Abstract
The chaperone-like activity of αA-crystallin has an important role in maintaining lens transparency. Previously we identified residues 70–88 as a chaperone site in αA-crystallin. In this study, we deleted the chaperone site residues to generate αAΔ70–76 and αAΔ70–88 mutants and investigated if there are additional substrate-binding sites in αA-crystallin. Both mutant proteins when expressed in E. coli formed inclusion bodies, and on solubilizing and refolding, they exhibited similar structural properties, with a 2- to 3-fold increase in molar mass compared to the molar mass of wild-type protein. The deletion mutants were less stable than the wild-type αA-crystallin. Functionally αAΔ70–88 was completely inactive as a chaperone, while αAΔ70–76 demonstrated a 40–50% reduction in anti-aggregation activity against alcohol dehydrogenase (ADH). Deletion of residues 70–88 abolished the ADH binding sites in αA-crystallin at physiological temperature. At 45 °C, cryptic ADH binding site(s) became exposed, which contributed subtly to the chaperone-like activity of αAΔ70–88. Both of the deletion mutants were completely inactive in suppressing aggregation of βL-crystallin at 53 °C. The mutants completely lost the anti-apoptotic property that αA-crystallin exhibits while they protected ARPE-19 (a human retinal pigment epithelial cell line) and primary human lens epithelial (HLE) cells from oxidative stress. Our studies demonstrate that residues 70–88 in αA-crystallin act as a primary substrate binding site and account for the bulk of the total chaperone activity. The β3 and β4 strands in αA-crystallin comprising 70–88 residues play an important role in maintenance of the structure and in preventing aggregation of denaturing proteins.
Keywords: Crystallin, chaperone, deletion, structure-function, aggregation, apoptosis
1. Introduction
α-Crystallin, is a major protein in all vertebrate eye lenses [1] and a member of the small heat shock protein family [2]. It consists of two subunits, A and B, of a molecular mass of 20 kDa and having over 55% sequence homology. The chaperone function of α-crystallin was discovered in the 1990s [3–5]. Chaperone function plays a role in the prevention of aggregation of various substrate proteins induced by thermal stress, di-sulfide bond cleavage and ultraviolet (UV) light–induced stress. The α-crystallin domain is the primary hallmark of the αA- and αB-crystallin subunits, which puts them in the family of small heat shock proteins. This domain is comprised of 7 to 8 β strands organized in two sheets: one with β2, β3, β8 and β9 strands and the other with β4, β5, β6 and β7 strands [6, 7]. The search to identify substrate binding sites in αA-and αB-crystallin has been the focus of numerous studies [8–14]. Our laboratory showed that substrate binding sites identified by bis-ANS labeling overlap with sites that cross-link to substrate proteins [14, 15]. Earlier we identified two possible substrate binding regions of αB-crystallin; one is 57–69 and the other is 93–107, with both regions identified by cross linking studies with denatured ADH [14]. In another study, region 78–99 in αA-crystallin and regions 73–82 and 93–103 in αB-crystallin were identified as bis-ANS binding sites of α-crystallin [15]. The 70–88 amino acid residues region in αA-crystallin is a putative chaperone site and that the 19-residue peptide representing this region, known as mini-αA, can act independently as a molecular chaperone [16]. This α-crystallin chaperone site corresponds to the β3 and β4 region [5–7]. Studies of the significance of the β3 strand of αB-crystallin, studied by point mutation and double mutation on the β3 strand, revealed that β3 has an important role in chaperone activity as well as client protein selectivity [17]. This study focused on the surface-exposed Asn-78, Lys-82 and His-83 residues in the β3 strand. Domain swapping of the β3 strand of αB-crystallin with the β3 strand of αA-crystallin had no effect on chaperone activity [17]. In another study, age-related cataract-forming mutation F71L was identified in the β3 sequence of αA-crystallin [18]. This mutation does not appear to significantly alter the molecular mass, secondary and tertiary structures, and hydrophobicity of αA-crystallin [18, 19].
Our previous studies show that maximal chaperone activity depends on the substrate proteins used in the aggregation assay. Chaperone substrate interaction occurs primarily via hydrophobic and ionic interactions [14, 15]. Molten globule–like reduced substrate proteins are recognized by αA-crystallin [20]. Such substrate proteins are on an irreversible path to aggregation and precipitation. We also have shown that αAF71G mutant is totally inactive as a chaperone at physiological temperature [21]. However, the αAF71G mutant exhibits marginal chaperone activity at a high temperature of 55 °C, suggesting exposure of the cryptic site on structural perturbations. These studies suggest the presence of multiple substrate binding sites on α-crystallin.
We investigated in detail the structure–function alteration of αA-crystallin after deletion of the primary chaperone site 70–88. We prepared two deletion mutants by deletion of 70–76 and 70–88 sequences from αA-crystallin. Loss of chaperone function in both of these mutants confirms our hypothesis that the primary chaperone site in α-crystallin is residues 70–88. We found that deletion of amino acid residues 70–76 as well as deletion of amino acid residues 70–88 results in higher oligomeric mass, poor stability and partial or complete loss of chaperone function.
2. Materials and methods
2.1. Deletion of αA70–76 and αA70–88 Sequences from Human αA-Crystallin cDNA
Deletion mutants were prepared using ExSite PCR-Based Site-Directed Mutagenesis Kit (Stratagene, Santa Clara, CA). The following primer sets were used. Primers for making αAΔ70–76: 5’pGTCCCGGTCGGATCGAACCTCAG3’ and 5’GTGAAGCACTTCTCCCCGGAGGAC3’; primers for making αAΔ70–88: 5’pGTCCCGGTCGGATCGAACCTCAG3’ and 5’GTGCAGGACGACTTTGTGGAGATC3’. The full-length human αA-crystallin cDNA clone in vector pET23d was used as a template.
2.2. Overexpression and Purification of Proteins
Recombinant wild-type and mutant proteins were expressed and purified as described previously [13, 21]. In brief, wild-type protein and deletion mutants were overexpressed in E. coli BL21(DE3)pLysS cells (Invitrogen). The cells were lysed in 50 mM Tris-HCl, 2 mM EDTA, and 0.1M NaCl (pH 7.5) containing lysozyme (0.1 mg/ml) and treated with benzonase (Sigma) for removal of DNA. Both of the mutant proteins went into inclusion bodies. Therefore, we used 6 M urea to solubilize inclusion bodies and then precipitated the recombinant proteins with 35% ammonium sulfate. The pellet was then dissolved in cell lysis buffer and purified using ion-exchange chromatography. Wild-type αA-crystallin was initially purified using gel filtration chromatography (Superdex G200 column). The fraction containing wild-type protein was treated with 6 M urea and processed further, as described for mutants. A stepwise gradient of 0 to 1 M NaCl was used for elution of proteins from the ion-exchange column. Refolding and purification of the mutant protein was achieved by on-column refolding on a Q-Sepharose ion-exchange column (GE Biosciences) [22]. The fractions were subjected to SDS-PAGE, and fractions containing crystallins were dialyzed against 50 mM Tris-HCl, concentrated and stored in presence of 10 mM dithiothreitol at −80 °C for further use.
