Abstract
One pathological hallmark of Alzheimer’s disease (AD) is the accumulation of amyloid-β peptide (Aβ) in the affected brain. While there are numerous deleterious effects of Aβ accumulation, there is general agreement that a sustained inflammatory response to aggregated Aβ contributes to progressive neurodegeneration in AD and microglial cells play a significant role in this process. Our laboratory and others have shown that small soluble aggregates of Aβ activate a microglia-mediated inflammatory response. One component of the response involves internalization of extracellular Aβ, and this process is likely very sensitive to Aβ structure. In this study we analyzed the proclivity of microglia for internalization of Aβ42 monomers and protofibrils using fluorescently-labeled Aβ. Both Aβ42 species were labeled directly via amino linkage with an Alexa Fluor 488 tetrafluorophenyl ester (AF488-TFP) and then isolated individually by chromatography. Aβ42 protofibrils retained their size and morphological properties after labeling but monomers had a much higher stoichiometry of labeling compared to protofibrils. Primary murine microglia internalized AF488-Aβ42 protofibrils rapidly and in significant amounts compared to AF488-Aβ42 monomers. Microglial internalization of protofibrils was dependent on time and concentration, and corresponded with tumor necrosis factor α secretion. In competition studies, unlabeled Aβ42 protofibril internalization, detected by immunostaining, did not diminish AF488-protofibril uptake. Internalized AF488-Aβ42 protofibrils were found widely dispersed in the cytosol with some lysosomal accumulation but little degradation. These studies highlight the sensitivity that microglia exhibit to Aβ structure in the internalization process and emphasize their affinity for soluble Aβ protofibrils.
Keywords: Amyloid-beta protein, aggregation, protofibrils, microglia, uptake, internalization, inflammation
1. Introduction
Microglial cells play an important role in Alzheimer’s disease-related (AD) neuroinflammation by responding to accumulations of amyloid-β peptide (Aβ) (Heneka, et al., 2015). Activated microglia and proinflammatory cytokines such as tumor necrosis factor α (TNFα) and interleukin 1-β (IL-1β) can be observed clustering around extracellular Aβ plaques in the human AD brain and in transgenic AD mice (Apelt and Schliebs, 2001; Dickson, et al., 1993; McGeer, et al., 1987). One key problem in AD is that the phagocytic microglia are not completely efficient at removing the plaques, however they may work in some manner to restrict plaque growth (Meyer-Luehmann, et al., 2008). The form of Aβ present in, or surrounding, the deposits likely affects the way microglia respond to the peptide accumulation.
Soluble Aβ peptides, primarily 40 or 42 residues in length, are released via enzymatic cleavage of the amyloid precursor protein (APP). Both peptides are ultimately found in the plaque deposits with the dense plaque core comprised of the more aggregation-prone Aβ42. The biophysical mechanisms by which plaque formation occurs in the brain are not fully understood however significant in vitro work has increased our understanding of the Aβ aggregation process. These studies have shown that unstructured Aβ monomer will undergo non-covalent self-assembly (Jarrett, et al., 1993) to form a polydisperse mixture of soluble oligomers (Bitan, et al., 2003; Kayed, et al., 2003; Mittag, et al., 2014) and/or protofibrils (Harper, et al., 1999; Mittag, et al., 2014; Walsh, et al., 1999; Walsh, et al., 1997) and ultimately insoluble fibrils (Harper, et al., 1997). Aβ assembly occurs via a nucleation-dependent polymerization process (Jarrett and Lansbury, 1993) and the rate-limiting nucleation step is characterized by a lag phase followed by rapid polymerization. More recent information on plaque composition suggests that there is greater structural diversity within the plaques than previously thought. Hyman and colleagues observed a halo of oligomeric Aβ surrounding Aβ plaques after immunostaining with the oligomer-selective NAB61 antibody (Koffie, et al., 2009). Although the plaques contain fibrillar Aβ at the core (Terry, et al., 1964), these data suggest that microglial cells may interact with multiple Aβ species within the plaque environment.
Numerous studies have demonstrated the ability of microglia (primary and cell lines) to internalize and traffic Aβ to the lysosome (Ard, et al., 1996; Chu, et al., 1998; Halle, et al., 2008; Li, et al., 2012; Mandrekar, et al., 2009; Paresce, et al., 1997; Sheedy, et al., 2013). Multiple receptors appear to regulate the Aβ internalization process including scavenger receptor (Paresce, et al., 1996), complement receptor type 3 (CR3, Mac-1), signal regulatory protein-β1 (SIRPβ1) receptor (Gaikwad, et al., 2009), P2Y4 receptor (Li, et al., 2013), and a receptor complex of CD36, α6β1 integrin and CD47 (Koenigsknecht and Landreth, 2004). Many of these, and other, studies utilized either fibrillar, “soluble”, or both forms of Aβ (Chung, et al., 1999; Fu, et al., 2012; Gaikwad, et al., 2009; Koenigsknecht and Landreth, 2004; Mandrekar, et al., 2009). The term “soluble” likely encompasses mixtures of aggregates and monomers. Cumulatively, the results indicate microglia internalization of Aβ is sensitive to structure or oligomerization state, and that different intracellular degradation pathways are utilized for different Aβ species.
Aβ protofibrils are well-recognized soluble intermediates in Aβ fibrillogenesis (Harper, et al., 1997; Harper, et al., 1999; Walsh, et al., 1999; Walsh, et al., 1997). We have previously demonstrated Aβ42 protofibril formation in several physiological buffering systems including artificial cerebrospinal fluid (aCSF) (Paranjape, et al., 2012; Paranjape, et al., 2013). These structures possess a classic curvilinear morphology with lengths <100 nm, and are robust stimulators of tumor necrosis factor α (TNFα) secretion in microglia. Our earlier work utilized cytokine production, immunochemistry, and confocal microscopy imaging to show preferential activation of microglia by protofibrils (Paranjape, et al., 2012) and BV-2 microglia cell-surface binding of protofibrils (Paranjape, et al., 2013) compared to monomers and fibrils. In the current study, we expand the biophysical characterization of Aβ-fluorophore conjugation and demonstrate that soluble fluorescently-labeled Aβ42 protofibrils are taken up rapidly by primary murine microglia, and in much greater quantity, than Aβ42 monomers.
