition. Different frequencies (6) and conditions (3) were used as independent variables in the repeated measures analysis of variance (ANOVA). Planned comparisons between specific frequency/intensity combinations were assessed with Fisher’s Least Significant Difference (LSD) post-hoc tests. Linear regressions were used to evaluate correlations between PPI and ABR data. Values throughout the manuscript are specified as means or medians ± standard error of the mean (SEM). We used an alpha level of .05 for all statistical tests. P values significant at the .05 level are indicated by one symbol while those significant at the .001 level or lower are indicated by two symbols. Due to the complexity of some figures, various symbols have been used to represent significant differences: * = significant difference between the control condition and one day after exposure; # = significant difference between the control condition and three months after exposure; @ = significant difference between one day after exposure and three months after exposure.
3. Results
3.1. Consistency of PPI audiometry
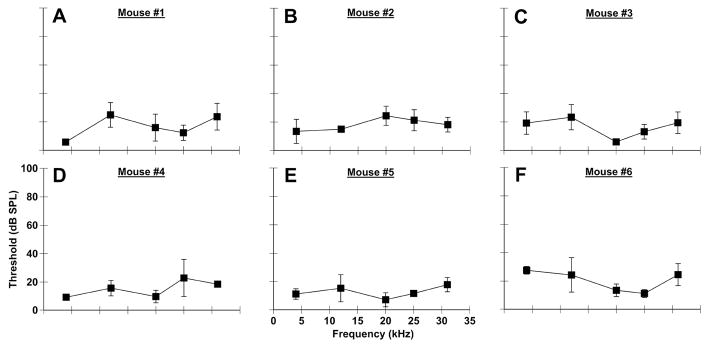
Accuracy and precision are crucial for any methodology that is assessing hearing loss. As such, we tested whether thresholds identified with PPI audiometry were comparable to thresholds determined by behavioral audiograms (Heffner & Masterson, 1980; Radizwon et al., 2009). We also assessed both the variation between mice as well as the variation within individual mice across multiple testing sessions. On three consecutive days of testing, thresholds at all frequencies tested (4, 12.5, 20, 25, 31.5 kHz) were below 40 dB SPL with inter-animal differences of usually less than 10 dB (Fig. 3). Importantly, these data confirmed results from a previous prepulse modulation study conducted on humans (Reiter, 1981). The variations in PPI thresholds for individual mice across days of testing and different frequencies did not exceed 5.77 dB (Fig. 3A–F). Interestingly, the Reiter study found that audiograms collected by the startle reflex are more “flattened” across frequency in comparison to more traditional audiograms, which is confirmed by our data in mice (Fig. 3, 4A–C control).
Figure 3.
PPI audiometric functions are consistent across time and subjects. PPI audiometric functions from six mice (A–F) over 3 days of testing. Average thresholds were different based on the particular mouse and frequency tested. However, the variation between all conditions was only 5.77 dB (SEM), which suggests low threshold variability.
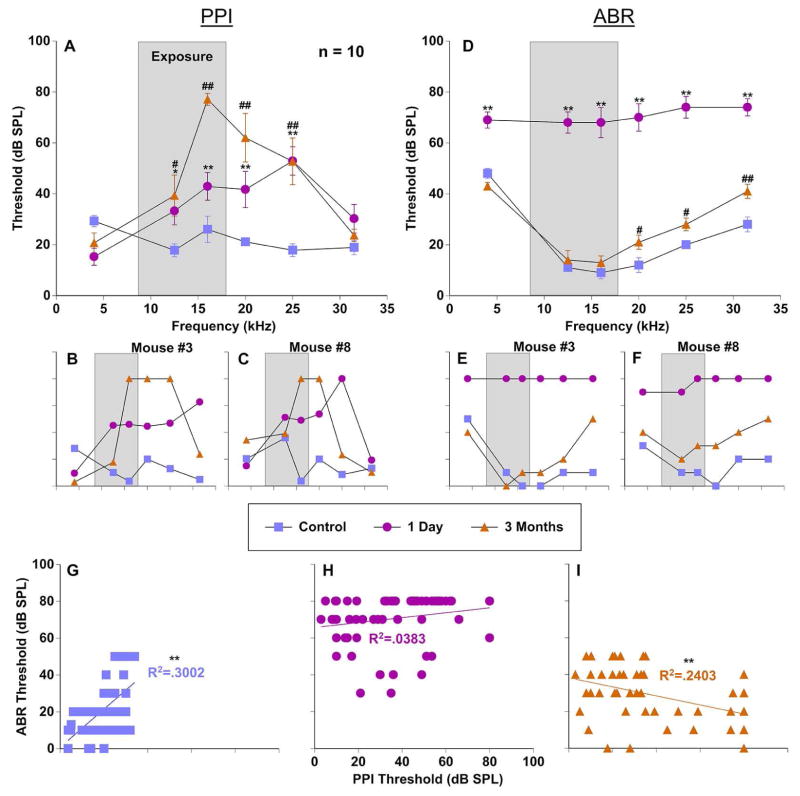
Figure 4.
Comparison of PPI and ABR audiometric thresholds in the assessment of sound exposure. Thresholds from a group of 10 mice were recorded before (control; blue), 1 day after (pink), and 3 months after exposure (orange). The narrow band exposure stimulus is represented by a grey box. Error bars show SEMs. A: Group PPI threshold averages as a function of frequency (4–31.5 kHz). B–C: PPI thresholds for two individual mice. D: Group ABR threshold averages as a function of frequency (4–31.5 kHz). E–F: ABR thresholds for two individual mice. G–I: Correlations between PPI and ABR thresholds from 10 mice in each condition. * = significant difference between the control condition and one day after exposure; # = significant difference between the control condition and three months after exposure. * = significant difference between the control condition and one day after exposure; # = significant difference between the control condition and three months after exposure; @ = significant difference between one day after exposure and three months after exposure.
3.2. Comparing PPI and ABR audiometric thresholds for hearing assessment
To determine if PPI audiometry is sensitive to both temporary and permanent threshold shifts we exposed 10 mice to a one octave narrowband noise centered at 12.5 kHz and presented at 116 dB SPL for 1 hour. Immediately after exposure, PPI thresholds were dramatically increased which resulted from a “flatter” cubic spline function in comparison to the “steep” monotonic function in the control condition, thus contributing to increased detection thresholds (Fig. 1D).
The effect of noise-induced trauma on hearing was evaluated with PPI and ABR thresholds collected before, one day, and three months after exposure. A repeated measures ANOVA found that both PPI and ABR thresholds were significantly elevated one day (PPI: F(1,53) = 75.8, p < .001; ABR: F(1,53) = 18.3, p < .001) and three months following exposure (Fig. 4A, D). Least Significant Difference posthoc pairwise comparisons checking for frequency specific differences found significant PPI threshold elevations at 12.5, 16, 20, and 25 kHz one day after exposure (Fig. 4A) (12.5 kHz: p < .05; 16, 20, 25 kHz: p < .001). ABR thresholds at almost all frequencies were maximally elevated one day after exposure (Fig. 4D) (4–31.5 kHz: p < .001). Interestingly, at the three month time point PPI threshold differences remained elevated at 12.5 and 25 kHz (12.5 kHz: p < .05; 25 kHz: p < .001) yet were further elevated at 16 and 20 kHz (16–20 kHz: p < .001), the frequencies directly surrounding the spectral characteristics of the acoustic trauma (Fig. 4A). However, ABR thresholds nearly recovered to baseline levels for most frequencies at three months, while some of them remained statistically elevated (Fig. 4D) (20–25 kHz: p < .05; 31.5 kHz: p < .001). Representative examples of PPI (Fig. 4B, C) and ABR (Fig. 4E, F) audiometric functions from two individual mice show typical patterns of damage. One day after exposure both mice demonstrated broad temporary threshold shifts. Mouse #3 and #8 showed PPI deficits in the range of 12.5 to 31.5 kHz and 12.5 to 25 kHz, respectively (Fig. 4B, C). These deficits roughly correspond to the non-specific frequency deficits which were observed with ABRs (Fig. 4E, F). At three months after exposure the results of hearing threshold assessments for both measurements were dramatically different. While PPI assessment showed narrow frequency specific deficits at the high edge of the range of exposure, ABR thresholds returned to near pre-exposed levels (Fig. 4D). Interestingly, despite nearly identical sound exposure, the individual mice exhibited deficits at slightly different frequency ranges (compare Fig. 4B and C).
