Abstract
Cochlear implantation is an effective, established procedure for patients with profound deafness. Although implant electrodes have been considered as biocompatible prostheses, surgical insertion of the electrode induces various changes within the cochlea. Immediate changes include insertional trauma to the cochlea. Delayed changes include a tissue response consisting of inflammation, fibrosis and neo-osteogenesis induced by trauma and an immunologic reaction to a foreign body. The goal of this study was to evaluate the effect of these delayed changes on the word recognition scores achieved post-operatively. Seventeen temporal bones from patients who in life had undergone cochlear implantation were prepared for light microscopy. We digitally calculated the volume of fibrous tissue and new bone within the cochlea using Amira® three-dimensional reconstruction software and assessed the correlations of various clinical and histologic factors. The postoperative CNC word score was positively correlated with total spiral ganglion cell count. Fibrous tissue and new bone were found within the cochlea of all seventeen specimens. The postoperative CNC word score was negatively correlated with the % volume of new bone within the scala tympani, scala media/vestibuli and the cochlea, but not with the % volume of fibrous tissue. The % volume of new bone in the scala media/vestibuli was positively correlated with the degree of intracochlear insertional trauma, especially trauma to the basilar membrane. Our results revealed that the % volume of new bone as well as residual total spiral ganglion cell count are important factors influencing post-implant hearing performance. New bone formation may be reduced by limiting insertional trauma and increasing the biocompatibility of the electrodes.
Keywords: Cochlear implant, fibrous tissue, new bone, insertional trauma, word recognition score, human inner ear
1. Introduction
Cochlear implantation is an effective procedure for auditory rehabilitation of patients with severe to profound deafness (Somdas et al., 2007; Benatti et al., 2013; Seyyedi and Nadol, 2014a), and implant electrodes have been considered as biocompatible prostheses with a low complication rate (Seyyedi and Nadol, 2014a). However, surgical insertion of the electrode induces immediate and delayed changes within the cochlea (Li et al., 2007; Somdas et al., 2007; Fayad et al., 2009). Immediate intracochlear changes are due to trauma during surgical insertion of the electrode including fracture of the osseous spiral lamina, disruption of the basilar membrane, dissection into the spiral ligament and stria vascularis, and injury to the lateral cochlear wall and modiolus (Li et al., 2007; Somdas et al., 2007). Delayed changes are due to both insertional trauma and to a host tissue response to the electrode consisting of inflammation, fibrosis and neo-osteogenesis (Li et al., 2007; Somdas et al., 2007; Fayad et al., 2009). These changes have been reported in several histopathological studies of temporal bone specimens from patients who in life had undergone cochlear implantation (Nadol and Eddington, 2004; Nadol et al., 2008; Fayad et al., 2009; Nadol et al., 2014; Seyyedi and Nadol, 2014a). Nadol and Eddington (2004) reported a robust fibrous and bony tissue response in all 21 ears at the cochleostomy site and an inflammatory cellular response in 12 of the 21 temporal bones. Nadol et al. (2008) have suggested an immunologic response to the electrode as a possible explanation in some cases of “soft failure” of cochlear implantation. Nadol et al. (2014) have suggested that a foreign body response may in certain cases result in migration or even extrusion of the electrode. Seyyedi and Nadol (2014a) reported fibrosis and new bone formation in all 28 temporal bones and a foreign body giant cell infiltration and granulomatous reaction in 27 of 28 temporal bones. Somdas et al. (2007), Li et al. (2007), and Fayad et al. (2009) have reported quantitative assessments of fibrosis and new bone formation in the cochlea following cochlear implantation in the human, but Li et al. (2007) have reported that the total volume of intra cochlear new tissue did not correlate with word recognition scores. However, other studies suggest that fibrous tissue and new bone may affect patterns of stimulation around the electrode (Kawano et al., 1998; Choi and Oghalai, 2005; O’Leary et al., 2013). Kawano et al. (1998) demonstrated that auditory thresholds of individual electrodes were increased and dynamic ranges were decreased with increasing amounts of fibrous tissue and new bone in a temporal bone study of five patients with Nucleus 22-channel cochlear implants. Choi and Oghalai (2005) and O’Leary et al. (2013) have suggested that the severity of local tissue response may be negatively correlated with both performance after implantation and preservation of the residual acoustic hearing using mathematical (Choi and Oghalai, 2005) and animal (O’Leary et al., 2013) models. However, other than the report by Li et al (2007) there have been few quantitative studies of correlations between postoperative hearing performance and the volume of fibrous tissue and new bone in human subjects. The objectives of this study were to quantitatively evaluate the % volumes of fibrosis and of new bone within the cochlea of patients who in life had undergone cochlear implantation using three-dimensional reconstruction software, to assess correlations between the calculated volumes and postoperative word recognition scores, and to evaluate the factors which may influence the generation of fibrous tissue and new bone.
2. Materials and Methods
2.1. Subjects
Seventeen human temporal bones from the collection of the Otopathology Laboratory of the Massachusetts Eye and Ear Infirmary from patients who in life had undergone cochlear and Case 7. In Case 1, the CNC word score was estimated based on the postoperative last-recorded HINT (Hearing In Noise Test) score, and in Case 7, NU6 (Northwestern University Auditory Test #6) was used. Timplantation using various electrode designs were evaluated. Cases with new bone formation before implantation based on evaluation of preoperative CT scans and operative records were excluded. Postoperative last-recorded word recognition scores (CNC [Consonant-Vowel Nucleus-Consonant Word Test] scores) were available in all cases except in Case 1 he word recognition score, cause of deafness, age at onset of hearing loss and deafness, implant type and ear, age at implantation, and duration of implantation in all seventeen specimens are presented in Table 1.
Table 1.
Demographics of cases studied.
| Case | Age at death (years) /sex |
Otologic disease | Age at onset of hearing loss (years) |
Age at onset of deafness (years) |
Cochlear implant | Implanted ear |
Postoperative word recognition score (CNC, %) |
Age at the last-recorded word recognition score (years) |
Age at implant (years) |
Duration of implantation (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 94/F | Genetic (suspected) | 56 | 82 | Advanced Bionics CII + P | AD | 10* | 90 | 82 | 143 |
| 2 | 89/F | Presbycusis | 60 | 79 | Advanced Bionics CII | AS | 10 | 87 | 80 | 97 |
| 3 | 82/M | Presbycusis/ Noise exposure |
45 | 61 | Advanced Bionics CII + P | AS | 15 | 82 | 73 | 105 |
| 4 | 96/M | Genetic (suspected) | 50 | 90 | Nucleus Freedom | AD | 20 | 93 | 91 | 61 |
| 5 | 66/M | Mondini’s deformity | 8 | 40 | Nucleus 22 | AS | 26 | 64 | 47 | 223 |
| 6 | 57/M | Superficial siderosis | 36 | 51 | Nucleus 24 | AD | 28 | 51 | 51 | 80 |
| 7 | 84/F | Presbycusis | 63 | 71 | Nucleus 22 | AD | 34** | 78 | 72 | 151 |
| 8 | 83/M | Otosclerosis | 16 | 52 | Nucleus 22 | AS | 36 | 72 | 60 | 276 |
| 9 | 89/F | Genetic (suspected) | 30 | 72 | Nucleus 24 contour | AS | 41 | 84 | 79 | 127 |
| 10 | 82/F | Chronic otitis media/ ISSNHL |
36 | 72 | Advanced Bionics CII | AS | 44 | 81 | 74 | 102 |
| 11 | 81/F | ISSNHL | 29 | 29 | Nucleus 22 | AD | 54 | 79 | 60 | 354 |
| 12 | 89/M | Unknown | 57 | 72 | Nucleus 24 contour | AD | 54 | 88 | 83 | 68 |
| 13 | 71/F | Bulbar polio/ISSNHL | 9 | 59 | Nucleus 24 contour | AD | 56 | 70 | 59 | 139 |
| 14 | 83/M | Meniere’s syndrome | 65 | 71 | Nucleus 24 | AD | 57 | 79 | 71 | 147 |
| 15 | 88/M | Meniere’s syndrome/ Noise exposure |
26 | 70 | Nucleus 24 contour | AD | 60 | 84 | 82 | 74 |
| 16 | 74/F | Otosclerosis | 6 | 53 | Advanced Bionics CII + P | AS | 66 | 69 | 66 | 95 |
| 17 | 89/M | Genetic (suspected)/ Noise exposure |
25 | 85 | Advanced Bionics Hi Res 90K |
AD | 74 | 86 | 86 | 33 |
| Average | 82 | 36 | 65 | 40 | 79 | 72 | 134 | |||
AS indicates left; AD, right; F, female; M, male; P, Positioner; CNC, Consonant-Vowel Nucleus-Consonant Word Test; ISSNHL, idiopathic sudden sensorineural hearing loss.
