Monitoring forced expiratory volume in 1 second (FEV1) is a valuable component to determine asthma control1, 2 and indicate risk of future exacerbation3 in children. However, use of spirometry by patients outside of healthcare facilities is limited by accessibility, staffing, time and cost.
The Electronic Peak Flow & FEV1 Meter (PIKO-1, NSPIRE Health, Longmont, CO) is a handheld personal device for measuring FEV1 values. It has the potential to offer personalized monitoring for children with asthma in non-clinical settings (home, school, or travel) and may be particularly beneficial to patients who have poor perception of their asthma symptoms.
The few available published studies report conflicting results regarding the utility of PIKO devices4–6, and are limited to either healthy volunteers or small numbers of asthmatics. Therefore, we aimed to determine the utility of PIKO in a large prospective cohort of children with asthma by determining its correlation and concordance of FEV1 with traditional spirometry.
We performed serial side–by-side spirometry and PIKO-1 forced exhalation maneuvers in 242 school age children with asthma enrolled in the School Inner City Asthma Study7. Pneumotach spirometry (Koko spirometer, nSpire Health, Inc., Longmont, CO, USA), performed per American Thoracic Society guidelines8, and PIKO-1 data were collected at a baseline (research facility) and follow-up visit (subject’s elementary school) by trained research staff. Best efforts recorded at each visit were compared by Pearson correlation and intraclass correlation coefficient (ICC). However, two continuous measures can have similar distributions but render quite different actual values, leading to high correlation but poor concordance between the two measures. We used the Bland-Altman method to evaluate concordance of values between devices. The Bland-Altman method plots mean difference between each matched PIKO and spirometry measurement to identify systematic bias between devices measuring the same quantity (i.e. one consistently reads 20 units lower) or proportional bias (differences between devices do not agree equally through the range of measurements). Absolute liter flow was used for comparison rather than percent predicted to eliminate any discrepancies that might be imposed by prediction models.
In total, 441 paired FEV1 measures, collected from 242 subjects, were analyzed. Subjects ranged in age from 4–12 (mean = 8) years and 50% female. The majority (n=166) of subjects self-identified as Black or Hispanic and 45% reported household income < $25,000/year. On average, subjects had normal lung function at baseline (mean FEV1 = 101% +/−19%), and lacked airflow obstruction (Mean FEV1/FVC =0.87 +/−0.08).
Spirometry FEV1 and PIKO-1 FEV1 were well correlated with Pearson coefficient = 0.80 (P<0.0001) (Figure) and ICC=0.75. Bland-Altman analysis demonstrated a mean difference between spirometry FEV1 and PIKO-1 FEV1 of 0.14L (95% limits of agreement = −0.40 to 0.68L) (Figure). Within session PFT variability was 0.4L for spirometry at 2 standard deviations, a much smaller range than seen in the PFT - PIKO confidence limits (1.1L), indicating that differences are due to distinctions in the devices themselves and not the within person techniques of using them. PIKO-1 FEV1 was moderately biased to underestimate FEV1 with increasing volumes, such that for every one liter increase in spirometry-FEV1 the mean difference between spirometry and PIKO-1 increased by 0.19L (95% confidence interval = 0.12 to 0.25, p<0.001). There was no effect on the order of PFT or PIKO performance(p=0.88)
Figure. Relationship between PIKO-1 and Spirometry FEV1.
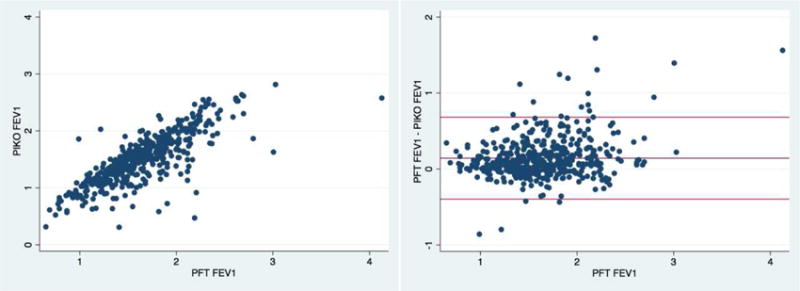
left panel: Scatterplot demonstrating correlation between PIKO-FEV1 and PFT-FEV1 (Pearson correlation = 0.8); right panel: Bland-Altman plot of PIKO and spirometry-derived FEV1 (mean difference FEV1 = 0.14L, 95% limits of agreement = −0.40 to 0.68L)
Overall, we found that PIKO-1 underestimated FEV1 compared to pneumotach spirometry by an average of 0.14L, representing a 10% discrepancy from mean baseline FEV1, but may have varied by more than 1L based on the limits of agreement. In context, 43% of values were greater than 150mL, the ATS/ERS standard for repeatability8. Additionally, we found that the difference in measures was not constant along the range of FEV1 values. These differences pose a substantial limitation to PIKO-1 use as a surrogate for measuring FEV1 in clinical or research applications in asthmatic children.