The relationship between PPI and ABR thresholds was directly compared with a linear regression for each condition. In the control condition (pre-exposure) PPI and ABR thresholds had a significant weakly positive correlation (R2 = 0.3 (F(1,57) = 24.4, p < .001)) (Fig. 4G). This suggests that these measures can be compared with some confidence in normal unexposed animals. However, one day after exposure this correlation disappeared (R2 = 0.038) (Fig. 4H), as might be expected by such drastic changes in ABR thresholds and only moderate changes in PPI thresholds. Interestingly, a weak negative correlation (R2 = 0.24 (F(1,57)= 18, p < .001)) was seen at three months after exposure due to the fact that significantly elevated PPI deficits were centered around the exposure frequencies whereas ABR thresholds recovered nearly to baseline levels (Fig. 4I).
3.3. Comparing PPI and ABR amplitudes for hearing assessment
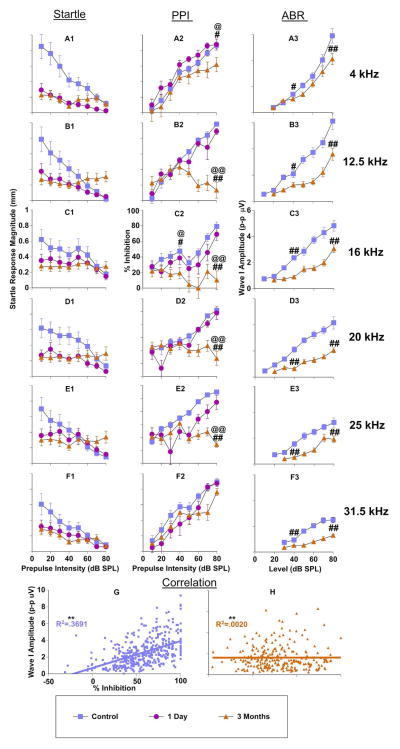
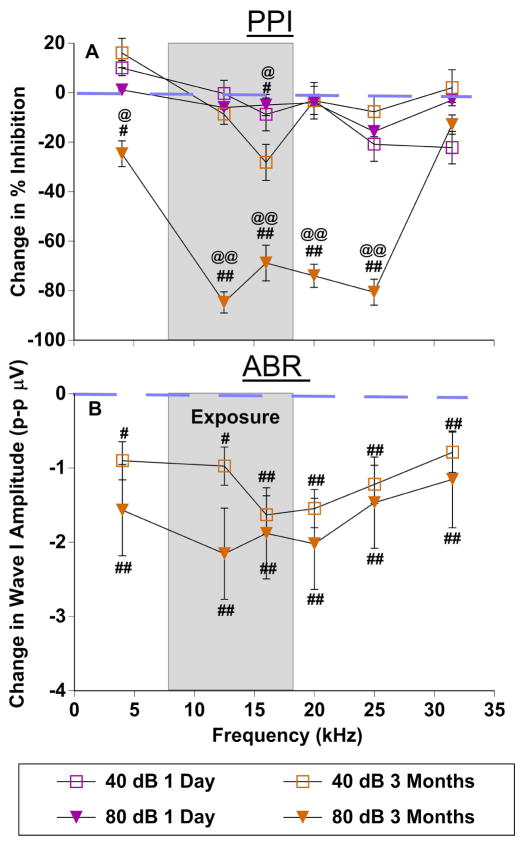
Recent studies have promoted the importance of examining ABR amplitudes instead of thresholds when evaluating noise induced hearing loss because of the strong link to the loss of suprathreshold auditory nerve fibers which are the most vulnerable during acoustic trauma (Kujawa & Liberman, 2009; Lin et al., 2011). For this reason we compared prepulse modulated startle magnitudes and the resulting inhibition (PPI) with wave one ABR amplitudes (Fig. 5) in the same mice that were tested for thresholds (Fig. 4). After exposure, a repeated measures ANOVA showed that prepulse modulated startle magnitudes were reduced significantly across all frequencies tested (4kHz: F(1,64) = 79.7, p < .001; 12.5kHz: F(1,64) = 29.9, p < .001; 16kHz: F(1,64) = 18.6, p < .001; 20kHz: F(1,64) = 41.2, p < .001; 25kHz: F(1,64) = 6.7, p < .05; 31.5kHz: F(1,64) = 25.7, p < .001) (Fig. 5A1-F1, pink 1 day post-exposure compared with blue, pre-exposure). The differences were most pronounced for low intensity prepulses, when the startle stimulus was inhibited the least. This pattern for prepulse modulated startle magnitudes changed somewhat at three months, in that for most frequencies tested, the audiometric function flattened due to a decrease in startle magnitude at low intensity prepulse conditions, but an increase of startle magnitudes at high prepulse intensities (Fig. 1D; Fig. 5A1-F1 orange). This might be explained by decreased startle values as shown later (Fig. 7). The prepulse inhibition for each prepulse modulated startle magnitude was calculated by dividing the SO trials by each prepulse trial. The resulting % inhibition curves show clear frequency-specific deficits most prominently expressed between 12.5 and 25 kHz only three months after exposure (Fig. 5A2-F2) (4kHz: F(1,64) = 34.7, p < .001; 12.5kHz: F(1,64) = 52.93, p < .001; 16kHz: F(1,64) = 58.68, p < .001; 20kHz: F(1,64) = 612.84, p < .001; 25kHz: F(1,64) = 433.773, p < .001. Interestingly, these inhibition changes were mostly observed with high intensity prepulses, while low intensity prepulses remained mostly intact (this is examined further below). The notable exception to this pattern was seen with inhibition at 16 kHz, in which low intensity prepulses also resulted in reduced inhibition from control levels (Fig. 5C2). In conjunction with prepulse modulated startle magnitudes and PPI changes, after exposure, a repeated measures ANOVA showed that ABR amplitudes were reduced significantly across all frequencies (Fig. 5A3-F3, orange 3 month post-exposure compared with blue, pre-exposure) (4kHz: F(1,72) = 23.8, p < .001; 12.5kHz: F(1,72) = 88.9, p < .001; 16kHz: F(1,72) = 104.8, p < .001; 20kHz: F(1,72) = 169.7, p < .001; 25kHz: F(1,72) = 82.8, p < .05; 31.5kHz: F(1,72) = 233.6, p < .001). It is important to mention that wave one ABR amplitudes were not measured one day after exposure because no ABR response was present at that time, likely due to a very strong temporary threshold shift.
Figure 5.
Comparison of prepulse modulated startle responses and resulting inhibition to ABR wave one amplitudes in the assessment of sound exposure. Measures from a group of 10 mice were recorded before (control; blue), 1 day after (pink), and 3 months after exposure (orange). Startle (A1-F1): Prepulse modulated startle response magnitudes as a function of prepulse intensity, with each panel representing data from one pure tone frequency (4 kHz A1 – 31.5 kHz F1). PPI (A2-F2): Resulting inhibition curves from the prepulse modulated startle responses in Startle. Inhibition was calculated as Startle Only (SO) divided by prepulse+startle for each stimulus frequency/intensity combination. ABR (A3-F3): ABR wave one amplitudes (peak to peak) as a function of stimulus level, with each panel representing data from one pure tone frequency (4 kHz A2 – 31.5 kHz F2). G–H: Correlations between PPI and ABR amplitudes from 10 mice in each condition. * = significant difference between the control condition and one day after exposure; # = significant difference between the control condition and three months after exposure; @ = significant difference between one day after exposure and three months after exposure.
Figure 7.
