Abstract
Measures of core cognitive processes (fluid abilities) are highly correlated with measures of knowledge (crystallized abilities) in healthy adults. In early stages of Alzheimer’s disease (AD), fluid abilities, however, decline more rapidly than crystallized abilities. We hypothesized that cognitively-normal older adults who evidenced lower fluid ability compared with crystallized ability (an ability discrepancy) would show evidence of early AD neuropathology indexed via in vivo measures of beta-amyloid (Aβ) deposition and cortical thickness in AD-vulnerable regions. A sample of older adults (n = 112) aged 65 to 89 underwent a cognitive battery, structural MRI, and a subset (n = 75) also completed PET scanning to measure Aβ deposition using F-18 Florbetapir. Of this sample, 60 older adults (43 with available PET scans) evidenced a discrepancy where fluid ability was lower than crystallized ability. The magnitude of the ability discrepancy was independently associated with a greater Aβ deposition and thinner cortex in AD-vulnerable regions, as well as age. The data suggest that such a discrepancy may be a marker of preclinical AD.
Keywords: ability discrepancy, aging, beta-amyloid, cortical thickness, dementia, preclinical
1.1 Introduction
Detecting Alzheimer’s disease (AD) early is of great importance, especially since potential interventions are likely to be most effective at the earliest stages of the disease (Sperling et al., 2011b). AD is characterized by marked declines in episodic memory and aggregation of plaques composed of amyloid-beta (Aβ), neuronal tangles (composed of tau protein filaments), and cortical atrophy. Both Aβ imaging and autopsy results have yielded evidence that a significant proportion of cognitively-normal older adults (approximately 25–45%) harbor Aβ deposits (e.g., Bennett et al., 2006; Mintun et al., 2006; Pike et al., 2007; Price & Morris, 1999)—a phase characterized as preclinical AD (e.g., Jack et al., 2012, 2013; Sperling et al., 2011a). Studies have increasingly reported that cognitively-normal older adults with elevated levels of Aβ show subtle behavioral deficits on cognitively challenging tasks, particularly episodic memory (e.g., Hedden et al., 2013; Resnick et al., 2010). A number of researchers also have reported effects in other domains, including processing speed, executive function, and reasoning (e.g., Rodrigue et al., 2012; Snitz et al., 2013; Wirth et al., 2013a, 2013b). Collectively, these deficits are all in cognitive domains that comprise fluid abilities and, like episodic memory, evidence decline in early stages of AD (e.g., Albert et al., 2011; Bastin & Salmon, 2014; Kaszniak, 1986; Rosen, 1983). In the present study, we utilized behavioral theories and measures commonly used in studies of cognitive aging as an important predictor of preclinical AD. Specifically, we used a “discrepancy” score based on the subtraction of fluid from crystallized ability and correlated this score with two AD brain markers: Aβ deposition and cortical thinning (Bakkour et al., 2009; Dickerson et al., 2009; Wong et al., 2010).
Fluid ability represents a set of core processes underlying intelligence that includes processing speed, working memory, and reasoning (e.g., Wechsler, 1944, 1997). These core abilities are drawn upon to engage in complex thought, solve problems, and function in everyday life (e.g., Johnson et al., 2004; Kaszniak, 1986; Lezak, 1995; Wechsler, 1944). Fluid ability tasks are typically unfamiliar and do not rely on past experience to perform the required mental operations. In contrast, people do bring accrued knowledge and experience to many situations and this experiential component of intelligence has been termed “crystallized ability.” Crystallized ability is usually assessed by vocabulary scores (e.g., Ekstrom et al., 1976; Wechsler, 1944; Zachary & Shipley, 1986) or word pronunciation scores (e.g., the National Adult Reading Test; Blair & Spreen, 1989).1 In cognitively-normal adults, fluid and crystallized abilities are highly correlated with one another (r = .70; Cattell, 1965, see also Kaufman et al., 1996), suggesting that individuals with higher fluid ability also have higher crystallized ability. At the same time, it is well-known that individuals afflicted with AD show declines in fluid ability faster than declines in crystallized ability (e.g., O’Carroll & Gilleard, 1986; Wechsler, 1944) and that fluid ability decline is predictive of the rate of symptom progression in AD (e.g., Albert et al., 2011; Bastin & Salmon, 2014; McKhann et al., 2011; Schmid et al., 2013; Tabert et al., 2006). Because crystallized ability is spared initially in AD (e.g., O’Carroll & Gilleard, 1986; Wechsler, 1944), it is considered to be a good measure of premorbid cognitive ability (e.g., Lehrl et al., 1995; Wechsler, 1944; Yates, 1956).
These differential rates of AD-related cognitive decline led us to reason that older adults who had a higher crystallized ability compared to their fluid ability (a positive ability discrepancy score) might harbor AD neuropathology. Consistent with this idea, a greater ability discrepancy has been shown not only to differentiate healthy adults from those with probable AD, but these discrepancies also have been shown to increase as disease severity worsens (e.g., Dierckx et al 2008; Lezak, 1995; McCarthy et al., 2005). Hence, we hypothesized that larger discrepancy scores between crystallized versus fluid abilities in healthy adults would be associated with individuals in a preclinical stage of AD as evidenced by elevated levels of Aβ and more cortical thinning in AD-sensitive brain regions.
Our primary aim was to use ability discrepancy scores to distinguish between normal and pathological aging. It is important to note, however, that ability discrepancies also have been observed in older relative to younger adults in cross-sectional analyses (e.g., Eisdorfer et al., 1959; Rabbitt, 1993). These age discrepancies are small in magnitude and could occur because the samples used included adults at the preclinical AD stage. In a similar way, consider that normal aging is characterized by a relatively modest memory deficit compared with young adults (e.g., Hertzog et al., 2003; Park et al., 1996), but AD is characterized by a very large memory deficit (e.g., McKhann et al., 2011). Perhaps differences in ability discrepancy parallel memory deficits in that a small discrepancy characterizes normal older adults, but a large discrepancy is associated with elevated levels of Aβ and more cortical thinning—both signs of preclinical AD. Thus, the modest ability discrepancy effect that occurs in normal older adults does not preclude that large differences in this marker might be diagnostic of AD pathology.
We hypothesized that ability discrepancy might covary with the aforementioned brain markers of preclinical AD. To address this issue, 1) the analyses between ability discrepancy and AD brain markers controlled for age, and 2) we focused the analyses on older adults that had positive ability discrepancy scores while also conducting supplemental analyses to test whether individuals with an exaggerated ability discrepancy were more likely to be associated with AD brain markers relative to other older adults in the sample.