Prior reports evaluating PIKO-1 device applications were conducted in either healthy volunteers5, 6 or small number of asthmatics over short time periods4. To our knowledge, this is the first study to evaluate the validity PIKO-1 FEV1 in a large number of asthmatic schoolchildren across formal (research clinic) and informal (school) settings.
Our findings are consistent with those presented by Rothe and colleagues5 for healthy volunteers and Aguilar-Fernandez9 in children with asthma in which good overall correlation was found with a consistent underestimation of FEV1 by the PIKO device. While systematic bias was also present in those studies, the confidence of limitations was narrow suggesting that despite the numerical inaccuracy, once calibrated to the difference, the PIKO may offer reliable data. Within a pediatric cohort of asthmatics and healthy volunteers, Gochicoa-Rangel et al.4 found the concordance between PIKO-1 and spirometry measurements to be lower in patients with partially controlled or uncontrolled asthma; precisely the population for whom the device would be most clinically useful. Similar to our findings, there was nearly a liter of variability in FEV1 between devices.
We performed a comparative investigation of PIKO and spirometry in children with asthma recruited from the general community with minimal airflow obstruction. It is difficult to exclude the effect of within-subject variability when determining variability of the devices; however this was minimized by: 1) both maneuvers were overseen by trained research personnel, 2) produced at the same visit, and 3) only best efforts were analyzed after exclusion of poor quality spirometry. Additionally, by the nature of recruitment from a general, poor, urban population, with overall mild asthma it is likely that few subjects had spirometry experience prior to enrollment, unlike at least one study that found a more reliable relationship among pulmonary specialty clinic patients9. The technical performance may improve with practice.
In conclusion, the findings from this study suggest that the PIKO-1 device has limited utility in assessing FEV1 in clinical or research settings in children with asthma. However, there may be applications for use of handheld devices longitudinally as a marker of clinical outcomes. Further investigation of its use in this respect and with different populations may prove the device more valuable.
Acknowledgments
Funding source:
This work was supported by NIH grants R01 AI 073964, R01 AI 073964-02S1, K24 AI 106822, SICAS2 U01 AI 110397, and U10HL098102 (PI: Phipatanakul). Authors are also supported in part by grants K23AI106945 (PI: Gaffin), K23 AI 104780 (PI: Sheehan). This work was conducted with support from Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health. We also thank Lincoln Diagnostics, Inc., Decatur, IL, USA, Greer Laboratories, Inc., and Lenoir, NC, USA, for their generous donation or discounts on skin testing devices and supplies.
Abbreviations
- FEV1
forced expiratory volume in 1 second
- ICC
intraclass correlation coefficient
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: Not applicable.
Conflict of interest: All authors declare no potential conflicts of interest related to this work.
References
- 1.Expert Panel Report 3 (EPR-3) Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. The Journal of allergy and clinical immunology. 2007;120:S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 2.Nair SJ, Daigle KL, DeCuir P, Lapin CD, Schramm CM. The influence of pulmonary function testing on the management of asthma in children. The Journal of pediatrics. 2005;147:797–801. doi: 10.1016/j.jpeds.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Fuhlbrigge AL, Kitch BT, Paltiel AD, et al. FEV(1) is associated with risk of asthma attacks in a pediatric population. The Journal of allergy and clinical immunology. 2001;107:61–67. doi: 10.1067/mai.2001.111590. [DOI] [PubMed] [Google Scholar]
- 4.Gochicoa-Rangel L, Larios-Castaneda PJ, Miguel-Reyes JL, et al. PIKO-6(R) vs. forced spirometry in asthmatic children. Pediatric pulmonology. 2014;49:1170–1176. doi: 10.1002/ppul.22996. [DOI] [PubMed] [Google Scholar]
- 5.Rothe T, Karrer W, Schindler C. Accuracy of the piko-1 pocket spirometer. The Journal of asthma: official journal of the Association for the Care of Asthma. 2012;49:45–50. doi: 10.3109/02770903.2011.643522. [DOI] [PubMed] [Google Scholar]
- 6.Brouwer AF, Roorda RJ, Duiverman EJ, Brand PL. Reference values for peak flow and FEV1 variation in healthy schoolchildren using home spirometry. The European respiratory journal. 2008;32:1262–1268. doi: 10.1183/09031936.00148107. [DOI] [PubMed] [Google Scholar]
- 7.Phipatanakul W, Bailey A, Hoffman EB, et al. The school inner-city asthma study: design, methods, and lessons learned. The Journal of asthma: official journal of the Association for the Care of Asthma. 2011;48:1007–1014. doi: 10.3109/02770903.2011.624235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. The European respiratory journal. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 9.Aguilar-Fernandez AJ, Villa-Asensi JR, Castro-Codesal M, Almeria-Gil E, Gonzalez-Alvarez MI, Romero-Andujar F. Concordance between the Piko - 1 portable device and pneumotachography in measuring PEF and FEV(1) in asthmatic children. Allergologia et immunopathologia. 2009;37:244–248. doi: 10.1016/j.aller.2009.03.004. [DOI] [PubMed] [Google Scholar]