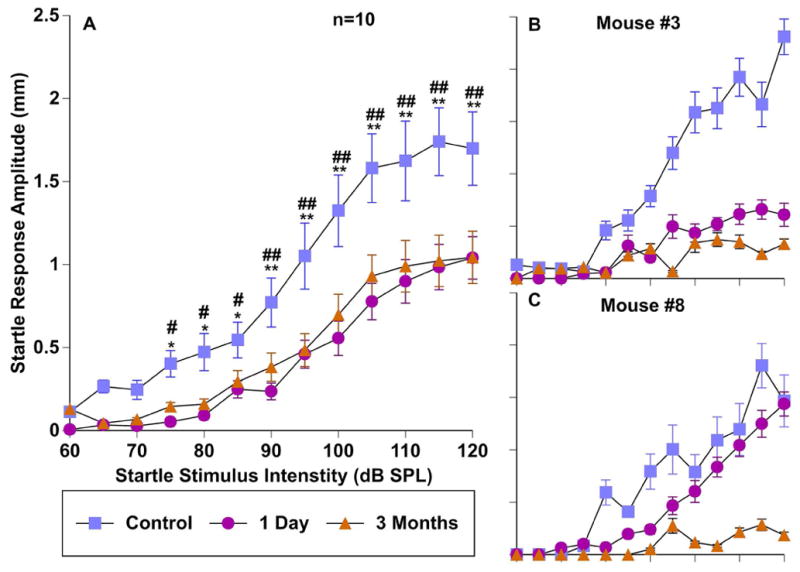
Effect of sound exposure on startle response magnitudes. Startle responses across a range of startle intensities were measured before (control; blue), 1 day after (pink), and 3 months after exposure (orange). A: Group input/output function averages of 10 mice. B–C: input/output functions from two individual mice. Error bars show SEMs. * = significant difference between the control condition and one day after exposure; # = significant difference between the control condition and three months after exposure.
Interestingly, when PPI and ABR wave one amplitude deficits were plotted across frequencies as a function of difference from control amplitudes, unique patterns of damage were observed (PPI Fig. 5A2-F2, ABR wave one amplitudes Fig. 5A3-F3, compared in Fig. 6A, B respectively). Planned comparisons between testing epochs at 40 and 80 dB prepulse levels were assessed with LSD post hoc tests, for both ABR amplitudes and PPI. These intensities were chosen to test the contribution between high and low threshold auditory nerve fibers. ABR wave one amplitude deficits were centered at or around the upper end of the exposure frequency range, with 80 dB SPL wave one amplitudes significantly more affected by exposure than 40 dB SPL amplitudes (F(1,53) = 75.3, p < .001) (Fig. 6B). This is consistent with decreased synapse density for high threshold nerve fibers. PPI deficits are shown in Figure 6A. One day after exposure (Fig. 6A purple), only minor differences in prepulse inhibition were observed in response to low (40 dB SPL) or high intensity prepulse stimuli (80 dB SPL). However, three months after exposure (Fig. 6A orange), the 40 dB SPL prepulse modulation was nearly identical to the one day after exposure, whereas the high intensity 80 dB SPL prepulse showed large magnitude increases (Fig. 6A orange square vs triangles), again suggesting severe hearing loss as a result of decreased synapse density from high threshold nerve fibers.
Figure 6.
Frequency specific deficits in PPI and ABR wave one amplitudes after sound exposure. A: Change of PPI after exposure (values from 40 and 80 dB SPL prepulse levels Fig. 5A2-F2). B: Change of ABR wave one amplitudes (values from 40 and 80 dB SPL stimulus levels Fig. 5A3-F3). The narrow band exposure stimulus is represented by a grey box. The dashed line represents no change from control condition. Error bars show SEMs. * = significant difference between the control condition and one day after exposure; # = significant difference between the control condition and three months after exposure.
The relationship between PPI and ABR wave one amplitudes was directly compared with a linear regression for each condition. A very similar pattern was seen between PPI/ABR amplitude correlations (Fig. 5G, 5H) and threshold correlations (Fig. 4G, I). In the control condition (unexposed) PPI and ABR amplitudes had a significant weakly positive correlation (R2 = 0.3691 (F(1,478) = 279.81, p < .001)) (Fig. 5G). Generally, increasing inhibition (as a result of increasing prepulse intensities) followed a pattern similar to ABR wave one amplitudes at corresponding stimulus intensities. However, three months after exposure this correlation (R2 = 0.0200), was weakened substantially (F(1,478) = 0.930, p = .335) (Fig. 5H). This can be explained by the fact that inhibition to low intensity prepulses at the three month time point generally followed control levels of inhibition, while inhibition to high intensity prepulses was reduced markedly as described above. Overall, the amplitude assessments between PPI and ABR tests differentially correlate based on condition, but are generally predictive of one another in control conditions.
3.4. Effects of sound exposure on startle magnitude and probability
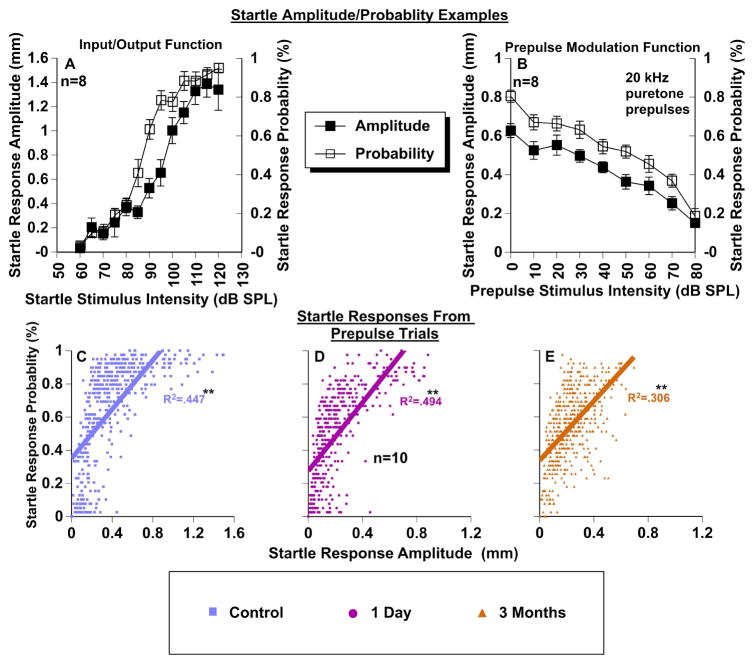
The magnitude of the startle response is often decreased either after sound exposure (Longenecker & Galazyuk, 2011; Longenecker et al., 2014; Lobarinas et al., 2013) or due to habituation. Both may confound experimental findings by causing a “floor effect”. To test for this possibility, startle-only input/output functions were collected before, one day after, and three months after sound exposure for the group of ten exposed mice (Fig. 7A). Since our PPI testing used a 100 dB SPL startle stimulus intensity, we wanted to determine if this startle intensity could evoke a sufficiently strong startle response to ensure that it could still be inhibited by a prepulse. Although the startle response amplitude was significantly lowered one day (F(1,104) = 172.9, p < .001), and three months (F(1,117) = 97.7, p < .001) after exposure, the startle magnitude remained more than 2 standard deviations above baseline of animal background movement, a concept we previously introduced (Longenecker & Galazyuk, 2012; Fig. 7A). Importantly, the damage due to sound exposure reduced the startle reflex to varying degrees in each mouse, which is important when considering both ABR related pathologies and prepulse inhibition (Fig. 7B, C). This can effectively reduce the dynamic range of inhibition and should be monitored closely in each animal. An example of this dynamic range change is shown in Figure 1D.
To further ensure that startle responses are properly assessed, our automatic startle waveform identification software provided us with an assurance that only true startle responses were included in our PPI data analysis (Grimsley et al., 2015). This technology is especially important for separating small startle waveforms from non-stimulus-related movements. The percentage of non-startles is stable across the session. The startle probability is directly correlated with the startle magnitude, so if the startle magnitude is stable (lack of habituation), then the startle probability will also remain constant. As mentioned above, each stimulus (prepulse level or startle only) was presented 39 times per session. We found that the startle response magnitude changes that occur within input/output functions (Fig. 8A), or within prepulse modulation functions (Fig. 8B) are closely correlated to startle probability both in the control (R2 = 0.447 (F(1,478) = 387, p < .001)) and in exposed animals at one day (R2 = 0.494 (F(1,430) = 421, p < .001)) or three months (R2 = 0.306 (F(1,478) = 213, p < .001)) post exposure (Fig. 8C–E). This result is very robust across sessions, animals, and conditions. In Figure 8A the startle probability elicited from a 110 dB SPL was about 80%. That implies that 20% of the trials were excluded because they were not determined to be startles by our waveform classifier system. This tool allowed us to not only accurately identify startle waveforms, but also to determine whether the startle response probability was constant or changing with prepulse parameters. This is most critical when the startle reflex is severely depressed as a byproduct of sound exposure, or when high intensity prepulses are presented.