Estimated CNC score based on HINT (Hearing In Noise Test) score.
NU6 (Northwestern University Auditory Test #6) score
2.2. Histological techniques
All temporal bones were removed and fixed in 10% buffered formalin. After decalcification in ethylene diamine tetra-acetic acid (EDTA) the electrode array was removed and, in those cases with a positioner, the positioner was retained within the cochlea during the remainder of the histologic preparation. The specimens were dehydrated in alcohol, embedded in celloidin, and sectioned at a thickness of 20 µm in a horizontal plane. Every tenth section was stained with hematoxylin and eosin, and mounted on a glass slide for subsequent reconstruction and study.
2.3. 2-D reconstruction of the cochlea
Every tenth section was studied by light microscopy. The serial sections of the temporal bones were reconstructed, and the numbers of spiral ganglion cells in Rosenthal’s canal were calculated by conventional 2-D methods (Guild, 1921; Schuknecht, 1993). The most apical section of the cochlea containing the electrode tip in which there was histologic evidence, such as fibrous sheath around the electrode (Li et al., 2007; Lee et al., 2011), and the entry point into the cochlea were determined. The electrode length within the cochlea and the electrode length located in the scala media/vestibuli or spiral ligament were also determined using the 2-D reconstructions.
2.4. 3-D reconstruction of the cochlea
The technique for 3-D reconstruction has been described previously (Li et al., 2007; Somdas et al., 2007). Digital images of each mounted slide were captured at low power (1.25×) under a light microscope fitted with a high-resolution camera (Olympus BX51, Olympus DP71). These images were cropped, resampled, registered, and segmented using the Amira® reconstruction and modeling software (ver. 6.0.0., FEI, Hillsboro, OR). Every tenth section was captured for all seventeen cases, and there was an average of 40 images per case. To determine the dimensions of the voxels, we used an image of a scale taken with the same objective, uploaded it with ImageJ software (http:/rsbweb.nih.gov/ij/), and used Set Scale of ImageJ software to determine the distance per pixel.
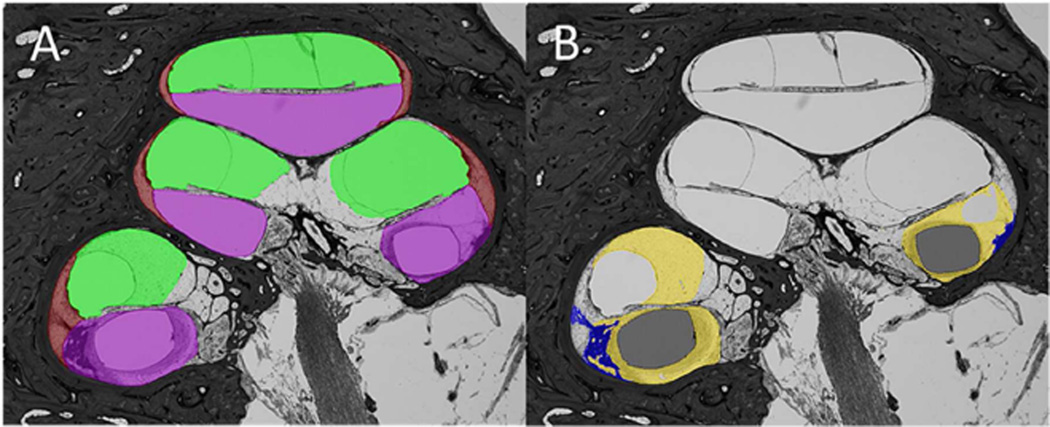
Each 2-D image was segmented by identifying and tagging the different structures within the cochlea duct as follows: purple was used for shading of the scala tympani; green for the scala media/vestibuli; brown for the spiral ligament; yellow for fibrous tissue; blue for new bone; black for the electrode of the implant (Figure 1A, B). Surface renderings of the segmented materials were generated and used to visualize the 3-D reconstruction (Figure 2A – E). Using surface rendering, the volume of each material (scala tympani, scala media/vestibuli, new fibrous tissue, and new bone) was edited with the Amira’s Volume Edit Tool, and measured using the Amira’s Surface Area/Volume Tool, and the % volume of the electrode, fibrous tissue, new bone and total new tissue (total of new fibrous tissue and new bone) within the scala tympani, scala media/vestibuli, and the cochlear lumen were calculated.
Figure 1.
Digitally segmented image of a histological section of the implanted cochlea of Case 17.
A: Showing scala tympani (purple), scala media/vestibuli (green), and spiral ligament (brown).
B: Showing electrode (black), new bone (blue), and new fibrous tissue (yellow).
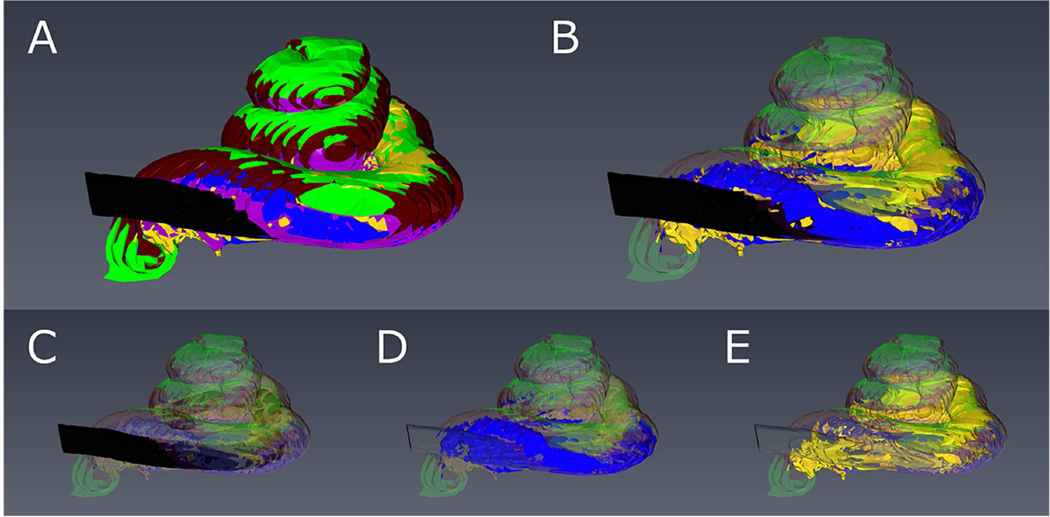
Figure 2.
