Abstract
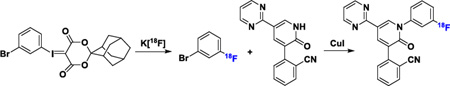
To prompt the development of 18F-labeled positron emission tomography (PET) tracers for the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, we have prepared 18F-labeled 2-(1-(3-fluorophenyl)-2-oxo-5-(pyrimidin-2-yl)-1,2-dihydropyridin-3-yl)benzonitrile ([18F]8). The radiosynthesis was achieved by a one-pot two-step method that utilized a spirocyclic hypervalent iodine (III) mediated radiofluorination to prepare the 18F-labeled 1-bromo-3-fluorobenzene ([18F]15) intermediate with K18F. A subsequent copper (I) iodide mediated coupling reaction was carried out with 2-(2-oxo-5-(pyrimidin-2-yl)-1,2-dihydropyridin-3-yl)benzonitrile (10) to [18F]8 in 10 ± 2% uncorrected radiochemical yield relative to starting 18F-fluoride with > 99% radiochemical purity and 0.8 ± 0.2 Ci/µmol specific activity at the end of synthesis. PET imaging studies with the title radiotracer in normal mice demonstrated good brain uptake (peak standardized uptake value (SUV) = 2.3 ± 0.1) and warrants further in vivo validation.
Keywords: AMPA receptor, Positron emission tomography, Fluorine-18, Radiotracer, Perampanel
Graphical Abstract
Glutamate has been recognized as a principle excitatory neurotransmitter in the brain since late 1970s.1 It exerts postsynaptical effects primarily through the metabotropic and ionotropic glutamate receptors (mGluRs and iGluRs, respectively).2 The iGluRs structurally incorporate ligand-gated tetrametric ion channels and allow fast synaptic responses to the glutamate. The iGluRs are composed of three families, namely, N-methyl-D-aspartate (NMDA), kainite, and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors.3–6 Modulation of these receptors can be used in the treatment of psychiatric disorders and neurodegenerative diseases, including epilepsy, ischemia, stroke, Parkinson’s disease and Alzheimer’s disease.7–8 In particular, competitive AMPA receptor antagonists have been the focus of drug discovery, with the first reported compound, 2, 3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline-2, 3-dione (NBQX), in 1991,9–10 which led to the two clinically translated drugs, namely, Becampanel (AMP397) and Selurampanel (BGG492).11–12 To date, there has not been an AMPA receptor antagonist in this class of compounds to reach the market. Meanwhile, non-competitive AMPA receptor antagonists, which occupy the less polar allosteric binding site, are not expected to be significantly influenced by endogenous glutamate levels, as compared to the competitive antagonists.13–14 In this category, Talampanel (GYKI-537773) was studied in a Phase II clinical trial as an antiepileptic agent.15–16 Irampanel (BIIR561) reached Phase I/IIa clinical trials for the treatment of stroke.17 Perampanel (Fycompa®) has been approved by the United States Food and Drug Administration for treating refractory partial onset seizures in 2012, and as an adjunctive treatment for primary generalized tonic-clonic seizures in 2015.18–21