2.1 Material and Methods
2.2 Participants
We drew our sample from 112 adults aged 65–89 who underwent MRI scanning, genotyping, and cognitive testing to measure fluid and crystallized ability as a part of the Dallas Lifespan Brain Study (DLBS). From these 112, we selected only subjects who had an ability discrepancy score (z-score crystallized minus z-score fluid ability) greater than zero (n = 60). Subjects with negative scores were excluded, as they were unrelated to our research question. In fact, they might be associated with non-dementia-related impairments due to early deficits in vocabulary ability (e.g., Copet et al., 2010; Satz, 1976). All 60 subjects selected had structural MRI data, and those that had behavioral data collected within 12 months also were invited to undergo PET scanning (43 of the 60; for more PET recruitment details, see Rodrigue et al., 2012).
All subjects were community-dwelling volunteers who were free from neurological conditions, major cardiovascular and psychiatric disorders, and no reported head injury with loss of consciousness > 10 min. Subjects also were excluded for reported drug/alcohol abuse, and major heart surgery or chemotherapy within 5 years of testing. All subjects were fluent English speakers, right-handed, and provided written informed consent as approved by the university human investigations committee guidelines. A summary of the sample characteristics is provided in Table 1.
Table 1.
Demographic Characteristics as a Function of Sample
| Full Sample | Ability Discrepancy > 0 |
Ability Discrepancy > 0 with PET |
Ability Discrepancy ≤ 0 |
|
|---|---|---|---|---|
| N | 112 (75 with PET) |
60 | 43 | 52 (32 with PET) |
| Age (years) | 75.58 (7.1) | 76.28 (7.2) | 76.14 (7.0) | 74.77 (7.0) |
| Age Range | 65–89 | 65–89 | 65–89 | 65–88 |
| Sex (M/F) | 41/71 | 29/31a | 23/20 | 12/40 |
| Education (years) | 16.13 (2.5) | 16.75 (2.5)a | 16.93 (2.5) | 15.41 (2.4) |
| Education Range | 12–21 | 12–21 | 12–21 | 12–21 |
| MMSE Score | 27.75 (1.3) | 27.78 (1.2) | 27.84 (1.2) | 27.71 (1.3) |
| MMSE Range | 26–30 | 26–30 | 26–30 | 26–30 |
| Mean Cortical Thickness |
2.69 (0.16) | 2.70 (0.16) | 2.71 (0.13) | 2.68 (0.17) |
| Mean Cortical Thickness Range |
2.00–3.03 | 2.00–3.03 | 2.39–3.03 | 2.15–2.95 |
| Mean Cortical Aβ | 1.23 (0.18) | − | 1.25 (0.19) | 1.22 (0.17) |
| Mean Cortical Aβ Range |
0.97–1.77 | − | 1.07–1.77 | 0.97–1.58 |
| N Aβ+ (%) | 31 (41%) | − | 17 (40%) | 14 (44%) |
| Discrepancy Score | −0.01 (1.0) | 0.69 (0.6) | 0.69 (0.6) | −0.82 (0.7) |
| Discrepancy Score Range |
−2.97–2.32 | 0.01–2.32 | 0.01–2.32 | −2.97–−0.03 |
Notes. Standard deviation is in parentheses; Mean cortical thickness and Aβ deposition is calculated across all regions of interest;
Subjects with an ability discrepancy score greater than zero were more educated and were more likely to be male than those with a score zero or less (p’s < .01).
2.3 Overall Protocol
All subjects had completed two neuropsychological assessment visits and one visit for MRI scanning. For those subjects with Aβ data, a fourth visit for a PET scan was completed.
2.3.1 Neuropsychological Assessment
To assess fluid and crystallized abilities, we selected measures available from the DLBS that were highly reliable, represented multiple domains of cognition, and are commonly used across many studies (e.g., Johnson et al., 2004; Wechlser, 1997). The six fluid measures spanned the cognitive domains of processing speed, working memory, and reasoning. Processing speed was measured using the number of correct items on the Digit Symbol task (Wechsler, 1997) and the Digit Comparison task (Hedden et al., 2002; adapted from Salthouse & Babcock, 1991). Working memory was measured using the number of correct items on the Letter-Number Sequencing task (Wechsler, 1997) and the sum of the number of words in each set on perfectly recalled trials on the Operation Span task (Turner & Engle, 1989). Reasoning was measured using the total score on the ETS Letter Sets task (Ekstrom et al., 1976) and accuracy on the Raven’s Progressive Matrices task (Raven, 1938). Crystallized ability was measured using the number of correct items for both the ETS Advanced Vocabulary Test I-V4 (Ekstrom et al., 1976) and the Shipley Vocabulary (Zachary & Shipley, 1986). We submitted all of these measures to a factor analysis, confirming that the six fluid measures and two crystallized measures would generate separate factor loadings (see section 2.4.1).
2.3.2 Structural MRI Acquisition & Processing
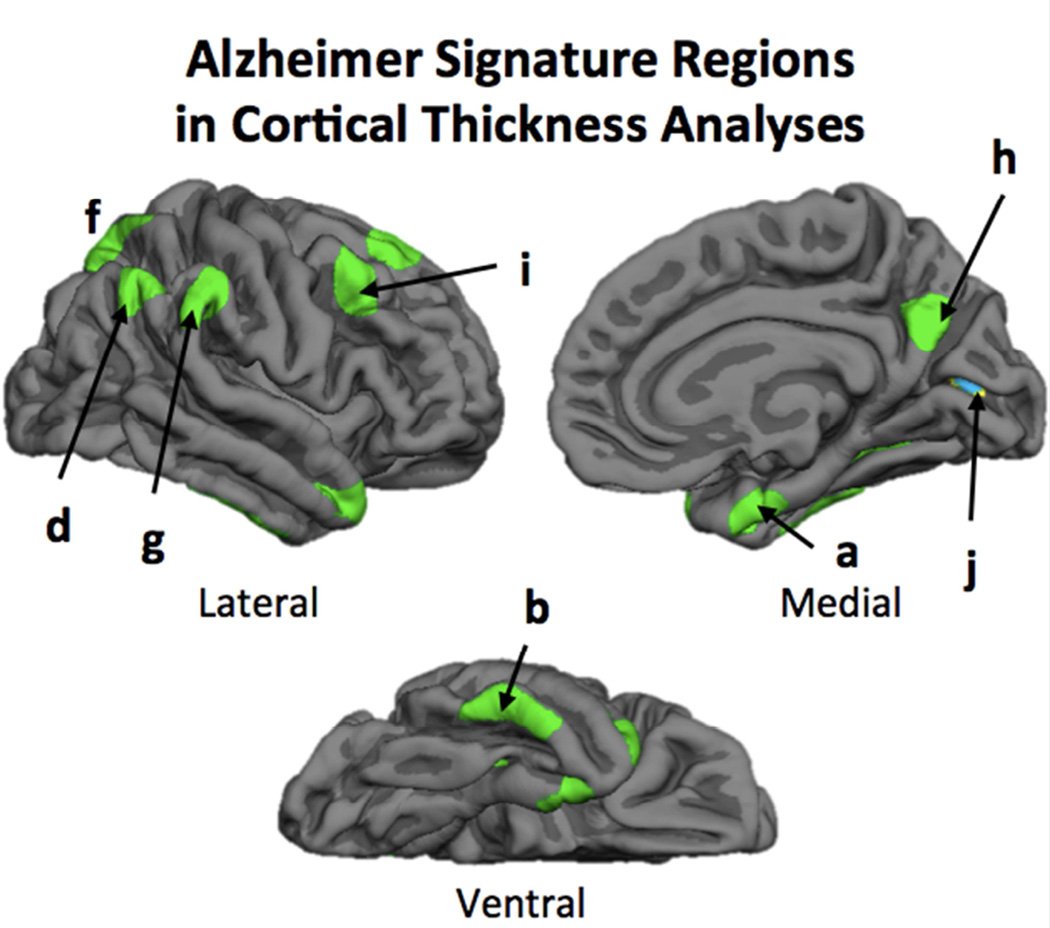
Subjects underwent MRI scanning on a 3T Philips Achieva scanner equipped with an 8-channel head coil. High-resolution anatomical images were collected using a T1-weighted MP-RAGE sequence with 160 sagittal slices, 1 × 1 × 1 mm3; 256 × 256 × 160 matrix, TR = 8.18 ms, TE = 3.76 ms, flip-angle = 12°, FOV = 220 mm. To es timate cortical thickness, T1-weighted images were processed using a surface-based processing stream provided by FreeSurfer v5.1 (http://surfer.nmr.mgh.harvard.edu), including bias-field correction, intensity normalization, skull-stripping (Dale et al., 1999; Segonne et al., 2004). Following these steps, FreeSurfer segments of gray/white matter and pial surfaces were used to estimate distance between boundaries, which were checked for accuracy and edited, if necessary, by extending white matter boundaries or removing non-brain tissue. The resulting surface maps were then transformed to a common space and smoothed using 10 mm FWHM Gaussian filter. From these maps, nine bilateral regions of interest (ROIs) were hand traced on the cortical surface and averaged across hemispheres and included anterior medial temporal lobe, inferior temporal gyrus, temporal pole, angular gyrus, superior frontal gyrus, superior parietal lobule, supramarginal gyrus, precuneus, and inferior frontal sulcus (see Figure 1). These ROIs were selected based on prior research identifying them as regions vulnerable to AD, also known as AD signature regions (Bakkour et al., 2009; Dickerson et al., 2009). An additional bilateral region in the occipital cortex served as a control ROI.