3-D reconstruction of the implanted right cochlea of Case 17 showing scala tympani (purple), scala media/vestibuli (green), spiral ligament (brown), electrode (black), new bone (blue), and new fibrous tissue (yellow).
A: All elements are shaded. B: Electrode, new bone and fibrous tissue are shaded, and the others are transparent. C: Only the electrode is shaded. D: Only new bone is shaded. E: Only new fibrous tissue is shaded.
2.5. Quantification of intracochlear damage caused by electrode insertion
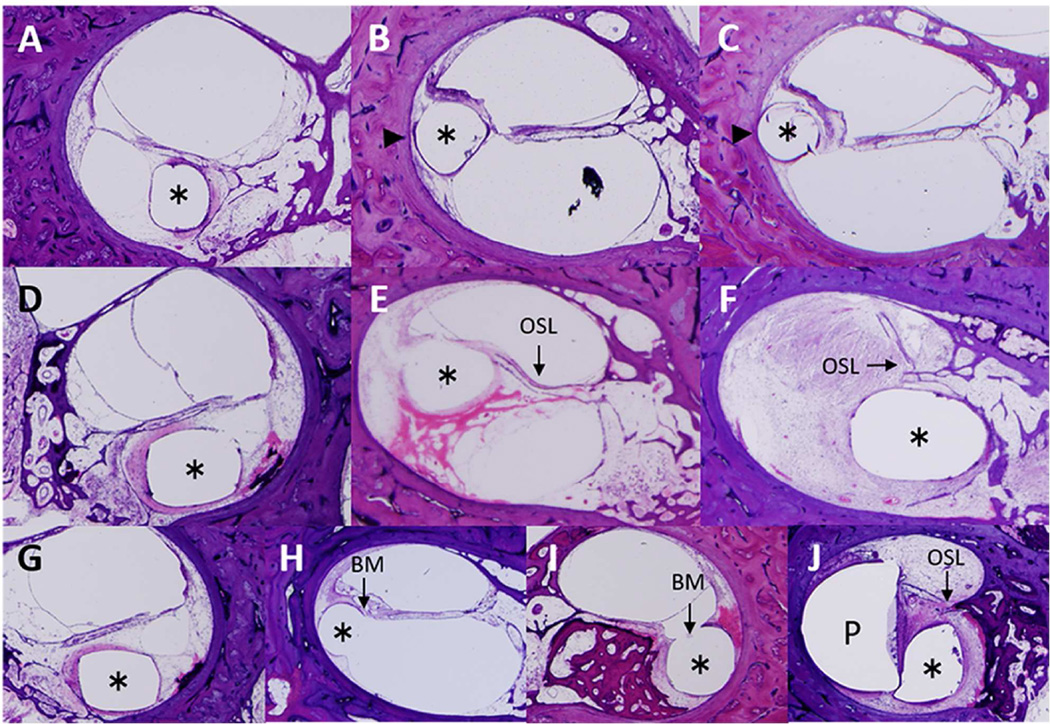
The degree of electrode insertional trauma was assessed by adapting a method previously described (Li et al., 2007; Lee et al., 2011) and presented in Table 2. Damage to the osseous spiral lamina, lateral cochlear wall and basilar membrane was used as markers for insertional trauma. To provide an overall index of damage in each temporal bone, in every tenth section along the electrode track a score was assigned indicating the severity of insertional trauma from 0 to 2 as described and illustrated in Figure 3A – J. These values were summed along the cochlear duct to generate a total damage score for each subject.
Table 2.
Quantification of new tissue formation and damage caused by implant.
| Case | % Volume of New Tissue | Total Spiral Ganglion Cell counts (cells) |
Damage Score | Electrode Length Located in SM/SV or SL (mm) |
Intracochlear Electrode Length (mm) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scala Tympani | Scala Media/Vestibuli | Total | Lateral Cochlear Wall (LCW) |
Osseous Spiral Lamina (OSL) |
Basilar Membrane |
LCW + OSL |
Total | ||||||||||
| New Fibrous Tissue |
New Bone |
Total New Tissue |
New Fibrous Tissue |
New Bone |
Total New Tissue |
New Fibrous Tissue |
New Bone |
Total New Tissue |
|||||||||
| 1 | 32.03 | 20.97 | 53.00 | 31.30 | 8.99 | 40.29 | 31.68 | 15.30 | 46.98 | 2040 | 118 | 67 | 109 | 185 | 294 | 24 (EL+P) 9 (only EL) |
25 |
| 2 | 32.68 | 43.33 | 76.01 | 18.96 | 11.71 | 30.67 | 24.79 | 25.13 | 49.92 | 3257 | 84 | 21 | 95 | 105 | 200 | 17 26 (EL+P) |
17 |
| 3 | 27.66 | 14.40 | 42.06 | 15.87 | 2.74 | 18.61 | 20.32 | 7.14 | 27.46 | 5226 | 120 | 51 | 124 | 171 | 295 | 26 (only EL) | 26 |
| 4 | 42.98 | 45.90 | 88.88 | 11.64 | 5.61 | 17.25 | 25.22 | 23.07 | 48.29 | 6467 | 12 | 32 | 0 | 44 | 44 | 22 | 22 |
| 5 | 57.40 | 37.74 | 95.14 | 82.39 | 4.55 | 86.94 | 73.77 | 16.01 | 89.78 | 5718 | 47 | 2 | 78 | 49 | 127 | 12 | 12 |
| 6 | 55.51 | 13.20 | 68.71 | 0.08 | 0.00 | 0.08 | 26.20 | 6.22 | 32.42 | 1298 | 38 | 0 | 25 | 38 | 63 | 5 | 13 |
| 7 | 38.79 | 5.73 | 44.52 | 0.00 | 0.00 | 0.00 | 20.49 | 3.03 | 23.52 | 11527 | 46 | 11 | 37 | 57 | 94 | 9 | 18 |
| 8 | 47.32 | 25.99 | 73.31 | 7.59 | 2.32 | 9.91 | 24.02 | 12.11 | 36.13 | 7310 | 54 | 12 | 36 | 66 | 102 | 17 | 17 |
| 9 | 22.12 | 26.12 | 48.24 | 0.03 | 0.00 | 0.03 | 10.89 | 12.85 | 23.74 | 4543 | 14 | 5 | 0 | 19 | 19 | 0 | 21 |
| 10 | 29.67 | 36.20 | 65.87 | 10.00 | 0.43 | 10.43 | 19.41 | 17.55 | 36.96 | 14375 | 61 | 8 | 45 | 69 | 114 | 9 | 17 |
| 11 | 61.54 | 2.69 | 64.23 | 25.47 | 1.19 | 26.66 | 42.12 | 1.88 | 44.00 | 14259 | 27 | 10 | 26 | 37 | 63 | 14 | 14 |
| 12 | 23.22 | 14.12 | 37.34 | 4.05 | 1.23 | 5.28 | 13.35 | 7.48 | 20.83 | 2856 | 53 | 46 | 45 | 99 | 144 | 13 | 21 |
| 13 | 16.37 | 15.07 | 31.44 | 3.64 | 3.43 | 7.07 | 10.36 | 9.57 | 19.93 | 18918 | 38 | 14 | 33 | 52 | 85 | 9 | 21 |
| 14 | 9.93 | 4.55 | 14.48 | 5.93 | 0.79 | 6.72 | 7.88 | 2.62 | 10.50 | 7690 | 74 | 9 | 58 | 83 | 141 | 15 | 21 |
| 15 | 51.92 | 4.92 | 56.84 | 6.95 | 0.88 | 7.83 | 35.56 | 3.45 | 39.01 | 9854 | 16 | 46 | 27 | 62 | 89 | 0 | 17 |
| 16 | 35.25 | 10.68 | 45.93 | 10.80 | 2.53 | 13.33 | 23.74 | 6.84 | 30.58 | 12322 | 106 | 29 | 105 | 135 | 240 | 16 (EL+P) 3 (only EL) |
21 |
| 17 | 30.23 | 20.65 | 50.88 | 15.10 | 0.00 | 15.10 | 22.81 | 10.53 | 33.34 | 11912 | 20 | 24 | 8 | 44 | 52 | 0 | 20 |
| Average | 36.15 | 20.13 | 56.29 | 14.69 | 2.73 | 17.42 | 25.45 | 10.63 | 36.08 | 8210 | 54.6 | 22.8 | 50.1 | 77.4 | 127.4 | 11 (EL+P) 9 (only EL) |
19 |
SM, scala media; SV scala vestibuli; SL, spiral ligament; EL, electrode; P, positioner.