Figure 8.
Relationship between startle response amplitudes and startle probability. A: Representative input/output startle-only function from one control mouse comparing startle response amplitudes (left Y axis) and corresponding startle response probabilities (right Y axis) as a function of startle stimulus intensity. B: Representative prepulse modulation function (20 kHz pure tone prepulse) from one control mouse comparing startle response amplitudes (left Y axis) and corresponding startle response probabilities (right Y axis) as a function of prepulse stimulus intensity. C–E: Correlations between startle response amplitudes and startle response probability for each condition measured with PPI audiometry (data from Fig. 6A1-F1). Note: x-axis scales are different to accommodate data because of net losses in startle response magnitudes after sound exposure. * = significant correlation.
Habituation to continually presented startle stimuli is also a concern during extended testing paradigms. To ensure habituation was not a significant confounding factor for the ASR in PPI, we monitored the startle magnitudes throughout the testing sessions. A Spearman correlation between startle magnitude and time of testing was run to identify whether magnitudes decreased during testing. The correlation was very small but significant (rs = .146, p < 0.001), suggesting that the startle response magnitudes were actually slightly elevated during recording sessions. This data is supported by previous findings that many strains of mice do not habituate to the acoustic startle reflex (Bullock et al. 1997). This has also been shown specifically for CBA/CaJ mice (Ison, 2001).
4. Discussion
We found that after several refinements, PPI audiometry was capable of measuring pre-pulse detection thresholds in mice much faster (90 minutes vs 9240 minutes) than previously shown in rats (Fechter et al., 1988). It requires roughly as much time as standard ABR measurements, but can be conducted on multiple animals simultaneously without any anesthesia. Spectrally broad temporary threshold shifts, resulting from sound exposure, can be detected by PPI audiometry and are similar to those detected by ABRs (Fig. 4A, D) and behavioral audiograms (Heffner et al., 2008; Heffner & Masterson, 1980; Radizwon et al., 2009). However, while high frequency permanent ABR threshold shifts were observed, lower frequency ABR thresholds recovered to baseline levels. This differed substantially from PPI thresholds which were elevated more broadly across frequencies and narrowed to substantial frequency-specific threshold shifts focused at the upper limit of the sound exposure frequencies. Importantly, PPI audiometry and ABR wave one amplitudes were both able to detect noise-induced hearing damage many months after sound exposure. Furthermore, PPI audiometry may reveal elements of higher-level plasticity in the auditory pathway, as discussed below. This suggests that each of these methods could be useful in assessing hearing related dysfunctions due to noise exposure, but might work better in conjunction, as they each are measuring different aspects of the auditory system.
4.1. What do ABRs and PPI audiometry measure?
The auditory brainstem response is by far the most popular methodology to assess the auditory system, as it can rapidly evaluate the functional connectivity of the auditory pathway. These recordings are able to identify gross malfunctions of the auditory system and are a useful tool for assessing the efficacy of sound trauma. The ABR is a non-invasive evoked potential usually conducted in anesthetized animals. It consists of five positive waves, each representing a synchronized neural output generated by auditory structures at and below the level of the inferior colliculus (Melcher & Kiang, 1996). This method allows for an in depth frequency specific synopsis of the cochlea and how it changes as a result of acoustic insults. A major advantage of this technique is that it can be conducted on humans, which allows comparison to animal models. Additionally, it can be done non-invasively and rather quickly, which allows screening of multiple animals in a short period of time. However, perhaps the biggest disadvantage of ABRs is the need for anesthesia in animal models which can alter the neural output in confounding ways (Chambers et al., 2012; Cederholm et al., 2012). Additionally, ABRs can become more difficult to collect in older/larger animals due to increased fat to muscle ratios (Zhou et al., 2006). Lastly, while it is known that ABRs can assess neural plastic changes that occur in the auditory brainstem (Skoe et al., 2013; Skoe et al., 2014), it is less certain if they directly measure neural computations above the level of the inferior colliculus. Sensorimotor gating experiments utilizing PPI of the acoustic startle reflex can also monitor neural plastic changes in these lower auditory structures but importantly, also have the ability to monitor cortical structures which are critically involved in modulating prepulse inhibition (Davis, 1984; Du et al., 2011). Thus, ABR audiometric functions provide some advantages, but may offer a more complete picture of the auditory system function if combined with a behavioral measure like PPI audiometry.
PPI audiometry is effective because the acoustic startle reflex (ASR) is greatly influenced by preceding stimuli (Hoffman & Searle, 1965; Buckland et al., 1969; Willott & Carlson, 1995). We have confirmed what was previously found, that prepulses presented at threshold levels can be used to assess an animal’s hearing by modulating the startle motor output (Fig. 4A–C; 5A2-F2) (Fechter et al., 1988). However, before PPI audiometry can be used routinely for hearing assessment in laboratory animals, it is important to have a clear understanding of what underlying circuitry is being assessed.
The neuronal circuitry underlying the ASR is straightforward and has been studied extensively (Yeomans & Frankland, 1996; Koch, 1999). The primary startle circuit includes the serial connections between the auditory nerve fibers, cochlear root neurons (in some species), and the nucleus reticularis pontis caudalis (PnC) region (Lee et al., 1996). The motor output is then sent to the interneurons of the spinal cord resulting in a startle. The simple relationship between evoked stimulus amplitude and the associated startle response magnitude (Fig. 7) and probability (Fig. 8) can be explained by the output of the nucleus reticularis pontis caudalis. Furthermore, it has been shown that if one ear is compromised (Longenecker & Galazyuk, 2011; Gómez-Nieto et al., 2014), that the startle magnitude is reduced, as a direct result of the neural response inputs to the PnC. Interestingly, our study found that startle response magnitude and probability are considerably well correlated, which suggests that even in low startle magnitude conditions, which can be observed in animals with hearing loss (Fig 8E), it is possible to include only valid startle data. This is important to consider when using PPI audiometry on sound-exposed animals. We controlled for the low startle confound in this study by using our advanced startle waveform analysis (Grimsley et al. 2015) and by monitoring the probability of startles in various conditions (Fig. 8C–E).
When prepulses are added to the ASR construct, the neural circuitry is far more complex (Swerdlow et al., 2001). The amount of inhibition that a prepulse has on the startle results from complicated neural computations that arise from various brain regions. The primary circuitry for mediating a prepulse’s effect on a startle resides below the level of the colliculi (Davis and Gendelman, 1977; Fox, 1979). These nuclei send GABAergic projections that terminate at the nucleus reticularis pontis caudalis (Fendt et al., 2001). Although this simple circuitry is directly responsible for generating the inhibitory effects on the startle, many higher nuclei can modulate these effects as well. Prepulse modulatory mechanisms are far more complicated and depend on behaviorally salient factors such as inter-stimulus interval, frequency, stimulus duration, prior auditory experience, and habituation. It has been shown that a large degree of top-down modulation can influence prepulses (Du et al., 2011; Swerdlow et al., 2001; Larrauri & Schmajuk, 2006). The amygdala, hippocampus, prefrontal cortex, auditory cortex and many other structures have been flagged as the source of this top-down modulation. To this extent, it seems that PPI audiometry has the potential to assess large portions of the auditory neuraxis and other neocortical regions. Therefore it is likely that PPI audiometry could be a useful tool to assess neuroplastic changes in awake behaviorally responsive animals. PPI audiometry would be especially suited for labs that already employ the ASR for other purposes such as tinnitus detection (Turner et al., 2006; Tziridis et al., 2012; Ropp et al., 2014; Koehler et al., 2013, Lowe & Walton, 2015) or psychopharmaceutical studies (Phillips et al., 2000; Davis & Menkes, 1982). It should be noted that PPI tests are performed on multiple animals concurrently with no prerequisite training for the animals or the handlers. In this study we found that we could assess auditory thresholds at 6 different frequencies in less than two hours, and future work will aim to reduce this length of time even further. This rate of testing would be exceptionally valuable for drug testing which requires large sample sizes.