Figure 1.
The nine AD signature regions were hand-traced in FreeSurfer and included a) anterior medial temporal lobe, b) inferior temporal gyrus, c) temporal pole, d) angular gyrus, e) superior frontal gyrus, f) superior parietal lobule, g) supramarginal gyrus, h) precuneus, and i) inferior frontal sulcus. The occipital cortex (j) was used as a control region.
2.3.3 PET Imaging Acquisition and Processing
To measure Aβ deposition, subjects underwent PET scanning with 18F-Florbetapir (Avid Radiopharmaceuticals). Subjects were injected with a 370 MBq (10 mCi) bolus of 18F-Florbetapir for a 10 min emission and a 10 min transmission scan. A 2-frame by 5-min each dynamic emission acquisition protocol was started 50 min post-injection. An internal rod source transmission scan was acquired for 7 min. The transmission image was reconstructed using back projection and a 6 mm FWHM Gaussian filter. The emission images were processed by iterative reconstruction, 4 iterations and 16 subsets with a 3 mm FWHM ramp filter. Each subject’s PET scan was spatially normalized to a Florbetapir uptake template (2 × 2 × 2 mm3 voxels) using SPM8 and in-house MATLAB scripts and visually inspected for registration quality. Aβ counts were assessed from eight bilateral ROIs in MNI space in regions where amyloid typically aggregates in AD patients (Wong et al., 2010). These ROIs included subregions in orbital frontal, dorsolateral prefrontal, anterior cingulate, posterior cingulate, lateral temporal, lateral parietal, and occipital cortex, as well as precuneus. To minimize inclusion of nonspecific white matter binding in these ROIs, we identified the gray/white matter boundary threshold in the PET images in a separate group of young adults (age < 35, n = 9), and eroded each ROI by the resulting binarized white matter mask. Within these final ROIs, counts were extracted, averaged across voxels within each ROI, and then normalized by counts in the gray matter of the cerebellum to produce standardized uptake value ratios (SUVRs).
2.4 Statistical Analysis
All statistical analyses were performed using SPSS version 20 (IBM Corp., Armonk, NY). Bias-corrected and accelerated confidence intervals were determined using 1,000 bootstrap samples and significance was set at p < .05.
2.4.1 Deriving Measures of Fluid and Crystallized Ability
Fluid and crystallized measures were first calculated using factor analysis. Factor analysis was chosen because it is a widely used technique that captures similar variance across measures to help reduce data to a smaller set of measures. Specifically, the eight neuropsychological measures (two each for processing speed, working memory, reasoning, and vocabulary) were z-transformed and then entered into a factor analysis using direct oblimin rotation (Kline, 1994). This rotation was chosen because it allows the resulting factors to be correlated with one another, thus better representing the true underlying nature of the data. We intentionally constrained the model to two factors because of our a priori interest in fluid and crystallized abilities, but we also inspected the resulting eigenvalues to ensure that two factors were the best fit for the dataset.
2.4.2 Calculating the Ability Discrepancy Score
Using the results from the factor analysis, we subtracted the fluid score from the crystallized score to create an ability discrepancy score for each subject. Increasingly positive values represented an increasingly greater discrepancy in ability (e.g. Kaufman, 1990; Matarazzo & Herman, 1985; Schretlen et al, 1994). Our primary analyses focused on individuals that had an ability discrepancy score greater than zero.
2.4.3 The Effects of Ability Discrepancy across Regions on Cortical Thickness
Here, we tested the hypothesis that older adults with higher positive discrepancy scores would have lower cortical thickness across the nine AD signature regions. We conducted a mixed analysis of covariance (ANCOVA) with ability discrepancy score treated as a continuous independent variable, ROIs as a within-subjects independent variable, and their interaction. The dependent measure was cortical thickness. This analysis allowed us to test whether ability discrepancy exerted a consistent effect across all nine ROIs or whether there was an interaction, suggesting that ability discrepancy exerted a different relationship across the ROIs. Testing this interaction enabled a closer inspection of the robustness of this relationship, and could potentially reveal particularly sensitive regions to preclinical AD. In addition, we controlled for age and sex in the analysis to generalize the results to all older age groups (65–90 year olds) and both sexes. These analyses provided input for which ROIs to include as summary scores to simplify the data when testing for independent associations of ability discrepancy and preclinical AD brain markers (see section 2.4.4).
