Abstract
Effects of sorafenib in hepatocellular carcinoma (HCC) are frequently transient due to tumor-acquired resistance, a phenotype that could be targeted by other molecules to reduce this adaptive response. Because melatonin is known to exert antitumor effects in HCC cells, this study investigated whether and how melatonin reduces resistance to sorafenib. Susceptibility to sorafenib (10 nM to 50 μM) in the presence of melatonin (1 and 2 mM) was assessed in HCC cell lines HepG2, HuH7 and Hep3B. Cell viability was reduced by sorafenib from 1 μM in HepG2 or HuH7 cells, and 2.5 μM in Hep3B cells. Co-administration of melatonin and sorafenib exhibited a synergistic cytotoxic effect on HepG2 and HuH7 cells, while Hep3B cells displayed susceptibility to doses of sorafenib that had no effect when administrated alone. Co-administration of 2.5 μM sorafenib and 1 mM melatonin induced apoptosis in Hep3B cells, increasing PARP hydrolysis and BAX expression. We also observed an early colocalization of mitochondria with lysosomes, correlating with the expression of mitophagy markers PINK1 and Parkin and a reduction of mitofusin-2 and mtDNA compared with sorafenib administration alone. Moreover, increased reactive oxygen species production and mitochondrial membrane depolarization were elicited by drug combination, suggesting their contribution to mitophagy induction. Interestingly, Parkin silencing by siRNA to impair mitophagy significantly reduced cell killing, PARP cleavage and BAX expression. These results demonstrate that the pro-oxidant capacity of melatonin and its impact on mitochondria stability and turnover via mitophagy increase sensitivity to the cytotoxic effect of sorafenib.
Keywords: sorafenib, melatonin, hepatocarcinoma, mitophagy, oxidative stress, apoptosis
Introduction
Hepatocellular carcinoma (HCC) is the sixth most prevalent cancer and the third most frequent cause of cancer-related death worldwide. Patients with HCC diagnosed in early stages are the best candidates for surgical resection or liver transplantation, with a 5-year survival success higher than 50% compared with 20% in untreated patients [1, 2]. Unfortunately, most HCC patients are diagnosed in advanced stages when curative treatments are not applicable and the benefits of conventional chemotherapy do not reach expected results. Currently, the only available non-curative approaches that have shown improvement in survival rate in advanced HCC are transarterial chemoembolization and sorafenib administration [3]. Sorafenib is a multikinase inhibitor that modulates cancer development and progression by targeting specific molecular pathways. The drug blocks tumor cell proliferation and angiogenesis through inhibition of the Raf/Mitogen-activated protein (MAP)/extracellular signal-regulated kinase (ERK) kinase (MEK)/ERK signaling cascade, and the kinase activity of vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor-β (PDGFR) [4]. The usefulness and safety of sorafenib in advanced HCC has been tested in different phase III randomized, double-blind, placebo-controlled trials [5]. Although results obtained from these clinical studies have shown survival benefits, the efficacy of sorafenib treatment is frequently transient, and the activation of compensatory pathways in response to drug administration leads to tumor acquired resistance, having a negative impact on the therapeutic outcome [6]. Therefore, overcoming acquired resistance is required to potentiate cytotoxic effects of sorafenib, and the combination with other molecules could be a manageable approach to reduce adaptive response of tumor cells to the action of the drug [7].
Melatonin (N-acetyl-5-metoxitriptamine), the main product of the pineal gland, exhibits antioxidant, inmunomodulatory and oncostatic features [8-10], playing a protective role in a wide range of liver diseases [11-13]. Studies with HepG2 cells have shown that melatonin induces mitogen-activated protein kinase (MAPK) signalling pathways, increases the expression of p53 and p21 proteins, and causes cell cycle arrest [14, 15]. Moreover, the indole modulates apoptosis by increasing BIM levels through forkhead box class O(FoxO)3a activation [16], and reduces VEGF expression in hypoxic conditions by blockade of hypoxia inducible factor 1 alpha (Hif1α) and signal transducer and activator of transcription (STAT3) [17]. In addition, melatonin modulates motility and invasiveness through molecular mechanisms that involve inhibition of matrix metalloproteinase (MMP)-9 and nuclear factor kappa B (NF-kB) [18]. Recently, it has been reported that ceramide metabolism controlled by melatonin plays an important role in autophagy regulation as well as in apoptotic cell death in HepG2 cells [19]. Data obtained in a murine model of HCC confirm that induction of apoptosis and endoplasmic reticulum stress contribute to the beneficial effects of melatonin [20] The indole shows oncostatic and pro-apoptotic properties not only in HCC cell lines, but also in different types of cancers, and it has been found that the combination of chemotherapeutics or other drugs with melatonin increases cytotoxicity while enhancing apoptotic cell death [20-25]. Recently, it has been found that concomitant treatment with melatonin and cisplatin in HeLa cells induces mitochondrial apoptosis because of reactive oxygen species (ROS) overproduction [22], stressing the link between ROS, mitochondria and apoptosis in cancer. Regardless of its well-known role as anti-oxidant molecule, melatonin occasionally stimulates the production of ROS, depending on cell type, concentration or duration of the treatment [10, 26-28]. Several studies have established that mitochondria are important targets of melatonin, and it is widely recognized that these organelles are the main source of ROS production. Indeed, mitochondria alterations caused by the indole could be responsible for the elevation of ROS levels, contributing to the pro-apoptotic effects observed in cancer cells [29].
The accumulation of mitochondrial ROS induces oxidation of proteins, lipids and DNA, leading in part to mitochondrial dysfunction. Mitophagy, a clearly distinct form of autophagy, promotes turnover of damaged mitochondria entrapped in autophagolysosomes through interactions of specific proteins at the outer mitochondrial membrane (OMM) with microtubule-associated protein light chain 3 (LC3) [30, 31]. This process is mediated by two different molecular pathways: NIP3-like protein X (NIX)/BCL2/adenovirus E1B 19 kDa protein-interacting protein 3-like (BNIP3L), regulated mainly by hypoxia, and Parkin (PARK2)/PTEN induced putative kinase 1 (PINK1). In response to mitochondrial membrane depolarization due in part to ROS generation, PINK1 is stabilized, accumulates at the OMM and selectively recruits Parkin to damaged mitochondria [32, 33]. Parkin ubiquitinates key protein substrates in order to promote its interaction with p62 that, in turn, facilitates interaction with LC3 [34, 35]. Numerous studies have analyzed the effects of mitophagy deficiency on tumorigenesis, revealing that inhibition of mitophagy promotes tumor progression. Parkin null mice (Parkin−/−) exposed to gamma radiation are more susceptible to liver tumors formation, and Parkin−/− MEF (mouse embryo fibroblast) significantly enhances glucose uptake, the rate of glycolysis and lactate production, leading to the Warburg effect [36]. Moreover, in human tumor samples, Parkin controls the stability of both cyclin D and cyclin E, and regulates cell growth [37], which could explain the enhancement in hepatocyte proliferation and development of macroscopic hepatic tumors with the characteristics of HCC in Parkin−/− mice.
Considering this background, this study evaluated if melatonin is able to reduce resistance to sorafenib in HCC cells and the potential contribution of ROS production and activation of the mitophagy pathway to the beneficial effect of the indole. Results obtained demonstrate that the pro-oxidant capacity of melatonin and its impact on mitochondria stability and mitophagy increase sensitivity to the citotoxic effect of the multikinase inhibitor.