2. Results
Characterization of fluorophore-labeled Aβ
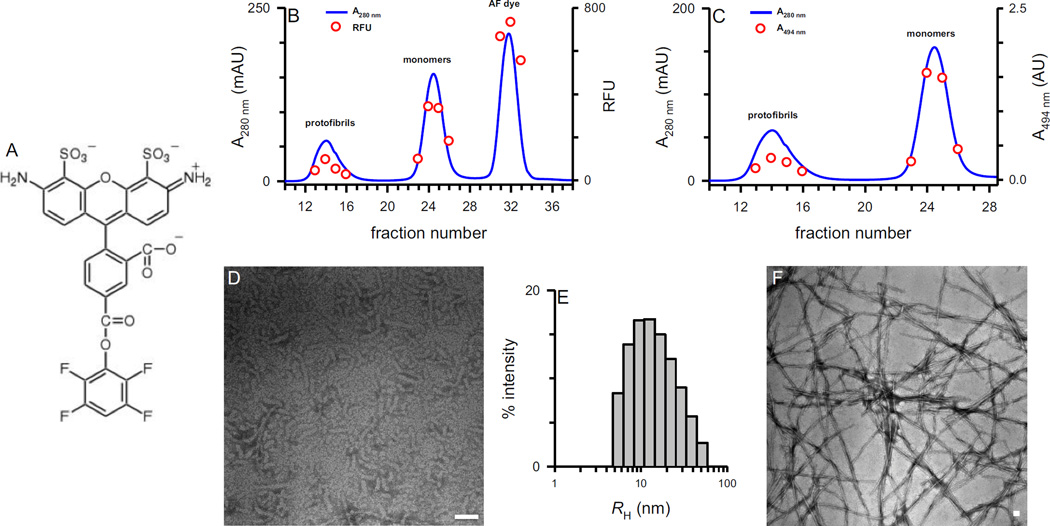
Aβ42 protofibrils and monomers were labeled simultaneously using a unique adaptation of a previously-described procedure by LaDu and colleagues (Jungbauer, et al., 2009). Aβ42 protofibrils were allowed to form in aCSF as outlined in the Methods. This Aβ42 preparation typically produces a roughly equal distribution of protofibrils and monomers. The Aβ solution mixture was then incubated with amine-reactive Alexa Fluor® 488 (AF488) TFP ester dye (Figure 1A). Potential Aβ conjugation sites for AF488 were the primary amines at K16, K28, and the N-terminus. Since the typical protofibril preparation also contains monomers, both species can be labeled with fluorophore simultaneously. Size exclusion chromatography (SEC) separation of the Aβ42 labeling solution on Superdex 75 yielded AF488-labeled Aβ42 protofibrils and monomers (Figure 1B). Monitoring of the elution in-line with UV absorbance (A280 nm) produced three peaks: Aβ42 protofibrils, Aβ42 monomers, and the unconjugated AF488 dye peak. AF488 by itself has a contribution to the A280 nm absorbance that is equal to 11 % of the AF488 A494 nm absorbance. This contribution was taken into account in subsequent Aβ concentration determinations. Both Aβ42 protofibrils and monomers were labeled with AF488 as fluorescence measurements of collected fractions showed the expected emission for the fluorophore in both peaks (Figure 1B).
Figure 1.
Preparation and characterization of AF488-Aβ42. Panel A. Structure of the AF488 TFP compound structure (Life Technologies). Panel B. AF488-labeled protofibrils, monomers and unincorporated dye were separated on Superdex 75 and eluted in aCSF. The elution trace (blue line) was acquired from in-line Abs280 nm readings, and peak fraction fluorescence (red circles) was determined by integrating the AF488 emission from 505–550 nm. Panel C. SEC elution trace (blue line) overlaid with peak fraction Abs494 nm measurements (red circles) subsequently used in stoichiometry calculations. Panel D. Protofibrils (10 µL, 38 µM) applied to a copper formvar grid and imaged by TEM at a magnification of 59,000, scale bar = 50 nm. Panel E. AF488-Aβ42 protofibrils were analyzed by DLS directly after SEC isolation and a representative regularized histogram of percent intensity versus RH is presented. Panel F. TEM of AF488-Aβ42 fibrils (10 µL, 37 µM) that formed during incubation of AF488-Aβ42 monomer fractions for 53 days at 4 °C. Magnification = 18,000, scale bar = 50 nm.
In order to quantitate the amount of AF488 conjugated to each Aβ42 species, absorbance scans (600–220 nm) were conducted on the same SEC fractions. A280 nm values obtained from these SEC fraction scans directly overlaid the SEC absorbance trace (data not shown) and were used in concert with A494 nm values to calculate both AF488 and Aβ concentrations as described in the Methods. These calculations allowed determination of labeling stoichiometry for Aβ42 protofibrils and monomers. Qualitatively, it was observed that Aβ42 monomers were labeled more efficiently than Aβ42 protofibrils. This was not unexpected as Aβ monomers are unstructured leaving the amine groups more exposed for fluorophore conjugation. Quantitatively, for the representative data shown in Figure 1, AF488:Aβ stoichiometry was 0.10 for the peak protofibril fraction and 0.36 and 0.49 for the two peak monomer fractions (#24 and #25 respectively). Overall, in n=5 separate labeling preparations the average AF488:Aβ labeling stoichiometry was 0.12 ± 0.04 standard deviation (SD) for Aβ42 protofibrils and 0.45 ± 0.13 SD for Aβ42 monomers. Thus, monomers were labeled at almost 4 times the efficiency of protofibrils and this difference in the extent of labeling between the two species had implications for data analysis in subsequent uptake experiments. Morphological analysis with transmission electron microscopy (TEM) showed that AF488-Aβ42 protofibrils possessed structural characteristics similar to unlabeled Aβ42 protofibrils with lengths centering around 50–100 nm (Figure 1D). Further characterization by dynamic light scattering (DLS) revealed AF488-labeled Aβ42 protofibrils with hydrodynamic radii (RH) ranging from 5–50 nm with an intensity-weighted mean RH of 16 nm. This value was obtained from the regularized histogram shown in Figure 1E. These findings indicated that labeled and unlabeled Aβ42 protofibrils were similar in their structural characteristics and the process of labeling did not dramatically alter the structure. This determination was consistent with that observed by LaDu and colleagues (Jungbauer, et al., 2009). Furthermore, the additional SEC purification step served to enrich the protofibril population and allowed careful separation of labeled monomeric and protofibrillar Aβ42 pools. Of note, Jungbauer et al. found that while N-terminal-labeled Aβ42 maintained similar aggregation properties to unlabeled peptide, limited solubility was observed for the AF488-N-terminal-labeled Aβ42 particularly at physiological pH (Jungbauer, et al., 2009). In the paradigm described in the current studies, solubility issues were not encountered during SEC isolation of AF488-labeled Aβ42 protofibrils and monomers in aCSF. Interestingly, despite potential labeling at K16 and K28, AF488-Aβ42 monomers were able to aggregate over time and form fibrils (Figure 1F). The TEM image depicts fibrils collected by centrifugation after incubation of AF488-Aβ42 monomers for 53 d at 4 °C. Subsequent fluorescence analysis confirmed that the fibrils were labeled with AF488 (data not shown). Conventional fluorescent techniques for monitoring Aβ aggregation, such as Thioflavin T and bis ANS, were unable to be used due to overlap and interference with the AF488 fluorophore emission.
Uptake of SEC-isolated AF488-Aβ42 monomers and protofibrils in primary murine microglia
Numerous earlier reports have utilized the term “soluble Aβ” to describe solutions prepared directly from reconstituted Aβ peptide. These solutions likely contain a mixture of monomeric and soluble aggregated Aβ species, thereby confounding the interpretation of the findings. For the current study, AF488-Aβ42 monomers and AF488-Aβ42 protofibrils were separated by SEC and the ability of primary murine microglia to take up the two distinct species was evaluated. AF488-Aβ42 protofibrils were rapidly and extensively internalized by microglia compared to AF488-Aβ42 monomers (Figure 2A). AF488-Aβ42 protofibrils (10 µM) were observed inside the microglia at the earliest time point (5 min), which represents an almost immediate entry into the cell. Much lower levels of AF488-Aβ42 monomers (10 µM) were found inside the microglia despite the fact that monomers were labeled with AF488 at nearly 4 times the stoichiometry of protofibrils. Internalized AF488-Aβ42 protofibrils were dense and widespread within the cytosol of the microglia, yet not completely homogenous or diffuse. A closer inspection showed numerous globular, and some punctate, accumulations throughout the intracellular milieu. Microglia were able to internalize AF488-Aβ42 monomers but the amount relative to AF488-Aβ42 protofibrils was strikingly low and demonstrated the overall proclivity of the microglia for protofibril internalization. Confocal images from multiple experiments of microglia treated with AF488-Aβ42 monomers and AF488-Aβ42 protofibrils were quantified using ImageJ software and demonstrated the significant difference in the speed and extent of uptake between the two Aβ42 species (Figure 2B).