2.3. Far- and Near-UV Circular Dichroism (CD) Spectroscopy
Estimation of the secondary and tertiary structure in proteins was carried out using Jasco 815 CD spectrometer. Protein solution (0.2 mg/ml in 50 mM phosphate buffer, pH 7.4) was taken for far-UV CD analysis in a 0.1-cm path length cuvette, and 2 mg/ml protein solution was taken for near-UV CD analysis in a 1-cm path length cuvette. Circular dichroic spectra were collected in the far-UV wavelength region, from 190 nm to 250 nm, for estimation of secondary structure, as described previously [13]. The CD spectra are expressed as molar ellipticity in degree cm2 dmol−1.
2.4. Urea Equilibrium Unfolding Study of αA-Crystallin Deletion Mutants
Stability of mutant αA-crystallin was determined by equilibrium chemical denaturation experiments. Mutant proteins (0.1 mg/ml in PBS, pH 7.4) were incubated for 4 h at 25 °C in various urea concentrations (0–8 M). Tryptophan fluorescence spectra of all samples were determined by using excitation wavelength of 295 nm and emission wavelength of 310–400 nm. Both the excitation and emission band passes were set at 5 nm. The ratio of fluorescence intensity at 337 nm and 350 nm was plotted against various urea concentrations. The urea equilibrium unfolding profile was fitted according to the sigmoidal fit and the C1/2 values for denaturation of 50% protein were estimated as described previously [23].
2.5. Surface Hydrophobicity Measurements by Bis-ANS Binding
Wild-type αA-crystallin and deletion mutant proteins (0.1 mg/ml each) in PBS were separately incubated with 10 µM bis-ANS at 37 °C for 1 h. At the end of incubation, the fluorescence emission spectrum was recorded between 420 nm and 600 nm, using 390 nm as the excitation wavelength. The excitation and emission band passes were 5 nm each.
2.6. Tryptophan Fluorescence of αA-Crystallin Deletion Mutants
Intrinsic tryptophan fluorescence spectra were recorded for wild-type αA-crystallin and deletion mutant proteins (0.1 mg/ml each) in phosphate buffer saline. The samples were excited at 295 nm and the emission spectra were recorded in the wavelength range of 310 nm to 400 nm by using a Jasco spectrofluorometer.
2.7. Chaperone Function of Wild-type αA-Crystallin and Deletion Mutants
The chaperone activity of wild-type αA-crystallin and deletion mutants was measured using different substrates, such as ADH (Worthington), Citrate Synthase (CS) (Sigma) and βL-crystallin (purified in the lab from bovine lens). Protein aggregation was measured by monitoring the light scattering at 360 nm in Shimadzu UV-VIS spectrophotometer equipped with a temperature-controlled multi-cell transporter. The assays were done with increasing concentrations of chaperone as a function of time. The metal chelating agent EDTA (100 µM) was used to induce aggregation of 250 µg of ADH in 1ml PBS at 37 °C. CS (75 µg) aggregation assay was performed in 40 mM HEPES-NaOH buffer, pH 7.3 at 43 °C, and βL (250 µg) aggregation was carried out in PBS at 55 °C.
2.8. Transmission Electron Microscopy (TEM)
Protein solution, 5 µl (0.01 mg/ml), was loaded onto carbon-coated copper grids. After 5 min, excess sample was removed using filter paper. The grid was then quickly washed 10 times with diluted PBS buffer, 15 seconds each time, and negatively stained with 2% uranyl acetate. The excess stain was wicked away with filter paper, and the sample was air dried and observed in the JEOL 1400 electron microscope. The grids were glow discharged for 30 seconds to make them negatively charged prior to deposition of all of the proteins.
2.9. Multi-Angle Light Scattering and Size Exclusion Chromatography
Molar mass (Mw) and hydrodynamic radius (Rh) of the proteins were determined by multi-angle light scattering (MALS) and dynamic light scattering (DLS) detectors (Wyatt Technology) using Astra (6.1) software. The protein samples were fractionated on a TSK gel G5000PWXL (Tosoh) size-exclusion column connected to an HPLC (Shimadzu) equipped with UV and RID detectors and coupled with MALS-DLS detectors. PBS was used as the solvent and the flow rate was set at 0.75 ml/min.
2.10. Substrate Binding Studies
Substrate binding to chaperone proteins was investigated using labeled ADH as substrate. Cysteine in ADH was labeled with Alexa Fluor 568 (Molecular Probes) and the unreacted dye was removed by passing the sample through a Sephadex G25 column. Aggregation of labeled ADH (0.25 mg) was induced by incubating the protein at 37 °C in PBS (0.5 ml) containing 100 mM EDTA (pH 7.3) and in the presence of wild-type or mutant proteins (0.5 mg). After 30 min of incubation, the samples were filtered through 0.2 µM filter and an aliquot of the sample was injected into a TSK gel G4000PWXL connected to an HPLC. The flow rate was set at 0.75 ml/min. Elution of the labeled ADH was followed using the fluorescence detector with excitation set at 578 nm and emission at 603 nm. In a separate experiment, unlabeled ADH was used as the substrate, and aggregation of the protein was induced by heating the protein at 45 °C in PBS and in the presence of wild-type αA-crystallin and mutant chaperone proteins. After 30 min, an aliquot of the sample was passed through a TSK gel G5000PWXL column connected to MALS-DLS detectors, and the complex peak was analyzed as described earlier. Incubation mixtures of ADH and chaperone proteins at 0 min served as control.
2.11. Cell Culture Studies
Retinal pigment epithelial cell line (ARPE-19), obtained from ATCC, and human primary lens epithelial (HLE) cells, collected from a 16-year-old donor eye, were cultured in DMEM supplemented with 5% FBS, 100 units of penicillin and 100 µg/ml streptomycin and incubated at 37 °C with 5% CO2 atmosphere. Experiments were conducted in 96-well plates. Cells were seeded at a density of 30,000 cells/well and allowed to grow for 24 h. Cells were then exposed to 0–6 µg/ml wild-type and mutant αA-crystallins for 4 h in serum-free media. The transduced cells were then treated with and without 150 µM H2O2 for 16 h. The anti-apoptotic activity of wild-type and mutant αA-crystallins was determined using Premix WST-1 Cell Proliferation Assay System from Clonetech. The assay was performed according to the instructions provided in the assay kit. Formazan dye formed by metabolically active cells was quantitated by measuring the absorbance at 440 nm on a SpectraMax i3 (Molecular Devices) plate reader.
3. Results
3.1. Deletion of Residues αA70–76 and αA70–88 from αA-Crystallin
The residues deleted in this study are identified in Figure 1A. Deletion of residues 70–76 and 70–88 in αA-crystallin was confirmed by plasmid DNA sequencing (Figure 1B) and by SDS-PAGE analysis (Figure 1C). Deletion of residues from the 70–88 region affected the solubility of the expressed proteins, which formed inclusion bodies. Isolation of protein from inclusion bodies using 6 M urea and purification through ion-exchange column Q-Sepharose resulted in proteins with a purity of over 95%.
FIGURE 1. Preparation of chaperone-site deletion mutants.
A, Amino acid sequence of human αA-crystallin showing the α-crystallin domain (blue shaded) flanked by the N-terminal region (red shaded) and a C-terminal tail (yellow shaded). The residues of the chaperone site that were deleted to generate α AΔ70–76 and αAΔ70–88 mutants are highlighted. B, Plasmid DNA sequencing confirming the deletion of nucleotides that code for the amino acids of the chaperone-site region. C, SDS-PAGE analysis of the proteins. Lane 1, molecular mass marker; lane 2, water-soluble extract of cells expressing αAΔ70–88; lane 3, urea-solubilized cell pellet from cells expressing α AΔ70–76; lane 4, purified αAΔ70–88; lane 5, purified αAΔ70–76; lane 6, purified wild-type protein. The bands above the crystallin bands in lanes 4, 5 and 6 are contaminants (<5%).