Materials and methods
Cell culture and reagents
The HepG2, HuH7 and Hep3B human hepatocarcinoma cell lines were obtained from the American Type Culture Collection (Manassas, VA). They were cultured under controlled conditions (37°C, 5% CO2) and grown in Dulbecco's modified Eagle's Medium-high glucose (Sigma, St Louis, MO) containing 10% fetal bovine serum and 100 U/mL penicillin/streptomycin. Cells were plated in 9.6 cm2 culture dishes at a density of 0.25 × 106 cells/well. Twenty four hours after plating, cells were treated with melatonin (0.1, 0.5, 1, 2 mM) (Sigma) and sorafenib (0.01, 0.05, 0.1, 1, 2.5, 5, 10, 50 μM) (Santa Cruz Biotechnology, Dallas, TX).
Cell viability assay
HepG2, HuH7 and Hep3B cells were seeded on 96-well plates at 5,000 cells/well 24 hours before being treated with different concentrations of melatonin and sorafenib for 48 hours. Cells were incubated for 3 hours with 0.5 mg/ml of 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) (Sigma) dissolved in serum free medium.[14] After this interval, cells were washed with PBS followed by the addition of DMSO. The optical densities were measured at 560 nm spectral wavelength using microtitre plate reader (SynergyTM HT Multi-Mode Microplate Reader, Bio-Tek Instruments, Inc., Winooski, VT, USA).
Annexin V-propidium iodide assay
Apoptosis was assessed by Alexa Fluor 488 annexin V/Dead apoptosis kit (Invitrogen, Carlsbad, CA) [19]. Hep3B cells were seeded in a 6-well plate at density of 0.25 × 106 cells/well. Next day, the cells were treated with melatonin (1 mM, 2 mM) and sorafenib (2.5 μM) for 48 hours. Cell pellets were resuspended in 100 μL buffer with 5 μl annexin V and 1 μL of propidium iodide, and incubated for 15 min at 25°C in the dark. 400 μL of buffer were added for a final volume of 500 μL. Cells were immediately analyzed by FACS SCAN flow Cytometer (Becton-Dickinson, San Jose, United States). 10,000 cells per sample were acquired and percentage of cell death was analyzed using Cell Quest software.
Western blot analysis
After treatments, cultured cells were washed twice with ice cold PBS and lysed by adding ice cold RIPA buffer containing 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 2 mM EDTA, 0.1% Triton 100X, 10% sodium deoxycholate, 10% SDS, 1 mM NaF and protease cocktail inhibitor (Roche, Basel, Switzerland) and scraped off the plate. Extracts were transferred to a microfuge tube and centrifuged for 10 min at 15,000g. Equal amounts of the supernatant protein (20 μg) were separately subjected to SDS-PAGE and transferred to a PVDF membrane (Bio-Rad, Hercules, CA)[18]. Primary antibodies (Ab) were diluted in blocking solution and incubated overnight at 4°C with polyclonal PINK, PARKIN, OPA-1, hFIS1, and Mitofusin-2 (1:1000 dilution; from Abcam, Cambridge, UK), poly ADP ribose polymerase (PARP) and BAX (1:100, Santa Cruz Biotechnology), LC3(1:1000, Cell Signalling Beverly, MA, USA) . Equal loading of protein was demonstrated by probing the membranes with a rabbit polyclonal anti β-actin antibody (Sigma). After washing with PBS-T, the membranes were incubated for 1 hour at room temperature with secondary HRP-conjugated antibody (1:5,000; Dako, Glostrup, Denmark) and visualized using ECL detection kit (Amersham Pharmacia, Uppsala, Sweden) .The density of the specific bands was quantified employing the software ImageJ (National institute of Mental Health, Bethesda, MD) with an imaging densitometer (Scion Image, Maryland, MA).
Mitochondrial DNA content
Speedtools tissue DNA extraction (Biotools B&M Labs, Madrid, Spain) was used for total DNA extraction and purification following the vendor instructions. Changes in mitochondrial DNA content was measured in comparison with nuclear DNA.[38] Mitochondrial DNA: mtMinArc Fw 5’ CTAAATAGCCCACACGTTCCC 3’, Rv 5’ AGAGCTCCCGTGAGTGGTTA 3’ (GenBank # NC_012920). Nuclear DNA: β2M Fw 5’ GCTGGGTAGCTCTAAACAATGTATTCA 3’, Rv 5’ CCATGTACTAACAAATGTCTAAAATGGT 3’ (GenBank # NT_010194.17). Quantitative real-time PCR analysis was performed using SYBR Green (Invitrogen). Relative changes in gene expression levels were determined using the 2−△△CT method.
Immunofluorescence and laser confocal imaging
Immunofluorescence colocalization of mitochondria with lysosomes was performed as described before [39]. For immunofluorescence labelling cells were cultured on 24 wells culture plates containing glass coverslips at a seeding density of 1×104. Briefly, Hep3B were incubated with melatonin and sorafenib. After that, cells were fixed for 15 minutes with 4% paraformaldehyde and washed twice with PBS 1X. Cells were blocked and permeabilized with PBS 1X + 0.2 % saponin and 1% fatty acid free BSA (FFA-BSA) for 15 minutes at room temperature. After washing twice with PBS 1X, cells were incubated with a mouse monoclonal [H4B4] LAMP2 antibody (Abcam) and a rabbit polyclonal Tom20 antibody (FL-145) (Santa Cruz) diluted both 1:300 in 1X PBS with 1 % ffa-BSA O/N at 4°C and washed twice with PBS 1X followed by incubation with a secondary anti-mouse IgG antibody, conjugated to Alexa 488 (1:1000) and anti-rabbit IgG antibody, conjugated to Alexa 647 (1:1000) for 1 hour at 25°C. Coverslips were washed twice with PBS 1X and mounted on glass slides with fluorescent mounting medium Fluoroshield™ with DAPI (Sigma) and visualized in a Leica SPE confocal laser-scanning microscope.
ROS measurement
Hep3B were plated in 24-well plates and treated with sorafenib and/or melatonin for 1, 3 and 6h. After treatment, cells were incubated with 20 μM 2 2’,7’ –dichlorofluorescin diacetate (DCFDA) in PBS during 30 minutes at 37°C. Fluorescence was immediately measured in a Microplate Fluorescence Reader using an exciting wavelength of 485 nm and an emission wavelength of 520 nm. Hydrogen peroxide was utilized as a positive control. Results are expressed as percentage of intensity of fluorescence versus control.
Mitochondrial transmembrane potential analysis by JC-1 staining assay
To evaluate the mitochondrial depolarization induced by drug treatment, we plated 6×105 Hep3B cells in 24-well plates. After 24 hours, cells were treated with sorafenib and/or melatonin for 1, 3 and 6h and stained for 20 minutes in medium containing C5,5',6,6'-tetrachloro- 1,1',3,3'-tetraethylbenzimidazolyl-carbocyanine iodide (JC-1) (Mitochondrial Membrane Potential Assay Kit, Cayman) following manufacturer's recommendations. Fluorescence was measured in a Microplate Fluorescence Reader using an exciting/emission wavelength of 550/600 nm for red and 485/535 nm for green fluorescence. Results are presented as a ratio of red/green fluorescence. Mitochondrial depolarization is indicated by a decrease in the red/green fluorescence intensity ratio. Fluorescence microscopy and MCCP as positive control were performed in parallel to validate fluorescence measurement.