Figure 2.
AF488-Aβ42 protofibrils are internalized by primary microglia in greater quantities than AF488-Aβ42 monomers. Primary microglia were plated in Mat-Tek cell culture dishes and treated with 10 µM AF488-Aβ42 monomers and protofibrils (green) at 37 °C for the times indicated. Cells were washed and fixed with 3.7 % formaldehyde as described in the Methods, and then stained with DAPI (blue). Panel A. Representative confocal images of time-dependent microglial exposure to AF488-Aβ42 (Panel A) were acquired at 40X magnification using identical power and pinhole settings for each fluorophore with a Zeiss LSM 700 laser scanning confocal microscope. Scale bar = 10 µm. Panel B. Quantitation of the AF488 fluorescence intensity (fluor int) in the confocal images with ImageJ as described in the Methods was carried out on data from multiple fields of two separate experiments utilizing identical conditions. Panel C. Tumor necrosis factor α (pg/ml) secretion measured by ELISA from samples in Panel A.
The time-dependent microglial uptake of AF488-Aβ42 protofibrils correlated with production of TNFα by the microglia (Figure 2C). The time course for TNFα production was similar to what we have observed previously for unlabeled Aβ42 protofibrils and monomers (Paranjape, et al., 2012; Paranjape, et al., 2013; Terrill-Usery, et al., 2014) wherein protofibrils, but not monomers, are robust stimulators of microglial TNFα secretion. Although uptake of AF488-Aβ42 protofibrils occurs rapidly, there appears to be sufficient cell-surface interaction to trigger signaling pathways for TNFα synthesis and secretion.
Comparisons between AF488-labeled and unlabeled Aβ
Amino-labeling of Aβ42 may interfere with some of the cellular processes that recognize and traffic Aβ42 species, particularly in light of the higher AF488 labeling stoichiometry for Aβ42 monomers. However, unlabeled Aβ42 monomers displayed the same characteristics as AF488-labeled Aβ42 monomers and were not taken up readily by primary murine microglia (Figure 3A–B). Internalization of unlabeled Aβ42 protofibrils by primary microglia was rapid and extensive similar to AF488-labeled protofibrils (Figure 3C–D) confirming that the presence of the AF488 label did not hinder or modulate microglial Aβ internalization. We have previously shown avid binding of Aβ42 protofibrils, but not monomers, to BV-2 microglia (Paranjape, et al., 2013). In order to confirm that the AF488 label did not block protofibril interactions with the microglial cell surface, binding studies were conducted using both immunostaining and direct fluorescence confocal microscopy. Both unlabeled and AF488-labeled Aβ42 protofibrils accumulated around the BV-2 cell surface (Figure 3E–F). The cumulative findings indicated that labeling of either Aβ42 monomers or Aβ42 protofibrils did not alter cellular interactions with the peptides.
Figure 3.
AF488-labeling of Aβ42 does not interfere with microglia uptake or surface interaction. Primary microglia (A–D) or BV-2 microglia (E, F) were treated with 5 µM unlabeled Aβ42 (A, C, E) or AF488-Aβ42 (B, D, F) monomers or protofibrils, washed, fixed and stained with DAPI (blue). AF488-Aβ42 samples were imaged directly (green), while unlabeled samples were immunostained as described in Methods using an AF488-conjugated secondary antibody (green). Scale bar = 10 µm.
Alternative approaches to confocal microscopy were employed to further ascertain and confirm differences in microglia internalization between AF488-labeled Aβ42 protofibrils and Aβ42 monomers. Immuno-dot blot analyses were conducted on lysates prepared after exposure of the primary microglia cells to Aβ for increasing times. Microglial cells were thoroughly washed before cell lysis including an acid wash to remove cell surface-bound Aβ. The findings were similar to those obtained from confocal microscopy and showed the extensive uptake of protofibrils compared to monomeric Aβ (Figure 4A). Due to the low levels of internalized monomers, cell lysates were also probed with a glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody as an immunoblot lysate control (Figure 4A). Densitometry quantitation of cumulative dot blot data from three separate experiments demonstrated the time-dependent uptake of Aβ42 protofibrils compared to Aβ42 monomers (Figure 4B). The dot blot analysis also provided information on the stability of internalized Aβ42 protofibrils and susceptibility to cellular degradation. No loss of internalized Aβ42 protofibril material was noted during the longer 6 and 24 h exposure times (Figure 4A–B). This indicated that although Aβ42 protofibrils were taken up rapidly by microglia, they were quite resistant to proteolysis.
Figure 4.
Aβ42 protofibrils are internalized by microglia in a time-dependent manner and to a greater extent than Aβ42 monomers. Primary microglia were incubated in a Nunc black 96-well cell culture plate and treated with 2 µM AF488-Aβ42 for the times indicated. Microglia cells were washed extensively, including an acid wash to remove external cell-surface-associated Aβ42, prior to fluorescence plate reading and lysate preparation. Panel A. Representative immuno-dot blot analysis of microglia lysates using anti-Aβ (top) or anti-GAPDH (bottom). Panel B. Densitometry quantitation (in pixel intensity) using ImageJ of dot blots from 3 separate time-dependent uptake experiments. Panel C. Following extensive washing and prior to microglial lysate preparation, internalized AF488-Aβ42 protofibrils or monomers were measured by fluorescence plate reader. The fluorescence data for the internalized AF488-Aβ42 represents the averages and standard error from 3 separate experiments and is shown as a percentage of the total AF488-Aβ42 fluorescence present in the microglia assay medium for each condition.
The AF488 labeling of Aβ42 protofibrils and Aβ42 monomers also permitted measurement of primary microglia uptake by fluorescence plate reader. After exposure of adherent primary microglia to AF488-Aβ42 protofibrils and monomers for increasing times, PBS and acid-washed microglia were assessed for intracellular AF488-Aβ42 accumulation. Percent fluorescence uptake was determined by the 535 nm emission intensity of internalized AF488-Aβ divided by the 535 nm emission intensity of the AF488-Aβ remaining in the medium after treatment of microglia. This was done to normalize the fluorescence between the higher stoichiometry-labeled monomers and lower stoichiometry-labeled protofibrils. The fluorescence plate reader findings supported those from confocal microscopy and immuno-dot blot showing the rapid and extensive uptake of protofibrillar Aβ42 (Figure 4C).