Figure 3.
Quantification and representative images of damage to the lateral cochlear wall, osseous spiral lamina and basilar membrane caused by the electrode. Asterisk indicates the track of the electrode in all ten images. LCW, lateral cochlear wall; OSL, osseous spiral lamina; BM, basilar membrane.
Lateral cochlear wall A: Damage score 0: No dissection into the spiral ligament as seen in Case 9.
B: Damage score 1: Dissection into the spiral ligament (arrowhead) as seen in Case 14.
C: Damage score 2: Dissection into the spiral ligament and contact with the bony lateral cochlear wall (arrowhead) as seen in Case 14.
Osseous spiral lamina D: Damage score 0: Neither displacement, fracture nor dislocation of the osseous spiral lamina as seen in Case 17.
E: Damage score 1: Displacement of the osseous spiral lamina (arrow) as seen in Case 7.
F: Damage score 2: Fracture and dislocation of the osseous spiral lamina (arrow) as seen in Case 15.
Basilar membrane G: Damage score 0: No displacement of the basilar membrane as seen in Case 17.
H: Damage score 1: Displacement of the basilar membrane (arrow) as seen in Case 13.
I: Damage score 2: Disruption of the basilar membrane (arrow) as seen in Case 10.
J: Damage score 2: Loss of the basilar membrane (P: positioner) as seen in Case 1.
2.6. Statistical analysis
Bivariate analyses and Pearson’s coefficients of correlation were performed to investigate relationships between clinical and histologic variables, using Mini StatMate software (ATMS Co. Ltd., Tokyo, Japan). P < 0.05 was considered significant.
3. Results
3.1. Clinical and histopathological variables (Tables 1, 2)
The clinical variables in the seventeen specimens are presented in Table 1. There were eight females and nine males with an average age at death of 82 years and ranging from 57 to 96 years. There were six Advanced Bionics electrodes (Sylmar, CA, USA) and eleven Cochlear Nucleus electrodes (Cochlear Corp., Sydney, Australia). The average duration of implantation was 134 months, ranging from 33 to 354 months. There were no re-implantation cases. The histologic variables are presented in Table 2. New bone and fibrous tissue were present in all seventeen temporal bones. In Case 6, 7, 9 and 17, no new bone was seen in the scala media/vestibuli, and in Case 7, no fibrous tissue was seen in the scala media/vestibuli. The average total residual spiral ganglion cell count was 8210, ranging from 1298 to 18918. The average damage scores of the lateral cochlear wall (LCW), osseous spiral lamina (OSL), basilar membrane, LCW + OSL, and the total were 54.6, 22.8, 50.1, 77.4, and 127.4 respectively, ranging from 12 to 120, from 0 to 67, from 0 to 124, from 19 to 185, and from 19 to 295 respectively. The average electrode length located in the scala media/vestibuli or spiral ligament was 11 mm (electrode and positioner) and 9 mm (electrode only), ranging from 0 to 26 mm. The average intracochlear electrode length was 19 mm, ranging from 12 to 26 mm.
3.2. Correlation Coefficients between clinical and histopathologic variables (Tables 3, 4)
Table 3.
Correlation coefficients between clinical and histopathologic variables.
| Postoperative CNC score |
Total SPG cell counts (Cells) |
Age at Implant (years) |
Duration of implantation (months) |
Intracochlear % Volume of New Tissue |
Electrode Length Located in SM/SV or SL (mm) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Fibrous Tissue |
New bone | Total | |||||||
| Postoperative CNC score | 1 | 0.6230** | 0.0008 | −0.0596 | −0.2309 | −0.5623* | −0.4218 | −0.6191** | |
| Total SPG cell counts | 1 | −0.1564 | 0.2035 | −0.1063 | −0.2811 | −0.203 | −0.2685 | ||
| Age at Implant (years) | 1 | −0.5869* | −0.4261 | 0.2889 | −0.2551 | 0.0456 | |||
| Duration of implantation (months) | 1 | 0.4171 | −0.2974 | −0.2762 | 0.2066 | ||||
| Intracochlear % | Fibrous Tissue | 1 | 0.1381 | 0.9205*** | 0.0561 | ||||
| Volume of | New Bone | 1 | 0.5141* | 0.2780 | |||||
| New Tissue | Total | 1 | 0.1582 | ||||||
| Electrode Length Located in SM/SV or SL (mm) |
1 | ||||||||
CNC, Consonant-Vowel Nucleus-Consonant Word Test; ST, scala tympani; SM, scala media; SV, scala vestibuli; SL, spiral ligament; SPG, spiral ganglion.
P < 0.05;
P < 0.01;
P < 0.001.
Table 4.
Correlation coefficients between CNC scores and the % volume of new tissue.
| % Volume of New Tissue |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Scala tympani (ST) |
Scala Media/Vestibuli (SM/SV) |
Total (ST + SM/SV) |
|||||||
| Fibrous Tissue |
New bone | Total | Fibrous Tissue |
New bone | Total | Fibrous Tissue |
New bone | Total | |
| CNC score | −0.1736 | −0.5265* | −0.4767 | −0.2956 | −0.6548** | −0.3781 | −0.2309 | −0.5623* | −0.4218 |
P < 0.05;
P < 0.01.