2.4.4 The Effects of Ability Discrepancy across Regions on Cortical Aβ Deposition
In this analysis, we tested the hypothesis that ability discrepancy scores would exert a main effect on Aβ deposition. We also assessed whether the effects exerted a consistent effect across the eight regions utilized from which Aβ SUVRs were extracted. We conducted an ANCOVA with ability discrepancy as a continuous variable, ROI as a within-subjects variable, and their interaction. The dependent measure was a continuous measure of Aβ deposition. We also controlled for age and sex. In addition to treating Aβ deposition as a continuous variable, we also used a threshold approach, whereby Aβ was treated as a categorical value and subjects were classified as Aβ+ or Aβ−. Using the threshold value of 1.22 (see Rodrigue et al., 2012), 17 (40%) were classified as Aβ+ and 26 (60%) as Aβ- (out of 43 subjects) for older adults in the positive ability discrepancy group. In the negative discrepancy group, 14 (44%) were classified as Aβ+ and 18 (56%) as Aβ- (out of 32 subjects). These proportions are consistent with previous research suggesting that approximately 25–45% of cognitively-normal older adults harbor Aβ deposits (e.g., Bennett et al., 2006; Mintun et al., 2006; Pike et al., 2007; Price & Morris, 1999). This categorical approach is commonly used (e.g., Dickerson et al., 2009; Landau et al., 2012; Okello et al., 2009; Resnick et al., 2010; Snitz et al., 2013; Wirth et al., 2013a, 2013b) and provided additional reliability of the results.
2.4.5 Independent Contributions of Cortical Thickness and Aβ Deposition on Ability Discrepancy
Because cortical thickness and Aβ deposition are both markers of preclinical AD, they might not provide independent information regarding the status of preclinical AD. On the other hand, models of preclinical AD (e.g., Jack et al., 2012, 2013) propose that Aβ deposition precedes neurodegeneration (e.g., cortical thinning), potentially leading to independent contributions of each measure. To assess whether the two types of AD brain markers independently affected ability discrepancy, we also conducted a multiple regression analysis. We treated cortical thickness and Aβ deposition as predictor variables and ability discrepancy as the outcome variable. ROIs that were significant in the ANCOVAs described above were averaged separately to derive a summary measure of cortical thickness and Aβ deposition. As in the previous analyses, age and sex were included as covariates. To maximize degrees of freedom, backward regression was used to eliminate insignificant higher-order factors.
In addition to the primary regression analysis, a supplemental regression analysis was conducted on the full sample with Group (Positive and Negative Discrepancy) as a between subjects factor. An interaction with Group and either of the AD brain markers would provide evidence that any of the relationships with the AD brain markers depended on which end of the ability discrepancy scale older adults fell.
3.1 Results
3.2 Factor Analysis of Neuropsychological Assessment
The factor analysis yielded two factors that, as expected, clearly differentiated crystallized from fluid abilities. For the first factor, the zero-order factor loadings (i.e., structure coefficients) were largest for Shipley Vocabulary (.91) and ETS Advanced Vocabulary (.84), thus representing crystallized ability. This factor explained 32.20% of the variance in the data. For the second factor, the factor loadings, in order of magnitude, were Digit Symbol (.74), Digit Comparison (.69), ETS Letter Sets (.65), Letter Number Sequencing (.46), Raven’s (.45), and Operation Span (.45). The second factor was designated as the fluid ability factor and explained an additional 16.57% of the variance. The other eigenvalues were less than 1.0 (the third eigenvalue had a value of .88), and explained little variance beyond the first two factors. Consistent with prior work (e.g., Cattell, 1965; Kaufman et al., 1996), the crystallized and fluid ability factors were positively correlated with one another, r (112) = .47, p < .001, CI (.32, .61).
3.3 Calculating Ability Discrepancy Scores and Partitioning Groups
Subtracting fluid from crystallized ability resulted in an ability discrepancy score for each subject that ranged from −2.97 to 2.32 (M = −0.01). Sixty of the 112 subjects had an ability discrepancy score greater than zero as well as MRI scans and hence were retained for the primary analyses pertaining to cortical thickness. Of these 60 subjects, 43 subjects were retained for the Aβ analysis as they had completed both MRI and PET scans. A comparison of the positive and negative discrepancy groups indicated that the two groups did not differ in chronological age, MMSE, cortical thickness averaged across the nine ROIs, or Aβ averaged across the eight ROIs (all p’s > .26). However, the positive group had higher levels of education (M = 16.82 vs. 15.57; t (110) = 2.75, p = .007) and relatively more men than women (48% male/52% females vs. 23% male/77% female; χ2 (112) = 7.66, p = .006) compared with the negative group. In the negative group, mean cortical thickness was not related to mean cortical Aβ (r(28) = .02, p = .94). We next assessed the same associations within the positive group. No relationship was observed between mean cortical thickness and mean cortical Aβ (r(39) = −.23, p = .15). Among age, sex, education, and MMSE, only age significantly correlated with ability discrepancy (r(60) = .26, p = .05; for the other variables all p’s > .49).
3.4 Effects of Ability Discrepancy on Cortical Thickness
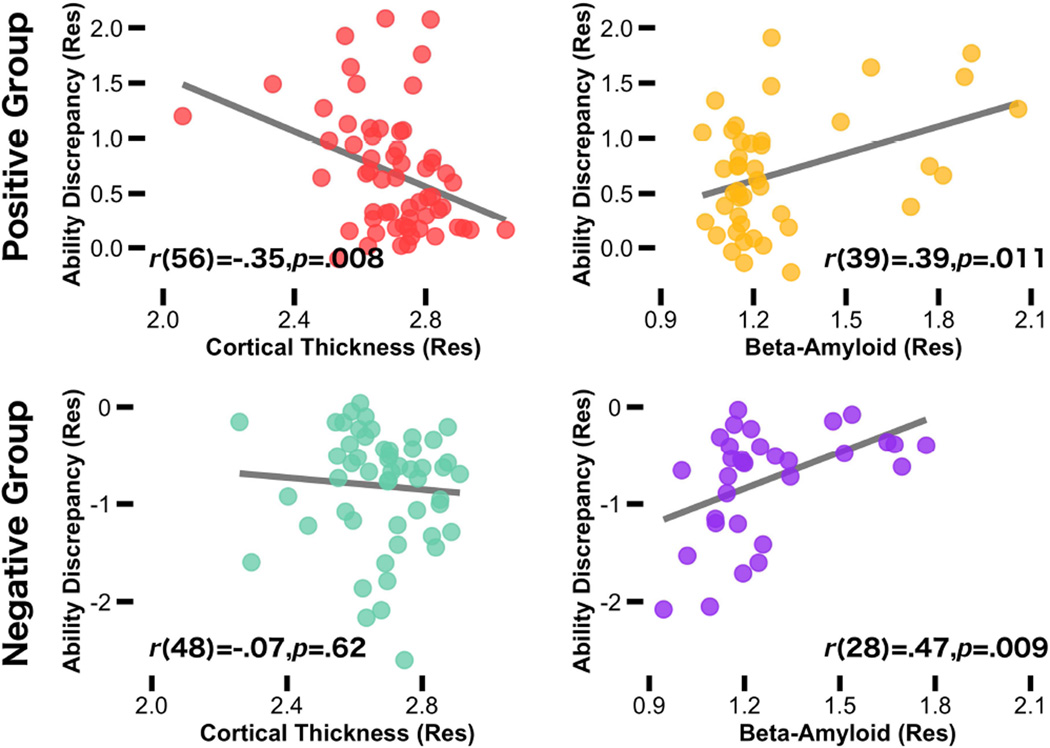
We conducted an Ability discrepancy × ROI ANCOVA on cortical thickness. This ANCOVA yielded a main effect of ability discrepancy and of ROI, but no interaction. The ability discrepancy effect was significant (F (1, 56) = 7.54, p = .008, ηp2 = .12); subjects with a greater ability discrepancy had lower cortical thickness. The accompanying parameter estimates for the main effect of ROI (F (8, 448) = 5.49, p < .001, ηp2 = .09) indicated that temporal regions were thicker than frontal and parietal regions (t (59) = 16.42, p < .001, d = 2.36 and t (59) = 26.64, p < .001, d = 3.77, respectively), and frontal regions were thicker than parietal regions (t (59) = 15.67, p < .001, d = 1.63). However, the absence of a significant interaction (F (8, 448) = 1.15, p = .33, ηp2 = .02) suggested that the ability discrepancy effect generalized across regions.2 Thus, the cortical thickness values in each of the nine AD signature regions were averaged together to form a composite cortical thickness score for subsequent analyses. Cortical thickness as a function of ability discrepancy is displayed in Figure 2 (top left).3 Notably, thickness in the control region (primary visual cortex) was not associated with ability discrepancy (p = .38).