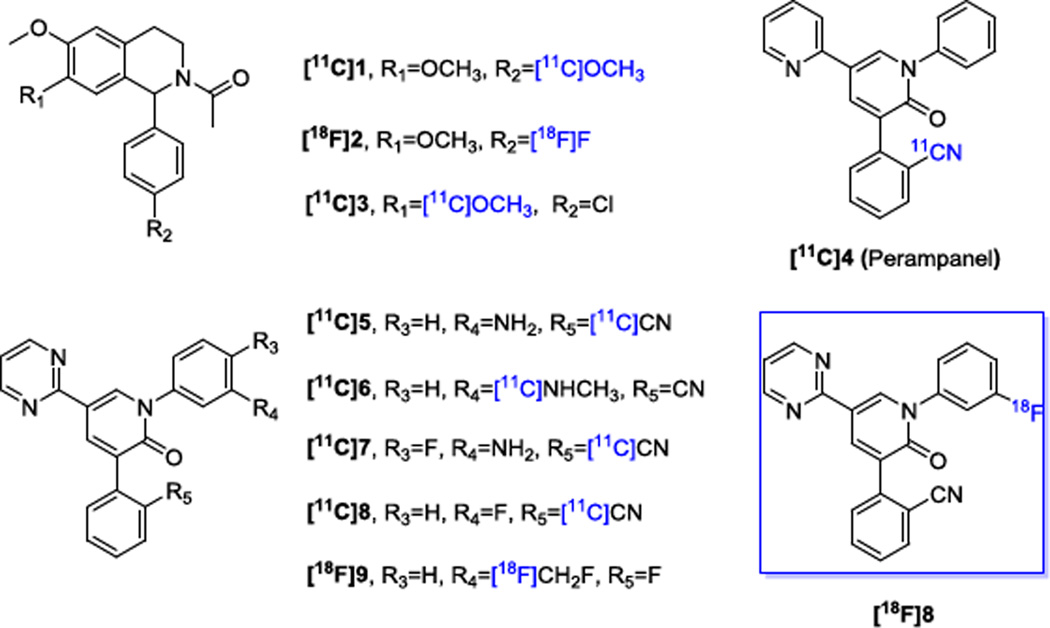
As a non-invasive imaging technology, positron emission tomography (PET) is capable of in vivo quantification of biochemical and pharmacological progress via radiolabeled targeted molecular probes. Quantification of AMPA in the living brain by PET would enable investigations of the AMPA system under normal and disease conditions, assessment of AMPA distribution in the brain and periphery, and target engagement for validation of promising drug candidates in clinical trials. To date, only a handful of AMPA PET radiotracers, most of which are 11C-labeled molecules, have been reported (Fig. 1). The radiosyntheses of isoquinoline derivatives [11C]1, [18F]2 and 11C-labeled Perampanel ([11C]4) have been reported, but their potential as PET tracers needs to be further evaluated in vivo.22–23 The isoquinoline derivative [11C]3, showed rapid clearance from the central nervous system (CNS) and low specific binding in rats.24 Among the recently disclosed PET tracers [11C]5–8 and [18F]9, [11C]5 and [11C]7 showed very low brain penetration in rhesus monkey (i.e. standardized uptake value (SUV)max = 0.5), and [18F]9 exhibited inconsistent distribution with the localization of AMPA receptors in rat brain. Whereas, [11C]6 demonstrated good radioactivity uptake in both rat and monkey brains (i.e. SUVmax = 1.6), consistent binding patterns with the AMPA localization and target specific binding features, hence, this radiotracer shows potential as PET tracer for AMPA receptors. A fluorinated isoquinoline derivative [11C]8 was radiolabeled via [11C]HCN, displayed even higher radioactivity uptake in rhesus monkey brain (i.e. SUVmax = 2.7), and maintained the promising properties demonstrated with [11C]6.25–26 In combination with the advantages of fluorine-18 that are attributed to its longer half-life (t1/2 = 109.7 min) compared with carbon-11 (t1/2 = 20.4 min), the goal of the present work is to develop a practical method for the radiosynthesis of the 18F-labeled isotopologue of 8 for imaging AMPA receptors with PET. Herein, we describe a novel one-pot two-step radiosynthesis of [18F]8, enabled by a spirocyclic hypervalent iodine (III) activated radiofluorination of the non-activated arene, and subsequent CuI mediated cross coupling with 2-(2-oxo-5-(pyrimidin-2-yl)-1, 2-dihydropyridin-3-yl) benzonitrile 10. We further report preliminary PET imaging studies with [18F]8 in mice.
Figure 1.
Chemical Structures of the reported PET tracers for AMPA receptors.