Concentration-dependence of AF488-Aβ42 monomers and protofibrils for microglial uptake
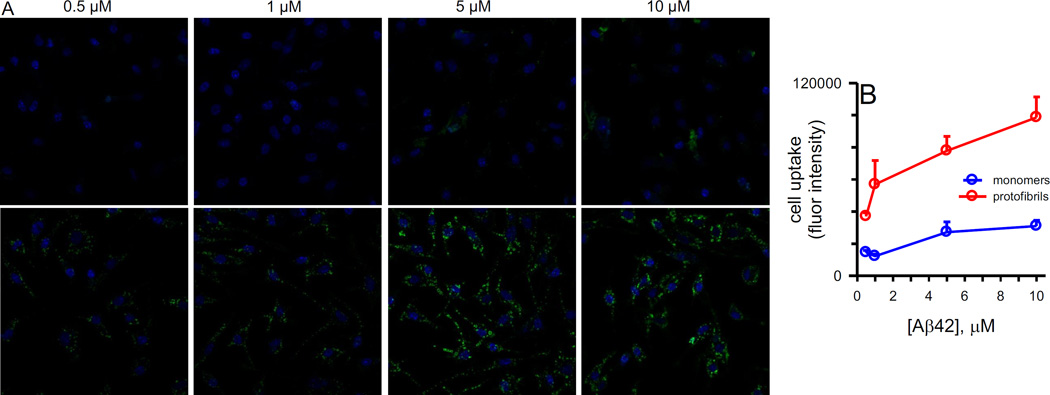
Exposure of the primary murine microglia for 10 min to a range of AF488-Aβ42 monomers and protofibril concentrations again showed the predilection of the microglia for Aβ42 protofibril uptake compared to Aβ42 monomers (Figure 5A). Even at the lowest concentration tested (500 nM), observable levels of AF488-Aβ42 protofibrils were visible within the microglia. Microglial uptake of protofibrils was concentration-dependent with the highest levels found at 5–10 µM. It was apparent from our studies that microglia respond rapidly, and internalize, soluble Aβ42 protofibrils, but not Aβ42 monomers. Fluorescence intensities were quantified from images obtained from multiple concentration-dependent primary microglia uptake experiments and the results indicated that internalization of Aβ42 protofibrils did not saturate within the concentration range and cell density used for these experiments (Figure 5B).
Figure 5.
Internalization of AF488-Aβ42 by primary microglia is concentration-dependent. Panel A. Primary microglia were treated as described in Figure 2 legend with AF488-Aβ42 monomers or protofibrils (green fluorescence) for 10 min at the concentrations indicated. Cells were counterstained with DAPI (blue) and confocal fluorescence images (40X) were captured as described. Panel B. Quantitation of the fluorescence intensity in the A488 channel for the images in panel A and 2 additional concentration-dependent experiments. For each field, the integrated density of fluorescence measured with ImageJ was divided by the number of cell nuclei.
Uptake of AF488-Aβ42 protofibrils is not diminished by competition with unlabeled Aβ42 protofibrils
Although microglia internalization of Aβ42 protofibrils did not appear to saturate, it was possible that the internalization process was receptor-mediated or driven by transport machinery with specific binding sites that may limit the number of protofibrils that can be internalized. It was reasoned that if the number of internalization or transport sites were saturated, competition experiments between AF488-labeled, and unlabeled, Aβ42 protofibrils would reveal this. However, images obtained after exposure of the microglia to solution mixtures containing 10 µM AF488-Aβ42 protofibrils and 0, 5, or 10 µM unlabeled Aβ42 protofibrils were not significantly different (Figure 6A). Additional, and multiple, experiments at 5 µM AF488-Aβ42 protofibrils also did not show competitive inhibition by unlabeled Aβ42 protofibrils (Figure 6B). Specifically the presence of unlabeled Aβ42 protofibrils did not diminish the uptake of AF488-Aβ42 protofibrils indicating that the process was either not competitive or non-saturable at the concentrations used in these experiments.
Figure 6.
Competition with unlabeled Aβ42 protofibrils does not significantly reduce internalization of AF488-Aβ42 protofibrils. Panel A. Primary microglia were treated with solution mixtures of AF488-labeled (L) and unlabeled (U) Aβ42 protofibrils at the concentrations depicted for 15 min at 37 °C. Cells were fixed, stained with DAPI (blue), and imaged (40X magnification) as described earlier in Fig 1. Panel B. In a separate set of experiments (n=2), primary microglia were treated with lower concentrations of AF488-labeled Aβ42 protofibrils (5 µM) for 10 min at 37 °C. Ratios depicted in panel B are in µM. Confocal images were obtained and uptake (fluorescence intensity) was quantified using ImageJ as previously described.
Cellular localization of AF488-Aβ42 protofibrils following microglial internalization
Numerous studies have demonstrated partial localization of Aβ within lysosomes (Halle, et al., 2008; Li, et al., 2012; Liu, et al., 2010; Mandrekar, et al., 2009). Our initial studies using Lyso-Tracker Red to image lysosomes showed significant internalization of AF488-Aβ42 protofibrils but no lysosome-Aβ42 protofibril colocalization (data not shown). Due to weak Lyso-Tracker confocal fluorescence signals, another lysosomal marker, Lamp1, was targeted by immunostaining. The greater sensitivity of the Lamp1 assay revealed some colocalization of AF488-Aβ42 protofibrils with lysosomal compartments. However, significant amounts of AF488-Aβ42 protofibrils were not in the lysosomes and many lysosomes were devoid of protofibrils (Figure 7).
Figure 7.
Partial colocalization of AF488-Aβ42 protofibrils with lysosomes. Primary microglia were cultured as described in Methods and treated with 5 µM A488-Aβ for 30 min at 37 °C, washed, fixed, and and permeabilized with 0.5 % Triton X-100. Cells were immunostained with 6E2 anti-Lamp1 primary and anti-mouse IgG-NL637 secondary antibody. Three different fields are shown and white arrows indicate lysosomes devoid of A488-Aβ42 protofibrils. Yellow arrows indicate areas of colocalization for A488-Aβ42 protofibrils and lysosomes. Green: A488-Aβ; Red: Lamp1; Blue: DAPI. Scale bar = 10 µm.
3. Discussion
The question of how the aggregation of Aβ impacts its biological activity has been front and center for almost three decades. Answering these questions has been hindered in many circumstances by the use of unseparated solution mixtures that may contain fibrillar Aβ material as well as a variety of soluble Aβ species (both monomeric and aggregated). In this report, we fluorescently labeled an Aβ preparation containing both Aβ42 protofibrils and monomers and then chromatographically separated the two Aβ species. This allowed direct comparison of microglial internalization propensity and kinetics between Aβ42 protofibrils and Aβ42 monomers. Primary microglia significantly favored internalization of Aβ42 protofibrils as compared to Aβ42 monomers both in total amount and speed, demonstrating that an aggregated form of Aβ is readily and preferentially taken up by microglia within the milieu of soluble Aβ. Furthermore, new insights were gained during the amine-dependent AF488 labeling of Aβ. Protofibrils were labeled less effectively than monomers, likely due to the restricted accessibility of lysine residues 16 and 28 in the β-sheet conformation.
We have previously investigated the microglia-Aβ42 interaction in BV-2 murine microglial cells. That particular study indicated a preferential binding of Aβ42 protofibrils to the cell surface compared to Aβ42 monomers and Aβ42 fibrils (Paranjape, et al., 2013). An important observation from that earlier study, relative to the current investigation, was that primary microglia were far more active at internalizing Aβ than BV-2 cells. However, the propensity to interact preferentially with protofibrillar Aβ42 was consistent in both studies. There is not a consensus in the field on the relationship between Aβ structure and microglial internalization. Several studies have reported internalization of fibrillar Aβ (Chung, et al., 1999; Fu, et al., 2012; Gaikwad, et al., 2009; Koenigsknecht and Landreth, 2004; Mandrekar, et al., 2009), while others have demonstrated internalization of soluble or oligomeric Aβ (Chung, et al., 1999; Mandrekar, et al., 2009). The findings are not always consistent as Pan et al. showed that oligomeric Aβ is not internalized by microglia, yet interferes with fibrillar Aβ phagocytosis (Pan, et al., 2011). Less is known about protofibrillar Aβ, but one study found that protofibrils, but not fibrils, were internalized by microglia in the AD CRND8 mouse model (Liu, et al., 2010).