3.2. Structural Characterization of Wild-Type αA-Crystallin and Deletion Mutants
The structural features of wild-type αA-crystallin and αAΔ70–76 and αAΔ70–88 mutant proteins were compared by looking at their secondary and tertiary structures, surface hydrophobicity and tryptophan fluorescence (Figure 2). Secondary structure, measured using far-UV CD spectroscopy, showed a characteristic β-sheet structure with minima at 217 nm for wild-type αA-crystallin [24]. The α-crystallin domain in αA-crystallin is composed of eight β strands (β2 through β9), with β3 and β4 situated in the chaperone-site region [6]. Deletion of residues 70–76 resulted in the loss of β3 strand, and deletion of residues 70–88 removed both β3 and β4 strands, which is reflected by a shift in the negative band from 217 nm to 212 nm in the far-UV CD analysis (Figure 2A). The spectrum of αAΔ70–88 showed two negative bands, a feature normally seen in proteins with increased α-helical content. The secondary structural contents, estimated from the far-UV CD data, using the method described by Sreerama and Woody, [25, 26] confirm an increase in α-helix and a decrease in β-sheet with αAΔ70–76 and αAΔ70–88 as compared to wild-type protein (Table 1). Both deletion mutants showed an overall decrease in intensity of near-UV CD spectra (Figure 2B). Wild-type αA-crystallin has 5 different minima and 5 different maxima, representing the microenvironments of the aromatic amino acid residues [27, 28]. Signals in the 250–270 nm range represent phenylalanine microenvironments. The region between 270 nm and 300 nm arises from tyrosine and tryptophan. The near-UV CD spectra for both deletion mutants showed loss of signal for the region between 270 nm and 290 nm, indicating a change in the microenvironment of tryptophan and/or tyrosine residues.
FIGURE 2. Spectroscopic characterization of wild-type and αA-crystallin deletion mutants.
A, Far-UV CD spectra. Spectra were recorded at a protein concentration of 0.2 mg/ml, taken in 1-mm path length cuvette. B, Near-UV CD spectra. Spectra were collected using protein solutions (2mg/ml), taken in 10 mm path length cuvette. The spectra shown are the average of five scans C, Tryptophan fluorescence of wild-type, αAΔ70–76 and αAΔ70–88 proteins. Intrinsic tryptophan fluorescence spectra were recorded between 310 nm to 400 nm by using an excitation wavelength of 295 nm. The spectra reported are the average of five different scans. D, Bis-ANS binding fluorescence spectra of wild-type and deletion mutant αA-crystallins.
Fluorescence emission spectra of 10 µM bis-ANS-bound protein solutions were recorded from 420 nm to 600 nm, with an excitation wavelength of 390 nm. The spectra reported are the average of five different scans.
Table 1.
Properties of wild-type and deletion mutants of αA-crystallin
| Protein | Monomeric mass (theoretical) (kDa) |
Quaternary structural properties a | Secondary structural elements b | |||||
|---|---|---|---|---|---|---|---|---|
| Average oligomeric mass (Mw) (kDa) |
Hydrodynamic radius (Rh) (nm) |
Polydispersity index (PDI) |
α-helix (%) |
β-sheet (%) |
β-turn (%) |
Unordered (%) |
||
| Wild-type αA | 19.8 | 585 (± 0.4%) | 8.5 (± 0.4%) | 1.026 (0.652%) | 3.9 | 38.8 | 24.2 | 33.1 |
| αAΔ70–76 | 18.9 | 1464 (± 0.8%) | 13.7 (± 1.4%) | 1.091 (1.07%) | 4.1 | 37.3 | 23.0 | 35.6 |
| αAΔ70–88 | 17.6 | 2496 (± 0.7%) | 16.6 (± 0.9%) | 1.128 (0.55%) | 5.1 | 36.2 | 23.5 | 35.2 |
Determined from multi-angle light scattering and quasi-elastic light scattering analysis using Astra software (Wyatt Technologies). Mw and Rh values are determined at peak apex. The value in parenthesis is the percent of deviation.
Estimated from CD measurements using CDPRO software. All three programs (SELCON3, CDSSTR and CONTINLL) included in the package were used with IBasis 4 reference set for structural prediction. The values obtained from the three analyses were compared for accuracy and averaged.
We recorded the tryptophan fluorescence spectra of wild-type αA-crystallin and deletion mutants to assess the changes in the microenvironment of the lone tryptophan residue in the N-terminal region of the proteins. A 20–30% increase in tryptophan fluorescence occurred for αAΔ70–76 and αAΔ70–88 mutant proteins as compared to the wild-type protein (Figure 2C). We also investigated the structural differences among the proteins by analyzing bis-ANS binding. Bis-ANS is an environment-sensitive fluorescent probe that binds to hydrophobic sites of proteins. Such interactions enhance the quantum yield of the probe, enabling analysis of relative hydrophobicity and conformational changes of the proteins [29]. There was a 2-fold increase in bis-ANS binding for αAΔ70–76 and αAΔ70–88 mutants when compared to wild-type protein (Figure 2D).
3.3. Increased Oligomeric Mass after Deletion of 70–76 and 70–88 Residues from αA-Crystallin
We examined the morphology of wild-type αA-crystallin, and αAΔ70–76 and αAΔ70–88 deletion mutants under TEM (Figure 3A). The oligomers in wild-type αA-crystallin were spherical and distinct, with a diameter of 8 – 9 nm. In the case of αAΔ70–76, besides single oligomers, aggregates of two to three oligomers joined together was seen. The αAΔ70–88 mutant showed a more heterogenous oligomeric assembly, with many three to four oligomers attached together, with a particle size in the range of 20–30 nm in addition to few single oligomers. The increased oligomeric mass of the deletion mutants was also confirmed by early elution of the mutants in size-exclusion column (Figure 3B). MALS analysis showed that the average molar mass (Mw) of wild-type protein was 585 (kDa). The average Mw increased by 2.5-fold and 4.2-fold, respectively, in αAΔ70–76 and αAΔ70–88 mutants (Table 1). Concomitant with the increase in molar mass, the deletion mutants also showed an increase in hydrodynamic radius (Rh) and polydispersity when compared to wild-type protein (Table 1).
FIGURE 3. Deletion of residues of the chaperone-site region increased the oligomerization of αA-crystallin.
A, TEM images of wild-type, αAΔ70–76 and αAΔ70–88 proteins. A drop of 0.01 mg/ml protein was negatively stained with 2% uranyl acetate and the images were captured in JEOL 1400 at 30000 magnifications. Scale bar is 100 nm for all the images. The experiments were repeated five times and the images shown are representative of one such observation. The arrow points to an aggregate of wild-type αA-crystallin or deletion mutant. B, Multi-angle light scattering analysis of wild-type, αAΔ70–76 and αAΔ70–88 proteins. Purified protein samples were passed through TSK G5000PWXL size exclusion column connected to a MALS-DLS instrument and the data were analyzed as described under methods. The analysis was done multiple times and the results were consistent. The molar mass (Mw) distribution across the refractive index (RI) peak is shown. The average oligomeric mass calculated from the analysis is given in Table 1.