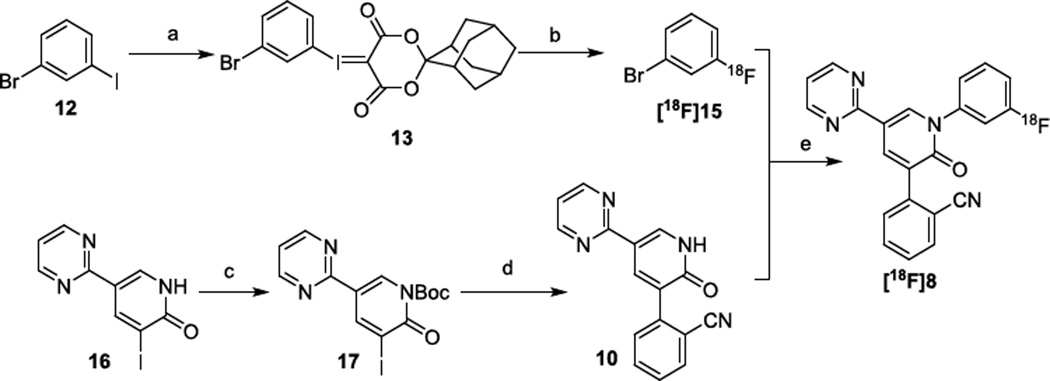
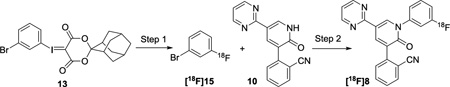
Radiofluorination of non-activated arenes still represents a challenging problem, despite extensive efforts devoted to this field.27–31 Unsurprisingly, attempts to achieve conventional nucleophilic aromatic substitution of the electron-deficient 2-(1-(3-nitrophenyl)-2-oxo-5-(pyrimidin-2-yl)-1, 2-dihydropyridin-3-yl)benzonitrile 11 (Scheme S1, Supporting Information, SI) with K[18F] or [18F]tetraethylammonium fluoride (TEA[18F]) led to no reaction.32,26 These results led us to utilize our recent developed radiofluorination method, which takes advantage of spirocyclic hypervalent iodine (III) species as the 18F-radiolabeling precursors.33–37 To prepare the precursor for radiofluorination, aryl iodide 12 was first converted to the hypervalent spirocyclic iodine (III) compound, 13, under oxidative conditions, and subsequently reacted with (1r,3r,5r,7r)-spiro[adamantane-2,2'-[1,3]dioxane]-4',6'-dione (SPIAD; 14) (Scheme S2, SI).37 Compound 10 was obtained from the coupling reactions between N-Boc-protected compound 17, derived from compound 16, and 2-cyanophenylboronic acid 1, 3-propanediol ester (18) under modified Ullmann reactions (Scheme S2, SI).26
Our initial studies established the feasibility for our radiosynthetic approach for [18F]8 with coupling reactions between compound 10 and 1-bromo-3-fluorobenzene 15 (Scheme 1 and Scheme S3, SI). As shown in Step 1, Table 1, optimization of the radiofluorination of the iodine(III) precursor 13, was carried out to yield [18F]15. Compound [18F]15 was obtained in 72 ± 3% (n > 10) radiochemical conversion (RCC) under the optimal conditions, where compound 13 (3.9 µmol), K2CO3 (7.2 µmol), and K222 (Kryptofix®, 13.3 µmol) were heated for 10 min at 120 °C in N,N-dimethylmethanamide (DMF, 0.2 mL) (Scheme S4 and Table S1, entries 4, SI).
Scheme 1.
De novo radiosynthesis of [18F]8a.
aReagents and conditions: (a) (i) NaBO3·4H2O, glacial acetic acid, 50 °C, Overnight; (ii) (1r,3r,5r,7r)-spiro[adamantane-2,2'-[1,3]dioxane]-4',6'-dione (SPIAD) 14,10% Na2CO3 (aq) (w/v), ethanol/dichloromethane; (b) K[18F] or TEA[18F], DMF, 120 °C, 10 min. (c) di-tert-butyl dicarbonate, 4-dimethylaminopyridine, THF, 0 °C to room temperature, Overnight; (d) 2-cyanophenylboronic acid 1,3-propanediol ester 18, Cesium carbonate, Pd(PPh3)4, DMF, 110°C, overnight. (e) CuI, K3PO4, trans-N,N'-dimethylcyclohexane-1, 2-diamine, DMF, 110 °C, 20 min.
Table 1.