Mammalian cells ingest material by either phagocytosis (larger particles such as bacteria, yeast, and cellular debris) or fluid-phase pinocytosis (smaller particles) (Conner and Schmid, 2003). Pinocytosis can occur through one of five routes: macropinocytosis, clathrin- or caveolin-mediated endocytosis, and clathrin- or caveolin-independent endocytosis. Macropinocytosis is associated with particles that are more than 1 µm in size, while the other four mechanisms are associated with smaller particles ranging in size from 60–120 nm (Conner and Schmid, 2003). Clathrin and caveolin are proteins that promote the formation of vesicles at the cell surface. These vesicles are then pinched off from the membrane as they enter the cell. During fluid-phase pinocytosis, membrane ruffles form on the membrane surface of microglial cells and when the ruffles close some of the extracellular medium is taken into the cell (Swanson and Watts, 1995). The resulting vesicle is fully inside the cell in less than 15 minutes (Swanson and Watts, 1995). During our microglia internalization studies, we have observed Aβ42 protofibrils appear to accumulate around the microglia membrane ruffles. This is evidenced by hollow circular Aβ42 structures localized at the cell surface in some of the images. Furthermore, the rapidity of Aβ42 protofibril internalization by microglia is consistent with fluid-phase pinocytosis. Landreth and coworkers have demonstrated distinct mechanisms of uptake for fibrillar Aβ by receptor-mediated nonclassical phagocytosis (Koenigsknecht and Landreth, 2004) and soluble Aβ (primarily monomer and small oligomers) by macropinocytosis (Mandrekar, et al., 2009).
In the course of the in situ fluorescence plate reader experiments we found that a significant percentage of the Aβ42 protofibrils were not taken up by the microglia under those concentration and time conditions. Some of this may be explained by Aβ adsorption to plate surfaces and Aβ association with the cell surface, which was removed by the acid wash (~8% for protofibrils at 24 h, very little for monomers, data not shown). Nevertheless this suggests that the rate of Aβ42 protofibril uptake by microglia may be limited in some manner. The fluorescence plate reader findings indicated that the amount of internalized Aβ protofibrils continued to increase over time as measured by fluorescence plate reader. Although the immuno-dot blot data (Figure 4B) appears to reach a steady level, this is likely due to the smaller range of the dot blot assay with respect to densitometry quantitation. The concentration-dependent experiments in Figure 5 indicated that further increases in [Aβ42 protofibril] increased the amount of internalized Aβ. Figure 4C suggests there is plenty of available Aβ42 protofibrils (>95%) at an initial concentration of 2 µM. However, an increase in [Aβ42 protofibrils] in the medium surrounding the microglia to 5 µM and 10 µM (Figure 5) favored increased uptake based on membrane gradient dynamics. The competition experiments in Figure 6 confirmed that inclusion of unlabeled Aβ42 protofibrils with AF488-Aβ42 protofibrils prior to microglia treatment did not diminish the amount of internalized AF488-Aβ42 protofibrils and saturation was not achieved. Further investigation into the mechanism of uptake microglia internalization of Aβ protofibrils will be needed to fully understand the transport or diffusion process.
Internalization by microglia and trafficking to intracellular lysosomal compartments has been shown to be a key pathway for aggregated Aβ (Chu, et al., 1998; Fu, et al., 2012; Halle, et al., 2008; Li, et al., 2012; Liu, et al., 2010; Mandrekar, et al., 2009; Paresce, et al., 1997). This process may or may not be degradative (Chu, et al., 1998; Paresce, et al., 1997). Our studies indicated that some, but only a small percentage, of Aβ42 protofibrils were trafficked to the lysosome. This finding, and the observation that Aβ42 protofibrils are quite stable (Coalier, et al., 2013), may help to explain our observation that Aβ42 protofibrils were not degraded and cleared after internalization by primary microglial cells.
Our investigations over a number of years have pointed to soluble Aβ42 protofibrils as robust stimulators of microglia compared to fibrils and monomers. We have conducted numerous studies of protofibril properties and the ensuing cellular response. While there are more studies of “oligomeric” Aβ, there are fewer on microglial-protofibril interactions. Due to the substantial interest in soluble Aβ aggregates such as protofibrils and oligomers, it is important to discern what is being studied when a solution of Aβ is described as “soluble” since these solutions likely contain multiple Aβ species. Few investigations rigorously separate Aβ mixtures into soluble aggregates and monomers, thus confusing the interpretation of the results. For our studies, the critical factor in ascertaining the difference between the two Aβ species was the isolation of each population by SEC. Our studies demonstrated rapid and widespread internalization of Aβ42 protofibrils by microglia and the difference in microglia uptake was striking between Aβ42 protofibrils and monomers. The internalization of Aβ by microglia is an important part of AD and these in vitro findings shed light on the sensitivity of microglia to distinct Aβ species.
4. Experimental procedures
4.1. Aβ peptide preparation
Aβ1–42 (Aβ42) was obtained from ERI Amyloid Laboratory, LLC (Oxford, CT) (formerly W. M. Keck Biotechnology Resource Laboratory) in lyophilized form and stored at −20° C. Aβ42 peptide was dissolved in 100% hexafluoroisopropanol (HFIP) (Sigma-Aldrich, St. Louis) at 1 mM, separated into aliquots in sterile microcentrifuge tubes, and evaporated uncovered at room temperature overnight in a fume hood. The following day the aliquots were vacuum-centrifuged to remove any residual HFIP and stored in dessicant at −20° C.
4.2. Aβ labeling
Aβ42 protofibrils were prepared as previously described (Paranjape, et al., 2012). Briefly, lyophilized Aβ42 (1 mg) was dissolved in 50 mM NaOH to yield a 2.5 mM Aβ42 solution followed by dilution to 250 µM Aβ42 in prefiltered artificial cerebrospinal fluid (aCSF, 15 mM NaHCO3, 1 mM Na2HPO4, 130 mM NaCl, 3 mM KCl, pH 7.8). The Aβ42 solution was incubated at room temperature for 30 min-4 h to allow the formation of protofibrils. For unlabeled Aβ42 preparations, the solution was centrifuged at 18,000g for 10 min and the supernatant was chromatographically separated. For fluorophore labeling of Aβ42, 500 µL of the Aβ protofibril preparation was added to a vial containing 100 µg of Alexa Fluor® 488 carboxylic acid, 2,3,5,6-tetrafluorophenyl ester (AF488-TFP) dry powder (Life Technologies), mixed gently by pipet, and transferred to a 1.8 mL glass vial containing a small magnetic stir bar. A second 500 µL aliquot of Aβ42 solution was added to the dye solution to bring the final volume to 1 mL. The solution was gently stirred for 1 h at 25 °C, transferred to a siliconized Eppendorf tube, and centrifuged and separated as described above.
4.3. Size exclusion chromatography
Both unlabeled and fluorophore-labeled Aβ centrifugation supernatants were fractionated on a Tricorn Superdex 75 10/300 GL column (GE Healthcare) using an AKTA FPLC system (GE Healthcare). Prior to injection of Aβ, the Superdex 75 column was coated with sterile bovine serum albumin (Sigma) to prevent any non-specific binding of Aβ to the column matrix. Following a 1 mL loading of the sample, Aβ was eluted at 0.5 mL min−1 in aCSF and 0.5 mL fractions were collected and immediately placed on ice. Elution was monitored in-line by UV absorbance (A280). Aβ concentrations were determined as described below.