Figure 2.
Scatterplot shows the relationship between AD brain markers and ability discrepancy in all participants as a function of ability discrepancy group (positive and negative). In the positive discrepancy group, discrepancies in ability (crystallized minus fluid) were associated with lower mean cortical thickness across brain regions previously shown to be vulnerable to AD (red), and elevated Aβ deposition (yellow). In the negative discrepancy group, discrepancies in ability were associated with greater Aβ deposition (purple), but no relationship was found for cortical thickness (green). Age and sex were controlled in all variables. Removing the subject with the lowest mean cortical thickness score did not change the strength of the correlation.
3.4 Effects of Ability Discrepancy on Cortical Aβ Deposition
We conducted an Ability discrepancy × ROI ANCOVA on Aβ Deposition. This ANCOVA yielded a main effect of ability discrepancy (F (1, 39) = 6.40, p = .016, ηp2 = .14), which was significant because, as predicted, subjects with a higher ability discrepancy score had more Aβ deposition. The main effect of ROI was not significant (p > .38). However, an ability discrepancy × ROI interaction was significant (F (7, 273) = 4.38, p < .001, ηp2 = .10), suggesting that the relationship between ability discrepancy and Aβ deposition differed across the ROIs. The parameter estimates from the ANCOVA indicated that the ability discrepancy effect was observed across only four of the eight ROIs: precuneus, temporal cortex, posterior cingulate, and anterior cingulate (Table 2). Marginal significance was observed for dorsolateral prefrontal cortex (p = .053), parietal (p = .077) and orbitofrontal cortex (p = .10) and non-significance was observed for occipital cortex (p > .16).4 Because of these observed regional differences, Aβ values were averaged across only the four significant regions to form a composite cortical Aβ score for subsequent analysis. Figure 2 (top right) displays the aggregated values for Aβ as a function of ability discrepancy. Using a threshold approach, the Aβ+ group had larger ability discrepancy scores than the Aβ- group (MAβ+ = .91 & MAβ- = 0.54; t (41) = 2.16, SEM = .18, p = .037). When including subjects in the full sample of 112 individuals, ability discrepancy was numerically larger in the Aβ+ than Aβ- group (MAβ+ = 0.30 & MAβ- = −0.089), but did not reach significance t (73) = 1.78, SEM = .22, p = .078).
Table 2.
Relationship Ability Discrepancy and Cortical Aβ across the Significant Regions of Interest
| Factor | β | SE | Lower CI | Upper CI | p-value | ηp2 |
|---|---|---|---|---|---|---|
| Precuneus | .27 | .089 | .09 | .45 | .004 | .19 |
| Temporal Cortex | .12 | .046 | .03 | .22 | .011 | .15 |
| Posterior Cingulate | .18 | .070 | .03 | .32 | .016 | .14 |
| Anterior Cingulate | .19 | .090 | .006 | .37 | .044 | .10 |
Notes. β = Standardized beta values; SE = Standard error; CI = Confidence intervals. Age and Sex were controlled.
3.5 Independent Contributions of Cortical Thickness and Aβ on Ability Discrepancy
Using backward regression, we entered chronological age, sex, mean cortical thickness, mean cortical Aβ, and mean cortical thickness × mean cortical Aβ interaction to predict ability discrepancy. As shown in Table 3, the final regression model was significant (F (4, 38) = 5.74, MSE = .24, p = .001) and explained 37.7% of the variance in ability discrepancy. Mean cortical thickness (p = .05) and mean cortical Aβ (p = .03) were each significantly related to ability discrepancy and accounted for 6.5% and 8.1% unique variance in the model, respectively. The interaction term (Thickness × Aβ) was not significant (p = .57), and was removed from the model. Age (p = .02) also accounted for unique variance (9.7%), but sex did not (p = .83).5
Table 3.
Effects of Chronological Age, Mean Cortical Aβ, and Mean Cortical Thickness in Multiple Regression Models Associated with Ability Discrepancy
| Factor | β | b | SE | Lower CI | Upper CI | p-value |
|---|---|---|---|---|---|---|
| Sex | −.031 | −.036 | .17 | −.37 | .30 | .83 |
| Chronological Age | .35 | .030 | .012 | .005 | .055 | .02 |
| Mean Cortical Thickness | −.28 | −1.27 | .64 | −2.56 | .018 | .05 |
| Mean Cortical Aβ | .30 | .67 | .30 | .059 | 1.29 | .03 |
Notes. β = Standardized beta values; b = Unstandardized beta values; SE = Standard error; CI = Confidence intervals; the model explained a total of 37.2% of the variance in ability discrepancy.
These findings are based on individuals that had a small to large positive ability discrepancy (Range = 0.01 – 2.32). To test the extent that the relationship between ability discrepancy and AD brain markers was greater in older adults with a positive ability discrepancy compared with those with a negative ability discrepancy, we conducted the final multiple regression on the full sample (n = 112) with group (Positive and Negative Discrepancy) as a factor and interactions with each of the AD brain markers to predict ability discrepancy (Figure 2, bottom panel). We found a significant group × mean cortical thickness interaction (β = 1.71, p = .045), but not a significant group × mean cortical Aβ interaction (β = 0.44, p = .42). Follow-up partial correlations (controlling for age × sex) indicated that the relationship between ability discrepancy and cortical thickness was specific to the positive ability discrepancy group (r(39) = −.37, p = .018 and r(28) = .08, p = .67, for the positive and negative ability discrepancy group, respectively). This finding suggests that all ranges of ability discrepancy scores are associated with increased Aβ deposition, but only exaggerated ability scores (in the positive direction) are associated with declines in cortical thickness.