Small interfering RNA transfection
HepG2 cells (0.6 × 105 cells/ml) were seeded in 12 wells culture plates with DMEM medium without antibiotics and 24 hours later 100μl of Lipofectamine® RNAiMAX reagent was added into each plate. The cells were transfected with PARKIN small interfering (siRNA) (sc-42158) (Santa Cruz Biotechnology) according to the manufacturer's instructions. A non-targeting siRNA-A sc-37007 was used as a negative control. At 24h after transfection, medium was replaced for complete DMEM and cells were treated with or without melatonin and imipramine.
Statistical analysis
Results are expressed as mean values ± SD of the indicated number of experiments. One-way ANOVA followed by Bonferroni post hoc test was used to measure differences between mean values of the different treated groups. p <0.05 was considered significant. Values were analyzed using the statistical package GraphPad Prism 5.
Results
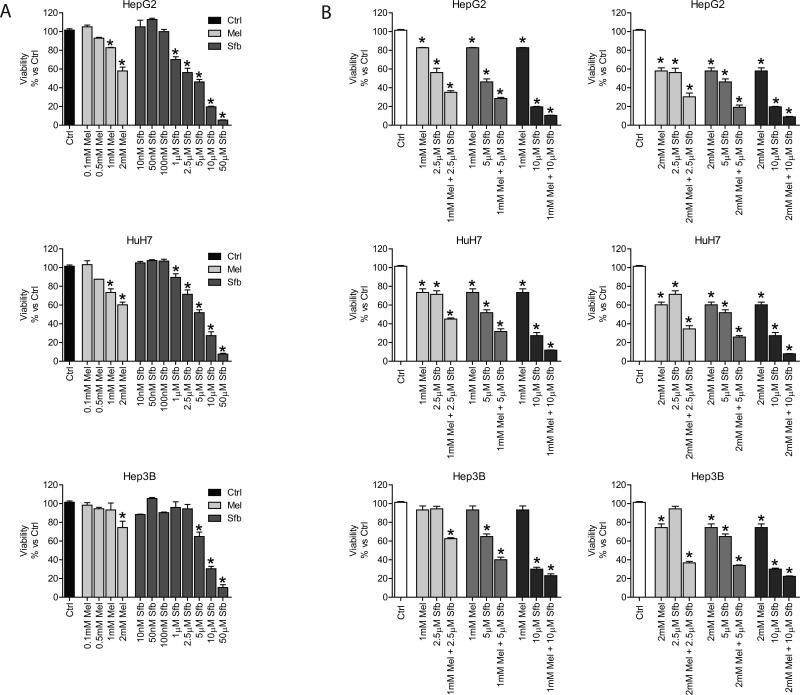
It is known that sorafenib and melatonin kill HCC cells and reduce tumor development, but the impact of combinatorial treatment with both molecules has not been investigated. Here, we studied for the first time the effect of sorafenib in combination with melatonin on the viability of several HCC cell lines. MTT assay was performed after 48 hours of treatment with different doses of both molecules. HepG2 and HuH7 cells responded to melatonin administration (1 and 2 mM) with a significant reduction in viability; however, Hep3B cells were sensitive only to the highest concentration on the indole (Fig. 1A). Nanomolar doses of sorafenib did not exert cytotoxicity in any of the three cell lines, but doses from 1 to 50 μM of sorafenib were lethal for HepG2 and HuH7 cells. Similarly to melatonin, only highest doses of the kinase inhibitor affected cell viability in Hep3B cells (Fig. 1A). Viability was significantly reduced in Hep3B cells when melatonin and sorafenib were concomitantly added using doses of sorafenib that had no effect when administrated alone (Fig. 1B). Because of the results obtained in Hep3B cells, next studies were carried out in this cell line with sorafenib and melatonin doses of 2.5 μM and 1 mM, respectively, which had deleterious effects when combined.
Fig. 1.
Dose-dependent viability response of different HCC cell lines to sorafenib and melatonin treatment. (A) HepG2, HuH7 and Hep3B cells were treated with melatonin (0.1, 0.5, 1 and 2mM) and sorafenib (0.01, 0.05, 0.1, 1, 2.5, 5, 10 and 50μM). Viability was analized by MTT assay after 48 hours. (B) HepG2, HuH7 and Hep3B cells were treated with melatonin (1 and 2mM), sorafenib (2.5, 5 and 10 μM) and melatonin plus sorafenib for 48 hours and MTT assay was performed to evalute the effect on viability. Data are expressed as a percentage of mean values ± S.E.M. of experiments performed in triplicate. *p < 0.05 versus control cells.
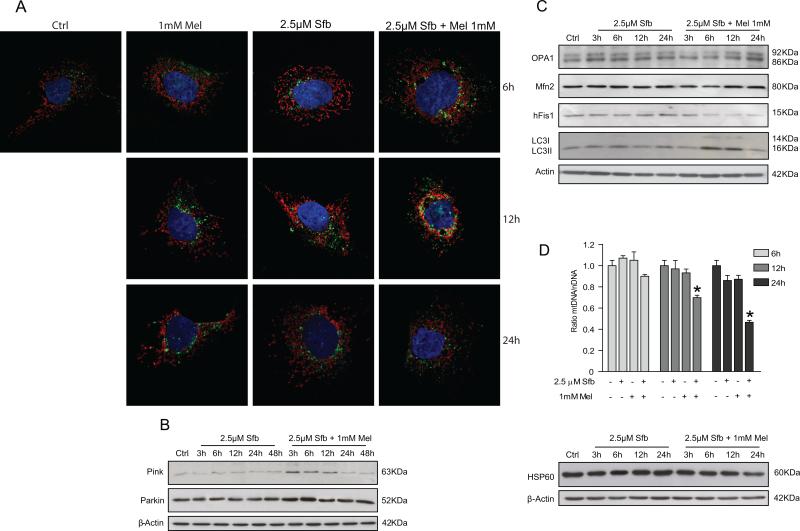
Mitochondria are the main organelles in the maintenance of energy, control of apoptosis-mediated cell death and oxidative stress production. All these functions have been observed during tumor initiation and promotion, suggesting that mitochondria could be a good target for therapeutic combinations. Mitophagy promotes turnover of damaged mitochondria entrapped in autophagolysosomes, and its inhibition has been observed to promote tumor progression. Confocal microscopy imaging denoted that sorafenib plus melatonin treatment for 6 and 12 hours stimulated mitochondria and lysosome colocalization, suggesting that mitochondria were delivered to lysosomes for degradation (Fig. 2A). We evaluated time course of PINK1 and Parkin protein levels in response to sorafenib or sorafenib plus melatonin treatment. Sorafenib administration (2.5 μM) did not induce significant changes in PINK1 expression over time, but its levels increased when melatonin was added to the treatment from 3 to 12 hours, declining thereafter (Fig. 2B). Levels of the related-protein Parkin were not modified with sorafenib alone; when the drug was combined with melatonin, Parkin showed a transient elevation from 3 to 6 hours, returning to control levels after 12 hours of combined treatment. (Fig. 2B). PINK1 and Parkin changes were in accordance to the increase observed from 6 to 12 hours in LC3II expression (Fig. 2C). Because mitophagy is closely related to mitochondrial dynamics, including organelle fusion and fission, we assessed protein levels of mitofusin-2 (Mfn-2), OPA1 (Optic atrophy 1) and Mitochondrial fission 1 protein (hFis). On one hand, fusion proteins were not affected by sorafenib treatment, while the combination of both drugs depleted Mfn and particularly OPA1 up to 6 hours after treatment. On the other hand, hFis, a fission protein, did not show changes with sorafenib treatment alone and exhibited a progressive reduction over time when cells were treated with sorafenib plus melatonin (Fig. 2C). To analyze changes in the number of mitochondria as a consequence of mitophagy induced by treatment, we examined alterations in the mitochondrial DNA copy number. As observed in figure 2D, reduced mtDNA levels were found in sorafenib plus melatonin treatment after 6 hours. In addition, levels of heat shock protein 60 (Hsp60), a mitochondrial chaperone implicated in biogenesis, decreased from 6 to 24 hours of treatment, suggesting that melatonin was able to inhibit mitochondrial genesis when it was combined with sorafenib (Fig. 2D).