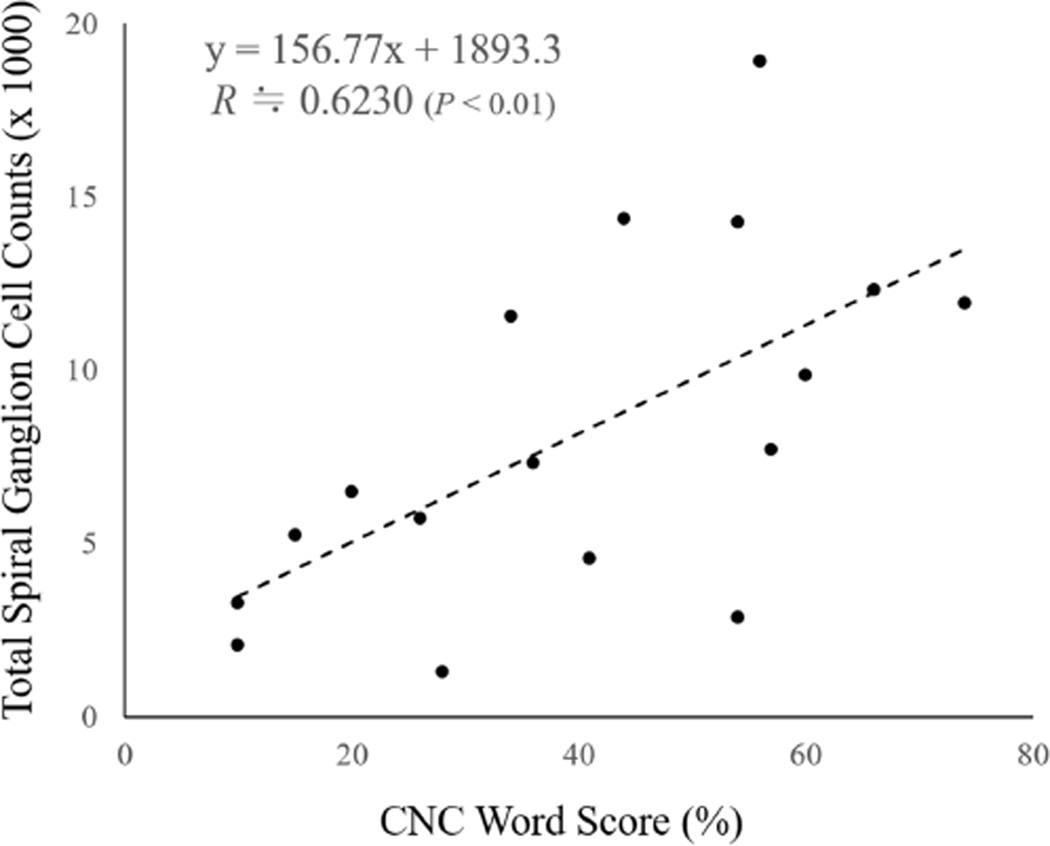
The correlation coefficients between CNC word score, spiral ganglion cell counts, age at implantation, duration of implantation, % volume of intracochlear new tissue and electrode length located in the scala media/vestibuli or spiral ligament are shown in Table 3. CNC word score had a significant positive correlation with total residual spiral ganglion cell counts (R ≒ 0.6230; P < 0.01, Figure 4) and a significant negative correlation with the % volume of new bone within the cochlea and the length of electrode located in the scala media/vestibuli and spiral ligament (R ≒ −0.5623, −0.6191 respectively; P < 0.05 and P < 0.01 respectively). The correlation coefficients between CNC word score and the % volume of new tissue within scala tympani, scala media/vestibuli and the cochlea (scala tympani + scala media/vestibuli) are shown in Table 4. CNC word score had a significant negative correlation only with the % volume of new bone in scala tympani, scala media/vestibuli and the cochlea (R ≒ −0.5265, −0.6548, −0.5623 respectively; P < 0.05, 0.01, 0.05 respectively).
Figure 4.
Scatter plot of the total spiral ganglion cell counts -vs- CNC word scores.
3.3. Correlation coefficients between the % volume of new tissue, damage score and electrode length (Tables 5, 6)
Table 5.
Correlation coefficients between damage scores and the % volume of new tissue within the scala tympani (A), scala media/vestibuli (B) and the cochlea (scala tympani + scala media/vestibuli, C).
| A | |||||||
|---|---|---|---|---|---|---|---|
| Damage Score |
Electrode Length Located in SM/SV or SL (mm) |
||||||
| Lateral Cochlear Wall (LCW) |
Osseous Spiral lamina (OSL) |
Basilar Membrane (BM) |
LCW + OSL |
Total (LCW+OSL +BM) |
|||
| % Volume of New Tissue within ST |
Fibrous Tissue | −0.3003 | −0.1678 | −0.1405 | −0.2918 | −0.2305 | −0.0638 |
| New Bone | −0.0539 | −0.1023 | −0.0012 | −0.0822 | −0.0471 | 0.2090 | |
| Total | −0.2515 | −0.1888 | −0.1016 | −0.2643 | −0.1968 | 0.0940 | |
| B | |||||||
|---|---|---|---|---|---|---|---|
| Damage Score |
Electrode Length Located in SM/SV or SL (mm) |
||||||
| Lateral Cochlear Wall (LCW) |
Osseous Spiral lamina (OSL) |
Basilar Membrane (BM) |
LCW + OSL |
Total (LCW+OSL +BM) |
|||
| % Volume of New Tissue within SM/SV |
Fibrous Tissue | 0.1479 | −0.0217 | 0.3771 | 0.1007 | 0.2319 | 0.2386 |
| New Bone | 0.4467 | 0.3714 | 0.5313* | 0.4843* | 0.5207* | 0.5959* | |
| Total | 0.2079 | 0.0388 | 0.4337 | 0.1702 | 0.2975 | 0.3157 | |
| C | |||||||
|---|---|---|---|---|---|---|---|
| Damage Score |
Electrode Length Located in SM/SV or SL (mm) |
||||||
| Lateral Cochlear Wall (LCW) |
Osseous Spiral lamina (OSL) |
Basilar Membrane (BM) |
LCW + OSL |
Total (LCW+OSL +BM) |
|||
| % Volume of New Tissue within the Cochlea |
Fibrous Tissue | −0.0756 | −0.0768 | 0.1798 | −0.0877 | 0.0335 | 0.0561 |
| New Bone | 0.0534 | 0.0391 | 0.0882 | 0.0557 | 0.0725 | 0.2780 | |
| Total | −0.0444 | −0.0511 | 0.1905 | −0.054 | 0.0576 | 0.1582 | |
CNC indicates Consonant-Vowel Nucleus-Consonant Word Test; ST, scala tympani; SM, scala media; SV, scala vestibuli; SL, spiral ligament; LCW, lateral cochlear wall; OSL, osseous spiral lamina; BM, basilar membrane.
P < 0.05.
Table 6.
Correlation coefficients between the length of the implant electrode located in the scala media/vestibuli or spiral ligament and the damage scores.
| Damage Score |
|||||
|---|---|---|---|---|---|
| Lateral Cochlear Wall (LCW) |
Osseous Spiral Lamina (OSL) |
Basilar Membrane (BM) |
LCW + OSL |
Total (LCW+OSL+ BM) |
|
| Electrode length Located in SM/SV or SL (mm) |
0.6959** | 0.4471 | 0.6538** | 0.7004** | 0.6999** |
P < 0.01.