4.2. Comparison of PPI audiometry to ABR assessments
4.2.1. Comparing PPI and ABR thresholds
The ASR has been shown to be greatly influenced by preceding stimuli and can be used for hearing assessment in laboratory animals (Hoffman & Searle 1965, Buckland et al. 1969, Willott & Carlson, 1995; Young & Fechter, 1983; Fechter et al., 1988) and humans (Reiter, 1981). In animals PPI can provide a behaviorally assessed, high throughput solution to assess auditory thresholds in normal hearing and sound exposed animals (Fig. 4A–C). To this point, however, the reliability of PPI audiometry to obtain thresholds across animals and days has not been tested. Previous work has shown that prepulse inhibition has only small variations from day to day (Willott et al., 2003), corroborating with our data showing consistency in PPI audiometry across animals and days (Fig. 3). Secondly, it is important to note that there were some differences in the audiogram generated by our PPI method and previous behavioral assessments in animals (Ehret, 1974; Radziwon et al. 2009). Although most of the thresholds matched within 10 dB SPL of behavioral paradigms, we notice that for some animals the 4 kHz threshold was much lower. This finding however was also seen in humans (Reiter, 1981). It is possible that the sounds inhibiting the startle reflex do not reflect the same circuitry as is involved with behavioral decision-making processes that lead to behavioral thresholds. Finally, we evaluated whether PPI could detect temporary and permanent threshold shifts due to noise exposure. Walter (2012) found that sound exposure caused increases in both PPI and ABR related thresholds, which is in good agreement with our data (Fig. 4A–F). However, it is well known that permanent threshold shifts will develop over some time after exposure and may have very different spectral characteristics than temporary threshold shifts. Three months after exposure ABR thresholds recovered to near baseline levels (Fig. 4D–F) (Kujawa & Liberman, 2009; Singer et al., 2013; Dehmel et al., 2012), whereas we found that PPI threshold became further increased at frequency specific regions of sound exposure compared with thresholds one day after exposure (Fig. 4A–C). This suggests that PPI thresholds are detecting some hearing deficits that ABR thresholds are not. Since top down PPI modulation of the startle reflex can come from auditory structures above the inferior colliculus (the furthest reach of ABRs), it is possible that changes in PPI audiometric thresholds represent higher order deficits as a result of sound exposure. It is well known that tonotopic maps of neuronal activity undergo plastic changes in response to sound exposure (Robertson & Irvine, 1989; Eggermont & Komiya, 2000; Kaltenbach et al., 2000; Middleton et al., 2011; Norena et al., 2010; Wang et al., 2009). Such reorganization may account for the poor correlation between PPI and ABR thresholds one day after exposure (Fig. 4H), and the negative correlation three months after exposure (Fig. 4I). However, it is possible that the precision of these correlations was not ideal, because while ABR thresholds are measured on a 10 dB interval scale, PPI thresholds were determined using a ratio scale via the cubic spline function (all rational numbers between 0–80). Despite this fact, that we are comparing two very distinct measures of hearing, correlations between these two hearing assessments are generally predictive of one another in control conditions (Fig. 4G).
4.2.2. Comparing startle modulated prepulse inhibition with ABR wave one amplitudes
It has been shown that suprathreshold prepulse modulation of the startle reflex can be diminished in mice with age-related hearing dysfunctions (Willott et al., 1994). This reduction was also demonstrated in the present study with prepulse modulated startle responses and associated inhibition after sound exposure (Fig. 5A1-F1). In agreement with previous studies (Kujawa & Liberman, 2009; Lin et al., 2011; Fernandez et al., 2015), ABR amplitudes in exposed mice decreased over time (Fig. 5A2-F2). The most important finding in this study was that the magnitude of these suprathreshold deficits were frequency specific when measured with both PPI audiometry and ABR wave one amplitudes (Fig. 6A, B respectively). Importantly, the greatest deficit was at the upper frequencies or slightly higher than the frequency range of the sound exposure. The damage caused by sound exposure typically localizes at 0.5 to 1 octave above the center frequency range of exposure (Cody & Johnstone, 1981). Responses to both the low intensity and high intensity sounds were examined using PPI and wave one ABR in an attempt to differentiate the contribution of high and low threshold fibers to hearing loss. The ABR deficits seen at three months were similar spectrally for both 40 and 80 dB SPL, however in agreement with previous work the wave one ABR amplitudes to 80 dB SPL sounds showed the highest extent of changes from control levels (Fig. 6B) (Kujawa & Liberman, 2009; Lin et al., 2011). The suprathreshold deficits assessed with PPI and ABR amplitudes were very similar. As mentioned previously, post-exposure PPI functions tend to “flatten” (Fig. 1D; 5A1-F1). This is presumably because the pure tone prepulses are less audible to the animal and thus, less able to modulate the startle stimulus. In unexposed animals high intensity prepulses will suppress the startle almost entirely (Fig. 5A1-F1 blue), however if the animal cannot detect the high intensity prepulses, the startle will not be inhibited, thus creating a flat startle function (Fig. 5A1-F1 orange). This can lead to decreased overall inhibition in response to these suprathreshold stimuli (Fig. 5A2-F2). This data, along with the frequency specific permanent threshold shifts detected by PPI audiometry (Fig. 4A), suggest that this method allows for accurate behavioral measurements of hearing. Furthermore, it is interesting to address that one day after sound exposure, overall startle responses were immediately reduced in both input-output functions (Fig. 7) and in prepulse modulated startle situations (Fig. 5A1-F1). This directly contrasts the change in inhibition which in most cases did not show a significant deficit (at frequencies neighboring sound exposure as seen in Fig. 6A) until three months after exposure. This suggests that ANF carrying broadband startle stimulus information are immediately affected by sound exposure, due to a temporary threshold shift, whereas, frequency specific auditory nerve fiber loss will develop after exposure. This result cannot be detected by ABR wave one amplitudes, as they are not able to be collected one day after exposure due to high temporary threshold shifts.
4.3 The PPI Model
4.3.1 Considerations and limitations of PPI threshold assessments
Several important factors must be considered when using the cubic spline function (Fechter et al., 1988) to assess auditory thresholds via PPI functions (Fig. 1). First, while the cubic spline function is a statistically appropriate method to assess large sample sizes at fine resolutions, for reasons mentioned above, it could possibly exaggerate beyond actual behavioral thresholds (Radizwon et al., 2009) when the magnitude of the startle response is very small, for example due to hearing loss. Future studies might apply alternatives to the cubic spline function to assess the prepulse modulated startle function. Our mouse data together with a previous study on rats (Fechter et al., 1988) advocate that the cubic spline is well-suited for threshold detection in this acoustic startle paradigm. Second, after noise exposure, the absolute startle magnitude is often reduced to at least half of its baseline levels (Fig. 7) (Longenecker & Galazyuk, 2011; Lobarinas et al., 2013). This is likely a two part process: first, at one day after exposure the ANF synapse is likely swollen, leading to a significant temporary threshold shift (Robertson, 1983), which is likely to contribute to the reduced startle magnitude (Lobarinas et al., 2013; Longenecker et al., 2014). Second, after a period of recovery from temporary threshold shift, the startle reduction seen at the three month time period is likely representing frequency specific detachment of ribbon synapses and concurrent declines of ANFs as a result of intense sound exposure, as evidenced by reduction of wave one ABR amplitudes (Fig. 5A3-F3; Fig. 6B) (see review Kujawa & Liberman, 2015), again contributing to reduced startle magnitude. A drop in startle magnitude after exposure can reduce the dynamic range of the prepulse modulated startle (Fig. 5A1-F1) and the resulting PPI (Fig. 5A2-F2), especially at three months after exposure when there is presumptive ANF loss. Thus both temporary and permanent changes contribute to a reduced startle amplitude. While the absolute levels of inhibition remain similar to those of unexposed mice at low prepulse intensities (Fig. 5A2-F2), it is important to remember that this inhibition is the result of a ratio that does not account for changes in dynamic range as a result of cochlear trauma (Viemeister, 1988). Psychoacoustically this would imply that inhibition can still occur for low intensity prepulses because the low threshold fibers are intact (Fig. 5A2-F2). However, due to net startle changes after cochlear loss, inhibition slopes are flatter, which would lead to artificial increases in detection thresholds (Fig. 1D). This is especially true for frequencies most affected by sound exposure (12.5–25 kHz) (Fig. 4A). Thus, it is important to consider changes in the startle reflex magnitude when making conclusions about prepulse inhibition following sound exposure (Lobarinas et al. 2013).