3.4. Deletion of Residues 70–76 and 70–88 Affects the Oligomeric Stability of αA-Crystallin
The stability of the deletion mutants was measured by equilibrium urea denaturation experiments [23]. Tryptophan fluorescence spectra at different urea concentrations were recorded. The unfolding data were plotted as the ratio of fluorescence intensities at 337 nm and 350 nm against increasing urea concentrations (Figure 4). An estimation of the transition midpoint (C1/2) value for 50% unfolding of the protein was done with sigmoidal fitting of the curve. Wild-type αA-crystallin showed a C1/2 of 3.1 M urea. With αAΔ70–76 and αAΔ70–88, the C1/2 values for the protein unfolding by urea were 1.91 M and 1.78 M, respectively, indicating the destabilized structure of deletion mutants.
FIGURE 4. Effect of deletion of 70–88 amino acids on the stability of αA-crystallin by equilibrium urea denaturation study.
Equilibrium urea unfolding profiles of wild-type αA-crystallin and αAΔ70–76 and αAΔ70–88 deletion mutants were obtained by plotting the ratio of tryptophan fluorescence emission at 337 nm and 350 nm with the excitation wavelength of 295 nm, at different urea concentrations at 25 °C. Protein solutions (0.1 mg/ml) were incubated with increasing concentrations of urea for recording the tryptophan fluorescence spectra. The data points are the average of three experiments.
3.5. Deletion of 70–76 and 70–88 Residues Leads to Loss of αA-Crystallin Chaperone Function
The chaperone-like activity of wild-type and deletion mutants was compared at and above physiological temperatures using different substrates. The percentage of substrate protein aggregation in the presence of various amounts of chaperone proteins at the 45 min assay point is shown in Figure 5. Wild-type protein showed a concentration-dependent protection of substrate proteins. Deletion of residues 70–76 decreased the chaperone activity by 50%, whereas deletion of residues 70–88 resulted in complete loss of αA-crystallin chaperone-like activity when ADH was used as the substrate. Higher concentrations of αAΔ70–88 were unable to protect against ADH aggregation at 37 °C. With CS, the αAΔ70–76 mutant was 40–50% less effective in suppressing aggregation, and the αAΔ70–88 mutant completely failed to prevent CS aggregation when used at a ratio similar to wild-type. However, when used at 5-fold molar excess, αAΔ70–88 showed 20% protection against CS aggregation. Both mutants, at all concentrations, completely lost their ability to suppress the aggregation of βL-crystallin (Figure 5). The mutants by themselves scattered light when incubated at 53 °C (data not shown), suggesting that heat stability of the protein is affected by the deletion of chaperone site residues.
FIGURE 5. Comparison of anti-aggregation activities of wild-type αA-crystallin, αAΔ70–76 and αAΔ70–88 using ADH, βL-crystallin and CS as substrates.
Aggregation of ADH (0.25 mg/ml) was induced at 37 °C by phosphate buffer containing 100 mM EDTA (pH 7.3). Aggregation assays with βL-crystallin (0.25 mg/ml PBS) were performed at 55 °C, and aggregation assays of the CS sample (0.075 mg/ml 40 mM HEPES-NaOH buffer, pH 7.3) were performed at 43 °C. The percentage of substrate proteins aggregated in the presence of various concentrations of wild-type and mutant proteins at the 45 min time point of assay was calculated and plotted. The aggregation of substrate protein in the absence of chaperone protein was considered 100% aggregation.
3.6. Inability of ADH to interact with αAΔ70–88 Mutant Protein during Chaperone Assay at 37 °C
To find out whether the loss of chaperone function in αAΔ70–88 is due to its inability to bind ADH, we induced the aggregation of Alexa 568–labeled ADH in the presence of chaperone proteins at 37 °C and looked for the complex formation by passing the incubation mixtures through a TSK gel 4000PWXL column. Incubation of Alexa 568-labeled ADH with wild-type αA-crystallin served as control. As a second control, we set up incubations of ADH568 with αAR116C mutant, which was prepared for another study. The αAR116C mutant has been shown by others to be 40% less effective, when compared to wild-type protein, in protecting the aggregation of ADH [30].
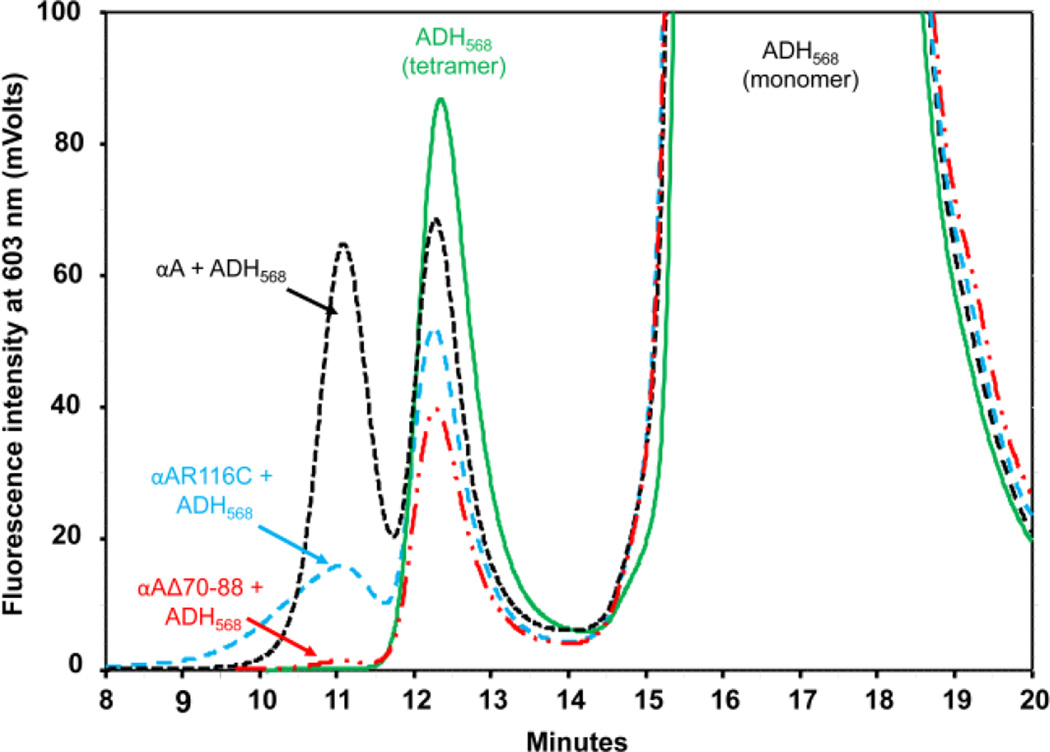
Using an incubation time to 30 min and an excess amount of chaperone protein, we were able to capture the complexes of chaperone proteins with early aggregating species of destabilized ADH and prevent any precipitates that could form if the chaperone protein fails to function. The fluorescence intensity of labeled ADH after fractionation of the samples through gel filtration column is shown in Figure 6. In the presence of EDTA, ADH elutes as two peaks: an oligomeric peak, with an elution time of 12–13 min, and a broad monomeric peak that elutes after 15 min. The wild-type and mutant αA-crystallins elute between 9 min and 12 min. If ADH is interacting with the chaperone proteins, we should see a fluorescent peak in 9–11 min. As we expected, a strong fluorescent peak elutes at 11 min with wild-type αA-protein, suggesting the binding of wild-type αA-crystallin with ADH. A fluorescent peak was also seen at 11 min in a sample containing αAR116C. However, the fluorescent peak was much smaller than that with wild-type αA-crystallin, suggesting less binding of ADH to αAR116C, which correlates with its chaperone function. Incubations of αAΔ70–88 did not show any peak eluting in the complex region, suggesting that ADH does not interact with the deletion mutant.