Fig. 2.
Effect of sorafenib and melatonin on mitochondria and lysosome localization, mitophagy and mitochondrial dynamics. Cells were treated with sorafenib (2.5μM), melatonin (1mM) and the combination of both compounds. (A) Confocal images show mitochondria and lysosome localization in the cell. Antibodies for Tom20 (red) and LAMP2 (green) detection were used for mitochondria and lysosomes analysis. Colocalization of both organelles are observed when red and green fluorescences are overlaped. (B and C) Pink and Parkin (mitophagic proteins), LC3 (autophagy protein) and Mfn-2, OPA1 and hFIS1 (fussion and fission proteins) were measured by Western blot at different times. (D) Mitochondria quantity was assessed by measurement of mtDNA content and Hsp60 protein levels. Images are representative for experiments performed in triplicate. Data are expressed as a percentage of mean values ± S.E.M. of three independent experiments. *p < 0.05 versus control cells .
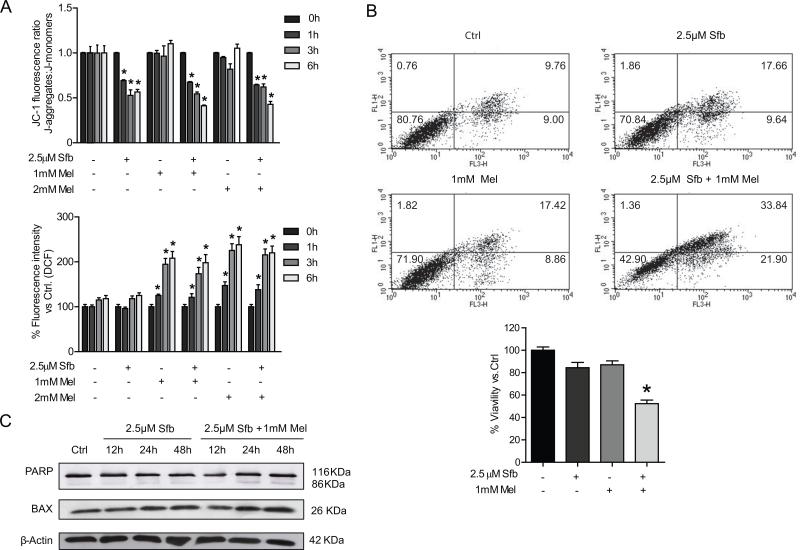
Pro-oxidant capacity of the indole could account for mitochondrial alterations observed by combination of melatonin and sorafenib. In order to assess oxidized environment, ROS production was measured in cells with a ROS-sensitive fluorescent probe. Although sorafenib did not disturb oxidant status, melatonin alone or in combination with sorafenib caused a time-dependent increase in ROS production as early as 1 hour post-treatment (Fig. 3A). These data suggest that the oxidative feature of melatonin could contribute, in part, of the recruitment of mitophagic proteins to mitochondrial membrane.
Fig. 3.
Analysis of membrane polarization status, oxidative stress and cell death in Hep3B under sorafenib and melatonin treatment. ROS production by DCF quantification and mitochondrial membrane potential by JC-1 determination were evaluated in Hep3B cells incubated with sorafenib (2.5μM), melatonin (1 and 2 mM) and the combination of both compounds at different times. (B) Flow cytometric assessment of cell viability was performed using an Annexin V–propidium iodide kit after 48 hours of treatment. (C). Representative immunoblots of PARP and BAX in Hep3B cells incubated with sorafenib (2.5μM) alone and combined with melatonin (1mM) at different times. (D) Images of immunoblots are representative for experiments performed in triplicate. Data are expressed as a percentage of mean values ± S.E.M. of three independent experiments. *p < 0.05 versus control cells.
Mitochondrial membrane depolarization has also been described as a necessary condition for mitophagy initiation. Evaluation of mitochondrial membrane potential was carried out measuring fluorescence changes caused in JC-1 staining in response to sorafenib or sorafenib plus melatonin treatment. Interestingly, sorafenib induced a decline in mitochondrial membrane potential that was not observed in cells treated with melatonin alone but was maintained in cells treated with the drug combination. (Fig. 3A). These observations suggest that both, ROS production and membrane depolarization, likely trigger mitophagy induction when melatonin and sorafenib are coadministered.
Due to the pro-apoptotic role of melatonin in HCC cell lines we evaluated apoptosis and cell death by annexin V/propidium iodide staining. Flow cytometry analysis revealed a similar pattern observed with MTT, confirming that cell viability was only reduced after sorafenib and melatonin co-administration (Fig. 3B). Time-dependent changes of the apoptotic markers Poly ADP-ribose polymerase (PARP) and BAX revealed increased expression 24 and 48 hours following melatonin co-administration with sorafenib, suggesting that the indol is necessary for apoptotic response (Fig. 3C). These results suggest that the combination of melatonin and sorafenib enhances apoptotic cell death.
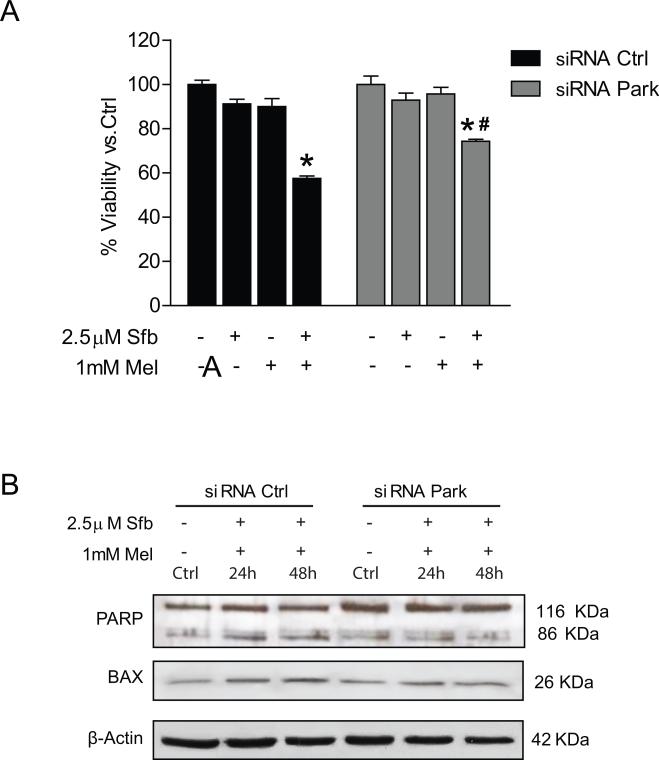
To address the impact of mitophagy on the susceptibility of HCC cells to cell death, we assessed the effect of Parkin silencing with siRNA on melatonin and sorafenib-induced apoptosis. Downregulation of Parkin reduced significantly the cytotoxic effect of melatonin in silenced cells after incubation with both drugs for 48 hours (Fig. 4A). Moreover, these findings were accompanied by a diminution in PARP cleavage and BAX levels (Fig. 4B). Results suggest that mitophagy induced by melatonin in sorafenib-treated cells is a mechanism that potentiates in part apoptotic cell death, enhancing the cytotoxic effect of the multikinase inhibitor.