Optimization of reaction conditions for the synthesis of [18F]8
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entrya | 10 (mg) | CuI (mg) | Amine (µL)c | K3PO4 (mg) | T (°C) | Time (min) | DMF (mL) | [18F]8 (%)d |
| 1 | 2 | 2 | 1.2 | 4.8 | 110 | 10 | 0.4 | 8 ± 4 (n = 2) |
| 2 | 4 | 2 | 1.2 | 4.8 | 130 | 10 | 0.4 | 20 ± 3 (n = 2) |
| 3 | 16 | 2 | 1.2 | 4.8 | 130 | 10 | 0.4 | 6 ± 3 (n = 2) |
| 4 | 4 | 4 | 2.4 | 4.8 | 130 | 10 | 0.4 | 48 ± 5 (n = 2) |
| 5 | 4 | 12 | 7.2 | 4.8 | 130 | 10 | 0.4 | 72 ± 5 (n = 5) |
| 6 | 4 | 12 | 7.2 | 15 | 130 | 10 | 0.4 | 20 ± 1 (n = 2) |
| 7b | 12 | 36 | 21.6 | 14.4 | 130 | 20 | 1.0 | 65 ± 10 (n = 5) |
Unless otherwise noted, the step 1 reaction mixture was from Table S1, entry 4, SI.
The step 1 reaction mixture was from Table S1, entry 13, SI.
Amine refers to trans-N, N′-dimethylcyclohexane-1, 2-diamine.
Determined by radio-HPLC integration of product peaks, relative to [18F]fluoride and 18F-side products if observed.
The CuI mediated cross coupling (c.f. step 2) between compound 10 and [18F]15 was initially optimized based on the amount of compound 13 (2 mg, Table S1, entry 4, SI). trans-N,N′-dimethylcyclohexane-1, 2-diamine was an effective amine ligand to accomplish this coupling (Table 1, entry 1). Exploration of amine ligands, including N,N,N′,N′-tetramethylethylenediamine (TMEDA), N,N′-dimethylethylenediamine (DMEDA) failed to produce [18F]8 (Table S2, entries 1–4, SI). Further optimization of reaction temperature (Table S2, entries 4–6, SI), stoichiometries of the coupling counterpart 10 (Table 1, entries 2–3 & Table S2, entries 7–9, SI), copper (I) iodide, its chelating amine and K3PO4 additive were carried out (Table 1, entries 4–6 & Table S2, entries 10–15, SI) to result in the 18F-incorporation yield up to 72 ± 5% (Table 1, entry 5). To our surprise, the above-mentioned optimized reaction conditions resulted in low isolated radiochemical yield (RCY) non-decay-corrected relative to starting 18F-fluoride (i.e. RCY < 3%). In order to improve the RCY, the amounts of reagents involved in both steps were scaled up by 3-fold (i.e. 6 mg of compound 13) and the 2nd step reaction was prolonged to 20 minutes As a result, the corresponding isolated RCY of [18F]8 was significantly improved to 10 ± 2% after a 60 minute synthesis time (n = 5).
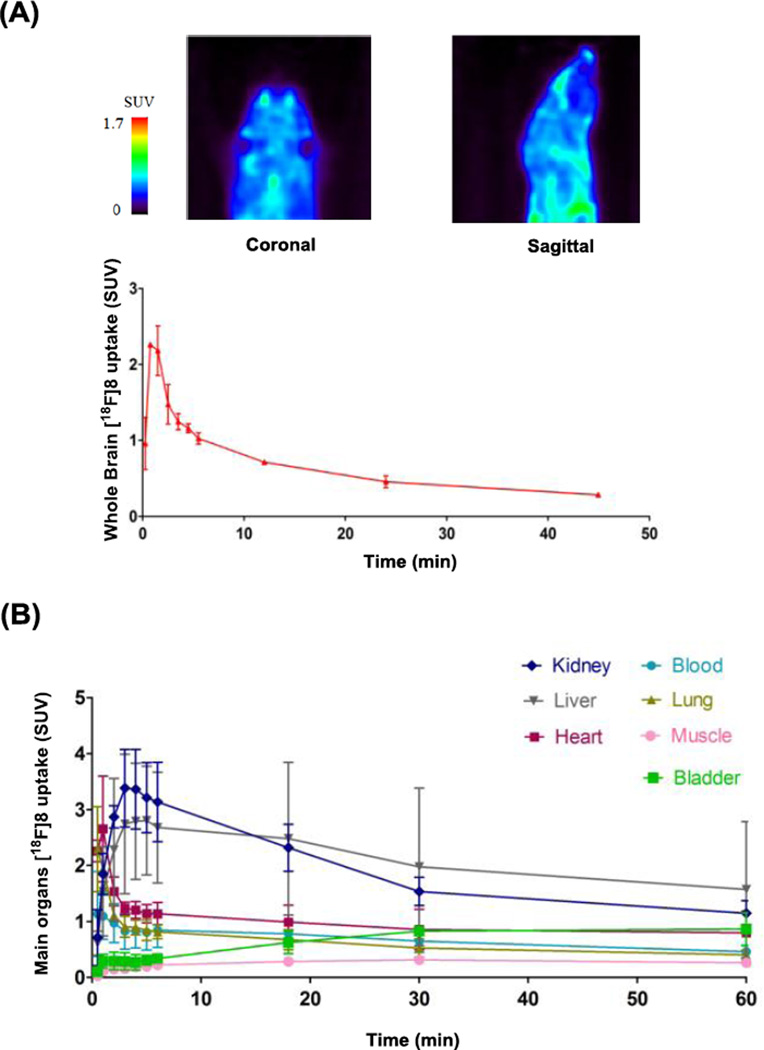