FIGURE 6. ADH is unable to interact with αAΔ70–88 during chaperone assay at 37 °C.
Interaction of Alexa568-labeled ADH with wild-type α-crystallin and αAR116C and αAΔ70–88 mutants was tested by inducing the aggregation of labeled ADH by 100 mM EDTA in the presence of chaperone proteins. After 30 min, the samples were passed through TSK gel G4000PWXL column and the elution of labeled ADH was monitored. The green line shows the elution of unincubated labeled ADH. The peak eluting between 9 min and 11.5 min is the complex peak for labeled ADH and chaperone protein incubation. The complex peak eluting region showed no appreciable fluorescence with αAΔ70–88 (red line). The black line is the fluorescent profile of wild-type-ADH complex and the blue line is the profile for αAR116C–ADH incubations.
3.7. Exposure of Minor Cryptic Chaperone Sites in αAΔ70–88 at Temperatures above Physiological Levels
We tested the interaction of wild-type αA-crystallin and of deletion mutants with unlabeled ADH to see if any additional cryptic chaperone sites in αA-crystallin get exposed at 45°C. The incubation mixtures of ADH and chaperone proteins were passed through a gel filtration column and the peak eluting between 7 min and 11 min that corresponds to complex/chaperone peak was analyzed by MALS-DLS at the start (0 min) and end (30 min) of incubation (Table 2). The elution profiles of the incubation mixtures and the molar mass distribution across the chaperone/complex peak and ADH peak (11.6 min) are shown in Figure 7. The complex/chaperone peak in αAΔ70–76 incubations shifted from 10 min at the beginning of the incubation to 9.5 min at the end of incubation. With αAΔ70–88, the complex/chaperone peak shifted from 9.5 min at the beginning of incubation to 8.8 min at the end of incubation. In contrast, the complex/chaperone peak for incubations with wild-type protein shifted only very slightly (10.5 to 10.4 min). The reduction in the elution time point of the deletion mutants during incubation with ADH cannot be due to the aggregation of the mutants per se, because we found an increase in the elution time point with a small decrease in molar mass when deletion mutants were incubated at 45 °C (data not shown). The complex peak area of deletion mutants significantly increased at the end of incubation, suggesting an interaction of ADH with deletion mutants at 45 °C. The wild-type protein peak showed only a small increase in molar mass on binding to ADH (Table 2). The mass of the αAΔ70–76 mutant peak increased 2-fold and the mass of αAΔ70–88 mutant increased about 3-fold by the end of incubation. Concomitant to the increase in molar mass, the hydrodynamic radius of the complex peak also increased by the end of incubation (Table 2).
Table 2.
Average molar mass (Mw) and hydrodynamic radius (Rh) of complex and ADH peaks at the start and end of incubation at 45 °C.
| Sample | Complex peak | ADH peak | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 min of incubation | 30 min of incubation |
0 min of incubation | 30 min of incubation |
|||||
| Mw (kDa) |
Rh (nm) |
Mw (kDa) |
Rh (nm) |
Mw (kDa) |
Rh (nm) |
Mw (kDa) |
Rh (nm) |
|
| ADH + αA | 607 (± 0.5%) |
8.4 (± 0.4%) |
726 (± 0.2%) |
9.7 (± 0.4%) |
217 (± 0.3%) |
5.64 (± 0.9%) |
261 (± 0.2%) |
6.11 (± 0.8%) |
| ADH + αAΔ70–76 | 1590 (± 0.8%) |
13.4 (± 0.4%) |
3130 (± 0.8%) |
17.4 (± 0.6%) |
258 (± 0.4%) |
6.99 (± 0.9%) |
366 (± 0.6%) |
8.56 (± 0.8%) |
| ADH + αAΔ70–88 | 2743 (± 0.4%) |
16.9 (± 0.3%) |
8419 (± 0.6%) |
25.6 (± 0.3%) |
256 (± 0.4%) |
6.89 (± 1.0%) |
692 (± 0.8%) |
9.08 (± 2.5%) |
Mw and Rh values are determined at peak apex. The value in parenthesis is the percent of deviation.
FIGURE 7. MALS-DLS analysis of complex formation by ADH with wild-type α-crystallin and deletion mutants at 45 °C.
ADH was incubated with chaperone proteins in PBS for 30 min at 45 °C. The samples were passed through TSK gel G5000PWXL column connected to a MALS-DLS detector. The molar mass (Mw) and size (Rh) of the complex and/or the chaperone protein peak eluting between 6 min and 11 min, at the beginning and at the end of incubation, were determined using Astra software and is given in Table 2. ADH elutes as a single oligomeric peak at 11 min in the absence of EDTA.
We also examined the change in molar mass and hydrodynamic radius of the ADH peak during incubation. With wild-type αA-crystallin, the oligomeric mass of ADH changed slightly (1.2-fold) at the end of incubation (Table 2). With αAΔ70–76, the molar mass of ADH peak increased 1.4-fold; with αAΔ70–88, the increase was 2.7-fold. The significantly increased oligomeric mass of the ADH peak in incubations with deletion mutants, especially αAΔ70–88, suggests that the mutants are not able to efficiently interact with ADH. Some of the ADH aggregates might have co-eluted with the complex peak and contributed to the increased peak area seen with deletion mutants (Figure 7). SDS-PAGE analysis showed an increased presence of ADH (data not shown) at the end of incubation in the fractions of the complex peak with deletion mutants. We were not able to discern if increased ADH in these samples came from self-aggregation or from complex formation with the deletion mutant. The shift in the trailing edge of the deletion mutant peak suggests that at least a portion of the ADH in the complex peak fractions is due to complex formation with deletion mutants.
3.8. Deletion of 70–88 Residues Abolished the Anti-Apoptotic Activity in αA-Crystallin
We studied the effect of chaperone site deletion on the anti-apoptotic function of αA-crystallin using ARPE-19 and HLE cells. Cytotoxicity was induced in these cells using 150 µM H2O2. The cells were treated with or without wild-type αA-crystallin and mutants for 4 h prior to the addition of H2O2 for 16 h. The percentage of dead cells was evaluated with the WST-1 (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate) cytotoxicity assay. In the absence of chaperone proteins, H2O2 caused apoptosis in about 12% of HLE cells and 30% of ARPE-19 cells (Figure 8). Wild-type protein protected both HLE and ARPE-19 cells from oxidative stress in a dose-dependent manner. Significant protection of HLE cells was seen with 2 µg wild-type protein, whereas 4 µg of wild-type protein was required to protect ARPE-19 cells. Both αAΔ70–76 and αAΔ70–88 mutants were unable to protect the cells from H2O2-mediated stress at all concentrations. Instead, increased apoptosis in both HLE and ARPE-18 cells occurred as compared to cells treated with H2O2 alone (positive control). The increased apoptosis observed with deletion mutants was not dose-dependent. Interestingly, the HLE and ARPE-19 cells treated with deletion mutants in the absence of H2O2 did not show a significant increase in apoptosis when compared to untreated cells (negative control) (data not shown). The data suggest that deletion of 70–88 residues in αA-crystallin not only abolishes the anti-apoptotic activity of the protein but also potentiates the apoptosis induced by H2O2.