The correlation coefficients between the % volume of new tissue, intracochlear insertional damage score, and electrode length located in the scala media/vestibuli and spiral ligament are shown in Table 5A–C. Only the % volume of new bone within the scala media/vestibuli was significantly positively correlated with the damage scores of the basilar membrane, the total of the lateral cochlear wall and osseous spiral lamina and the total damage score (R ≒ 0.5313, 0.4843, 0.5207 respectively; P < 0.05 each, Table 5B). There was no significant correlation between the % volume of new bone in the scala media/vestibuli and the damage score of the lateral cochlear wall or osseous spiral lamina alone (R ≒ 0.4467, 3714 respectively; P ≒ 0.0723, 0.1421 respectively, Table 5B). The insertional damage within the cochlea was not significantly correlated with the % volume of new tissue within the scala tympani and the cochlea (Table 5 A, C). The % volume of new bone in the scala media/vestibuli was significantly positively correlated with electrode length located in the scala media/vestibuli or spiral ligament (R ≒ 0.5959, P < 0.05).
The length of electrode located in the scala media/vestibuli or spiral ligament was significantly positively correlated with the damage scores of the lateral cochlear wall, basilar membrane, the total of the lateral cochlear wall and osseous spiral lamina and the total damage score (R ≒ 0.6959, 0.6538, 0.7004, 0.6999 respectively; P < 0.01 each, Table 6).
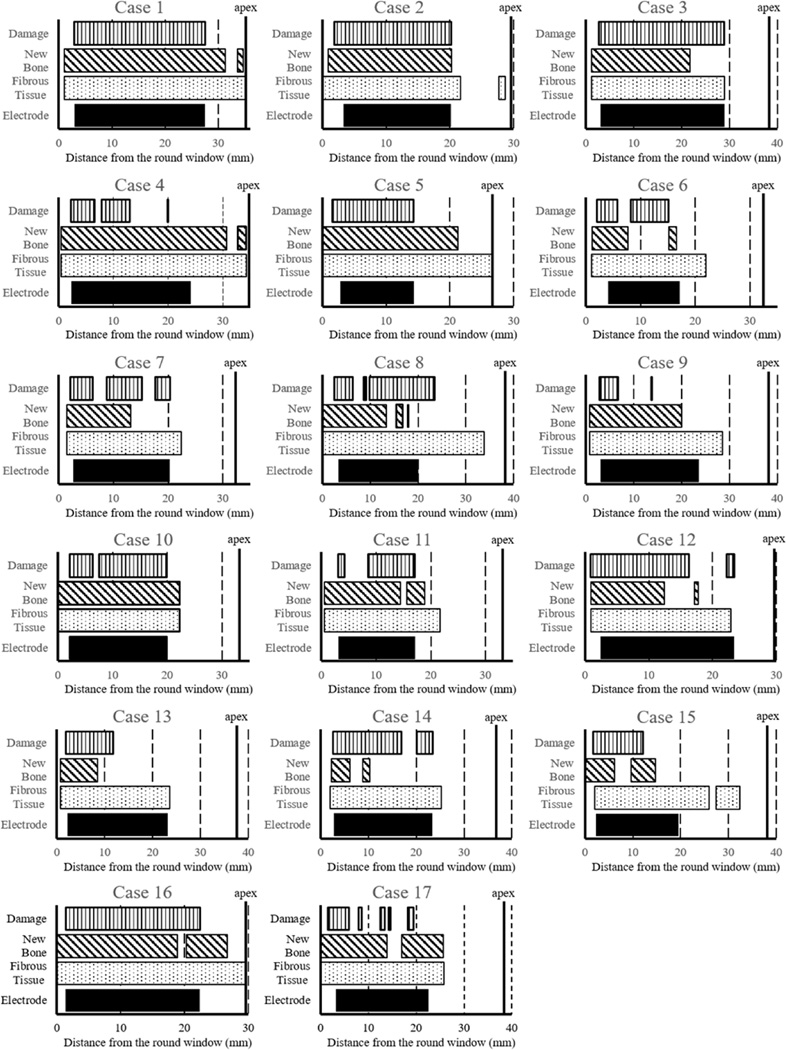
3.4. Distribution of new bone and fibrous tissue within the cochlea (Figure 5)
Figure 5.
Distribution of fibrous tissue, new bone, intracochlear damage and location of the electrode within the cochlea in all seventeen cases.
The distribution of new bone, fibrous tissue, intracochlear damage and the location of the implant electrode in all seventeen cases are shown in Figure 5. Fibrous tissue extended all along the electrode in almost all cases, whereas new bone extended for variable lengths along the electrode. Fibrous tissue extended beyond the tip of the electrode in most cases, and to the round window in seven cases (Case 2, 5, 8, 10, 15, 16 and 17). New bone extended apical to the tip of the electrode in eight cases (Case 1, 2, 4, 5, 10, 11, 16 and 17), and to the round window in six cases (Case 5, 8, 10, 15, 16 and 17). The area where new bone was seen was more extensive than the area where intracochlear damage was seen in nine cases (Case 1, 2, 4, 5, 9, 10, 11, 16, 17).
4. Discussion
4.1. Correlation between clinical and histopathologic variables
There have been inconclusive and conflicting results concerning a possible correlation between spiral ganglion cell counts and word recognition score after cochlear implantation (Nadol et al., 2001; Khan et al., 2005; Fayad and Linthicum, 2006; Li et al., 2007; Seyyedi et al., 2014b). Khan et al. (2005) concluded that spiral ganglion cell counts were not correlated with NU6 word scores in fifteen subjects. Li et al. (2007) also reported that spiral ganglion cell counts were not correlated with word recognition score for twelve subjects. On the other hand, Nadol et al. (2001) reported an apparent negative correlation between residual spiral ganglion cell count and hearing performance during life as measured by single-syllable word recognition for eight subjects. Fayad and Linthicum (2006) reported that spiral ganglion cell count in segment III showed significant negative correlations to post-implant speech discrimination scores for words and sentences as did segment IV and total ganglion cell count with word score in fourteen subjects. Seyyedi et al. (2014b) reported that the number of surviving spiral ganglion cell is significantly positively correlated with word recognition scores in within-subject comparison in six bilateral cochlear implant recipients. These conflicting results may be at least in part the results of the fact that the numbers of all studies including the current study are small, and also because the otologic history of patients were different. For example, in many cases the causes of deafness in the current study were peripheral sensorineural hearing loss such as presbycusis, Ménière’s disease, and genetic hearing loss. In contrast the causes of deafness as reported by Nadol et al. (2001) included two cases with head trauma and three cases with bacterial meningitis, which may have impaired central auditory function. Similarly, the causes of deafness as reported by Khan et al. (2005) included two cases with head trauma and two cases with bacterial meningitis. Moreover, the range of word recognition scores in the current study was 10% to 74% (average: 40.3%), compared to a smaller range (0%-30%, average: 18.4%) in the study by Nadol et al. (2001) and the average word recognition score (26.1%) reported in the study by Khan et al. (2005) (t-test, P < 0.05). Further studies will be needed to confirm the correlation between spiral ganglion cell counts and word recognition scores following cochlear implantation. Although the duration between last word recognition test and death may influence the correlation between word recognition scores and spiral ganglion cell counts, there was no significant difference between the mean duration (± SEM) of the current report (33.5 ± 5.7 months) and those of the reports by Nadol et al. (2001, 21.9 ± 14.1 months) and Khan et al. (2005, 27.8 ± 7.5 months) (t-test). In this study in seventeen subjects, post-implant hearing performance was positively correlated with residual spiral ganglion cell counts supporting the previous reports by Seyyedi et al. (2014b).