4.1 Discussion
The present study sought to assess whether a positive difference between crystallized and fluid ability in cognitively normal adults was associated with two brain markers of preclinical AD—cortical thinning and Aβ deposition. Results indicated that (a) decreased thickness and greater Aβ deposition in AD-related regions were associated with a larger discrepancy score; (b) both cortical thickness and Aβ deposition were significant and independent factors associated with a greater positive ability discrepancy score; (c) these relationships were significant in both positive and negative discrepancy groups for Aβ deposition, but only in the positive discrepancy group for cortical thickness; and (d) age was independently associated with ability discrepancy after accounting for cortical thickness and Aβ deposition.
The cortical thickness regions selected as ROIs have previously been found to be specific to mild AD in four independent samples by Dickerson and colleagues (2009). In Dickerson et al. (2009), cortical thickness was reduced in AD patients compared with cognitively-normal controls and was associated with the severity of cognitive symptoms in the AD patients. Moreover, cortical thickness in these regions also was negatively associated with an in vivo measure of Aβ deposition in cognitively-normal older adults (e.g., Bakkour et al., 2009; Dickerson et al., 2009). Our finding that cortical thinning in these same regions was associated with higher ability discrepancy scores suggests that ability discrepancy may be an early behavioral indicator of early stages of preclinical AD in currently normal adults. This relationship was only found in older adults with more exaggerated discrepancies in ability (i.e., the positive ability discrepancy group), suggesting that this marker is sensitive in later stages of preclinical AD, when neurodegeneration has already began. We also report that, consistent with Dickerson et al. (2009), the relationship between ability discrepancy and thickness did not occur in primary visual cortex, providing specificity of the thickness effects to AD-sensitive regions. Although recent models of AD focus on hippocampal volume as the primary structural measure that declines in the cascade of events towards AD (e.g., Jack et al., 2012, 2013), the present findings support the notion that cortical thinning in these AD-related regions might be included as a marker of preclinical AD as well (e.g., Bakkour et al., 2009; Dickerson et al., 2009).
Next we turn to the findings regarding Aβ deposition. The presence of Aβ at autopsy is one requirement for a diagnosis of AD (McKhann et al., 2011). There is growing evidence that Aβ deposition in cognitively normal adults is associated with subtle fluid processing decline (e.g., Hedden et al., 2013; Rodrigue et al., 2012; Snitz et al., 2013; Wirth et al., 2013a, 2013b) and later is related to the progression to mild cognitive impairment (e.g., Blasko et al., 2008; Okello et al., 2009), resulting in the categorization of such individuals as having preclinical AD. In the present study, we report that a greater magnitude of ability discrepancy was consistently associated with more Aβ deposition. We found this relationship both in the positive and negative ability discrepancy groups, suggesting this marker is sensitive to both early and later stages of preclinical AD.
This Aβ effect on ability discrepancy was specific to regions associated with AD such as the precuneus (e.g., Braak & Braak, 1991), but was absent in the occipital cortex, a region that shows little accumulation of Aβ in the earliest stages of AD (Rodrigue, 2012; Vlassenko et al., 2012). This specificity nicely dovetails with the cortical thickness findings that also showed the same dissociation with the occipital cortex. The convergence of the cortical thickness and Aβ deposition findings strengthen evidence for the two markers together serving as a behavioral index of preclinical AD.
4.3 Ability Discrepancy Scores: Normal Aging or Neuropathology?
The results suggest that an ability discrepancy score might provide unique information, in the absence of expensive imaging data, regarding the increased likelihood that an individual is harboring Aβ and is already exhibiting signs of neurodegeneration. In the context of this conclusion, it is important to consider whether ability discrepancy is a specific indicator of neuropathology or merely a general characteristic of normal aging. There is some evidence that ability discrepancy increases with age (e.g., Eisdorfer et al., 1959; Rabbitt, 1993). It is well-recognized that cross-sectional studies of aging typically yield a decrease in fluid abilities from young adulthood into old age, while crystallized ability remains stable or even increases with age (e.g., Baltes, 1987; Park et al., 1996). Recently, an impressive study sampling over 48,000 adults found that performance on fluid measures such as processing speed peaked as early as late teens/early 20’s, whereas crystallized abilities peaked later in the 50’s (Hartshorne & Germine, 2015). These different peaks in performance, on the surface, suggest that ability discrepancies are characteristic of normal aging and may not confer important information regarding early stages of pathology.
However, there are two important reasons that the present findings support the use of a greater discrepancy score as a potential indicator of preclinical AD. First, many subjects in presumably “normal” samples will have latent neuropathology, including Aβ deposition, and are on an initial trajectory of pathological decline (cf. Sliwinski et al., 1996). In such cases, those individuals might actually drive the discrepancies between fluid and crystallized abilities found in other studies, rather than the cognitively-normal adults without latent neuropathology. Second, even if such discrepancies do exist in normal aging, the present results show that the magnitude of ability discrepancy might separate normal from pathological aging. Just as a moderate memory decline occurs almost universally with advanced age (e.g., Hertzog et al., 2003; Park et al., 1996) and a precipitous memory decline is diagnostic of AD, the same appears to be true for an ability discrepancy; a moderate ability discrepancy occurs with advanced age, but a precipitous increase in discrepancy is diagnostic of latent pathology and early neurodegeneration. The fact that we found the effects of Aβ deposition and cortical thickness on ability discrepancy after chronological age was statistically controlled further bolsters this idea.
We are unaware of any studies that have related AD risk factors to ability discrepancy, but the extant literature does show that patients with AD evidence greater ability discrepancies than cognitively-normal adults and these discrepancies increase as disease severity worsens (e.g., Dierckx et al 2008; Lezak, 1995; McCarthy et al., 2005). In addition, longitudinal evidence has shown that the combination of increases in Aβ and greater cortical thinning is associated with steeper declines in cognition (e.g., Dickerson & Wolk, 2012; Landau et al., 2012; Resnick et al., 2010) and greater likelihood to convert to AD (e.g., Dickerson et al., 2011; Julkunen et al., 2010; Landau et al., 2012; Okello et al., 2009; Querbes et al., 2009). Together, these sources of information converge on the conclusion that a large ability discrepancy is, at the least, an additional signal to researchers and clinicians that an individual harbors AD brain markers and therefore might be in the preclinical stages of AD. In the presence of cognitive complaints or other symptoms, further investigation into an individual’s condition is warranted.