4.4. Absorbance measurements and concentration calculations
Chromatography samples were scanned from 600 to 220 nm on a Cary 50 (Varian) UV/Visible spectrophotometer and absorbance values at 280 nm (A280, tot) and 494 nm (A494, AF488) in particular were obtained. Since Aβ and the AF488 label (A280, AF488 = A494, AF488 × 0.11) both contribute to A280, tot, Aβ concentrations were obtained after subtraction of the A280, AF488 contribution. This was done using A494, AF488 and A280, tot from the absorbance scans and the following calculation derived from Beer’s Law (A = ε / c).
| (Equation 1) |
where l is the path length (1 cm) and ε280, Aβ is the extinction coefficient at 280 nm for Aβ (1450 M−1 cm−1). AF488 concentrations were determined using an extinction coefficient at 494 nm of 71,000 M−1 cm−1. The AF488:Aβ42 stoichiometry was also determined from the absorbance-determined concentrations calculations.
4.5. Fluorescence measurements
AF488-labeled Aβ42 fluorescence was determined in chromatography fractions using a Cary Eclipse fluorescence spectrophotometer. Emission scans (500–600 nm) were obtained with an excitation wavelength of 495 nm. The resulting curves were integrated from 505–550 nm to produce relative fluorescence units (RFU). In situ cellular fluorescence plate reader measurements were carried out using a Perkin Elmer Victor 3 multi-mode instrument. Excitation and emission filters were 485 nm and 535 nm, respectively, with a 0.1 second signal averaging time.
4.6. Dynamic light scattering
Hydrodynamic radius (RH) measurements were taken at room temperature with a DynaPro Titan instrument (Wyatt Technology, Santa Barbara, CA). Samples (30 µl) were placed into a quartz cuvette and light scattering intensity was collected at a 90° angle using a 5-second acquisition time. Particle diffusion coefficients were calculated from auto-correlated light intensity data and converted to RH with the Stokes-Einstein equation using Dynamics software (version 6.12.0.3). Histograms of percent intensity vs. RH were generated by data regularization and intensity-weighted mean RH values (nm) were derived from the regularized histograms.
4.7. Primary microglia isolation
Primary murine microglia were obtained from wild-type (WT) C57BL/6 (Harlan Laboratories). Microglia were isolated as previously described (Esen and Kielian, 2007; Paranjape, et al., 2012) from 3–4 day old mouse pups. Briefly, brains were isolated under sterile conditions, minced, and trypsinized. The brain tissue was then resuspended in complete DMEM growth medium containing 10% fetal bovine serum (FBS), 4 mM L-glutamine, 100 U/ml penicillin, 0.1 mg/ml streptomycin and 0.25 µg/mL amphotericin-B, OPI medium supplement (oxalocetate, pyruvate, insulin) (Sigma-Aldrich), and 0.5 ng/ml recombinant mouse granulocyte-macrophage colony-stimulating factor (GM-CSF) (Life Technologies). The cell suspension was filtered, centrifuged, resuspended in complete medium and seeded into 150 cm2 flasks. Cells were cultured at 37 °C in 5% CO2 until confluent (1–2 weeks) and microglia were selectively harvested from the adherent astrocyte layer by shaking of the flask for 6 h or overnight at 37 °C in 5% CO2 and collection of the medium.
4.8. Microglial uptake
For cell uptake studies, WT primary murine microglia, obtained as described above, were seeded in sterile glass-bottom Mat-Tek P35GC-1.5–14-C culture dishes (MatTek Corp., Ashland, MA) or 96-well cell culture plates overnight at a density of 5 × 105 cells/ml in complete growth medium. Prior to cell treatment, medium was replaced with growth medium lacking FBS and GM-CSF (assay medium). Cells were then incubated at 37 °C in 5% CO2 for the indicated times with AF488-labeled, unlabeled Aβ42, aCSF control, or AF488 dye fraction. Following incubation, the conditioned medium was collected and stored at −20 °C for subsequent analysis by enzyme-linked immunosorbent assay (ELISA). Adherent microglial cells were rinsed twice with assay medium and then fixed with 3.7 % formaldehyde for confocal microscopy analysis. For combination fluorescence plate and dot blot analysis, microglia were plated in Nunc F96 Nunclon sterile 96-well black plates. A more thorough wash protocol was utilized for these studies. This protocol involved removal of medium from treated cells followed by rinsing each well 3X with 100 µL sterile PBS. An acid wash (100 µL) was performed using a solution of 0.5 M NaCl and 0.2 M acetic acid for 15 minutes at 4 °C, then the cells were rinsed 2X with PBS. All PBS and acid washes were moved to another plate for both fluorescence and dot blot analysis. For intracellular cell extract preparation, microglia were lysed for 15 min at 25 °C with 35–50 µL radio immunoprecipitation assay (RIPA) buffer containing 150 mM NaCl, 20 mM Tris, 1 mM ethylenediaminetetraacetic acid (EDTA), 1% Triton X-100, 1% deoxycholic acid, 0.1% sodium dodecyl sulfate (SDS), and protease inhibitor cocktail (Sigma-Aldrich) at a 10x dilution. In some cases, multiple microglia wells were combined to yield higher concentration lysates. Microglial lysates were stored at −20 °C for further analysis.
4.9. BV-2 microglia cell culture and cell binding assay
BV-2 murine microglial cells were cultured as previously described (Paranjape, et al., 2012) in Dulbecco’s modified Eagle’s medium (DMEM, 4.5 g/L glucose) (Hyclone) containing 50 U/ml penicillin, 50 µg/ml streptomycin, 50 µM β-mercaptoethanol, and 5 % fetal bovine serum (FBS, Hyclone). The Aβ-BV-2 microglial binding assay was conducted as previously described (Paranjape, et al., 2013). BV-2 murine microglia cells (0.2 ml, 5 × 105 cells/mL) were plated in individual MatTek dishes overnight. Cells were treated with either AF488-Aβ or unlabeled Aβ (5 µM) or aCSF buffer control and incubated for 30 min at 25 °C. Cells were then washed 3x with PBS containing 0.05 % Tween 20 (PBST) before and after fixing with 3.7 % formaldehyde, and after each step in the immunoassay for unlabeled Aβ detection. All incubations were conducted with gentle shaking at 25 °C. Samples treated with unlabeled Aβ were incubated for 1 h each with blocking buffer (10 % w/v dried milk in PBST), anti-Aβ antibody Ab9 (1:5000 dilution in PBST with 5 % w/v dried milk), and donkey anti-mouse IgG antibody conjugated to Northern Lights (NL) 493 (R&D Systems). After a final wash step, 1 mL PBS was applied to the wells to avoid cellular dehydration.