CNC word score was negatively correlated with the % volume of new bone especially in the scala media/vestibuli, and not with the % volume of fibrous tissue and total new tissue. Li et al. (2007) reported that the volume of new tissue was not significantly correlated with word recognition score after implantation. They suggested that this may be due to the small sample size. Kawano et al. (1998) indicated that the auditory thresholds for individual electrodes were increased with larger amounts of intracochlear fibrous tissue and new bone, especially of fibrous tissue and that the dynamic ranges were negatively correlated with intracochlear pathology, especially with new bone. Chiba et al. (2000) concluded that speech recognition scores were more positively correlated with the dynamic ranges than with threshold (T-) levels and maximum comfort (C-) levels in a psycophysical study of 56 patients with Nucleus 22-channel cochlear implants. Similarly, Firszt et al. (2002) reported that one of the variables that differentiated the best performers from the other subjects was relatively large dynamic ranges in a study of 11 adults implanted with the Clarion cochlear implant. In the current study, there was no correlation between word recognition score and fibrous tissue in the scala tympani, which was principally located between the modiolus and the electrode (R ≒ −0.1736, P ≒ 0.5053; Table 4). Given the results of the current study and those of Kawano et al. (1998), Chiba et al. (2000) and Firszt et al. (2002), it may be hypothesized that although fibrous tissue increases the auditory thresholds, it may not have much influence on postoperative word recognition. The results of the current study support the findings of Kawano et al. (1998) as to the correlation between post-implant hearing performance and the % volume of new bone within the cochlea.
CNC word scores were negatively correlated with the length of electrode located in the scala media/vestibuli or spiral ligament. An electrode located in the scala media/vestibuli or spiral ligament is at a greater distance from the modiolus compared to the typical location in the scala tympani. Previous studies have reported that a perimodiolar positioning of the electrode achieved both lower thresholds of electronically evoked auditory brainstem response (EABR) and a wider dynamic range in the cat (Shepherd et al., 1993) and guinea pig (Marsh et al., 1981). The results of the current study are consistent with these findings, and cochlear implant manufacturers have introduced electrodes designed to achieve a juxtaposition of the implanted electrode and the modiolus.
Neither age at implantation nor duration of implantation was significantly correlated with CNC word score. The results of the current study provided additional data supporting the report by Li et al. (2007). Also, neither age at implantation nor duration of implantation was significantly correlated with the % volume of new tissue within the cochlea. The results of the current study provided additional data supporting the previous reports by Li et al. (2007) and Fayad et al. (2009).
4.2. Correlation between the % volume of new tissue, damage score and electrode length
There was a positive correlation between the % volume of new bone in the scala media/vestibuli and the damage scores of the basilar membrane, the total of the lateral cochlear wall and osseous spiral lamina, and the total damage score. The damage to the lateral cochlear wall alone was not correlated with the % volume of new bone in the scala media/vestibuli. Li et al. (2007) reported that the damage to the lateral cochlear wall was positively correlated with the volume of intracochlear new bone and fibrous tissue formation using a three dimensional analysis. O’Leary et al. (2013) reported that there was a positive correlation between fracture of the osseous spiral lamina and new bone formation using an animal cochlear implant model. Although damage to the lateral cochlear wall or osseous spiral lamina may not be an important determinant alone, the results of the current study suggest that damage to both the lateral cochlear wall and osseous spiral lamina tends to cause new bone formation within the cochlear lumen. Tinling et al. (2004) showed that fluorescent labeled osteoid extended from the cochlear endosteum into the lumen of the scala tympani in post-meningitic labyrinthitis in Mongolian gerbils. It is possible that severe insertional trauma by the electrode may expose the endosteum of the lateral bony cochlea wall or osseous spiral lamina and result in the promotion of ossification. Zehnder et al. (2005) reported that osteoprotegerin, a potent inhibitor of osteoclast formation and function, is expressed at extremely high levels within the soft tissue of the cochlea including the spiral ligament and Deiters’ cells of the organ of Corti in mice. Therefore damage to the spiral ligament and basilar membrane by electrode insertion may cause up-regulation of osteoprotegerin and secretion into the perilymph, resulting in inhibition of bone remodeling and consequent promotion of ossification within the cochlear lumen.
The length of the electrode located in the scala media/vestibuli or spiral ligament was also positively correlated with the % new bone in the scala media/vestibuli (Table 5B) and with damage scores of the lateral cochlear wall, basilar membrane and the cochlea (Table 6). These data support implantation of the electrode in the scala tympani in order to promote atraumatic insertion as well as to achieve a perimodiolar position. In addition, fracture of the osseous spiral lamina by traumatic insertion may promote otogenic meningitis after cochlear implantation (Wei et al., 2007; Kamakura and Nadol, 2016). The % volume of fibrous tissue was not correlated with the damage scores within the cochlea. This suggests that insertional trauma alone is not a critical determinant of formation of fibrous tissue. Alternatively a foreign body reaction to the electrode is common after cochlear implantation (Seyyedi and Nadol, 2014a), and may be involved in the formation of fibrous tissue. It is possible that intracochlear new fibrous tissue may promote new bone formation within the inner ear, which is negatively correlated with CNC word scores.
4.3. Distribution of new bone and fibrous tissue within the cochlea
New bone and fibrous tissue were distributed mainly along the electrode, consistent with the report by Li et al. (2007) and Somdas et al. (2007). The area where new bone was seen was more extensive than the area where intracochlear damage was seen in many cases. This may be because osteoprotegerin expressed within the soft tissue of the cochlea or osteoid from the cochlear endosteum may diffuse into the perilymph or endolymph as a result of insertional trauma.
5. Conclusions
We studied the clinical and otopathologic factors correlated with hearing performance after cochlear implantation. CNC word score was positively correlated with residual spiral ganglion cell counts, and negatively correlated with the % volume of intracochlear new bone and with the length of electrode located in the scala media/vestibuli and spiral ligament. The % volume of new bone in the scala media/vestibuli was positively correlated with intracochlear insertional trauma especially to the basilar membrane. Fibrous tissue alone was not significantly correlated with CNC word score or with intracochlear insertional trauma. Instead, a foreign body response to the electrode may be a cause of formation of fibrous tissue. These findings suggest that atraumatic insertion of the cochlear implant electrode into the scala tympani and other steps to reduce intracochlear new bone formation may promote improved word recognition using the implant.
Highlights.
The postoperative CNC word score was positively correlated with total spiral ganglion cell count.
The postoperative CNC word score was negatively correlated with the % volume of new bone within the scala tympani, scala media/vestibuli and the cochlea, but not with the % volume of fibrous tissue.
The % volume of new bone in the scala media/vestibuli was positively correlated with the degree of intracochlear insertional trauma.
Acknowledgments
We thank Haobing Wang (Eaton Peabody Laboratory, Massachusetts Eye and Ear Infirmary) for helpful technical assistance in the use of Amira software for 3-D reconstruction. We also thank Diane Jones, Barbara Burgess, Jennifer O'Malley and Meng Yu Zhu for their expert preparation of the temporal bone specimens, and Garyfallia Pagonis for helpful technical assistance in creating digitized images of the temporal bone sections.