Fig. 4.
Effect of Parking siliencing in Hep3B viability and cell death under sorafenib and melatonin treatment. Cells were treated with sorafenib (2.5μM), melatonin (1mM) and the combination of both compounds. (A) Viability was analyzed by MTT assays after 48hr. (B) Representative immunoblots of PARP and BAX at different times. Images of immunoblots are representative for experiments performed in triplicate. Data are expressed as a percentage of mean values ± S.E.M. of three independent experiments. *p < 0.05 significant differences versus nonsilenced cells, #p < 0.05 significant differences between treated and untreated silenced cells.
Discussion
Although several drugs have been tested for HCC treatment, most of them have failed in phase III clinical trials, and only the multikinase inhibitor sorafenib has been approved by the Food and Drug Administration (FDA) for HCC management [40]. Even so, sorafenib presents several limitations and response to treatment is lacking in many patients due to the acquired resistance that tumor cells develop. Since high doses of the drug have not shown efficacy in arresting tumor progression in long term, and side effects are a critical factor in survival [41], it is necessary to find strategies to increase chemosensitivity towards sorafenib. For instance, metformin, histone deacetylase inhibitors, CXC chemokine receptor 2 or cyclooxygenase 2 inhibitors, in combination with sorafenib reduce metastasis, proliferation or cell viability, and increase apoptosis both in vitro and in vivo studies [7]. Moreover, preclinical and clinical research has proven that sorafenib addition to conventional chemotherapy increases benefits in the treatment of different cancers [42].
Melatonin has been proposed as a potential drug for HCC treatment due to its anti-proliferative, pro-apoptotic, anti-angiogenic and anti-invasiveness properties in cultured cells [14-18]. Results from the present study show that response to sorafenib administration was different in three HCC cell lines, HepG2, HuH7 and Hep3B; low doses of the kinase inhibitor reduced viability of HepG2 and HuH7 cells, but only the highest doses were toxic to Hep3B cells. Sorafenib has been previously reported to induce autophagy in HuH7 but not in Hep3B cells, suggesting that events preceding autophagy activation might be altered in Hep3B [43]; this fact could be a possible reason beyond the different response to sorafenib of both cell lines. In any case, co-administration of melatonin plus sorafenib showed a synergistic effect in the reduction of cell viability in all HCC cell lines tested. Although melatonin has not been previously combined with sorafenib, it has been shown to reduce side effects of some chemotherapy treatments and to improve the cytotoxic effects of different chemotherapy agents in human cervical cancer, hepatoma or human lung cancer cell lines [22, 44, 45]. Moreover, positive effects of the combination of sorafenib with other oncostatic molecules derived from natural resources (such as resveratrol, quercitin or curcumin) have been tested in different cancer types [46-48].
Mitochondrial biogenesis and degradation through mitophagy are important events in the control of the mitochondria quality, and deletion of different regulators of mitophagy has been observed in cancer [49]. Parkin has been identified as a tumor suppressor gene for hepatocellular carcinoma, and mutations of Parkin gene have been described in cancer [50, 51]. In our study, sorafenib and melatonin co-administration stimulated Parkin expression 6 hours post-treatment, while sorafenib alone has no effect. Localization of Parkin to mitochondria is mediated by PINK1, which phosphorylates Parkin, allowing its translocation to mitochondrial membrane [31]. We found that PINK1 expression increased concomitant with Parkin induction under melatonin and sorafenib co-treatment. Expression of lipidated form of LC3, the main protein for autophagosome formation, was also elevated under melatonin co-administration, suggesting that Parkin-mediated mitochondrial degradation is performed, in part, by mitophagy, although proteasome could be also implicated due to the E3 ubiquitin ligase activity of Parkin [52]. Besides, melatonin administration to sorafenib-treated cells promoted colocalization of mitochondria and lysosomes. These findings suggest that melatonin induces mitochondria delivery to lysosomes for degradation, probably via autophagosome formation.
In addition, mitochondrial DNA content decreased 3 hours post co-treatment, indicating a reduction in mitochondria number. To confirm this data, we measured protein levels of Hsp60, a mitochondrial chaperone with a key role in mitochondrial biogenesis, which has been defined as a potential component on the PINK1/parkin mitophagy pathway [53]. Our results show that melatonin addition to sorafenib decreased Hsp60 protein content from 6 to 24h after treatment, indicating a possible reduction in mitochondria biogenesis. Data support that addition of melatonin to conventional sorafenib treatment induces mitochondrial degradation probably by a mechanism involving PINK1 and Parkin activities. Results differ from those in liver fibrosis mouse models, in which administration of the indole alleviates impairment of mitophagy and ameliorates mitochondrial biogenesis [54]. Therefore, melatonin modulation of mitophagy seems to be cell-type and context-dependent, similarly to is effects on other signaling pathways [55].
Mfn-2 belongs to a group of proteins necessary for mitochondrial fusion that links to mitophagy through Parkin activity, responsible for Mfn-2 ubiquitination and proteasomal degradation [56, 57]. Mfn-2 deficiency modifies mitochondrial dynamics leading to mitochondria fragmentation [58], and changes in its expression have been described in several diseases [59]. In the present research, melatonin combined with sorafenib reduced Mfn-2 protein levels from 3 to 6 hours of treatment, which correlated with Parkin induction. However, longer exposure time to these drugs restored and increased Mfn-2 protein. Analysis of HCC tissues has revealed that Mfn-2 is down-regulated, and its overexpression promotes apoptosis [60]. These findings suggest that the late increase observed in Mfn-2 expression after administration of sorafenib plus melatonin could be associated with an anti-proliferative function of this protein, since an increase in BAX and PARP cleavage was detected.
In addition to Mfn-2 activity as promoter of outer membrane fusion, inner membrane fusion is also required. This event is regulated by the activity of OPA1 [58], which is cleaved when depolarization occurs, causing the degradation of dysfunctional mitochondria [61]. In our study, melatonin in combination with sorafenib was able to decrease OPA1 levels from 3 to 6 hours after treatment, suggesting that inner membrane fusion of altered mitochondria is functional. Fragmentation of mitochondria, which favours its clearance by mitophagy, is regulated by dynamin-related protein Drp-1 and hFis1 [62]. Mitochondrial fragmentation by overexpression of hFis1 is known to promote autophagy markers accumulation and probably mitophagy [63]. Our results show that melatonin addition to sorafenib treatment increased hFis1 levels, suggesting that fission and the balance between fission and fusion could be implicated in mitophagy under melatonin treatment. Although the effect of the indole on mitophagy has not been previously studied in tumor cells, it has been described that melatonin prevents mitochondrial translocation of Drp-1 to the mitochondria in cadmium-treated neurons [64].