4.10. Confocal microscopy
Cells were treated in Mat-Tek dishes and fixed as described above, followed by three washes with PBST. Cell nuclei were visualized by incubation for 5 min with 0.2 mL of 0.3 µM 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI). After a final wash step, 1.0 mL PBS was applied to the wells to avoid cellular dehydration. LysoTracker studies were done prior to cell treatment by adding 0.100 mL of assay medium containing 0.100 µM LysoTracker and incubating 30 min at 37 °C in 5% CO2. Microglia treated with AF488-labeled Aβ were imaged directly without further manipulation. For microglia treated with unlabeled Aβ, fixed cells were immunostained as described above in Section 4.8. Lysosomal Lamp1 detection was done by immunostaining as described for unlabeled Aβ but with a 1:500 dilution of anti-Lamp1 primary antibody (Novus Biologicals) into PBS containing 1% BSA and a 1:100 dilution of anti-mouse IgG-NL637 secondary antibody (R & D Systems). Excitation wavelengths used were 405 nm (DAPI), 488 nm (AF488-labeled Aβ42 and anti-mouse IgG-AF488 secondary antibody), 561 nm (LysoTracker Red, Invitrogen), and 639 nm for anti-mouse IgG-NL637. Both two-dimensional and Z-stack images were obtained with a Zeiss LSM 700 confocal microscope at 40X magnification using ZEN 2009 imaging and analysis software. For comparison experiments, all optical and electrical settings were fixed at identical levels for each image to ensure that fluorescence intensity measurements were not influenced by different settings. Confocal fluorescence images were quantitated with ImageJ software using a procedure detailed by (Ansari, et al., 2013). Briefly, a region of interest was drawn around each cell using a polygon drawing tool. Area and integrated density were measured for each cell. Background fluorescence was measured by averaging 5 random areas between cells in the field. The corrected total cell fluorescence (CTCF) was calculated as follows: [integrated density of total cell area – (total cell area x mean background fluorescence)].
4.11. Dot blot
All steps in the dot blot were done at 25 °C on a platform rotary shaker set at 70 rpm. A nitrocellulose membrane was soaked in water and allowed to dry completely. Microglial cell lysates (1.7 µL) were spotted onto the membrane, allowed to dry for 20 min, and blocked with phosphate-buffered saline (PBS) containing 0.1% Tween 20 and 5% milk with gentle shaking for 1 hr. The membrane was washed 3x for 5 min with PBS containing 0.2% Tween at pH 7.4 (PBST). The membrane was treated with mouse anti-Aβ primary antibody (Ab9, 1:5000 dilution) followed by anti-mouse IgG-HRP secondary antibody (1:1000) with a multi-wash step in between. Both antibodies were diluted in PBS containing 1% milk and 0.1% Tween prior to membrane application. After washing, the membrane was treated with ECL western blotting substrate solution (Pierce), and washed for 1 min with accelerated shaking at 120 rpm. The membrane was subsequently dried and exposed to film for 60 seconds in an autoradiography cassette. The film was developed, fixed, washed in water, and dried for scan analysis. Duplicate dot blot membranes were probed for GAPDH as a control intracellular protein. The procedure only differed in the primary mouse anti-GAPDH antibody (1:1000 dilution). The primary and secondary antibody diluents in the GAPDH procedure were PBS containing 1% milk and 0.1% Tween. Densitometry was performed on dot blot film images using ImageJ software.
4.12. ELISA
Levels of murine TNFα were determined by ELISA as previously detailed (Udan, et al., 2008). Briefly, 96-well plates were coated overnight with monoclonal anti-mouse TNFα capture antibody (R&D Systems), washed with PBS containing 0.05% Tween-20 and blocked with PBS containing 1% bovine serum albumin (BSA), 5% sucrose and 0.05% NaN3 following by a wash step. Successive treatments with washing in between were done with samples or standards, biotinylated polyclonal anti-mouse TNFα detection antibody (R&D Systems) in 20 mM Tris with 150 mM NaCl and 0.1% BSA, streptavidin-horseradish peroxidase (HRP) conjugate, and equal volumes of HRP substrates 3,3’,5,5’-tetramethylbenzidine and hydrogen peroxide. The reaction was stopped by the addition of 1% H2SO4 solution. The optical density of each sample was analyzed at 450 nm with a reference reading at 630 nm using a SpectraMax 340 absorbance plate reader (Molecular Devices, Union City, CA). The concentration of TNFα in the experimental samples was calculated from a mouse TNFα standard curve of 15–2000 pg/ml. When necessary, samples were diluted to fall within the standard curve.
4.13. Electron microscopy
SEC-purified AF488-Aβ42 protofibrils or fibrils (10 µL) were applied to a 200-mesh formvar-coated copper grid (Ted Pella, Inc.). Samples were allowed to adsorb for 10 min at 25 °C, followed by removal of excess sample solution. Grids were washed three times by placing the sample-containing side down on a droplet of water. Heavy metal staining was done by incubation of the grid on a droplet of 2 % uranyl acetate (Electron Microscopy Sciences, Hatfield, PA) for 5 min, removal of excess solution, and air drying. Affixed samples were visualized with a JEOL JEM-2000 FX transmission electron microscope operated at 200 keV.
Highlights.
Aβ42 protofibrils and monomers were simultaneously labeled, then separated.
Aβ42 monomers were labeled at higher stoichiometry than protofibrils.
Microglia internalized Aβ42 protofibrils very rapidly and extensively.
Purified Aβ42 monomers were not readily taken up by microglia.
Significant colocalization of Aβ42 protofibrils with lysosomes was not observed.
Acknowledgments
This work was supported by Award Number R15AG033913 from the National Institute on Aging (MRN).
Abbreviations used
- AD
Alzheimer’s disease
- Aβ
amyloid-β protein
- aCSF
artificial cerebrospinal fluid
- HFIP
hexafluoroisopropanol
- PBS
phosphate-buffered saline
- SEC
size exclusion chromatography
- TEM
transmission electron microscopy
- TNFα
tumor necrosis factor α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Ansari N, Muller S, Stelzer EH, Pampaloni F. Quantitative 3D cell-based assay performed with cellular spheroids and fluorescence microscopy. Methods Cell Biol. 2013;113:295–309. doi: 10.1016/B978-0-12-407239-8.00013-6. [DOI] [PubMed] [Google Scholar]
- Apelt J, Schliebs R. β-amyloid-induced glial expression of both pro- and anti-inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain Res. 2001;894:21–30. doi: 10.1016/s0006-8993(00)03176-0. [DOI] [PubMed] [Google Scholar]
- Ard MD, Cole GM, Wei J, Mehrle AP, Fratkin JD. Scavenging of Alzheimer’s amyloid β-protein by microglia in culture. J Neurosci Res. 1996;43:190–202. doi: 10.1002/(SICI)1097-4547(19960115)43:2<190::AID-JNR7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Bitan G, Kirkitadze MD, Lomakin A, Vollers SS, Benedek GB, Teplow DB. Amyloid β-protein (Aβ) assembly: Aβ40 and Aβ42 oligomerize through distinct pathways. Proc. Natl. Acad. Sci. USA. 2003;100:330–335. doi: 10.1073/pnas.222681699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu T, Tran T, Yang F, Beech W, Cole GM, Frautschy SA. Effect of chloroquine and leupeptin on intracellular accumulation of amyloid-beta (Aβ) 1–42 peptide in a murine N9 microglial cell line. FEBS Lett. 1998;436:439–444. doi: 10.1016/s0014-5793(98)01161-2. [DOI] [PubMed] [Google Scholar]
- Chung H, Brazil MI, Soe TT, Maxfield FR. Uptake, degradation, and release of fibrillar and soluble forms of Alzheimer’s amyloid β-peptide by microglial cells. J. Biol. Chem. 1999;274:32301–32308. doi: 10.1074/jbc.274.45.32301. [DOI] [PubMed] [Google Scholar]