4.3.2 A behavioral representation of high threshold ANF depletion following sound exposure
Recent studies have discovered that while clinically normal threshold levels may be maintained following sound exposure, both mice (100 dB SPL 2 hours; Kujawa & Liberman, 2009) and guinea pigs (106 or 109 dB SPL 2 hours; Lin et al., 2011) display clear suprathreshold ABR wave one amplitude deficits associated with ANF degeneration. Similarly our exposed mice demonstrate reduced ABR wave one amplitudes (Fig. 5A3-F3). Our ABR wave one amplitudes at intense, suprathreshold sound levels were depressed further than at lower sound levels, which might be expected from an intense sound exposure (116 dB SPL 1 hour). The hidden hearing loss model proposed by Schaette and McAlpine, 2011 suggests that high threshold low spontaneous rate auditory nerve fibers are preferentially damaged after exposure, leaving the majority of low threshold fibers intact (Fig. 9). If so, we would expect that the exposed mice in our study would have difficulty perceiving high intensity prepulses at frequencies most affected by sound exposure. These deficits should be mostly evident within a half octave above the center of exposure (in our case 12.5–25 kHz) (Cody & Johnstone, 1981).
Figure 9.
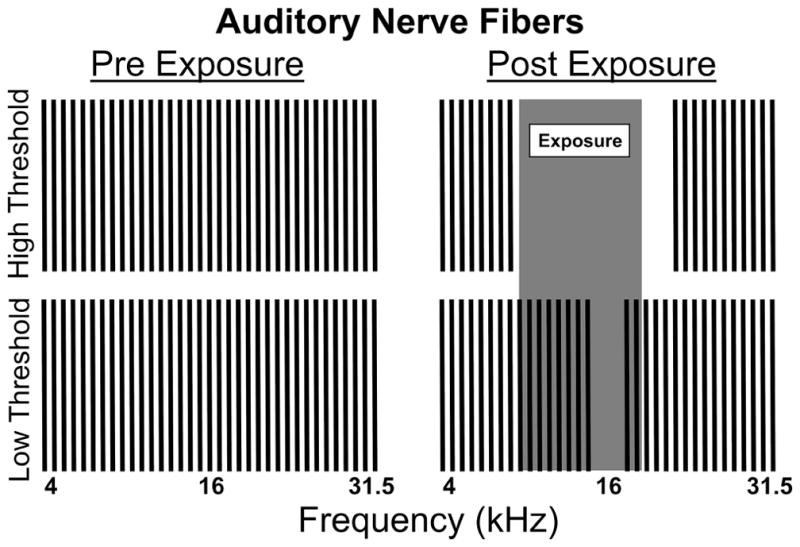
Model for auditory nerve fiber damage three months after sound exposure. Post exposure, high threshold fibers across the range of sound exposure are damaged. Low threshold fibers are mostly spared except for a 1/3-octave notch above the center frequency of exposure (16 kHz region). This damage explains both the PPI audiometric thresholds seen in Fig. 4A–C, as well as the PPI and ABR wave one amplitude curves in Fig. 5A2-F2 and Fig. 5A3-F3 respectively.
Importantly, our PPI methodology was able to test this hypothesis behaviorally. Both the frequency specific ANF damage as well as a general decrease in startle magnitude can be we explained by this model (Fig. 9). The level of inhibition dropped precipitously in response to sound levels above 40 dB SPL at the frequencies most affected by sound exposure (Fig. 5A2-F2). These sound levels are predominantly processed by high threshold auditory nerve fibers (Liberman & Kiang, 1978; Winter & Palmer, 1991). The loss of these fibers presumably produced the frequency specific prepulse inhibition deficits, a behavioral indication of hidden hearing loss (Fig. 9, high threshold ANF damage). It is also important to highlight the fact that some low threshold fibers were also likely damaged after a 116 dB SPL sound exposure, particularly those fibers responsible for frequencies near 16 kHz (Fig. 9, low threshold ANF damage). This was experimentally observed at 16 kHz with a unique PPI deficit at even at low intensity prepulses (Fig. 5C2), as well as the most elevated detection threshold (Fig. 4A), and the greatest 40 dB SPL stimulus ABR wave one deficit (Fig. 6B). Additionally, this model could also explain why the startle magnitude decreased after exposure (Fig. 5A1-F1, Fig. 7). Since a broadband startle stimulus was used, you would expect the reduced startle response reductions to be proportionate to the high threshold ANF loss across all frequencies showing prepulse inhibition deficits (Fig. 9, high threshold ANF damage).
Can PPI audiometry explain possible perceptual deficits after high intensity sound exposure? Above we discussed how decreases in inhibition might be explained by ANF fiber loss, but it is possible that these deficits have central origin. Interestingly, the inhibition curve is increasingly depressed at higher intensity prepulses (Fig. 5A2-F2), but not closely following the ABR wave one amplitude function (Fig. 5A3-F3). This implies that the behavioral significance of high threshold auditory nerve fibers might be more severe than previously thought. Clearly the direct loss of these fibers alone, which degenerate from the time of exposure until three months after exposure (as evidenced by changes in inhibition from one day after exposure compared to three months in Fig. 5A2-F2), cannot fully explain the intensity coding issues or dynamic range issues that lead to some of the perceptual PPI deficits shown here. Any number of additional plastic changes in the auditory system following ANF degeneration could lead to these extreme inhibition deficits at high intensity prepulses (Robertson & Irvine, 1989; Eggermont & Komiya, 2000; Kaltenbach et al., 2000; Middleton et al., 2011; Norena et al., 2010; Wang et al., 2009). Even after unilateral sound exposure it is possible that maladaptive plasticity could lead to bilateral changes throughout the auditory system (Rubio, 2006; Popescu & Polley, 2010) To our knowledge this is one of the first examples of behavioral evidence of hidden hearing loss in an animal model (Mehraei et al., 2016). Since a modified version of this PPI audiometric method has already been conducted on humans (Reiter, 1981), it might be possible to determine if these effects in mice are also observed in older adults or sound exposed populations. This would be expected based on recent studies showing that ANFs degenerate as a function of age in all humans (Viana et al., 2015).
Highlights.
PPI audiometry can reliably assess hearing in control and sound exposed animals.
PPI audiometry and ABR thresholds differ after exposure.
PPI inhibition and ABR wave one amplitudes show similar deficits after exposure.
Acknowledgments
This research was supported by grant R01 DC011330 to AVG, R01 DC013314 to MJR and 1F31 DC013498-01A1 to RJL from the National Institute on Deafness and Other Communication Disorders of the U.S. Public Health Service. The authors also thank Olga Galazyuk for developing software that allowed off-line data analysis and statistical evaluation.