Mitophagy has been described as a possible mechanism for removing impaired mitochondria and preventing ROS production [65]. Melatonin exerts antioxidant properties at low doses in liver cancerous cells, but at concentrations from 1 to 10 mM it has been reported to induce ROS overproduction and glutathione oxidation [10], Therefore, ROS generation by melatonin could be involved in mitophagy induction. Treatment with the indole increased ROS levels as measured by DCF fluorescence, and its combination with sorafenib strengthened the production of oxidative species. Mitochondrial depolarization is a sign for mitophagy induction related to ROS levels. In this condition, Parkin stimulates the formation of autophagosomes that engulfs damaged mitochondria and initiates Mfn-2 degradation. In our study, sorafenib acted as an uncoupler, reducing membrane potential; however, there was no effect on ROS production. Nevertheless, sorafenib has shown oxidative properties in HepG2 cells [66], suggesting that the effect on ROS production depend on the cell type or cellular context. Melatonin showed an opposite tendency, increasing ROS levels and having no effect on mitochondrial membrane potential. When sorafenib and the indole were combined, both effects were observed, and only in this case mitophagy was activated. Therefore, it is conceivable that both ROS and depolarization are necessary conditions for mitophagy induction in Hep3B cells.
Different pathways that modulate mitophagy have opposite influence on cancer progression and prognosis. On one hand, cell cycle is deregulated when Parkin is mutated, and this protein has been proposed to have a tumor suppressor role [37, 67]. In HCC, analysis of several cell lines has demonstrated that Parkin protein expression is reduced, and more than 50% of primary liver tumors have low levels of Parkin [68]. On the other hand, BNIP3 and NIX, proteins implicated in mitophagy under hypoxic conditions, correlate with invasiveness, but downregulation of BNIP3 is correlated with bad prognosis [69]. To clarify the involvement of Parkin and, thus, of mitophagy, we decided to knock down the expression of Parkin. Our data support that mitophagy potentiates apoptotic cell death and enhances the cytotoxic effect of the multikinase inhibitor, suggesting that in this context melatonin-induced mitophagy is lethal to Hep3B, similar to the lethal mitophagy induced by ceramide [70].
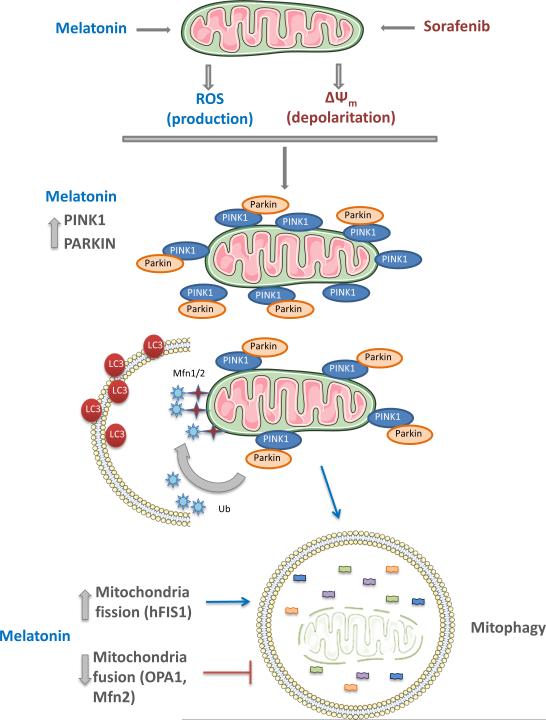
In summary, results from the present study indicate that co-administration of melatonin and sorafenib induces in HCC cells an early mitophagic response, with ROS overproduction and mitochondrial membrane depolarization that associate to an increase in apoptosis and a reduction in cell viability (Fig. 5). Although further studies are necessary for a better knowledge of the molecular mechanisms involved, our experiments suggest that melatonin could represent an interesting adjuvant in HCC treatment with sorafenib.
Fig. 5.
Proposed model of action of melatonin and sorafenib combination on mitochondria stability. Sorafenib administration to Hep3B cells causes a reduction in mitochondrial membrane potential and its depolarization. Melatonin is responsible for the over-production of ROS in the mitochondria. Both events promote the translocation of PINK1 to the outer mitochondrial membrane and the recruitment of Parkin. Fusion proteins are regulated by the ubiquin-ligase activity of Parkin, necessary for the interaction with LC3, located in the phagosome membrane. Enhancement of mitochondria fission is required for mitophagy induction since it alters mitochondrial membrane potential; contrary, fusion rescues mitochondria from its degradation. Under these circumstances, melatonin diminishes the expression of fusion proteins (OPA-1, Mfn-2) while increases transiently levels of hFIS1.
Acknowledgements
CIBEREHD is funded by Instituto de Salud Carlos III, Spain. The work was further supported from grants SAF-2014-57674-R and SAF2015-69966-R from Plan Nacional de I+D Spain, the center grant P50-AA-11999 Research Center for Liver and Pancreatic Diseases funded by NIAAA/NIH and the Generalitat de Catalunya grant 2014SGR785. NPD and RO are supported by the Ministry of Education of Spain (becas FPU, references FPU13/04173 and FPU12/01433, respectively).
References
- 1.TORRE LA, BRAY F, SIEGEL RL, et al. Global cancer statistics. CA Cancer J Clin 2015. 2012;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.EASL-EORTC CLINICAL PRACTICE GUIDELINES: MANAGEMENT OF HEPATOCELLULAR CARCINOMA European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 3.FORNER A, LLOVET JM, BRUIX J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 4.PASCUAL S, HERRERA I, IRURZUN J. New advances in hepatocellular carcinoma. World J Hepatol. 2016;8:421–438. doi: 10.4254/wjh.v8.i9.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.BRUIX J, TAKAYAMA T, MAZZAFERRO V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–1354. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 6.CHEN J, JIN R, ZHAO J, et al. Potential molecular, cellular and microenvironmental mechanism of sorafenib resistance in hepatocellular carcinoma. Cancer Lett. 2015;367:1–11. doi: 10.1016/j.canlet.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 7.CH'ANG HJ. Optimal combination of antiangiogenic therapy for hepatocellular carcinoma. World J Hepatol. 2015;7:2029–2040. doi: 10.4254/wjh.v7.i16.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MAURIZ JL, COLLADO PS, VENEROSO C, et al. A review of the molecular aspects of melatonin's anti-inflammatory actions: recent insights and new perspectives. J Pineal Res. 2013;54:1–14. doi: 10.1111/j.1600-079X.2012.01014.x. [DOI] [PubMed] [Google Scholar]
- 9.MANCHESTER LC, COTO-MONTES A, BOGA JA, et al. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J Pineal Res. 2015;59:403–419. doi: 10.1111/jpi.12267. [DOI] [PubMed] [Google Scholar]
- 10.OSSENI RA, RAT P, BOGDAN A, et al. Evidence of prooxidant and antioxidant action of melatonin on human liver cell line HepG2. Life Sci. 2000;68:387–399. doi: 10.1016/s0024-3205(00)00955-3. [DOI] [PubMed] [Google Scholar]
- 11.SAN-MIGUEL B, CRESPO I, SANCHEZ DI, et al. Melatonin inhibits autophagy and endoplasmic reticulum stress in mice with carbon tetrachloride-induced fibrosis. J Pineal Res. 2015;59:151–162. doi: 10.1111/jpi.12247. [DOI] [PubMed] [Google Scholar]