To determine the in vivo distribution and brain permeability of [18F]8, we performed preliminary PET imaging studies in mice. Administration of [18F]8 in 10% ethanolic saline (0.4 mCi radioactivity, > 99% radiochemical purity (Fig. S1 & S2, SI), and specific activity of 0.8 ± 0.2 Ci/µmol (Fig. S3) at the time of injection) was coincident with initiation of 60 minute dynamic brain PET scans to determine biodistribution. As shown in Figure 2, [18F]8 rapidly crossed the blood-brain barrier (BBB) and reached a maximum whole brain activity of 2.3 ± 0.1 SUV at approximately 70 seconds post injection of [18F]8 (Fig. 2A). This result is consistent with the reported 11C-isotopologue of 8, confirming similar brain permeability of the [18F]8. We also determined the logP7.4 of compound 8 to be 1.5 by an established HPLC method,38 which is similar to the experimental result of 1.7 in the report (Table S3 and Fig. S4, SI).26 Further whole body biodistribution analysis revealed high uptake (SUV > 1) in the blood, heart, lung, kidney and liver at 1 min post injection, but low uptake in muscle and bladder over the entire analysis. The activity washed out rapidly from blood, heart and lung within 3 min, but slowly declined in kidney and liver over 60 min (Fig. 1B and Fig. S5A, SI).
Figure 2.
PET imaging of [18F]8 in mice: (A) Summed image 0–60 min post tracer injection in the brain. (B) Time-activity curves (TACs) of whole body biodistribution. Data are expressed as SUV (mean ± SD, n = 2). For region of interest (ROI) placement, see supporting information (Figure S5A, SI).
In summary, we have synthesized the [18F]8 via a novel one-pot two-step reaction, starting from a spirocyclic hypervalent iodine (III) precursor 13, followed by a copper mediated N-arylation reaction. Preclinical PET imaging studies of [18F]8 demonstrated consistent high brain uptake as its 11C-isotopologue and showed reasonable uptake and clearance of activity in main organs, thereby representing a promising fluorine-18 labeled radiotracer for further evaluation as a potential AMPA receptor imaging agent.
Supplementary Material
Acknowledgments
We would like to thank the staff at the radiochemistry program, Gordon Center for Medical Imaging, Nuclear Medicine and Molecular Imaging, Massachusetts General Hospital, MA, and Department of Chemistry & Chemical Biology, Northeastern University for their generous support. We also thank Drs. Lee Collier, Benjamin H. Rotstein and Chongzhao Ran, Mr. Jian Yang and Ms. Jing Yang for their helpful discussions. S.H.L is a recipient of NIH career development award from the National Institute on Drug Abuse (DA038000).