Using an ability discrepancy score has an important advantage over other measures of cognition such as memory. Because crystallized ability often is used as a premorbid measure of ability, the difference between fluid and crystallized ability could serve as a proxy for longitudinal decline in a cross-sectional sample. Thus, unlike cross-sectional measures of memory, intra-individual variability is not confounded with longitudinal decline. As more longitudinal data become available, we would predict that older adults with an exaggerated ability discrepancy would exhibit faster rates of cognitive decline and eventual clinical symptomology of AD. However, until these predictions can be confirmed, it is premature to diagnose a cognitively-normal individual with a high ability discrepancy score as having preclinical AD, but it is more reasonable to consider a high discrepancy score as evidence for increased risk that an individual harbors Aβ deposition and cortical thinning in AD signature regions.
5.1 Conclusion
One of the most pressing questions in aging research is determining whether some older adults are on a pathological aging trajectory toward dementia or on a normal aging trajectory. The current study utilizes theoretical models of cognitive aging to direct attention toward discrepancies between fluid and crystallized abilities as a significant risk factor that has not been considered in the recent past. These discrepancies might be important in predicting who is more likely to be in the preclinical stages of AD in a cross-sectional sample.
Highlights.
A much lower fluid than crystallized ability may signify preclinical dementia.
A greater ability discrepancy was related to a thinner cortex and more amyloid.
Cortical thickness and amyloid independently correlated with ability discrepancy.
Acknowledgments
Role of funding source:
Contract grant sponsor: National Institute on Aging; Contract grant numbers: 5R37AG-006265-25 (D.C.P.); 3R37AG-006265-25S1 (D.C.P.); 4 R00-AG-036818-04 (K.M.K.); R00-AG-36848-04 (K.M.R.); Contract grant sponsor: Alzheimer’s Association; Contract grant number: IIRG-09-135087 (D.C.P.). Avid Radiopharmaceuticals provided the radiotracer for the study and supported some research costs, but did not provide stipends, travel, or salary to any of the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
The authors declare no competing financial interests.
Because vocabulary and word pronunciation measures are highly correlated with one another (r = .78; e.g., Blair & Spreen, 1989; O’Carroll & Gilleard, 1986), we consider them both under the term “crystallized ability.”
Controlling for APOE status and education did not change the significance of the results, nor was APOE status or education a significant factor (all p’s > .05). Because we had a modest age range (65–89), we also tested whether age interacted with ability discrepancy, but no interaction with age was significant (all p’s > .46).
One subject did have a mean cortical thickness value about 4 standard deviations below the group mean thickness level (subject mean thickness = 2.00 mm, group mean thickness = 2.70 mm). Visual inspection of the FreeSurfer segmentation did not reveal clear errors in the segmentation, but rather verified substantial atrophy throughout the cortex. Nevertheless, the above analyses were re-run excluding this subject with nearly identical results, suggesting that this person did not inflate the relationships between ability discrepancy and cortical thickness. This subject also did not undergo PET scanning, and so was not included in the subsequent PET analyses.
Controlling for APOE status did not change the significance of the results, nor was APOE status a significant factor (p = .64). Controlling for education changed the significance between ability discrepancy and Aβ in the anterior cingulate from p = .044 to p = .058, although education itself was not significant (p > .05). Ability discrepancy did not interact with age (all p’s > .50).
Adding APOE status in the model did not change the significance of the results, nor was APOE status a significant factor (p > .05). Controlling for education changed the significance between ability discrepancy and Aβ in the anterior cingulate from p = .050 to p = .055, although education itself was not significant (p > .05). Conducting separate regression analyses adding interactions with age yielded no significant interactions with age (all p’s > .16).
References
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkour A, Morris JC, Dickerson BC. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology. 2009;72:1048–1055. doi: 10.1212/01.wnl.0000340981.97664.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes PB. Theoretical Propositions of Life-Span Developmental-Psychology - on the Dynamics between Growth and Decline. Dev Psychol. 1987;23:611–626. [Google Scholar]
- Bastin C, Salmon E. Early neuropsychological detection of Alzheimer’s disease. Eur J Clin Nutr. 2014;68:1192–1199. doi: 10.1038/ejcn.2014.176. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Blair JR, Spreen O. Predicting premorbid IQ: A revision of the National Adult Reading Test. Clin Neuropsychol. 1989;3:129–136. [Google Scholar]
- Blasko I, Jellinger K, Kemmler G, Krampla W, Jungwirth S, Wichart I, Tragl KH, Fischer P. Conversion from cognitive health to mild cognitive impairment and Alzheimer’s disease: prediction by plasma amyloid beta 42, medial temporal lobe atrophy and homocysteine. Neurobiology of aging. 2008;29:1–11. doi: 10.1016/j.neurobiolaging.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Cattell RB. The scientific analysis of personality. Baltimore: Penguin Books; 1965. [Google Scholar]
- Copet P, Jauregi J, Laurier V, et al. Cognitive profile in a large French cohort of adults with Prader-Willi syndrome: differences between genotypes. J Intellect Disabil Res. 2010;54:204–215. doi: 10.1111/j.1365-2788.2010.01251.x. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Stoub TR, Shah RC, et al. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76:1395–1402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Wolk DA, Alzheimer’s Disease Neuroimaging I. MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults. Neurology. 2012;78:84–90. doi: 10.1212/WNL.0b013e31823efc6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierckx E, Engelborghs S, De Raedt R, et al. Differentiation between dementia and depression among older persons: can the difference between actual and premorbid intelligence be useful? J Geriatr Psychiatry Neurol. 2008;21:242–249. doi: 10.1177/0891988708324938. [DOI] [PubMed] [Google Scholar]
- Eisdorfer C, Busse EW, Cohen LD. The WAIS performance of an aged sample: the relationship between verbal and performance IQs. J Gerontol. 1959;14:197–201. doi: 10.1093/geronj/14.2.197. [DOI] [PubMed] [Google Scholar]
- Ekstrom R, French J, Harman H, Dermen D. Manual for Kit of Factor Referenced Cognitive Tests. Princeton: Educational Testing Service; 1976. [Google Scholar]
- Hartshorne JK, Germine LT. When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychol Sci. 2015;26:433–443. doi: 10.1177/0956797614567339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 2013;80:1341–1348. doi: 10.1212/WNL.0b013e31828ab35d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Park DC, Nisbett R, Ji LJ, Jing Q, Jiao S. Cultural variation in verbal versus spatial neuropsychological function across the life span. Neuropsychology. 2002;16:65–73. doi: 10.1037//0894-4105.16.1.65. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Dixon RA, Hultsch DF, et al. Latent change models of adult cognition: Are changes in processing speed and working memory associated with changes in episodic memory? Psych Aging. 2003;18:755–769. doi: 10.1037/0882-7974.18.4.755. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Weigand SD, et al. An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71:765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W, Bouchard TJ, Krueger RF, et al. Just one g: consistent results from three test batteries. Intelligence. 2004;32:95–107. [Google Scholar]