Abbreviations
- PPI
prepulse inhibition
- ABR
auditory brainstem response
- ASR
acoustic startle reflex
- SO
startle only
- ITI
inter-trial interval
- ANOVA
analysis of variance
- LSD
Least Significant Difference post hoc test
- ANF
auditory nerve fiber
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buckland G, Buckland J, Jamieson C, Ison JR. Inhibition of startle response to acoustic stimulation produced by visual prestimulation. Journal of Comparative and Physiological Psychology. 1969;67(4):493–496. doi: 10.1037/h0027307. [DOI] [PubMed] [Google Scholar]
- Bullock AE, Slobe BS, Vazquez V, Collins AC. Inbred mouse strains differ in the regulation of startle and prepulse inhibition of the startle response. Behavioral Neuroscience. 1997;111(6):1353–1360. doi: 10.1037//0735-7044.111.6.1353. [DOI] [PubMed] [Google Scholar]
- Cederholm JME, Froud KE, Wong ACY, Ko M, Ryan AF, Housley GD. Differential actions of isoflurane and ketamine-based anaesthetics on cochlear function in mouse. Hearing Research. 2012;292:71–79. doi: 10.1016/j.heares.2012.08.010. http://dx.doi.org/10.1016/j.heares.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AR, Hancock KE, Maison SF, Liberman MC, Polley DB. Sound-evoked olivocochlear activation in unanesthetized mice. JARO. 2012;13:209–2017. doi: 10.1007/s10162-011-0306-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody AR, Johnstone BM. Acoustic trauma: Single neuron basis for the “halfoctave shift”. J Acoust Soc Am. 1981;70(3):707–711. doi: 10.1121/1.386906. [DOI] [PubMed] [Google Scholar]
- Davis M. The mammalian startle response. In: Eaton RC, editor. Neural Mechanisms of Startle Behavior. New York, NY: Springer Science & Plenum Press; 1984. pp. 287–351. [DOI] [Google Scholar]
- Davis M, Gendelman PM. Plasticity of the acoustic startle response in the acutely decerebrate rat. Journal of Comparative and Physiological Psychology. 1977;91(3):549–563. doi: 10.1037/h0077345. [DOI] [PubMed] [Google Scholar]
- Davis M, Menkes DB. Tricyclic antidepressants vary in decreasing 2-adrenocepter sensitivity with chronic treatment: assessment with clonidine inhibition of acoustic startle. Br J Pharmacol. 1982;77:217–22. doi: 10.1111/j.1476-5381.1982.tb09288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmel S, Eisinger D, Shore SE. Gap prepulse inhibition and auditory brainstem-evoked potentials as objective measures for tinnitus in guinea pigs. Frontiers in Systems Neuroscience. 2012;6(42):1–15. doi: 10.3389/fnsys.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Wu X, Li L. Differential organized top-down modulation of prepulse inhibition of startle. J Neurosci. 2011;31(38):13644–13653. doi: 10.1523/JNEUROSCI.1292-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ, Komiya H. Moderate noise trauma in juvenile cats results in profound cortical topographic map changes in adulthood. Hearing Research. 2000;142:89–101. doi: 10.1016/s0378-5955(00)00024-1. [DOI] [PubMed] [Google Scholar]
- Ehret G. Age-dependent hearing loss in normal hearing mice. Naturwissenschaften. 1974;11:506. doi: 10.1007/BF00622976. [DOI] [PubMed] [Google Scholar]
- Fechter LD, Sheppard L, Young JS, Zeger S. Sensory threshold estimation from a continuous graded response produced by reflex modification audiometry. J Acoustic Soc Am. 1988;84(1):179–185. doi: 10.1121/1.396962. [DOI] [PubMed] [Google Scholar]
- Fendt M, Li L, Yeomans JS. Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology. 2001;156:216–224. doi: 10.1007/s002130100794. [DOI] [PubMed] [Google Scholar]
- Fernandez KA, Jeffers PWC, Lall K, Liberman MC, Kujawa SG. Aging after noise exposure: Acceleration of cochlear synaptopathy in “recovered” ears. J Neurosci. 2015;35(19):7509–7520. doi: 10.1523/JNEUROSCI.5138-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JE. Habituation and prestimulus inhibition of the auditory startle reflex in decerebrate rats. Physiology & Behavior. 1979;23:291–297. doi: 10.1016/0031-9384(79)90370-6. [DOI] [PubMed] [Google Scholar]
- Gerrard RL, Ison JR. Spectral frequency and the modulation of the acoustic startle reflex by background noise. J Experimental Psych: Animal Behavior Processes. 1990;16(1):106–112. [PubMed] [Google Scholar]
- Gómez-Nieto R, Horta-Junior JAC, Castellano O, Millian-Morell L, Rubio ME, Lopez DE. Origin and function of short-latency inputs to the neural substrates underlying the acoustic startle reflex. Front in Neurosci. 2014;8(216):1–18. doi: 10.3389/fnins.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham FK. The more or less startling effects of weak prestimulation. Psychophysiology. 1975;12:238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Grimsley CA, Longenecker RJ, Rosen MJ, Young JW, Grimsley JM, Galazyuk AV. An improved approach to separating startle data from noise. J Neurosci Methods. 2015;253:206–217. doi: 10.1016/j.jneumeth.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner H, Masterson B. Hearing in Glires: Domestic rabbit, cotton rat, feral house mouse, and kangaroo rat. J Acoust Soc Am. 1980;68(6):1584–1599. [Google Scholar]
- Heffner HE, Koay G, Heffner RS. Comparison of behavioral and auditory brainstem response measures of threshold shift in rats exposed to loud sound. J Acoustic Soc Am. 2008;124(2):1093–1104. doi: 10.1121/1.2949518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HS, Searle JL. Acoustic variables in the modification of startle reaction in the rat. Journal of Comparative and Physiological Psychology. 1965;60(1):53–58. doi: 10.1037/h0022325. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Wible BL. Role of weak signals in acoustic startle. J Acoustic Soc Am. 1970;47(2):489–497. doi: 10.1121/1.1911919. [DOI] [PubMed] [Google Scholar]
- Ison JR. The acoustic startle response: Reflex elicitation and reflex modification by preliminary stimuli. In: Willott James F., editor. Handbook of Mouse Auditory Research: from Behavior to Molecular Biology. CRC Press; Boca Raton: 2001. pp. 83–90. [Google Scholar]
- Ison JR, Allen PD. Pre- but not post- menopausal female CBA/CaJ mice show less prepulse inhibition than male mice of the same age. Behavioral Brain Research. 2007;185:76–81. doi: 10.1016/j.bbr.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J, Afman CE. Plasticity of spontaneous neural activity in the dorsal cochlear nucleus after intense sound exposure. Hearing research. 2000;147:282–292. doi: 10.1016/s0378-5955(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59(2):107–28. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Koehler SD, Shore SE. Stimulus timing-dependent plasticity in dorsal cochlear nucleus is altered in tinnitus. Journal of Neuroscience. 2013;33(50):19647–19656. doi: 10.1523/JNEUROSCI.2788-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hearing Research. 2015;330:191–199. doi: 10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Aasen I, Sharma T. Sex differences in prepulse inhibition deficits in chronic schizophrenia. Schizophrenia Research. 2004;69:219–235. doi: 10.1016/j.schres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Larrauri J, Schmajuk N. Prepulse inhibition mechanisms and cognitive processes: a review and model. Neurotransmitter Interactions and Cognitive Function. 2006;98:245–278. doi: 10.1007/978-3-7643-7772-4_12. [DOI] [PubMed] [Google Scholar]
- Lee Y, Lopez DE, Meloni EG, Davis M. A primary acoustic startle pathway: Obligatory role of cochlear root neurons and the nucleus reticularis pontis caudalis. J Neurosci. 1996;16(11):3775–3789. doi: 10.1523/JNEUROSCI.16-11-03775.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Kiang NYS. Acoustic trauma in cats: Cochlear pathology and auditory-nerve activity. Acta Otolaryngol Suppl. 1978;358:1–63. [PubMed] [Google Scholar]
- Liberman LD, Liberman MC. Dynamics of cochlear synaptopathy after acoustic overexposure. JARO. 2015;16:205–219. doi: 10.1007/s10162-015-0510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC. Primary neural degeneration in the guinea pig cochlea after reversible noise-induced threshold shift. JARO. 2011;12:605–616. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Hayes SH, Allman BL. The gap-startle paradigm for tinnitus screening in animal models: Limitations and optimization. Hearing Research. 2013;295:150–160. doi: 10.1016/j.heares.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker RJ, Galazyuk AV. Development of tinnitus in CBA/CaJ mice following sound exposure. JARO. 2011;12:647–658. doi: 10.1007/s10162-011-0276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker RJ, Galazyuk AV. Methodological optimization of tinnitus assessment using prepulse inhibition of the acoustic startle reflex. Brain Research. 2012;1485:54–62. doi: 10.1016/j.brainres.2012.02.067. [DOI] [PubMed] [Google Scholar]