This work was supported by grant R01-DC000152 from the National Institute of Deafness and Other Communication Disorders (NIDCD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
Takefumi Kamakura carried out the reconstructions and wrote the manuscript; Joseph B. Nadol Jr. designed the study and critically reviewed and revised the manuscript.
Conflicts of interest
The authors disclose no conflicts of interest.
References
- Benatti A, Castiglione A, Trevisi P, Bovo R, Rosignoli M, Manara R, Martini A. Endocochlear inflammation in cochlear implant users: case report and literature review. Int. J. Pediatr. Otorhinolaryngol. 2013;77(6):885–893. doi: 10.1016/j.ijporl.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Chiba H, Kawano A, Tomizawa A, Sato H, Ueda K, Suzuki M. The Relation T and C Level and Speech Perception Ability in Cochlear 22 Implanted Patients. Audiol. Jpn. 2000;43(4):261–265. (in Japanese) [Google Scholar]
- Choi CH, Oghalai JS. Predicting the effect of post-implant cochlear fibrosis on residual hearing. Hear. Res. 2005;205(1–2):193–200. doi: 10.1016/j.heares.2005.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayad JN, Linthicum FHJN., Jr Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope. 2006;116(8):1310–1320. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- Fayad JN, Makarem AO, Linthicum FH., Jr Histopathologic assessment of fibrosis and new bone formation in implanted human temporal bones using 3D reconstruction. Otolaryngol. Head Neck Surg. 2009;141(2):247–252. doi: 10.1016/j.otohns.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firszt JB, Chambers RD, Kraus N. Neurophysiology of cochlear implant users II: comparison among speech perception, dynamic range, and physiological measures. Ear Hear. 2002;23(6):516–531. doi: 10.1097/00003446-200212000-00003. [DOI] [PubMed] [Google Scholar]
- Guild SR. A graphic reconstruction method for the study of the organ of Corti. Anat. Rec. 1921;22:141–157. [Google Scholar]
- Kawano A, Seldon HL, Clark GM, Ramsden RT, Raine CH. Intracochlear factors contributing to psychophysical percepts following cochlear implantation. Acta Otolaryngol. 1998;118(3):313–326. doi: 10.1080/00016489850183386. [DOI] [PubMed] [Google Scholar]
- Kamakura T, Nadol JB., Jr Cochlear histopathology as observed, in two patients with a cochlear implant electrode with positioner. Otol. Neurotol. 2016;37(6):642–646. doi: 10.1097/MAO.0000000000000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AM, Handzel O, Burgess BJ, Damian D, Eddington DK, Nadol JB., Jr Is word recognition correlated with the number of surviving spiral ganglion cells and electrode insertion depth in human subjects with cochlear implants? Laryngoscope. 2005;115(4):672–677. doi: 10.1097/01.mlg.0000161335.62139.80. [DOI] [PubMed] [Google Scholar]
- Lee J, Nadol JB, Jr, Eddington DK. Factors associated with incomplete insertion of electrodes in cochlear implant surgery: a histopathologic study. Audiol. Neurootol. 2011;16(2):69–81. doi: 10.1159/000316445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li PM, Somdas MA, Eddington DK, Nadol JB., Jr Analysis of intracochlear new bone and fibrous tissue formation in human subjects with cochlear implants. Ann. Otol. Rhinol. Laryngol. 2007;116(10):731–738. doi: 10.1177/000348940711601004. [DOI] [PubMed] [Google Scholar]
- Marsh RR, Yamane H, Potsic WP. Effect of site of stimulation on the guinea pig's electrically evoked brain stem response. Otolaryngol. Head Neck Surg. 1981;89(1):125–130. doi: 10.1177/019459988108900127. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr, Eddington DK, Burgess BJ. Foreign body or hypersensitivity granuloma of the inner ear after cochlear implantation: one possible cause of a soft failure? Otol. Neurotol. 2008;29(8):1076–1084. doi: 10.1097/MAO.0b013e31818c33cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol JB, Jr, Eddington DK. Histologic evaluation of the tissue seal and biologic response around cochlear implant electrodes in the human. Otol. Neurotol. 2004;25(3):257–262. doi: 10.1097/00129492-200405000-00010. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr, O'Malley JT, Burgess BJ, Galler D. Cellular immunologic responses to cochlear implantation in the human. Hear. Res. 2014;318:11–17. doi: 10.1016/j.heares.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol JB, Jr, Shiao JY, Burgess BJ, Ketten DR, Eddington DK, Gantz BJ, Kos I, Montandon P, Coker NJ, Roland JT, Jr, Shallop JK. Histopathology of cochlear implants in humans. Ann. Otol. Rhinol. Laryngol. 2001;110(9):883–891. doi: 10.1177/000348940111000914. [DOI] [PubMed] [Google Scholar]
- O’Leary SJ, Monksfield P, Kel G, Connolly T, Souter MA, Chang A, Marovic P, O'Leary JS, Richardson R, Eastwood H. Relations between cochlear histopathology and hearing loss in experimental cochlear implantation. Hear. Res. 2013;298:27–35. doi: 10.1016/j.heares.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF. Pathology of the ear. 2. Philadelphia: Lea and Febiger; 1993. pp. 1–29. [Google Scholar]
- Seyyedi M, Nadol JB., Jr Intracochlear inflammatory response to cochlear implant electrodes in humans. Otol. Neurotol. 2014a;35(9):1545–1551. doi: 10.1097/MAO.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyyedi M, Viana LM, Nadol JB., Jr Within-subject comparison of word recognition and spiral ganglion cell count in bilateral cochlear implant recipients. Otol. Neurotol. 2014b;35(8):1446–1450. doi: 10.1097/MAO.0000000000000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Hatsushika S, Clark GM. Electrical stimulation of the auditory nerve: the effect of electrode position on neural excitation. Hear. Res. 1993;66(1):108–120. doi: 10.1016/0378-5955(93)90265-3. [DOI] [PubMed] [Google Scholar]
- Somdas MA, Li PM, Whiten DM, Eddington DK, Nadol JB., Jr Quantitative evaluation of new bone and fibrous tissue in the cochlea following cochlear implantation in the human. Audiol. Neurootol. 2007;12(5):277–284. doi: 10.1159/000103208. [DOI] [PubMed] [Google Scholar]
- Tinling SP, Colton J, Brodie HA. Location and timing of initial osteoid deposition in postmeningitic labyrinthitis ossificans determined by multiple fluorescent labels. Laryngoscope. 2004;114(4):675–680. doi: 10.1097/00005537-200404000-00015. [DOI] [PubMed] [Google Scholar]
- Wei BP, Shepherd RK, Robins-Browne RM, Clark GM, O'Leary SJ. Effects of inner ear trauma on the risk of pneumococcal meningitis. Arch. Otolaryngol. Head Neck Surg. 2007;133(3):250–259. doi: 10.1001/archotol.133.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder AF, Kristiansen AG, Adams JC, Merchant SN, McKenna MJ. Osteoprotegerin in the inner ear may inhibit bone remodeling in the otic capsule. Laryngoscope. 2005;115(1):172–177. doi: 10.1097/01.mlg.0000150702.28451.35. [DOI] [PubMed] [Google Scholar]