- Coalier KA, Paranjape GS, Karki S, Nichols MR. Stability of early-stage amyloid-β(1–42) aggregation species. Biochim Biophys Acta. 2013;1834:65–70. doi: 10.1016/j.bbapap.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Lee SC, Mattiace LA, Yen SHC, Brosnan C. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer disease. Glia. 1993;7:75–83. doi: 10.1002/glia.440070113. [DOI] [PubMed] [Google Scholar]
- Esen N, Kielian T. Effects of low dose GM-CSF on microglial inflammatory profiles to diverse pathogen-associated molecular patterns (PAMPs) J Neuroinflammation. 2007;4:10. doi: 10.1186/1742-2094-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Liu B, Frost JL, Hong S, Jin M, Ostaszewski B, Shankar GM, Costantino IM, Carroll MC, Mayadas TN, Lemere CA. Complement component C3 and complement receptor type 3 contribute to the phagocytosis and clearance of fibrillar Aβ by microglia. Glia. 2012;60:993–1003. doi: 10.1002/glia.22331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaikwad S, Larionov S, Wang Y, Dannenberg H, Matozaki T, Monsonego A, Thal DR, Neumann H. Signal regulatory protein-β1: a microglial modulator of phagocytosis in Alzheimer’s disease. Am J Pathol. 2009;175:2528–2539. doi: 10.2353/ajpath.2009.090147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat Immunol. 2008;9:857–65. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JD, Lieber CM, Lansbury PT., Jr Atomic force microscopic imaging of seeded fibril formation and fibril branching by the Alzheimer’s disease amyloid-β protein. Chem. Biol. 1997;4:951–959. doi: 10.1016/s1074-5521(97)90303-3. [DOI] [PubMed] [Google Scholar]
- Harper JD, Wong SS, Lieber CM, Lansbury PT., Jr Observation of metastable Aβ amyloid protofibrils by atomic force microscopy. Chem. Biol. 1997;4:119–125. doi: 10.1016/s1074-5521(97)90255-6. [DOI] [PubMed] [Google Scholar]
- Harper JD, Wong SS, Lieber CM, Lansbury PT., Jr Assembly of Aβ amyloid peptides: an in vitro model for a possible early event in Alzheimer’s disease. Biochemistry. 1999;38:8972–8980. doi: 10.1021/bi9904149. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, Khoury JE, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett JT, Berger EP, Lansbury PT., Jr The carboxy terminus of the β amyloid protein is critical for the seeding of amyloid formation: Implications for the pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32:4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- Jarrett JT, Lansbury PT., Jr Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer’s disease and scrapie? Cell. 1993;73:1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- Jungbauer LM, Yu C, Laxton KJ, LaDu MJ. Preparation of fluorescently-labeled amyloid-beta peptide assemblies: the effect of fluorophore conjugation on structure and function. J Mol Recognit. 2009;22:403–13. doi: 10.1002/jmr.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Koenigsknecht J, Landreth G. Microglial phagocytosis of fibrillar β-amyloid through a β1 integrin-dependent mechanism. J Neurosci. 2004;24:9838–9846. doi: 10.1523/JNEUROSCI.2557-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, Hyman BT, Spires-Jones TL. Oligomeric amyloid β associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci USA. 2009;106:4012–4017. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HQ, Chen C, Dou Y, Wu HJ, Liu YJ, Lou HF, Zhang JM, Li XM, Wang H, Duan S. P2Y4 receptor-mediated pinocytosis contributes to amyloid beta-induced self-uptake by microglia. Mol Cell Biol. 2013;33:4282–4293. doi: 10.1128/MCB.00544-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kanekiyo T, Shinohara M, Zhang Y, LaDu MJ, Xu H, Bu G. Differential regulation of amyloid-β endocytic trafficking and lysosomal degradation by apolipoprotein E isoforms. J Biol Chem. 2012;287:44593–601. doi: 10.1074/jbc.M112.420224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Condello C, Schain A, Harb R, Grutzendler J. CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-β phagocytosis. J Neurosci. 2010;30:17091–101. doi: 10.1523/JNEUROSCI.4403-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar S, Jiang Q, Lee CY, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE. Microglia mediate the clearance of soluble Aβ through fluid phase macropinocytosis. J Neurosci. 2009;29:4252–4262. doi: 10.1523/JNEUROSCI.5572-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987;79:195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-β plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittag JJ, Milani S, Walsh DM, Radler JO, McManus JJ. Simultaneous measurement of a range of particle sizes during Aβ1–42 fibrillogenesis quantified using fluorescence correlation spectroscopy. Biochem Biophys Res Commun. 2014;448:195–199. doi: 10.1016/j.bbrc.2014.04.088. [DOI] [PubMed] [Google Scholar]
- Pan XD, Zhu YG, Lin N, Zhang J, Ye QY, Huang HP, Chen XC. Microglial phagocytosis induced by fibrillar β-amyloid is attenuated by oligomeric β-amyloid: implications for Alzheimer’s disease. Mol Neurodegener. 2011;6:45. doi: 10.1186/1750-1326-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjape GS, Gouwens LK, Osborn DC, Nichols MR. Isolated amyloid-β(1–42) protofibrils, but not isolated fibrils, are robust stimulators of microglia. ACS Chem Neurosci. 2012;3:302–311. doi: 10.1021/cn2001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjape GS, Terrill SE, Gouwens LK, Ruck BM, Nichols MR. Amyloid-β(1–42) protofibrils formed in modified artificial cerebrospinal fluid bind and activate microglia. J Neuroimmune Pharmacol. 2013;8:312–22. doi: 10.1007/s11481-012-9424-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paresce DM, Chung H, Maxfield FR. Slow degradation of aggregates of the Alzheimer’s disease amyloid β-protein by microglial cells. J Biol Chem. 1997;272:29390–29397. doi: 10.1074/jbc.272.46.29390. [DOI] [PubMed] [Google Scholar]
- Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalize aggregates of the Alzheimer’s disease amyloid β-protein via a scavenger receptor. Neuron. 1996;17:553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, Moore KJ. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;8:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JA, Watts C. Macropinocytosis. Trends Cell Biol. 1995;5:424–8. doi: 10.1016/s0962-8924(00)89101-1. [DOI] [PubMed] [Google Scholar]
- Terrill-Usery SE, Mohan MJ, Nichols MR. Amyloid-β(1–42) protofibrils stimulate a quantum of secreted IL-1β despite significant intracellular IL-1β accumulation in microglia. Biochim Biophys Acta. 2014;1842:2276–2285. doi: 10.1016/j.bbadis.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD, Gonatas NK, Weiss M. Ultrastructural studies in Alzheimer’s presenile dementia. Am. J. Pathol. 1964;44:269–297. [PMC free article] [PubMed] [Google Scholar]
- Udan ML, Ajit D, Crouse NR, Nichols MR. Toll-like receptors 2 and 4 mediate Aβ(1–42) activation of the innate immune response in a human monocytic cell line. J Neurochem. 2008;104:524–533. doi: 10.1111/j.1471-4159.2007.05001.x. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A, Benedek GB, Selkoe DJ, Teplow DB. Amyloid b-protein fibrillogenesis: Structure and biological activity of protofibrillar intermediates. J. Biol. Chem. 1999;274:25945–25952. doi: 10.1074/jbc.274.36.25945. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Lomakin A, Benedek GB, Condron MM, Teplow DB. Amyloid b-protein fibrillogenesis: Detection of a protofibrillar intermediate. J. Biol. Chem. 1997;272:22364–22372. doi: 10.1074/jbc.272.35.22364. [DOI] [PubMed] [Google Scholar]