- 12.SAN-MIGUEL B, CRESPO I, VALLEJO D, et al. Melatonin modulates the autophagic response in acute liver failure induced by the rabbit hemorrhagic disease virus. J Pineal Res. 2014;56:313–321. doi: 10.1111/jpi.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.TUÑON MJ, SAN MIGUEL B, CRESPO I, et al. Melatonin attenuates apoptotic liver damage in fulminant hepatic failure induced by the rabbit hemorrhagic disease virus. J Pineal Res. 2011;50:38–45. doi: 10.1111/j.1600-079X.2010.00807.x. [DOI] [PubMed] [Google Scholar]
- 14.MARTIN-RENEDO J, MAURIZ JL, JORQUERA F, et al. Melatonin induces cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line. J Pineal Res. 2008;45:532–540. doi: 10.1111/j.1600-079X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 15.CARBAJO-PESCADOR S, GARCIA-PALOMO A, MARTIN-RENEDO J, et al. Melatonin modulation of intracellular signaling pathways in hepatocarcinoma HepG2 cell line: role of the MT1 receptor. J Pineal Res. 2011;51:463–471. doi: 10.1111/j.1600-079X.2011.00910.x. [DOI] [PubMed] [Google Scholar]
- 16.CARBAJO-PESCADOR S, STEINMETZ C, KASHYAP A, et al. Melatonin induces transcriptional regulation of Bim by FoxO3a in HepG2 cells. Br J Cancer. 2013;108:442–449. doi: 10.1038/bjc.2012.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CARBAJO-PESCADOR S, ORDOÑEZ R, BENET M, et al. Inhibition of VEGF expression through blockade of Hif1alpha and STAT3 signalling mediates the anti-angiogenic effect of melatonin in HepG2 liver cancer cells. Br J Cancer. 2013;109:83–91. doi: 10.1038/bjc.2013.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ORDOÑEZ R, CARBAJO-PESCADOR S, PRIETO-DOMINGUEZ N, et al. Inhibition of matrix metalloproteinase-9 and nuclear factor kappa B contribute to melatonin prevention of motility and invasiveness in HepG2 liver cancer cells. J Pineal Res. 2014;56:20–30. doi: 10.1111/jpi.12092. [DOI] [PubMed] [Google Scholar]
- 19.ORDOÑEZ R, FERNANDEZ A, PRIETO-DOMINGUEZ N, et al. Ceramide metabolism regulates autophagy and apoptotic cell death induced by melatonin in liver cancer cells. J Pineal Res. 2015;59:178–189. doi: 10.1111/jpi.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.BORIN TF, ARBAB AS, GELALETI GB, et al. Melatonin decreases breast cancer metastasis by modulating Rho-associated kinase protein-1 expression. J Pineal Res. 2016;60:3–15. doi: 10.1111/jpi.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MAO L, DAUCHY RT, BLASK DE, et al. Melatonin suppression of aerobic glycolysis (Warburg effect), survival signalling and metastasis in human leiomyosarcoma. J Pineal Res. 2016;60:167–177. doi: 10.1111/jpi.12298. [DOI] [PubMed] [Google Scholar]
- 22.PARIENTE R, PARIENTE JA, RODRIGUEZ AB, et al. Melatonin sensitizes human cervical cancer HeLa cells to cisplatin-induced cytotoxicity and apoptosis: effects on oxidative stress and DNA fragmentation. J Pineal Res. 2016;60:55–64. doi: 10.1111/jpi.12288. [DOI] [PubMed] [Google Scholar]
- 23.ALONSO-GONZALEZ C, GONZALEZ A, MARTINEZ-CAMPA C, et al. Melatonin enhancement of the radiosensitivity of human breast cancer cells is associated with the modulation of proteins involved in estrogen biosynthesis. Cancer Lett. 2016;370:145–152. doi: 10.1016/j.canlet.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 24.JU HQ, LI H, TIAN T, et al. Melatonin overcomes gemcitabine resistance in pancreatic ductal adenocarcinoma by abrogating nuclear factor-kappaB activation. J Pineal Res. 2016;60:27–38. doi: 10.1111/jpi.12285. [DOI] [PubMed] [Google Scholar]
- 25.LU JJ, FU L, TANG Z, et al. Melatonin inhibits AP-2beta/hTERT, NF-kappaB/COX-2 and Akt/ERK and activates caspase/Cyto C signaling to enhance the antitumor activity of berberine in lung cancer cells. Oncotarget. 2016;7:2985–3001. doi: 10.18632/oncotarget.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.BIZZARRI M, PROIETTI S, CUCINA A, et al. Molecular mechanisms of the proapoptotic actions of melatonin in cancer: a review. Expert Opin Ther Targets. 2013;17:1483–1496. doi: 10.1517/14728222.2013.834890. [DOI] [PubMed] [Google Scholar]
- 27.BEJARANO I, ESPINO J, BARRIGA C, et al. Pro-oxidant effect of melatonin in tumour leucocytes: relation with its cytotoxic and pro-apoptotic effects. Basic Clin Pharmacol Toxicol. 2011;108:14–20. doi: 10.1111/j.1742-7843.2010.00619.x. [DOI] [PubMed] [Google Scholar]
- 28.WOLFLER A, CALUBA HC, ABUJA PM, et al. Prooxidant activity of melatonin promotes fas-induced cell death in human leukemic Jurkat cells. FEBS Lett. 2001;502:127–131. doi: 10.1016/s0014-5793(01)02680-1. [DOI] [PubMed] [Google Scholar]
- 29.ZHANG HM, ZHANG Y. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res. 2014;57:131–146. doi: 10.1111/jpi.12162. [DOI] [PubMed] [Google Scholar]
- 30.KIM I, RODRIGUEZ-ENRIQUEZ S, LEMASTERS JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.YOULE RJ, NARENDRA DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MATSUDA N, SATO S, SHIBA K, et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.NARENDRA DP, JIN SM, TANAKA A, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.SARRAF SA, RAMAN M, GUARANI-PEREIRA V, et al. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature. 2013;496:372–376. doi: 10.1038/nature12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.GEISLER S, HOLMSTROM KM, SKUJAT D, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 36.ZHANG C, LIN M, WU R, et al. Parkin, a p53 target gene, mediates the role of p53 in glucose metabolism and the Warburg effect. Proc Natl Acad Sci U S A. 2011;108:16259–16264. doi: 10.1073/pnas.1113884108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.GONG Y, ZACK TI, MORRIS LG, et al. Pan-cancer genetic analysis identifies PARK2 as a master regulator of G1/S cyclins. Nat Genet. 2014;46:588–594. doi: 10.1038/ng.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.STEFANOVIC M, TUTUSAUS A, MARTINEZ-NIETO GA, et al. Targeting glucosylceramide synthase upregulation reverts sorafenib resistance in experimental hepatocellular carcinoma. Oncotarget. 2016;7:8253–8267. doi: 10.18632/oncotarget.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.BAULIES A, RIBAS V, NUNEZ S, et al. Lysosomal Cholesterol Accumulation Sensitizes To Acetaminophen Hepatotoxicity by Impairing Mitophagy. Sci Rep. 2015;5:18017. doi: 10.1038/srep18017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LLOVET JM, HERNANDEZ-GEA V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res. 2014;20:2072–2079. doi: 10.1158/1078-0432.CCR-13-0547. [DOI] [PubMed] [Google Scholar]
- 41.KUCZYNSKI EA, LEE CR, MAN S, et al. Effects of Sorafenib Dose on Acquired Reversible Resistance and Toxicity in Hepatocellular Carcinoma. Cancer Res. 2015;75:2510–2519. doi: 10.1158/0008-5472.CAN-14-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DAL LAGO L, D'HONDT V, AWADA A. Selected combination therapy with sorafenib: a review of clinical data and perspectives in advanced solid tumors. Oncologist. 2008;13:845–858. doi: 10.1634/theoncologist.2007-0233. [DOI] [PubMed] [Google Scholar]