- Julkunen V, Niskanen E, Koikkalainen J, et al. Differences in cortical thickness in healthy controls, subjects with mild cognitive impairment, and Alzheimer’s disease patients: a longitudinal study. J Alzheimers Dis. 2010;21:1141–1151. doi: 10.3233/jad-2010-100114. [DOI] [PubMed] [Google Scholar]
- Kaszniak AW. The neuropsychology of dementia. In: Grant I, Adams KM, editors. Neuropsychological assessment of neuropsychiatric disorders. Oxford: Oxford University Press; 1986. pp. 172–220. [Google Scholar]
- Kaufman AS. Assessing adolescent and adult intelligence. Boston: Allyn and Bacon; 1990. [Google Scholar]
- Kaufman AS, McLean JE, Lincoln A. The Relationship of the Myers-Briggs Type Indicator (MBTI) to IQ Level and the Fluid and Crystallized IQ Discrepancy on the Kaufman Adolescent and Adult Intelligence Test (KAIT) Assessment. 1996;3:225–239. [Google Scholar]
- Kline P. An easy guide to factor analysis. New York: Routledge; 1994. [Google Scholar]
- Landau SM, Mintun MA, Joshi AD, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72:578–586. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrl S, Triebig G, Fischer B. Multiple-Choice Vocabulary-Test MWT as a Valid and Short Test to Estimate Premorbid Intelligence. Acta Neurol Scand. 1995;91:335–345. doi: 10.1111/j.1600-0404.1995.tb07018.x. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 3rd. New York: Oxford University Press; 1995. [Google Scholar]
- Matarazzo JD, Herman DO. Clinical uses of the WAIS–R: Base rates of differences between VIQ and PIQ in the WAIS–R standardization sample. In: Wolman BB, editor. Handbook of Intelligence: Theories, measurements and applications. New York: Wiley; 1985. pp. 899–932. [Google Scholar]
- McCarthy F, Burns WJ, Sellers AH. Discrepancies between premorbid and current IQ as a function of progressive mental deterioration. Percept Mot Skills. 2005;100:69–76. doi: 10.2466/pms.100.1.69-76. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- O’Carroll RE, Gilleard CJ. Estimation of premorbid intelligence in dementia. Br J Clin Psychol. 1986;25:157–158. doi: 10.1111/j.2044-8260.1986.tb00690.x. [DOI] [PubMed] [Google Scholar]
- Okello A, Koivunen J, Edison P, et al. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C–PIB PET study. Neurology. 2009;73:754–760. doi: 10.1212/WNL.0b013e3181b23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Smith AD, Lautenschlager G, et al. Mediators of long-term memory performance across the life span. Psych Aging. 1996;11:621–637. doi: 10.1037//0882-7974.11.4.621. [DOI] [PubMed] [Google Scholar]
- Pike KE, Savage G, Villemagne VL, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Querbes O, Aubry F, Pariente J, et al. Early diagnosis of Alzheimer’s disease using cortical thickness: impact of cognitive reserve. Brain. 2009;132:2036–2047. doi: 10.1093/brain/awp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt P. Does It All Go Together When It Goes - the 19th Bartlett Memorial Lecture. Q J Exp Psychol-A. 1993;46:385–434. doi: 10.1080/14640749308401055. [DOI] [PubMed] [Google Scholar]
- Raven JC. Progressive matrices: A perceptual test of intelligence. London: HK Lewis; 1938. [Google Scholar]
- Resnick SM, Sojkova J, Zhou Y, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010;74:807–815. doi: 10.1212/WNL.0b013e3181d3e3e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy KM, Devous MD, et al. beta-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012;78:387–395. doi: 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen WG. Neuropsychological investigation of memory, visuoconstructional, visuoperceptual, and language abilities in senile dementia of the Alzheimer type. Adv Neurol. 1983;38:65–73. [PubMed] [Google Scholar]
- Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Dev Psychol. 1991;27:763–776. [Google Scholar]
- Satz P. Cerebral dominance and reading disability: An old problem revisited. In: Knights RM, Bakker DJ, editors. The neuropsychology of learning disorders: Theoretical approaches. 1976. pp. 273–294. [Google Scholar]
- Schmid NS, Taylor KI, Foldi NS, Berres M, Monsch AU. Neuropsychological signs of Alzheimer’s disease 8 years prior to diagnosis. J Alzheimers Dis. 2013;34:537–546. doi: 10.3233/JAD-121234. [DOI] [PubMed] [Google Scholar]
- Schretlen D, Benedict RH, Bobholz JH. Composite reliability and standard errors of measurement for a seven-subtest short form of the Wechsler Adult Intelligence Scale— Revised. Psychol Assess. 1994;6:188–190. [Google Scholar]
- Segonne F, Dale AM, Busa E, et al. A hybrid approach to the skull-stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Sliwinski M, Lipton RB, Buschke H, et al. The effects of preclinical dementia on estimates of normal cognitive functioning in aging. J Gerontol B Psychol Sci Soc Sci. 1996;51:217–225. doi: 10.1093/geronb/51b.4.p217. [DOI] [PubMed] [Google Scholar]
- Snitz BE, Weissfeld LA, Lopez OL, et al. Cognitive trajectories associated with beta-amyloid deposition in the oldest-old without dementia. Neurology. 2013;80:1378–1384. doi: 10.1212/WNL.0b013e31828c2fc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Jack CR, Jr, Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med. 2011;3:111–133. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabert MH, Manly JJ, Liu X, et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006;63:916–924. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]
- Turner M, Engle R. Is working memory capacity task dependent? J Mem Lang. 1989;28:127–154. [Google Scholar]
- Vlassenko AG, Benzinger TL, Morris JC. PET amyloid-beta imaging in preclinical Alzheimer’s disease. Biochim Biophys Acta. 2012;1822:370–379. doi: 10.1016/j.bbadis.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The measurement of adult intelligence. Baltimore: Williams and Wilkins; 1944. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale–III (WAIS-III) New York: Psychological Corporation; 1997. [Google Scholar]
- Wirth M, Madison CM, Rabinovici GD, Oh H, Landau SM, Jagust WJ. Alzheimer’s disease neurodegenerative biomarkers are associated with decreased cognitive function but not beta-amyloid in cognitively normal older individuals. J Neurosci. 2013;33:5553–5563. doi: 10.1523/JNEUROSCI.4409-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth M, Oh H, Mormino EC, Markley C, Landau SM, Jagust WJ. The effect of amyloid beta on cognitive decline is modulated by neural integrity in cognitively normal elderly. Alzheimers Dement. 2013;9:687–698. doi: 10.1016/j.jalz.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Rosenberg PB, Zhou Y, et al. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F–AV-45 (flobetapir F 18) J Nucl Med. 2010;51:913–920. doi: 10.2967/jnumed.109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates A. The use of vocabulary in the measurement of intelligence deterioration: A review. J Ment Sci. 1956;102:409–440. doi: 10.1192/bjp.102.428.409. [DOI] [PubMed] [Google Scholar]
- Zachary RA. Shipley WC. Shipley institute of living scale: Revised manual. Los Angeles: Western Psychological Services; 1986. [Google Scholar]