FIGURE 8. Protection of HLE and ARPE-19 cells from oxidative stress-induced apoptosis.
Serum-starved cells were pre-incubated separately with 2 – 6 µg/ml of wild-type, αAΔ70–76 and αAΔ70–88 chaperone proteins for 4 h and then treated with 150 µM hydrogen peroxide for 16 h. Cytotoxicity was measured by WST-1 assay kit. The percentage of apoptotic cells in the presence of different concentrations of chaperone protein is plotted. The values are the average of 4–6 wells. * indicates the values are significantly different (P< 0.005) when compared to H2O2-untreated (negative control) sample. H2O2-treated cells without the addition of chaperone protein served as positive control.
4. Discussion
Published reports on α-crystallin chaperone function indicate that multiple factors contribute to the chaperone-like activity in α-crystallin. For α-crystallin to function as chaperone, three main elements are essential: a recognition element that recognizes the substrates that are unfolding; a binding element that interacts with the unfolded substrates, and a solubilizing element that helps to keep the complexes in solution (Figure 9). Modifications affecting any one of these elements will alter the chaperone efficiency of the protein. In α-crystallin the components required for effective chaperoning are distributed throughout the molecule. The N-and C-termini of α-crystallin are important for the recognition, selection, and solubility of unfolding substrate proteins [31]. The conserved α-crystallin domain is mainly involved in substrate binding [32]. We previously identified residues 70–88 of αA-crystallin as a potential substrate binding site and demonstrated that a synthetic peptide having the substrate binding site sequence residues (KFVIFLDVKHFSPEDLTVK) functions like a molecular chaperone [16]. In this study we show that the deletion of the residues of the chaperone site completely abolish the chaperone-like activity of αA-crystallin confirming that residues 70–88 as the major substrate binding site. Studies of hydrophobic probe binding and chemical cross-linking indicate that the β3-β5 region of the α-crystallin domain participates in substrate binding [14, 15, 33, 34].
FIGURE 9. Schematic diagram showing the components essential for the function of a chaperone.
Multiple factors govern the chaperone-like activity of α-crystallin. The determinants of chaperone-like activity in α-crystallin are distributed throughout the molecule. Residues 70–88 in αA-crystallin seem to act as the major substrate-binding site (determined in this study) that contributes to the bulk of the chaperone activity at physiological temperatures. Other cryptic substrate binding sites get exposed at elevated temperatures.
The deletion of 70–76 residues (i.e., the β3 strand) disturbed the structural organization of α-crystallin as reflected by CD and urea denaturation studies. Deletion of 70–88 (i.e., β3 and β4 strands) further altered the secondary and quaternary structures slightly. Our observations suggest that β3 and β4 strands have a role in the structural stability of the αA-crystallin. The His79 in β4 strand of αA-crystallin can bind to metal ions, which stabilizes the structure of protein [35, 36]. Although His79 is not removed in the αAΔ70–76 mutant, the deletion of 70–76 residues probably affects the binding of metal ions with His79. In a previous study, αAΔ70–76 showed during Cu2+-induced ascorbic acid oxidation assay about a 75% loss of ascorbic acid protection compared to wild-type αA-crystallin, suggesting that the αAΔ70–76 mutant was not able to effectively sequester Cu2+, which led to the oxidation of ascorbic acid [37]. It was also shown that chaperone activity of αA-crystallin increases significantly upon Cu2+ binding [38].
Both mutants showed a 2-fold increase in surface hydrophobicity. Increased surface hydrophobicity is generally associated with enhanced chaperone activity [39, 40]. Some studies with mutant α-crystallins have shown decreased chaperone function with higher hydrophobicity [21, 41]. Reddy et al [42] found no correlation between hydrophobicity and chaperone-like activity, which led them to conclude that hydrophobicity may be one of the factors influencing chaperone-like activity but may not be the predominant one. Our study reiterates that not all bis-ANS binding sites contribute to chaperone-like activity of the protein. Structural alterations seen with αAΔ70–88 do not warrant the complete loss of its chaperone activity, unless the region required for the binding of the substrate protein is lost. Chaperone activity has been reported with mutants having much more severe structural loss than the αAΔ70–88 mutant [43]. We found that both αAΔ70–76 and αAΔ70–88 exhibit similar structural characteristics but only αAΔ70–88 fails to interact with the substrate. Therefore, it can be concluded that the inability of αAΔ70–88 to bind to ADH at 37 °C is not due to the altered structure of the chaperone protein but is because of the loss in the region required for substrate binding. We demonstrate this by showing that labeled ADH is unable to interact with αAΔ70–88 at the same time it was able to interact with αAR116C mutant, a protein showing similar structural changes.
Our study demonstrates that the 70–88 amino acid region of αA-crystallin is crucial for the structural stability of the protein as well as for its function as a chaperone. Previously we showed that a F71G mutant of rat αA-crystallin is totally inactive in suppressing the aggregation of substrate proteins and suggested that Phe71 in αA-crystallin is essential for chaperone function [21]. Surprisingly, deletion of 70–76 residues, which included the critical Phe71 residue, resulted in only a 50% loss in chaperone activity with ADH. The F71G mutant, however, was stable at 55 °C and suppressed the aggregation of βL-crystallin, while the αAΔ70–76 mutant was unstable and unable to protect βL-crystallin from aggregation. The loss of chaperone function in the F71G mutant with ADH is attributed to complex instability. F71G mutation might have led to improper binding of ADH, which resulted in the precipitation of complex. The loss of chaperone function seen with the deletion mutants is not because of the simple deletion of multiple residues. It has been shown that deletion of multiple residues from the N- or C-termini of α-crystallin does not prevent the interaction of the chaperone with substrate proteins [44]. Some reports [10, 33, 34] suggest that there are both high- and low-affinity substrate binding sites on small heat shock proteins. We observed that low-binding cryptic sites in αAΔ70–88 are exposed above physiological temperatures. As shown in Figure 7, both the deletion mutants exhibit increased binding of ADH at 45 °C as compared to wild-type protein. Only a 1.2-fold increase in the molar mass of α-crystallin was observed upon complex formation with ADH, and the mass amount of the wild-type peak had increased by 6% at the end of incubation. Previously, with αB-crystallin, we observed that the wild-type protein is very much resistant to increased molar mass upon complex formation with ADH. During chaperone action, the subunits in α-crystallin oligomers probably rearrange to resist the large increase in the molar mass which we think is essential for increased chaperone efficiency. The deletion mutants showed a 2- to 3-fold increase in the molar mass of the complex peak. The mass amount of the αAΔ70–76 and αAΔ70–88 peaks increased by 20% and 65%, respectively. A large part of the increased mass is probably due to the aggregation of ADH by itself, which co-elutes with the complex peak. The reduction in the ADH binding interfaces, due to the deletion of 70–88 residues in αA-crystallin, would lead to the aggregation of ADH. The progressive increase in the molar mass of the ADH peak (Table 2) suggests that there is a decrease in the binding interfaces with the mutants. We were not able to estimate the mass contributed by the complex in the first peak. The shift in the trailing edge of the complex peak with deletion mutants clearly suggests their interaction with ADH at 45 °C. Our data confirm that residues 70–88 constitute a major substrate binding site and there are minor cryptic substrate binding sites in αA-crystallin that get exposed above physiological temperatures.