- 43.FISCHER TD, WANG JH, VLADA A, et al. Role of autophagy in differential sensitivity of hepatocarcinoma cells to sorafenib. World J Hepatol. 2014;6:752–758. doi: 10.4254/wjh.v6.i10.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.FAN LL, SUN GP, WEI W, et al. Melatonin and doxorubicin synergistically induce cell apoptosis in human hepatoma cell lines. World J Gastroenterol. 2010;16:1473–1481. doi: 10.3748/wjg.v16.i12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.REITER RJ, TAN DX, SAINZ RM, et al. Melatonin: reducing the toxicity and increasing the efficacy of drugs. J Pharm Pharmacol. 2002;54:1299–1321. doi: 10.1211/002235702760345374. [DOI] [PubMed] [Google Scholar]
- 46.KIM C, BAEK SH, UM JY, et al. Resveratrol attenuates constitutive STAT3 and STAT5 activation through induction of PTPepsilon and SHP-2 tyrosine phosphatases and potentiates sorafenib-induced apoptosis in renal cell carcinoma. BMC Nephrol. 2016;17:19. doi: 10.1186/s12882-016-0233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.JAKUBOWICZ-GIL J, LANGNER E, BADZIUL D, et al. Quercetin and sorafenib as a novel and effective couple in programmed cell death induction in human gliomas. Neurotox Res. 2014;26:64–77. doi: 10.1007/s12640-013-9452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.HU B, SUN D, SUN C, et al. A polymeric nanoparticle formulation of curcumin in combination with sorafenib synergistically inhibits tumor growth and metastasis in an orthotopic model of human hepatocellular carcinoma. Biochem Biophys Res Commun. 2015;468:525–532. doi: 10.1016/j.bbrc.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 49.CHOURASIA AH, BOLAND ML, MACLEOD KF. Mitophagy and cancer. Cancer Metab. 2015;3:4. doi: 10.1186/s40170-015-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.FUJIWARA M, MARUSAWA H, WANG HQ, et al. Parkin as a tumor suppressor gene for hepatocellular carcinoma. Oncogene. 2008;27:6002–6011. doi: 10.1038/onc.2008.199. [DOI] [PubMed] [Google Scholar]
- 51.VEERIAH S, MORRIS L, SOLIT D, et al. The familial Parkinson disease gene PARK2 is a multisite tumor suppressor on chromosome 6q25.2-27 that regulates cyclin E. Cell Cycle. 2010;9:1451–1452. doi: 10.4161/cc.9.8.11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.YOSHII SR, KISHI C, ISHIHARA N, et al. Parkin mediates proteasome-dependent protein degradation and rupture of the outer mitochondrial membrane. J Biol Chem. 2011;286:19630–19640. doi: 10.1074/jbc.M110.209338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.RAKOVIC A, GRUNEWALD A, VOGES L, et al. PINK1-Interacting Proteins: Proteomic Analysis of Overexpressed PINK1. Parkinsons Dis. 2011;2011:153979. doi: 10.4061/2011/153979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.KANG JW, HONG JM, LEE SM. Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride-induced liver fibrosis. J Pineal Res. 2016;60:383–393. doi: 10.1111/jpi.12319. [DOI] [PubMed] [Google Scholar]
- 55.FERNANDEZ A, ORDONEZ R, REITER RJ, et al. Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis. J Pineal Res. 2015;59:292–307. doi: 10.1111/jpi.12264. [DOI] [PubMed] [Google Scholar]
- 56.CHEN Y, LV L, JIANG Z, et al. Mitofusin 2 protects hepatocyte mitochondrial function from damage induced by GCDCA. PLoS One. 2013;8:e65455. doi: 10.1371/journal.pone.0065455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.OSELLAME LD, BLACKER TS, DUCHEN MR. Cellular and molecular mechanisms of mitochondrial function. Best Pract Res Clin Endocrinol Metab. 2012;26:711–723. doi: 10.1016/j.beem.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.YIN XM, DING WX. The reciprocal roles of PARK2 and mitofusins in mitophagy and mitochondrial spheroid formation. Autophagy. 2013;9:1687–1692. doi: 10.4161/auto.24871. [DOI] [PubMed] [Google Scholar]
- 59.SEBASTIAN D, HERNANDEZ-ALVAREZ MI, SEGALES J, et al. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci U S A. 2012;109:5523–5528. doi: 10.1073/pnas.1108220109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.WANG W, LU J, ZHU F, et al. Pro-apoptotic and anti-proliferative effects of mitofusin-2 via Bax signaling in hepatocellular carcinoma cells. Med Oncol. 2012;29:70–76. doi: 10.1007/s12032-010-9779-6. [DOI] [PubMed] [Google Scholar]
- 61.HEAD B, GRIPARIC L, AMIRI M, et al. Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol. 2009;187:959–966. doi: 10.1083/jcb.200906083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.MOZDY AD, MCCAFFERY JM, SHAW JM. Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J Cell Biol. 2000;151:367–380. doi: 10.1083/jcb.151.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.GOMES LC, SCORRANO L. High levels of Fis1, a pro-fission mitochondrial protein, trigger autophagy. Biochim Biophys Acta. 2008;1777:860–866. doi: 10.1016/j.bbabio.2008.05.442. [DOI] [PubMed] [Google Scholar]
- 64.XU S, PI H, ZHANG L, et al. Melatonin prevents abnormal mitochondrial dynamics resulting from the neurotoxicity of cadmium by blocking calcium-dependent translocation of Drp1 to the mitochondria. J Pineal Res. 2016;60:291–302. doi: 10.1111/jpi.12310. [DOI] [PubMed] [Google Scholar]
- 65.KUBLI DA, GUSTAFSSON AB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res. 2012;111:1208–1221. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.CHIOU JF, TAI CJ, WANG YH, et al. Sorafenib induces preferential apoptotic killing of a drug- and radio-resistant Hep G2 cells through a mitochondria-dependent oxidative stress mechanism. Cancer Biol Ther. 2009;8:1904–1913. doi: 10.4161/cbt.8.20.9436. [DOI] [PubMed] [Google Scholar]
- 67.POULOGIANNIS G, MCINTYRE RE, DIMITRIADI M, et al. PARK2 deletions occur frequently in sporadic colorectal cancer and accelerate adenoma development in Apc mutant mice. Proc Natl Acad Sci U S A. 2010;107:15145–15150. doi: 10.1073/pnas.1009941107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.WANG F, DENISON S, LAI JP, et al. Parkin gene alterations in hepatocellular carcinoma. Genes Chromosomes Cancer. 2004;40:85–96. doi: 10.1002/gcc.20020. [DOI] [PubMed] [Google Scholar]
- 69.ERKAN M, KLEEFF J, ESPOSITO I, et al. Loss of BNIP3 expression is a late event in pancreatic cancer contributing to chemoresistance and worsened prognosis. Oncogene. 2005;24:4421–4432. doi: 10.1038/sj.onc.1208642. [DOI] [PubMed] [Google Scholar]
- 70.SENTELLE RD, SENKAL CE, JIANG W, et al. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat Chem Biol. 2012;8:831–838. doi: 10.1038/nchembio.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]