Abbreviations
- mGluRs
metabotropic glutamate receptors
- iGluRs
ionotropic glutamate receptors
- NMDA
N-methyl-D-aspartate
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- PET
positron emission tomography
- CNS
central nervous system
- TEA[18F]
[18F]tetraethylammonium fluoride
- SPIAD
(1r,3r,5r,7r)-spiro[adamantane-2,2'-[1,3]dioxane]-4',6'-dione
- RCC
radiochemical conversion
- K222
Kryptofix® or 4,7,13,16,21,24-Hexaoxa-1,10-diazabicyclo[8.8.8]hexacosanein
- DMF
N,N-Dimethylmethanamide
- TMEDA
N,N,N′,N′-tetramethylethylenediamine
- DMEDA
N,N′-Dimethylethylenediamine
- TEAB
tetraethylammonium bicarbonate
- SUV
standardized uptake value
- RCY
radiochemical yield
- DMSO
Dimethyl sulfoxide
- BBB
crossed the blood-brain barrier
- TAC
Time-activity curve
- ROI
region of interest
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Meldrum BS. J. Nutr. 2000;130:1007S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- 2.Niswender CM, Conn PJ. Annu. Rev. Pharmacool. Toxicol. 2010;50:295. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer ML, Armstrong N. Annu. Rev. Physiol. 2004;66:161. doi: 10.1146/annurev.physiol.66.050802.084104. [DOI] [PubMed] [Google Scholar]
- 4.Watkins JC, Jane DE. Brit. J. Pharmacol. 2006;147:S100. doi: 10.1038/sj.bjp.0706444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan HJ, Myers SJ, Dingledine R. Pharmacol. Rev. 2010;62:405. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kew JNC, Kemp JA. Psychopharmacology. 2005;182:320. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- 7.Calabresi P, Cupini LM, Centonze D, Pisani F, Bernardi G. Ann. Neurol. 2003;53:693. doi: 10.1002/ana.10603. [DOI] [PubMed] [Google Scholar]
- 8.Chang PKY, Verbich D, McKinney RA. Eur. J. Neurosci. 2012;35:1908. doi: 10.1111/j.1460-9568.2012.08165.x. [DOI] [PubMed] [Google Scholar]
- 9.Klockgether T, Turski L, Honore T, Zhang ZM, Gash DM, Kurlan R, Greenamyre JT. Ann. Neurol. 1991;30:717. doi: 10.1002/ana.410300513. [DOI] [PubMed] [Google Scholar]
- 10.Meldrum BS, Rogawski MA. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2007;4:18. doi: 10.1016/j.nurt.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ornstein PL, Arnold MB, Augenstein NK, Lodge D, Leander JD, Schoepp DD. J. Med. Chem. 1993;36:2046. doi: 10.1021/jm00066a016. [DOI] [PubMed] [Google Scholar]
- 12.Faught E. Expert Opin. Inv. Drug. 2014;23:107. doi: 10.1517/13543784.2014.848854. [DOI] [PubMed] [Google Scholar]
- 13.Barreca ML, Gitto R, Quartarone S, De Luca L, De Sarro G, Chimirri A. J. Chem. Inf. Comp. Sci. 2003;43:651. doi: 10.1021/ci025625q. [DOI] [PubMed] [Google Scholar]
- 14.Balannik V, Menniti FS, Paternain AV, Lerma J, Stern-Bach Y. Neuron. 2005;48:279. doi: 10.1016/j.neuron.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 15.Donevan SD, Yamaguchi S, Rogawski MA. J. Pharmacol. Exp. Ther. 1994;271:25. [PubMed] [Google Scholar]
- 16.Chappell AS, Sander JW, Brodie MJ, Chadwick D, Lledo A, Zhang D, Bjerke J, Kiesler GM, Arroyo S. Neurology. 2002;58:1680. doi: 10.1212/wnl.58.11.1680. [DOI] [PubMed] [Google Scholar]
- 17.Feigin V. Curr. Opin. Investig. Drugs. 2002;3:908. [PubMed] [Google Scholar]
- 18.Hanada T, Hashizume Y, Tokuhara N, Takenaka O, Kohmura N, Ogasawara A, Hatakeyama S, Ohgoh M, Ueno M, Nishizawa Y. Epilepsia. 2011;52:1331. doi: 10.1111/j.1528-1167.2011.03109.x. [DOI] [PubMed] [Google Scholar]