- Longenecker RJ, Chonko KT, Maricich SM, Galazyuk AV. Age effects on tinnitus and hearing loss in CBA/CaJ mice following sound exposure. SpringerPlus. 2014;3:542. doi: 10.1186/2193-1801-3-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe AS, Walton JP. Alterations in peripheral and central components of the auditory brainstem response: A neural assay of tinnitus. Plus One. 2015;10(2):1–23. doi: 10.1371/journal.pone.0117228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehraei G, Hickox AE, Bharadwaj HM, Goldberg H, Verhulst S, Liberman MC, Shinn-Cunningham BG. Auditory brainstem response latency in noise as a marker of cochlear synaptopathy. The Journal of Neuroscience. 2016;36(13):3755–3764. doi: 10.1523/JNEUROSCI.4460-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher JR, Kiang NYS. Generators of the brainstem auditory evoked potential in cat III: identified cell populations. Hearing Research. 1996;93:52–71. doi: 10.1016/0378-5955(95)00200-6. [DOI] [PubMed] [Google Scholar]
- Middleton JW, Kiritani T, Pedersen C, Turner J, Shepherd GMG, Tzounopoulos T. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. PNAS. 2011;108(18):7601–7606. doi: 10.1073/pnas.1100223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreña AJ, Moffat G, Blanc JL, Pezard L, Cazals Y. Neural changes in the auditory cortex of awake guinea pigs after two tinnitus inducers: Salicylate and acoustic trauma. Neuroscience. 2010;166:1194–1209. doi: 10.1016/j.neuroscience.2009.12.063. [DOI] [PubMed] [Google Scholar]
- Phillips MA, Langley RW, Bradshaw CM, Szabadi E. The effects of some antidepressant drugs on prepulse inhibition of the acoustic startle (eyeblink) responseand the N1/P2 auditory evoked response in man. J Psycopharmacol. 2000;14(1):40–5. doi: 10.1177/026988110001400105. [DOI] [PubMed] [Google Scholar]
- Plappert CF, Rodenbucher AM, Pilz PKD. Effects of sex and estrous cycle on modulation of the acoustic startle response in mice. Physiology & Behavior. 2005;84:585–594. doi: 10.1016/j.physbeh.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Popescu MV, Polley DB. Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex. Neuron. 2010;65:718–731. doi: 10.1016/j.neuron.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziwon KE, June KM, Stolzberg DJ, Xu-Friedman M, Salvi RJ, Dent ML. Behavioral measured audiograms and gap detection thresholds in CBA/CaJ Mice. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009;195(10):961–969. doi: 10.1007/s00359-009-0472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter LA. Experiments Re: Clinical application of reflex modulation audiometry. Journal of speech and hearing research. 1981;24(1):92–98. doi: 10.1044/jshr.2401.92. [DOI] [PubMed] [Google Scholar]
- Robertson D. Functional significance of dendritic swelling after loud sounds in the guinea pig cochlea. Hearing Research. 1983;9:263–278. doi: 10.1016/0378-5955(83)90031-x. [DOI] [PubMed] [Google Scholar]
- Robertson D, Irvine DRF. Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. The Journal of Comparative Neurology. 1989;282:456–471. doi: 10.1002/cne.902820311. [DOI] [PubMed] [Google Scholar]
- Ropp TF, Tiedemann KL, Young ED, May BJ. Effects of unilateral acoustic trauma on tinnitus-related spontaneous activity in the inferior colliculus. JARO. 2014;15:1007–1022. doi: 10.1007/s10162-014-0488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio ME. Redistribution of synaptic AMPA receptors at glutamatergic synapses in the dorsal cochlear nucleus as an early response to cochlear ablation in rats. Hearing Research. 2006;216–217:154–167. doi: 10.1016/j.heares.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Schaette R, McAlpine D. Tinnitus with a normal audiogram: Physiological evidence for hidden hearing loss and computational model. J Neurosci. 2011;31(38):13452–13457. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W, Zuccotti A, Jaumann M, Lee SC, Panford-Walsh R, Xiong H, Zimmermann U, Franz C, Geisler HS, Kopschall I, Rohbock K, Varakina K, Verpoorten S, Reinbothe T, Schimmang T, Ruttiger L, Knipper M. Noise-induced inner hair cell ribbon loss disturbs central arc mobilization: a novel molecular paradigm for understanding tinnitus. Mol Neurobiology. 2013;47:261–279. doi: 10.1007/s12035-012-8372-8. [DOI] [PubMed] [Google Scholar]
- Skoe E, Krizman J, Spitzer E, Kraus N. The auditory brainstem is a barometer of rapid auditory learning. Neuroscience. 2013;243:104–114. doi: 10.1016/j.neuroscience.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Skoe E, Chandrasekaran B, Spitzer ER, Wong PCM, Kraus N. Human brainstem plasticity: The interaction of stimulus probability and auditory learning. Neurobiology of Learning and Memory. 2014;109:82–93. doi: 10.1016/j.nlm.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Stapells DR, Oates P. Estimation of the pure-tone audiogram by the auditory brainstem response: A review. Audiol Neurootol. 1997;2:257–280. doi: 10.1159/000259252. [DOI] [PubMed] [Google Scholar]
- Stapells DR. Frequency-specific ABR and ASSR threshold assessment in young infants. In: Seewald RC, Tharpe AM, editors. Comprehensive handbook of pediatric audiology. San Diego: Plural Publishing, Inc; 2011. pp. 409–448. [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Tukey JW. Exploratory Data Analysis. Addison-Wesley Publishing Company; 1977. [Google Scholar]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behavioral Neuroscience. 2006;120:188–195. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- Tziridis K, Ahlf S, Schulze H. A low cost setup for behavioral audiometry in rodents. J Vis Exp. 2012;(68):e4433. doi: 10.3791/4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana LM, O’Malley JT, Burgess BJ, Jones DD, Oliveira CACP, Santos F, Merchant SN, Liberman LD, Liberman MC. Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hearing Research. 2015;327:78–88. doi: 10.1016/j.heares.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemeister NF. Intensity coding and the dynamic range problem. Hearing Research. 1988;34:267–274. doi: 10.1016/0378-5955(88)90007-x. [DOI] [PubMed] [Google Scholar]
- Walter M, Tziridis K, Ahlf S, Schulze H. Context dependent auditory thresholds determined by brainstem audiometry and prepulse inhibition in Mongolian Gerbils. Open Journal of Acousics. 2012;2:34–49. http://dx.doi.org/10.4236/oja.2012.21004. [Google Scholar]
- Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience. 2009;164:747–759. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF, Carlson S, Chen H. Prepulse inhibition of the startle response in mice: Relationship to hearing loss and auditory system plasticity. Behavioral Neuroscience. 1994;108:703–713. doi: 10.1037//0735-7044.108.4.703. [DOI] [PubMed] [Google Scholar]
- Willott JF, Carlson S. Modification of the acoustic startle response in hearing-impaired C57BL/6J Mice: Prepulse augmentation and prolongation of prepulse inhibition. Behavioral Neuroscience. 1995;109(3):396–403. doi: 10.1037//0735-7044.109.3.396. [DOI] [PubMed] [Google Scholar]
- Willott JF, Tanner L, O’Steen J, Johnson KR, Bogue MA, Gagnon L. Acoustic startle and prepulse inhibition in 40 inbred strains of mice. Behavioral Neuroscience. 2003;117(4):716–727. doi: 10.1037/0735-7044.117.4.716. [DOI] [PubMed] [Google Scholar]
- Winter IM, Palmer AR. Intensity coding in low-frequency auditory-nerve fibers of the guinea pig. J Acoust Soc Am. 1991;90(4):1958–1967. doi: 10.1121/1.401675. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Frankland PW. The acoustic startle reflex: neurons and connections. Brain Research Reviews. 1996;21:301–314. doi: 10.1016/0165-0173(96)00004-5. [DOI] [PubMed] [Google Scholar]
- Young JS, Fechter LD. Reflex inhibition procedures for animal audiometry: A technique for assessing ototoxicity. J Acoust Soc Am. 1983;73(5):1686–1693. doi: 10.1121/1.389391. [DOI] [PubMed] [Google Scholar]
- Zhou X, Jen PHS, Seburn KL, Frankel WN, Zheng QY. Auditory brainstem responses in 10 inbred strains of mice. Brain Res. 2006;1091(1):16–26. doi: 10.1016/j.brainres.2006.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]