The significance of the 70–88 region in αA-crystallin function is clearly indicated in our cytotoxicity studies, as both of the deletion mutants showed increased apoptosis when oxidative stress was applied to HLE and ARPE-19 cells. These findings strengthen our hypothesis that residues 70–88 serve as the major binding site of αA-crystallin. The anti-apoptotic function of αA-crystallin is directly related to its chaperone activity [45]. In a previous study, the anti-apoptotic activity of residues 70–88 was also demonstrated using mini-chaperones [46]. α-Crystallins have also been shown to interact with members of the Bcl-2 family that regulate apoptotic pathways [47]. Further study is needed to identify the role of 70–88 residues in the anti-apoptotic properties of αA-crystallin.
5. Conclusions
We conclude that residues 70–88 of the αA-crystallin chaperone region are essential for providing structural stability and interacting with substrates during chaperone function. The residues of this region contribute to the bulk of chaperone-like activity and can be considered the major substrate binding site in αA-crystallin. Other cryptic binding sites that are exposed above physiological temperatures contribute partly to the chaperone activity.
Highlights.
Residues 70–88 of the α-crystallin are needed for providing structural stability.
αAΔ70–88 mutant showed complete loss of chaperone-like activity.
Residues 70–88 in αA-crystallin is the primary substrate binding site.
Cryptic substrate binding sites are exposed at higher temperatures.
Acknowledgments
The authors thank Sharon Morey for her help in the preparation of the manuscript. This work was supported by EY023219 and EY011981 grants from NEI to KKS.
ABBREVIATIONS
- ADH
alcohol dehydrogenase
- ARPE-19
Retinal pigment epithelial cell line
- CD
Circular Dichroism
- CS
citrate synthase
- DLS
dynamic light scattering
- DMEM
Dulbecco’s Modified Eagle Medium
- EDTA
Ethylenediaminetetraacetic acid
- FBS
fetal bovine serum
- HLE
human lens epithelial
- HPLC
high-pressure liquid chromatography
- Mw
Molar mass
- Rh
hydrodynamic radius
- RI
refractive index
- MALS
multi-angle light scattering
- TEM
Transmission Electron Microscopy
- UV
ultraviolet
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bloemendal H. The vertebrate eye lens. Science. 1977;197:127–138. doi: 10.1126/science.877544. [DOI] [PubMed] [Google Scholar]
- 2.Ingolia TD, Craig EA. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc. Natl. Acad. Sci. U S A. 1982;79:2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. U S A. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horwitz J. Proctor Lecture. The function of alpha-crystallin. Invest. Ophthalmol. Vis. Sci. 1993;34:10–22. [PubMed] [Google Scholar]
- 5.Narberhaus F. Alpha-crystallin-type heat shock proteins: socializing minichaperones in the context of a multichaperone network. Microbiol. Mol. Biol. Rev. 2002;66:64–93. doi: 10.1128/MMBR.66.1.64-93.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim KK, Kim R, Kim SH. Crystal structure of a small heat-shock protein. Nature. 1998;394:595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- 7.van Montfort RL, Basha E, Friedrich KL, Slingsby C, Vierling E. Crystal structure and assembly of a eukaryotic small heat shock protein. Nat. Struct. Biol. 2001;8:1025–1030. doi: 10.1038/nsb722. [DOI] [PubMed] [Google Scholar]
- 8.Das KP, Petrash JM, Surewicz WK. Conformational properties of substrate proteins bound to a molecular chaperone alpha-crystallin. J. Biol. Chem. 1996;271:10449–10452. doi: 10.1074/jbc.271.18.10449. [DOI] [PubMed] [Google Scholar]
- 9.Das KP, Surewicz WK. On the substrate specificity of alpha-crystallin as a molecular chaperone. Biochem. J. 1995;311:367–370. doi: 10.1042/bj3110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaya N, Garcia V, Vierling E. Substrate binding site flexibility of the small heat shock protein molecular chaperones. Proc. Natl. Acad. Sci. U S A. 2009;106:15604–15609. doi: 10.1073/pnas.0902177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajaraman K, Raman B, Ramakrishna T, Rao CM. Interaction of human recombinant alphaA-and alphaB-crystallins with early and late unfolding intermediates of citrate synthase on its thermal denaturation. FEBS Lett. 2001;497:118–123. doi: 10.1016/s0014-5793(01)02451-6. [DOI] [PubMed] [Google Scholar]
- 12.Santhoshkumar P, Murugesan R, Sharma KK. Deletion of (54)FLRAPSWF(61) residues decreases the oligomeric size and enhances the chaperone function of alphaB-crystallin. Biochemistry. 2009;48:5066–5073. doi: 10.1021/bi900085v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santhoshkumar P, Sharma KK. Conserved F84 and P86 residues in alphaB-crystallin are essential to effectively prevent the aggregation of substrate proteins. Protein Sci. 2006;15:2488–2498. doi: 10.1110/ps.062338206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma KK, Kaur H, Kester K. Functional elements in molecular chaperone alpha-crystallin: identification of binding sites in alpha B-crystallin. Biochem. Biophys. Res. Commun. 1997;239:217–222. doi: 10.1006/bbrc.1997.7460. [DOI] [PubMed] [Google Scholar]
- 15.Sharma KK, Kumar GS, Murphy AS, Kester K. Identification of 1,1’-bi(4-anilino)naphthalene-5,5’-disulfonic acid binding sequences in alpha-crystallin. J. Biol. Chem. 1998;273:15474–15478. doi: 10.1074/jbc.273.25.15474. [DOI] [PubMed] [Google Scholar]
- 16.Sharma KK, Kumar RS, Kumar GS, Quinn PT. Synthesis and characterization of a peptide identified as a functional element in alphaA-crystallin. J. Biol. Chem. 2000;275:3767–3771. doi: 10.1074/jbc.275.6.3767. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh JG, Estrada MR, Houck SA, Clark JI. The function of the beta3 interactive domain in the small heat shock protein and molecular chaperone, human alphaB crystallin. Cell Stress Chaperones. 2006;11:187–197. doi: 10.1379/CSC-186.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhagyalaxmi SG, Srinivas P, Barton KA, Kumar KR, Vidyavathi M, Petrash JM, Bhanuprakash Reddy G, Padma T. A novel mutation (F71L) in alphaA-crystallin with defective chaperone-like function associated with age-related cataract. Biochim. Biophys. Acta. 2009;1792:974–981. doi: 10.1016/j.bbadis.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Validandi V, Reddy VS, Srinivas PN, Mueller NH, Bhagyalaxmi SG, Padma T, Petrash JM, Reddy GB. Temperature-dependent structural and functional properties of a mutant (F71L) alphaA-crystallin: molecular basis for early onset of age-related cataract. FEBS Lett. 2011;585:3884–3889. doi: 10.1016/j.febslet.2011.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindner RA, Kapur A, Carver JA. The interaction of the molecular chaperone, alpha-crystallin, with molten globule states of bovine alpha-lactalbumin. J. Biol. Chem. 1997;272:27722–27729. doi: 10.1074/jbc.272.44.27722. [DOI] [PubMed] [Google Scholar]