- 19.Hibi S, Ueno K, Nagato S, Kawano K, Ito K, Norimine Y, Takenaka O, Hanada T, Yonaga M. J. Med. Chem. 2012;55:10584. doi: 10.1021/jm301268u. [DOI] [PubMed] [Google Scholar]
- 20.French JA, Krauss GL, Steinhoff BJ, Squillacote D, Yang HC, Kumar D, Laurenza A. Epilepsia. 2013;54:117. doi: 10.1111/j.1528-1167.2012.03638.x. [DOI] [PubMed] [Google Scholar]
- 21.French JA, Krauss GL, Wechsler RT, Wang XF, DiVentura B, Brandt C, Trinka E, O'Brien TJ, Laurenza A, Patten A, Bibbiani F. Neurology. 2015;85:950. doi: 10.1212/WNL.0000000000001930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao M, Kong D, Clearfield A, Zheng QH. Bioorg. Med. Chem. Lett. 2006;16:2229. doi: 10.1016/j.bmcl.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 23.Lee HG, Milner PJ, Placzek MS, Buchwald SL, Hooker JM. J. Am. Chem. Soc. 2015;137:648. doi: 10.1021/ja512115s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arstad E, Gitto R, Chimirri A, Caruso R, Constanti A, Turton D, Hume SP, Ahmad R, Pilowsky LS, Luthra SK. Bioorg. Med. Chem. 2006;14:4712. doi: 10.1016/j.bmc.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 25.Oi N, Yamamoto N, Suzuki M, Nakatani Y, Suhara T, Zhang M-R, Fukumura T, Higuchi M, Minamimoto T, Maeda J, Tokunaga M, Nagai Y. WO2014143210 A1. 2014
- 26.Oi N, Tokunaga M, Suzuki M, Nagai Y, Nakatani Y, Yamamoto N, Maeda J, Minamimoto T, Zhang MR, Suhara T, Higuchi M. J. Med. Chem. 2015;58:8444. doi: 10.1021/acs.jmedchem.5b00712. [DOI] [PubMed] [Google Scholar]
- 27.Preshlock S, Tredwell M, Gouverneur V. Chem. Rev. 2016;116:719. doi: 10.1021/acs.chemrev.5b00493. [DOI] [PubMed] [Google Scholar]
- 28.Tredwell M, Gouverneur V. Angew. Chem. Int. Ed. Engl. 2012;51:11426. doi: 10.1002/anie.201204687. [DOI] [PubMed] [Google Scholar]
- 29.Brooks AF, Topczewski JJ, Ichiishi N, Sanford MS, Scott PJ. Chem. Sci. 2014;5:4545. doi: 10.1039/C4SC02099E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell MG, Ritter T. Chem. Rev. 2015;115:612. doi: 10.1021/cr500366b. [DOI] [PubMed] [Google Scholar]
- 31.Buckingham F, Gouverneur V. Chem. Sci. 2016;7:1645. doi: 10.1039/c5sc04229a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terence S. WO03047577A2. 2003
- 33.Rotstein BH, Stephenson NA, Vasdev N, Liang SH. Nat. Commun. 2014;5:4365. doi: 10.1038/ncomms5365. [DOI] [PubMed] [Google Scholar]
- 34.Stephenson NA, Holland JP, Kassenbrock A, Yokell DL, Livni E, Liang SH, Vasdev N. J. Nucl. Med. 2015;56:489. doi: 10.2967/jnumed.114.151332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobson O, Weiss ID, Wang L, Wang Z, Yang X, Dewhurst A, Ma Y, Zhu G, Niu G, Kiesewetter DO, Vasdev N, Liang SH, Chen X. J. Nucl. Med. 2015;56:1780. doi: 10.2967/jnumed.115.160960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Jacobson O, Avdic D, Rotstein BH, Weiss ID, Collier L, Chen X, Vasdev N, Liang SH. Angew. Chem. Int. Ed. Engl. 2015;54:12777. doi: 10.1002/anie.201505927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rotstein BH, Wang L, Liu RY, Patteson J, Kwan EE, Vasdev N, Liang SH. Chem. Sci. 2016;7:4407. doi: 10.1039/c6sc00197a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webster GRB, Friesen KJ, Sarna LP, Muir DCG. Chemosphere. 1985;14:12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.