- 21.Santhoshkumar P, Sharma KK. Phe71 is essential for chaperone-like function in alpha A-crystallin. J. Biol. Chem. 2001;276:47094–47099. doi: 10.1074/jbc.M107737200. [DOI] [PubMed] [Google Scholar]
- 22.Singh D, Raman B, Ramakrishna T, Rao CM. Mixed oligomer formation between human αA-crystallin and its cataract-causing G98R mutant: structural, stability and functional differences. Journal of Molecular Biology. 2007;373:1293–1304. doi: 10.1016/j.jmb.2007.08.062. [DOI] [PubMed] [Google Scholar]
- 23.Nagaraj RH, Nahomi RB, Shanthakumar S, Linetsky M, Padmanabha S, Pasupuleti N, Wang B, Santhoshkumar P, Panda AK, Biswas A. Acetylation of alphaA-crystallin in the human lens: effects on structure and chaperone function. Biochim. Biophys. Acta. 2012;1822:120–129. doi: 10.1016/j.bbadis.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surewicz WK, Olesen PR. On the thermal stability of alpha-crystallin: a new insight from infrared spectroscopy. Biochemistry. 1995;34:9655–9660. doi: 10.1021/bi00030a001. [DOI] [PubMed] [Google Scholar]
- 25.Sreerama N, Woody RW. A self-consistent method for the analysis of protein secondary structure from circular dichroism. Anal. Biochem. 1993;209:32–44. doi: 10.1006/abio.1993.1079. [DOI] [PubMed] [Google Scholar]
- 26.Sreerama N, Woody RW. Estimation of protein secondary structure from circular dichroism spectra: comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal. Biochem. 2000;287:252–260. doi: 10.1006/abio.2000.4880. [DOI] [PubMed] [Google Scholar]
- 27.Biswas A, Goshe J, Miller A, Santhoshkumar P, Luckey C, Bhat MB, Nagaraj RH. Paradoxical effects of substitution and deletion mutation of Arg56 on the structure and chaperone function of human alphaB-crystallin. Biochemistry. 2007;46:1117–1127. doi: 10.1021/bi061323w. [DOI] [PubMed] [Google Scholar]
- 28.Sun TX, Das BK, Liang JJ. Conformational and functional differences between recombinant human lens alphaA- and alphaB-crystallin. J. Biol. Chem. 1997;272:6220–6225. doi: 10.1074/jbc.272.10.6220. [DOI] [PubMed] [Google Scholar]
- 29.Sharma KK, Kaur H, Kumar GS, Kester K. Interaction of 1,1’-bi(4-anilino)naphthalene-5,5’-disulfonic acid with alpha-crystallin. J. Biol. Chem. 1998;273:8965–8970. doi: 10.1074/jbc.273.15.8965. [DOI] [PubMed] [Google Scholar]
- 30.Shroff NP, Cherian-Shaw M, Bera S, Abraham EC. Mutation of R116C results in highly oligomerized alpha A-crystallin with modified structure and defective chaperone-like function. Biochemistry. 2000;39:1420–1426. doi: 10.1021/bi991656b. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh JG, Shenoy AK, Jr, Clark JI. N- and C-Terminal motifs in human alphaB crystallin play an important role in the recognition, selection, and solubilization of substrates. Biochemistry. 2006;45:13847–13854. doi: 10.1021/bi061471m. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh JG, Estrada MR, Clark JI. Structure-based analysis of the beta8 interactive sequence of human alphaB crystallin. Biochemistry. 2006;45:9878–9886. doi: 10.1021/bi060970k. [DOI] [PubMed] [Google Scholar]
- 33.Ahrman E, Lambert W, Aquilina JA, Robinson CV, Emanuelsson CS. Chemical cross-linking of the chloroplast localized small heat-shock protein, Hsp21, and the model substrate citrate synthase. Protein Sci. 2007;16:1464–1478. doi: 10.1110/ps.072831607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee GJ, Roseman AM, Saibil HR, Vierling E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997;16:659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karmakar S, Das KP. Stabilization of oligomeric structure of alpha-crystallin by Zn+(2) through intersubunit bridging. Biopolymers. 2011;95:105–116. doi: 10.1002/bip.21540. [DOI] [PubMed] [Google Scholar]
- 36.Karmakar S, Das KP. Identification of histidine residues involved in Zn(2+) binding to alphaA-and alphaB-crystallin by chemical modification and MALDI TOF mass spectrometry. Protein J. 2012;31:623–640. doi: 10.1007/s10930-012-9439-0. [DOI] [PubMed] [Google Scholar]
- 37.Raju M, Santhoshkumar P, Henzl TM, Sharma KK. Identification and characterization of a copper-binding site in alphaA-crystallin. Free Radic. Biol. Med. 2011;50:1429–1436. doi: 10.1016/j.freeradbiomed.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmad MF, Singh D, Taiyab A, Ramakrishna T, Raman B, Rao Ch M. Selective Cu2+ binding, redox silencing, and cytoprotective effects of the small heat shock proteins alphaA- and alphaB-crystallin. J. Mol. Biol. 2008;382:812–824. doi: 10.1016/j.jmb.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 39.Das BK, Liang JJ. Detection and characterization of alpha-crystallin intermediate with maximal chaperone-like activity. Biochem. Biophys. Res. Commun. 1997;236:370–374. doi: 10.1006/bbrc.1997.6950. [DOI] [PubMed] [Google Scholar]
- 40.Srinivas V, Raman B, Rao KS, Ramakrishna T, Rao Ch M. Structural perturbation and enhancement of the chaperone-like activity of alpha-crystallin by arginine hydrochloride. Protein Sci. 2003;12:1262–1270. doi: 10.1110/ps.0302003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kundu B, Shukla A, Chaba R, Guptasarma P. The excised heat-shock domain of alphaB crystallin is a folded, proteolytically susceptible trimer with significant surface hydrophobicity and a tendency to self-aggregate upon heating. Protein Expr. Purif. 2004;36:263–271. doi: 10.1016/j.pep.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Reddy GB, Kumar PA, Kumar MS. Chaperone-like activity and hydrophobicity of alpha-crystallin. IUBMB Life. 2006;58:632–641. doi: 10.1080/15216540601010096. [DOI] [PubMed] [Google Scholar]
- 43.Murugesan R, Santhoshkumar P, Sharma KK. Cataract-causing alphaAG98R mutant shows substrate-dependent chaperone activity. Mol. Vis. 2007;13:2301–2309. [PubMed] [Google Scholar]
- 44.Thampi P, Abraham EC. Influence of the C-terminal residues on oligomerization of alpha A-crystallin. Biochemistry. 2003;42:11857–11863. doi: 10.1021/bi030129w. [DOI] [PubMed] [Google Scholar]
- 45.Pasupuleti N, Matsuyama S, Voss O, Doseff AI, Song K, Danielpour D, Nagaraj RH. The anti-apoptotic function of human alphaA-crystallin is directly related to its chaperone activity. Cell Death Dis. 2010;1:e31. doi: 10.1038/cddis.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sreekumar PG, Chothe P, Sharma KK, Baid R, Kompella U, Spee C, Kannan N, Manh C, Ryan SJ, Ganapathy V, Kannan R, Hinton DR. Antiapoptotic properties of alpha-crystallin-derived peptide chaperones and characterization of their uptake transporters in human RPE cells. Invest. Ophthalmol. Vis. Sci. 2013;54:2787–2798. doi: 10.1167/iovs.12-11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mao YW, Liu JP, Xiang H, Li DW. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11:512–526. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]